Impact of an Antimicrobial Stewardship Strategy on Surgical Hospital Discharge: Improving Antibiotic Prescription in the Transition of Care
Abstract
1. Introduction
2. Materials and Methods
2.1. Design, Setting, and Study Periods
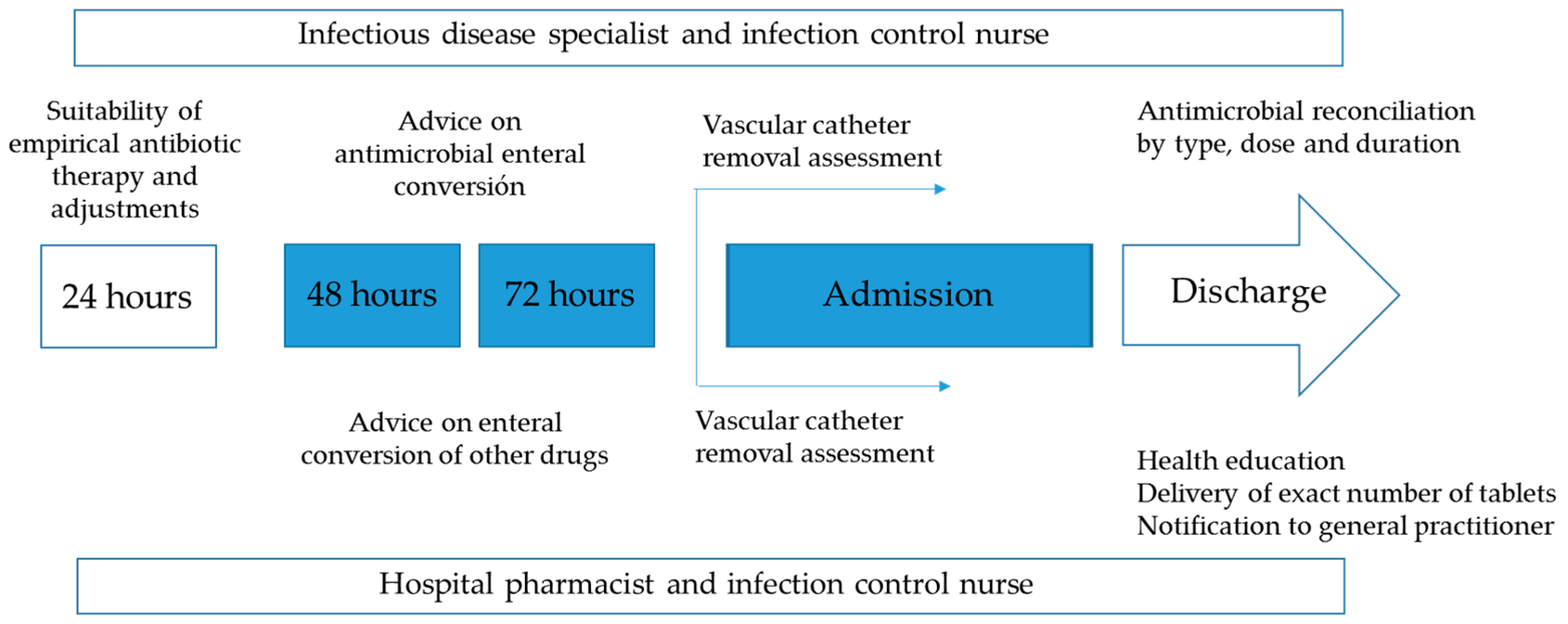
2.2. AMSP Actions
- -
- During hospitalization: 1. Developing and updating antibiotic treatment protocols of the most prevalent urinary infections, based on scientific evidence and local sensitivities, available on the mobile application (ProAPP Lleida) and intranet of the institution; 2. Sharing of AMSP actions with the urology department; 3. General and specific training of professionals; 4. Daily review of all positive microbiological results (blood cultures and any other samples), except weekends and holidays; 5. Daily written non-imposed advice for professionals in computerized SAP “Systems, Applications, Products in Data Processing” medical history, advice on-site or by telephone. The actions could take place in relation to any positive microbiological result and/or systemic antibiotic prescription made for admitted patients. 6. Promotion of enteral conversion at 48–72 h on possibilities and characteristics of the microorganism and oral bioavailability of the antibiotic, with clinical stability and respected digestive tract.
- -
- At the time of discharge: 1. Advice on the suitability of empirical or targeted oral antimicrobial therapy, dose, frequency, and duration; 2. Assess the absence of interactions, duplications, and allergy/intolerance; 3. Delivery of a recommended number of antibiotic tablets, submitting oral and written information to the patient; 4. Computerized notification of the reconciliation to the community primary care physician; 5. Periodic feedback of results to the team members. The flowchart is shown in Figure 1.
