The Assessment of Antimicrobial and Anti-Biofilm Activity of Essential Oils against Staphylococcus aureus Strains
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Material and Extraction of Essential Oils
4.2. Identification of the Chemical Composition of Essential Oils
4.3. Antimicrobial Susceptibility Test and Bacterial Strains
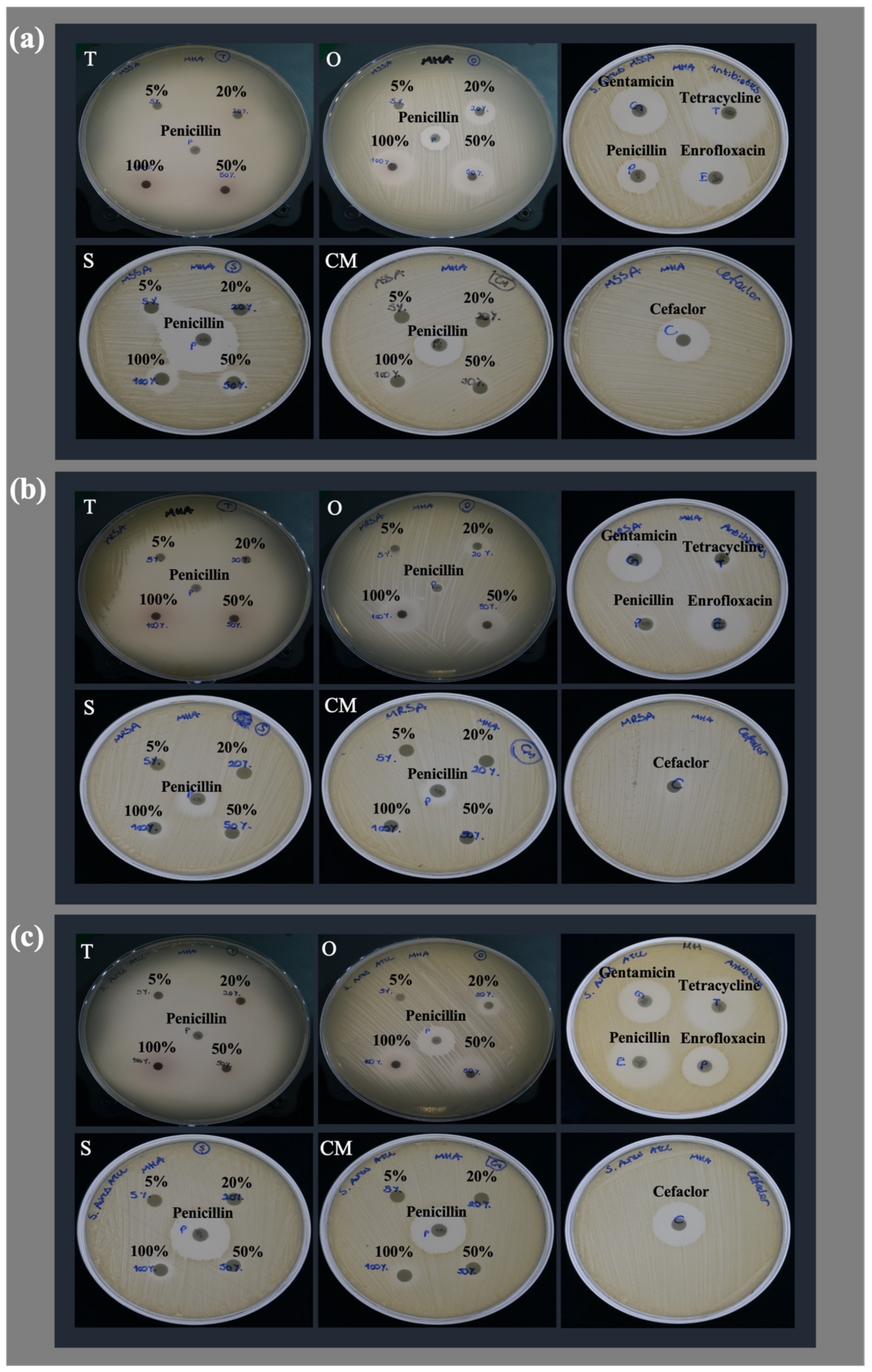
4.3.1. Antimicrobial Activity
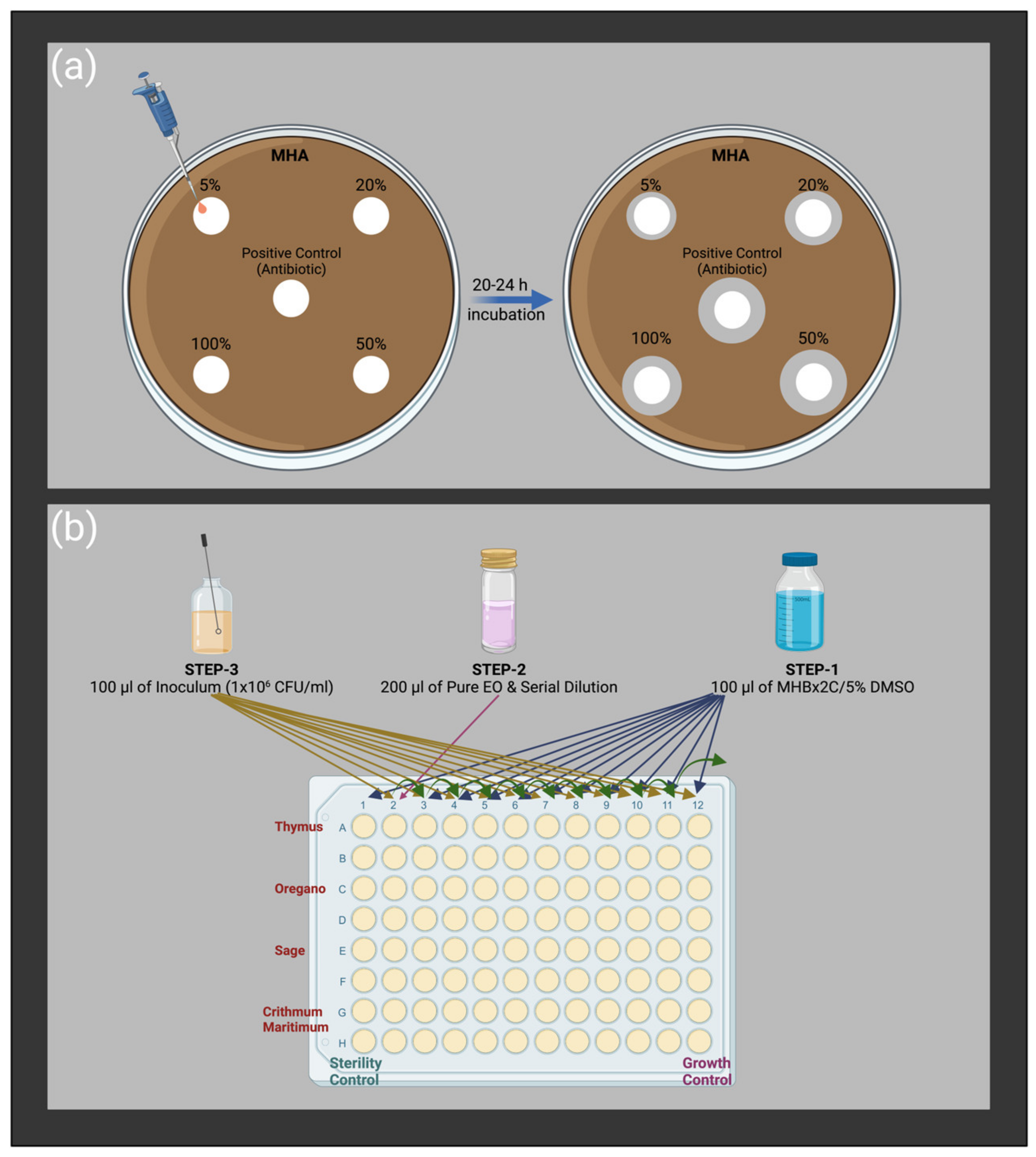
Disc Diffusion Method
The Modified Broth Microdilution Method