2.3. Measurement of Consumption and Economic Impact
2.4. Evaluation Methods and Sources of Information
2.5. Statistical Analysis
2.6. Ethical Declaration
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance. 2016. Available online: http://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 8 January 2023).
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- Bell, B.G.; Schellevis, F.; Stobberingh, E.; Goossens, H.; Pringle, M. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect. Dis. 2014, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Ya, K.Z.; Win, P.T.N.; Bielicki, J.; Lambiris, M.; Fink, G. Association between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2023, 6, e2253806. [Google Scholar] [CrossRef]
- Hammond, A.; Stuijfzand, B.; Avison, M.B.; Hay, A.D. Antimicrobial resistance associations with national primary care antibiotic stewardship policy: Primary care-based, multilevel analytic study. PLoS ONE 2020, 15, e0232903. [Google Scholar] [CrossRef] [PubMed]
- Gágyor, I.; Hay, A.D. Outcome selection in primary care antimicrobial stewardship research. J. Antimicrob. Chemother. 2021, 77, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Avent, M.L.; Cosgrove, S.E.; Price-Haywood, E.G.; van Driel, M.L. Antimicrobial stewardship in the primary care setting: From dream to reality? BMC Fam. Pract. 2020, 21, 134. [Google Scholar] [CrossRef] [PubMed]
- Barlam, T.F.; Cosgrove, S.E.; Abbo, L.M.; MacDougall, C.; Schuetz, A.N.; Septimus, E.J.; Srinivasan, A.; Dellit, T.H.; Falck-Ytter, Y.T.; Fishman, N.O.; et al. Implementing an Antibiotic Stewardship Program: Guidelines by the Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America. Clin. Infect. Dis. 2016, 62, e51–e77. [Google Scholar] [CrossRef]
- Rodríguez-Bano, J.; Pano-Pardo, J.R.; Alvarez-Rocha, L.; Asensio, Á.; Calbo, E.; Cercenado, E.; Cisneros, J.M.; Cobo, J.; Delgado, O.; Garnacho-Montero, J.; et al. Programs for optimizing the use of antibiotics (PROA) in Spanish hospitals: GEIH-SEIMC, SEFH and SEMPSPH consensus document. Enferm. Infecc. Microbiol. Clin. 2012, 30, 22.e1–22.e23. [Google Scholar] [CrossRef]
- Cercenado, E.; Rodríguez-Baño, J.; Alfonso, J.L.; Calbo, E.; Escosa, L.; Fernández-Polo, A.; García-Rodríguez, J.; Garnacho, J.; Gil-Navarro, M.V.; Grau, S.; et al. Antimicrobial stewardship in hospitals: Expert recommendation guidance document for activities in specific populations, syndromes and other aspects (PROA-2) from SEIMC, SEFH, SEMPSPGS, SEMICYUC and SEIP. Enferm. Infecc. Microbiol. Clin. 2023, 41, 238–242. [Google Scholar] [CrossRef]
- Tamma, P.D.; Avdic, E.; Keenan, J.F.; Zhao, Y.; Anand, G.; Cooper, J.; Dezube, R.; Hsu, S.; Cosgrove, S.E. What Is the More Effective Antibiotic StewardshipIntervention: Preprescription Authorization or Postprescription Review with Feedback? Clin. Infect. Dis. 2017, 64, 537–543. [Google Scholar]
- Dyer, A.P.; Ashley, E.D.; Anderson, D.J.; Sarubbi, C.; Wrenn, R.; Hicks, L.A.; Srinivasan, A.; Moehring, R.W. Total duration of antimicrobial therapy resulting from inpatient hospitalization. Infect. Control Hosp. Epidemiol. 2019, 40, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Hersh, A.L.; Spivak, E.S. Antibiotic Overuse and Stewardship at Hospital Discharge: The Reducing Overuse of Antibiotics at Discharge Home Framework. Clin. Infect. Dis. 2022, 74, 1696–1702. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, V.M.; Flanders, S.A.; Snyder, A.; Conlon, A.; Rogers, M.A.; Malani, A.N.; McLaughlin, E.; Bloemers, S.; Srinivasan, A.; Nagel, J.; et al. Excess antibiotic treatment duration and adverse events in patients hospitalized with pneumonia: A multihospital cohort study. Ann. Intern. Med. 2019, 171, 153–163. [Google Scholar] [CrossRef]
- Madaras-Kelly, K.J.; Burk, M.; Caplinger, C.; Bohan, J.G.; Neuhauser, M.M.; Goetz, M.B.; Zhang, R.; Cunningham, F.E.; Pneumonia Duration of Therapy Medication Utilization Evaluation Group. Total duration of antimicrobial therapy in veterans hospitalized with uncomplicated pneumonia: Results of a national medication utilization evaluation. J. Hosp. Med. 2016, 11, 832–839. [Google Scholar] [CrossRef]
- Suzuki, H.; Perencevich, E.N.; Alexander, B.; Beck, B.F.; Goto, M.; Lund, B.C.; Nair, R.; Livorsi, D.J. Inpatient Fluoroquinolone Stewardship Improves the Quantity and Quality of Fluoroquinolone Prescribing at Hospital Discharge: A Retrospective Analysis Among 122 Veterans Health Administration Hospitals. Clin. Infect. Dis. 2020, 71, 1232–1239. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Gandhi, T.; Conlon, A.; Chopra, V.; Malani, A.N.; Flanders, S.A. The Association of Antibiotic Stewardship with Fluoroquinolone Prescribing in Michigan Hospitals: A Multi-hospital Cohort Study. Clin. Infect. Dis. 2019, 69, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Joean, O.; Tahedl, D.; Flintrop, M.; Winkler, T.; Sabau, R.; Welte, T.; Kuczyk, M.A.; Vonberg, R.-P.; Rademacher, J. Clinical and Microbiological Effects of an Antimicrobial Stewardship Program in Urology—A Single Center Before-After Study. Antibiotics 2022, 11, 372. [Google Scholar] [CrossRef]
- Wagenlehner, F.; Tandogdu, Z.; Bartoletti, R.; Cai, T.; Cek, M.; Kulchavenya, E.; Köves, B.; Naber, K.; Perepanova, T.; Tenke, P.; et al. The Global Prevalence of Infections in Urology Study: A Long-Term, Worldwide Surveillance Study on Urological Infections. Pathogens 2016, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Liu, E.; Linder, K.E.; Kuti, J.L. Antimicrobial Stewardship at Transitions of Care to Outpatient Settings: Synopsis and Strategies. Antibiotics 2022, 11, 1027. [Google Scholar] [CrossRef]
- CDC. Core Elements of Hospital Antibiotic Stewardship Programs; US Department of Health and Human Services, CDC: Atlanta, GA, USA, 2019. Available online: https://www.cdc.gov/antibiotic-use/core-elements/hospital.html (accessed on 23 December 2022).
- Cossette, B.; Ricard, G.; Poirier, R.; Gosselin, S.; Langlois, M.F.; Imbeault, P.; Breton, M.; Couturier, Y.; Sirois, C.; Lessard-Beaudoin, M.; et al. Pharmacist-led transitions of care between hospitals, primary care clinics, and community pharmacies. J. Am. Geriatr. Soc. 2022, 70, 766–776. [Google Scholar] [CrossRef]
- Giesler, D.L.; Krein, S.; Brancaccio, A.; Mashrah, D.; Ratz, D.; Gandhi, T.; Bashaw, L.; Horowitz, J.; Vaughn, V. Reducing overuse of antibiotics at discharge home: A single-center mixed methods pilot study. Am. J. Infect. Control 2022, 50, 777–786. [Google Scholar] [CrossRef] [PubMed]
- COFARES. Available online: https://www.actasanitaria.com/documentos/observatorio-tendencias-de-cofares-sobre-uso-antibioticos_2004197_102.html (accessed on 8 February 2023).
- Jover-Sáenz, A.; Ramírez-Hidalgo, M.F.; Vidal, M.V.; González, M.G.; Marrón, S.M.C.; Arias, A.E.; Sacrest, M.F.; Castellana-Perelló, D.; Barcenilla-Gaite, F.; Translational Research Group on Infectious Diseases of Lleida (Head Group, TRIDLE- IRBLleida). Antimicrobial stewardship program at a tertiary care academic medical hospital: Clinical, microbiological and economic impact. A 5-year temporary descriptive study. Infect. Prev. Pract. 2020, 2, 100048. [Google Scholar] [CrossRef] [PubMed]
- Jover-Sáenz, A.; Grup d’estudi i equip PROAP. P-ILEHRDA -Programa integrat local extrahospitalari de racionalització, millora i desprescripció antibiòtica a Lleida-. Primers resultats d’un programa d’optimització d’ús d’antimicrobians (PROA) en atenció primària. Ann. Med. 2018, 4, 160–166. (In Catalan) [Google Scholar]
- Leja, N.; Collins, C.D.; Duker, J. Antimicrobial Stewardship by Transitions of Care Pharmacists at Hospital Discharge. Hosp. Pharm. 2021, 56, 714–717. [Google Scholar] [CrossRef] [PubMed]
- Paulson, C.M.; Handley, J.F.; Dilworth, T.J.; Persells, D.; Prusi, R.Y.; Brummitt, C.F.; Torres, K.M.; Skrupky, L.P. Impact of a Systematic Pharmacist-Initiated Antibiotic Time-Out Intervention for Hospitalized Adults. J. Pharm. Pract. 2022, 35, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Hicks, L.A.; Bartoces, M.G.; Roberts, R.M.; Suda, K.J.; Hunkler, R.J.; Taylor, T.H., Jr.; Schrag, S.J. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin. Infect. Dis. 2015, 60, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Khaw, C.; Oberle, A.D.; Lund, B.C.; Egge, J.; Heintz, B.H.; Erickson, B.; Livorsi, D.J. Assessment of Guideline Discordance with Antimicrobial Prophylaxis Best Practices for Common Urologic Procedures. JAMA Netw. Open 2018, 1, e186248. [Google Scholar] [CrossRef]
- Lebentrau, S.; Gilfrich, C.; Vetterlein, M.W.; Schumacher, H.; Spachmann, P.J.; Brookman-May, S.D.; Fritsche, H.M.; Schostak, M.; Wagenlehner, F.M.; Burger, M.; et al. Impact of the medical specialty on knowledge regarding multidrug-resistant organisms and strategies toward antimicrobial stewardship. Int. Urol. Nephrol. 2017, 49, 1311–1318. [Google Scholar] [CrossRef]
- Lebentrau, S.; Vetterlein, M.W.; May, M. The Urologist’s Role in Antibiotic Stewardship: Results from the MR2 Study. Eur. Urol. 2017, 71, 995–996. [Google Scholar] [CrossRef]
- Srinivasan, A.; Song, X.; Richards, A.; Sinkowitz-Cochran, R.; Cardo, D.; Rand, C. A Survey of Knowledge, Attitudes, and Beliefs of House Staff Physicians From Various Specialties Concerning Antimicrobial Use and Resistance. Arch. Intern. Med. 2004, 164, 1451–1456. [Google Scholar] [CrossRef]
- Vaughn, V.M.; Gandhi, T.N.; Chopra, V.; Petty, L.A.; Giesler, D.L.; Malani, A.N.; Bernstein, S.J.; Hsaiky, L.M.; Pogue, J.M.; Dumkow, L.; et al. Antibiotic Overuse After Hospital Discharge: A Multi-hospital Cohort Study. Clin. Infect. Dis. 2021, 73, e4499–e4506. [Google Scholar] [CrossRef] [PubMed]
- Tamma, P.D.; Miller, M.A.; Cosgrove, S.E. Rethinking How Antibiotics Are Prescribed: Incorporating the 4 Moments of Antibiotic Decision Making Into Clinical Practice. JAMA 2019, 321, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Kurotschka, P.K.; Fulgenzio, C.; Da Cas, R.; Traversa, G.; Ferrante, G.; Massidda, O.; Gágyor, I.; Aschbacher, R.; Moser, V.; Pagani, E.; et al. Effect of Fluoroquinolone Use in Primary Care on the Development and Gradual Decay of Escherichia coli Resistance to Fluoroquinolones: A Matched Case-Control Study. Antibiotics 2022, 11, 822. [Google Scholar] [CrossRef] [PubMed]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Etminan, M.; Sodhi, M.; Ganjizadeh-Zavareh, S.; Carleton, B.; Kezouh, A.; Brophy, J.M. Oral Fluoroquinolones and Risk of Mitral and Aortic Regurgitation. J. Am. Coll. Cardiol. 2019, 74, 1444–1450. [Google Scholar] [CrossRef]
- Jover-Sáenz, A.; Ramírez-Hidalgo, M.; Bellés Bellés, A.; Ribes Murillo, E.; Batlle Bosch, M.; Cayado Cabanillas, J.; Garrido-Calvo, S.; Gracia Vilas, M.I.; Gros Navés, L.; Javierre Caudevilla, M.J.; et al. Impact of a Primary Care Antimicrobial Stewardship Program on Bacterial Resistance Control and Ecological Imprint in Urinary Tract Infections. Antibiotics 2022, 11, 1776. [Google Scholar] [CrossRef]
- Schechner, V.; Fallach, N.; Braun, T.; Temkin, E.; Carmeli, Y. Antibiotic exposure and the risk of hospital-acquired diarrhoea and Clostridioides difficile infection: A cohort study. J. Antimicrob. Chemother. 2021, 76, 2182–2185. [Google Scholar] [CrossRef]
- Slimings, C.; Riley, T.V. Antibiotics and hospital-acquired Clostridium difficile infection: Update of systematic review and meta-analysis. J. Antimicrob. Chemother. 2014, 69, 881–891. [Google Scholar] [CrossRef]
- Al-Tawil, K.; Babu, A.; Loeffler, M.; Williams, T. Second generation cephalosporin antibiotic prophylaxis and Clostridium difficile infection in hip and knee arthroplasty. Ann. R. Coll. Surg. Engl. 2017, 99, 351–354. [Google Scholar] [CrossRef]
- Buckley, A.M.; Moura, I.B.; Altringham, J.; Ewin, D.; Clark, E.; Bentley, K.; Wilkinson, V.; Spittal, W.; Davis, G.; Wilcox, M.H. The use of first-generation cephalosporin antibiotics, cefalexin and cefradine, is not associated with induction of simulated Clostridioides difficile infection. J. Antimicrob. Chemother. 2021, 77, 148–154. [Google Scholar] [CrossRef]
- Sutton, J.D.; Stevens, V.W.; Chang, N.-C.N.; Khader, K.; Timbrook, T.T.; Spivak, E.S. Oral β-Lactam Antibiotics vs Fluoroquinolones or Trimethoprim-Sulfamethoxazole for Definitive Treatment of Enterobacterales Bacteremia From a Urine Source. JAMA Netw. Open 2020, 3, e2020166. [Google Scholar] [CrossRef]
- Bonkat, G.; Wagenlehner, F. In the Line of Fire: Should Urologists Stop Prescribing Fluoroquinolones as Default? Eur. Urol. 2019, 75, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B. The New Antibiotic Mantra-“Shorter Is Better”. JAMA Intern. Med. 2016, 176, 1254–1255. [Google Scholar] [CrossRef]
- Chavada, R.; Davey, J.; O’Connor, L.; Tong, D. ‘Careful goodbye at the door’: Is there role for antimicrobial stewardship interventions for antimicrobial therapy prescribed on hospital discharge? BMC Infect. Dis. 2018, 18, 225. [Google Scholar] [CrossRef]
- Brower, K.I.; Hecke, A.; Mangino, J.E.; Gerlach, A.T.; Goff, D.A. Duration of antibiotic therapy for general medicine and general surgery patients throughout transitions of care: An antibiotic stewardship opportunity for noninfectious disease pharmacists. Hosp. Pharm. 2021, 56, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.T.; Roque, F.; Falcão, A.; Figueiras, A.; Herdeiro, M.T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents 2013, 41, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Livorsi, D.; Comer, A.; Matthias, M.S.; Perencevich, E.N.; Bair, M.J. Factors Influencing Antibiotic-Prescribing Decisions Among Inpatient Physicians: A Qualitative Investigation. Infect. Control Hosp. Epidemiol. 2015, 36, 1065–1072. [Google Scholar] [CrossRef]
- Nelson, G. Another Key Moment for Antimicrobial Stewardship: Hospital Discharge. Clin. Infect. Dis. 2020, 71, 1240–1242. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, L.; Monnet, D.L.; Haaijer-Ruskamp, F.M.; Bonten, M.J.M.; Lundborg, S.; Verheij, T.J.M. Self-medication with antibiotics in Europe: A case for action. Curr. Drug Saf. 2010, 5, 329–332. [Google Scholar] [CrossRef]
- Lescure, D.; Paget, J.; Schellevis, F.; van Dijk, L. Determinants of Self-Medication with Antibiotics in European and Anglo-Saxon Countries: A Systematic Review of the Literature. Front. Public Health 2018, 6, 370. [Google Scholar] [CrossRef]
- Davar, K.; Clark, D.; Centor, R.M.; Dominguez, F.; Ghanem, B.; Lee, R.; Lee, T.C.; McDonald, E.G.; Phillips, M.C.; Sendi, P.; et al. Can the Future of ID Escape the Inertial Dogma of Its Past? The Exemplars of Shorter Is Better and Oral Is the New IV. Open Forum Infect. Dis. 2022, 10, ofac706. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, T.G.; Robertson, J.; van den Ham, H.A.; Iwamoto, K.; Bak Pedersen, H.; Mantel-Teeuwisse, A.K. Assessing the impact of law enforcement to reduce over-the-counter (OTC) sales of antibiotics in low- and middle-income countries; a systematic literature review. BMC Health Serv. Res. 2019, 19, 536. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; Lo, J.; Leung, V.; Brown, K.; Schwartz, K.L.; Daneman, N.; Garber, G.; Wu, J.H.; Langford, B.J. Estimating daily antibiotic harms: An umbrella review with individual study meta-analysis. Clin. Microbiol. Infect. 2022, 28, 479–490. [Google Scholar] [CrossRef] [PubMed]
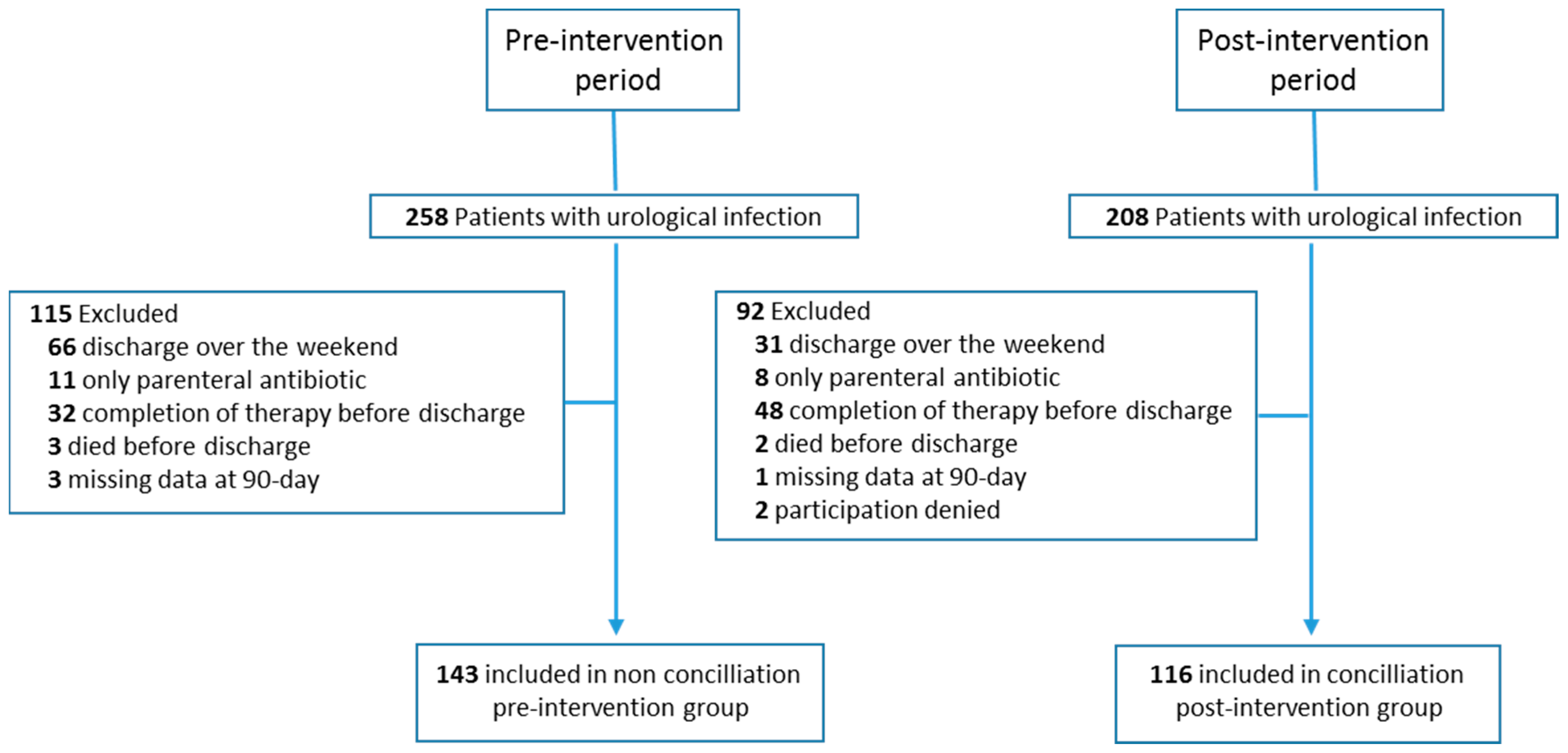
| All Cases (n = 259) N° (%) | Group Pre-Intervention (n = 143) N° (%) | Group Post-Intervention (n = 116) N° (%) | p Value | |
|---|---|---|---|---|
| Mean age (SD), year | 65.5 (16.8) | 64.4 (15.1) | 61.6 (16.1) | NS |
| Male gender | 213 (82.2) | 132 (92.3) | 81 (69.8) | <0.001 |
| Comorbidity index | ||||
| Charlson ≥ 2 | 103 (39.8) | 62 (46.4) | 41 (35.3) | NS |
| Length of hospital stays, mean (SD), days | 4.9 (3.1) | 4.7 (2.7) | 5.1 (3.5) | NS |
| Infectious urinary entity and group | ||||
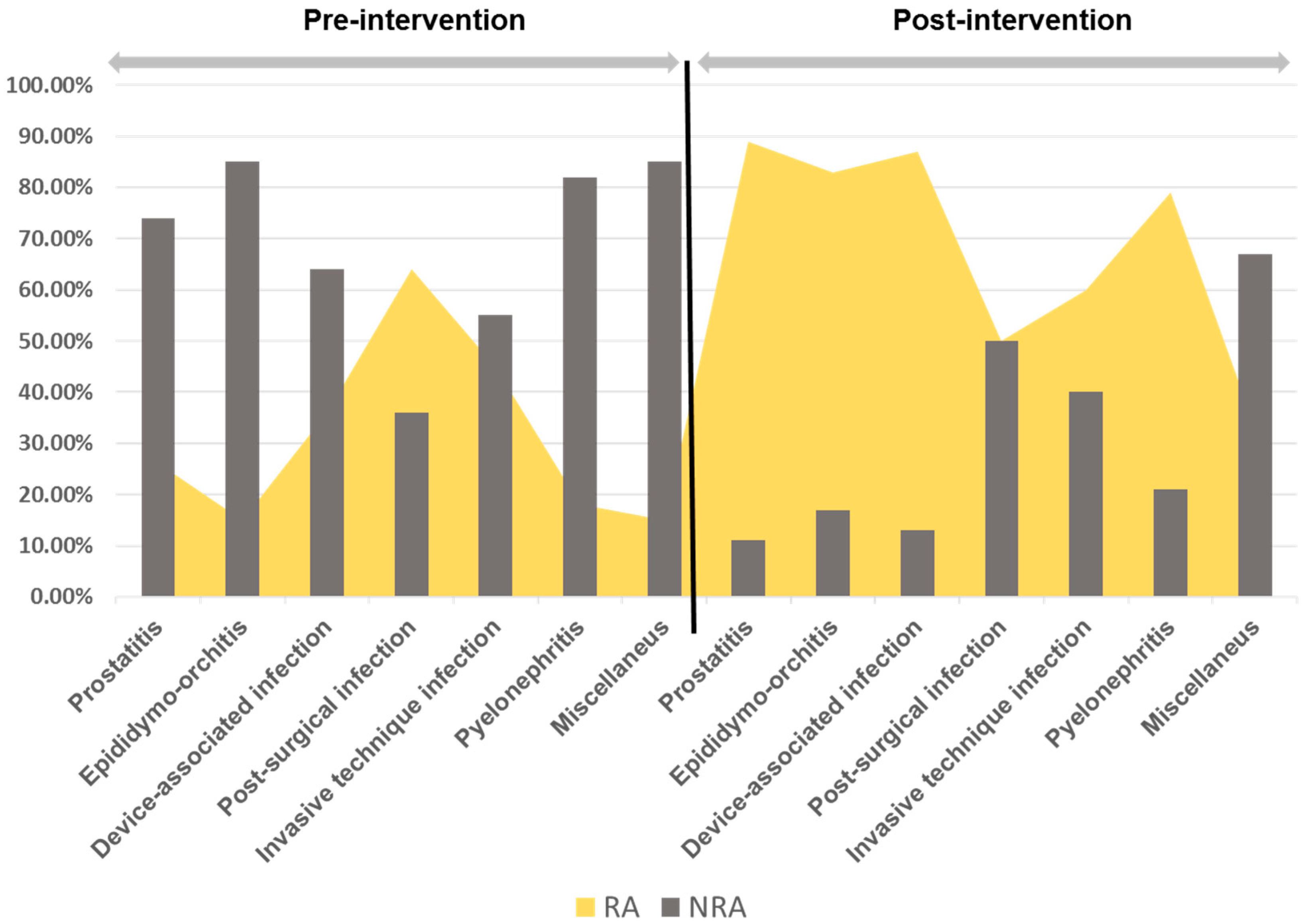
| Prostatitis | 87 (33.6) | 69 (48.2) | 18 (15.5) | <0.001 |
| Epididymo-orchitis | 23 (8.9) | 17 (11.9) | 6 (5.2) | NS |
| Device-associated infection | 29 (11.2) | 14 (9.8) | 15 (12.9) | NS |
| Post-surgical infection | 19 (7.3) | 11 (7.7) | 8 (6.9) | NS |
| Invasive technique infection | 16 (6.2) | 11 (7.7) | 5 (4.3) | NS |
| Pyelonephritis | 74 (28.6) | 17 (11.9) | 57 (49.1) | <0.001 |
| Miscellaneous | 10 (3.9) | 4 (2.8) | 6 (5.2) | NS |
| Group Preintervention n (%) | Group Postintervention n (%) | Absolute Difference 95% CI | p Value | |
|---|---|---|---|---|
| Quinolones (J01M) | 37 (25.9) | 14 (12.1) | −13.8 (−25.9 to 3.8) | 0.016 |
| Third-generation cephalosporins (J01DD) | 31 (21.7) | 23 (19.8) | −1.0 (−11.8 to 8.1) | NS |
| Co-amoxiclav (J01CR02) | 15 (10.5) | 17 (14.7) | 4.2 (−4.0 to 12.3) | NS |
| Cotrimoxazole (J01EE01) | 44 (30.8) | 26 (22.4) | −8.4 (−19.1 to 2.4) | NS |
| Cefuroxime (J01DC02) | 9 (6.3) | 32 (27.6) | 21.3 (12.2 to 30.5) | <0.001 |
| Amoxicillin (J01CR02) | 2 (1.4) | 1 (0.9) | −0.5 (2.0 to −3.1) | NS |
| Fosfomycin trometamol (J01XX01) | 5 (3.5) | 3 (2.6) | −0.9 (−5.1 to 3.3) | NS |
| Total recommended antibiotics (RA) | 60 (42.0) | 62 (53.4) | 11.5 (−0.7 to 23.6) | 0.043 |
| Patients n° (%) | Group Pre-Intervention n (%) | Group Post-Intervention n (%) | Absolute Difference 95% CI | p Value |
|---|---|---|---|---|
| 30-day overall mortality | 2 (1.4) | 1 (0.9) | −0.5 (−3.1 to 2.2) | NS |
| 30-day readmission | 19 (13.3) | 16 (13.8) | 0.5 (−7.9 to 8.9) | NS |
| 30-day Retreatment/emergency or community visit | 22 (15.4) | 8 (9.5) | −5.9 (−13.9 to 2.1) | NS |
| No clinical resolution | 17 (11.9) | 10 (8.7) | −3.3 (−10.6 to 4.1) | NS |
| Adverse drug event | 3 (2.1) | 0 (0) | −2.1 (−5.1 to 1.2) | NS |
| 90-day C. difficile infection | 3 (2.1) | 0 (0) | −2.1 (−5.1 to 1.2) | NS |
| Group Pre-Intervention (n = 143) | Group Post-Intervention (n = 116) | Absolute Difference 95% CI | p Value | |
|---|---|---|---|---|
| DOT in | 4.63 | 4.95 | 0.88 (−0.38 to 1.00) | NS |
| DOT out | 14.01 | 6.56 | −7.45 (−8.73 to −6.17) | <0.001 |
| DOT in + out | 18.67 | 11.54 | −6.16 (−8.66 to −5.60) | <0.001 |
| Mean DOT out by UD | 16.74 | 9.56 | −7.21 (−8.61 to −5.81) | <0.001 |
| Mean tablets out by UD | 26.00 | 13.46 | −12.54 (−15.64 to −9.43) | <0.001 |
| Mean tablets saved | 5.13 | 5.29 | 0.16 (−1.08 to 1.40) | NS |
| Mean DOT saved | 2.73 | 3.00 | 0.27 (−0.44 to 0.97) | NS |
| Average cost per MPM (€) | 7.42 | 6.02 | −1.40 (−2.44 to −0.35) | 0.009 |
| Average cost saved (€) | 1.11 | 1.66 | 0.56 (0.92 to 0.19) | 0.003 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jover-Sáenz, A.; Santos Rodríguez, C.; Ramos Gil, M.Á.; Palomera Fernández, M.; Invencio da Costa, L.F.; Torres-Puig-gros, J.; Castellana Perelló, D.; Montiu González, E.; Schoenenberger-Arnaiz, J.A.; Bordalba Gómez, J.R.; et al. Impact of an Antimicrobial Stewardship Strategy on Surgical Hospital Discharge: Improving Antibiotic Prescription in the Transition of Care. Antibiotics 2023, 12, 834. https://doi.org/10.3390/antibiotics12050834
Jover-Sáenz A, Santos Rodríguez C, Ramos Gil MÁ, Palomera Fernández M, Invencio da Costa LF, Torres-Puig-gros J, Castellana Perelló D, Montiu González E, Schoenenberger-Arnaiz JA, Bordalba Gómez JR, et al. Impact of an Antimicrobial Stewardship Strategy on Surgical Hospital Discharge: Improving Antibiotic Prescription in the Transition of Care. Antibiotics. 2023; 12(5):834. https://doi.org/10.3390/antibiotics12050834
Chicago/Turabian StyleJover-Sáenz, Alfredo, Carlos Santos Rodríguez, Miguel Ángel Ramos Gil, Meritxell Palomera Fernández, Liliana Filippa Invencio da Costa, Joan Torres-Puig-gros, Dolors Castellana Perelló, Elisa Montiu González, Joan Antoni Schoenenberger-Arnaiz, Juan Ramón Bordalba Gómez, and et al. 2023. "Impact of an Antimicrobial Stewardship Strategy on Surgical Hospital Discharge: Improving Antibiotic Prescription in the Transition of Care" Antibiotics 12, no. 5: 834. https://doi.org/10.3390/antibiotics12050834
APA StyleJover-Sáenz, A., Santos Rodríguez, C., Ramos Gil, M. Á., Palomera Fernández, M., Invencio da Costa, L. F., Torres-Puig-gros, J., Castellana Perelló, D., Montiu González, E., Schoenenberger-Arnaiz, J. A., Bordalba Gómez, J. R., Galindo Ortego, X., & Ramirez-Hidalgo, M. (2023). Impact of an Antimicrobial Stewardship Strategy on Surgical Hospital Discharge: Improving Antibiotic Prescription in the Transition of Care. Antibiotics, 12(5), 834. https://doi.org/10.3390/antibiotics12050834