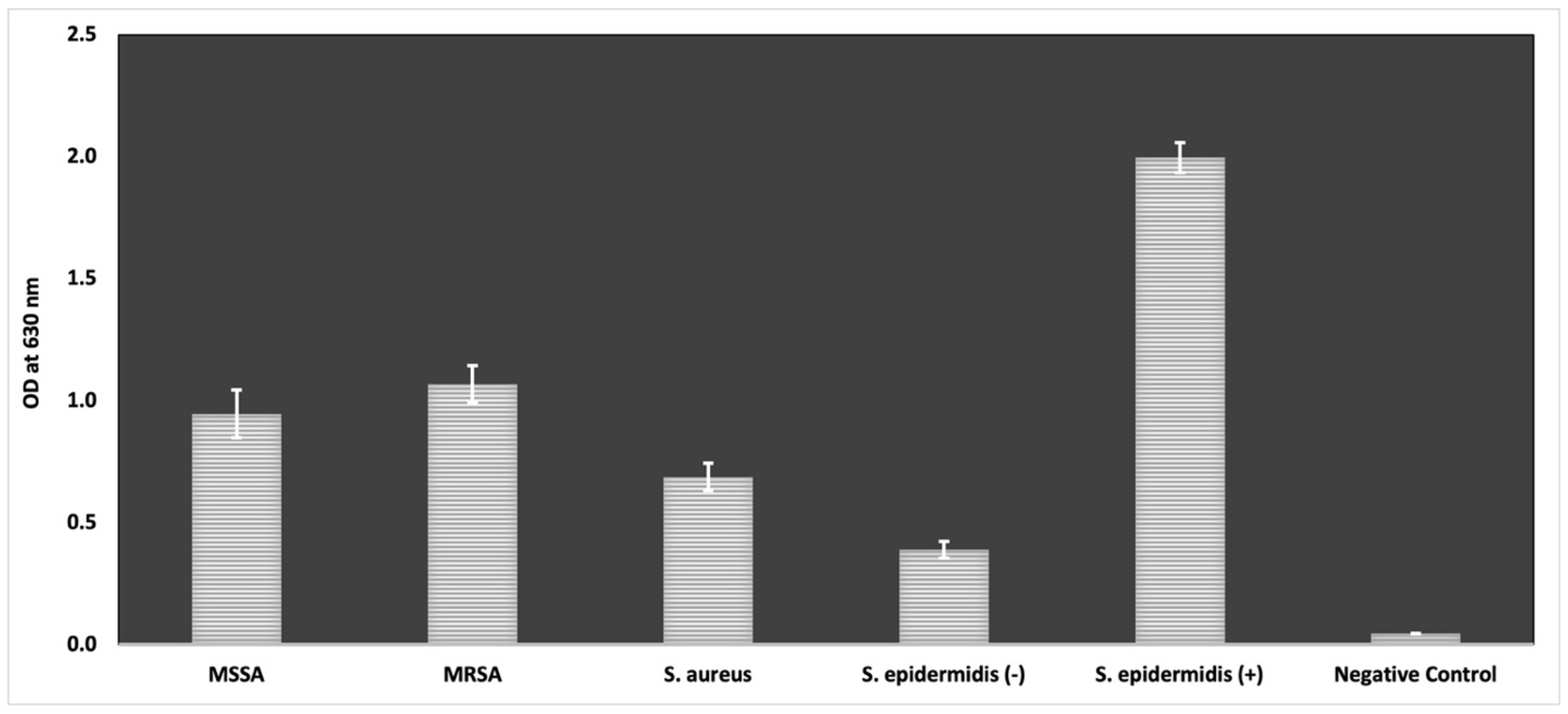
4.3.2. Anti-Biofilm Activity
Modified Microtiter Plate Biofilm Formation Assay
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harbarth, S.; Balkhy, H.H.; Goossens, H.; Jarlier, V.; Kluytmans, J.; Laxminarayan, R.; Saam, M.; Van Belkum, A.; Pittet, D. Antimicrobial resistance: One world, one fight! Antimicrob. Resist. Infect. Control. 2015, 4, 49. [Google Scholar] [CrossRef]
- Rozos, G.; Skoufos, I.; Fotou, K.; Alexopoulos, A.; Tsinas, A.; Bezirtzoglou, E.; Tzora, A.; Voidarou, C. Safety Issues Regarding the Detection of Antibiotics Residues, Microbial Indicators and Somatic Cell Counts in Ewes’ and Goats’ Milk Reared in Two Different Farming Systems. Appl. Sci. 2022, 12, 1009. [Google Scholar] [CrossRef]
- Christaki, E.; Marcou, M.; Tofarides, A. Antimicrobial resistance in bacteria: Mechanisms, evolution, and persistence. J. Mol. Evol. 2020, 88, 26–40. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H. Perspectives towards antibiotic resistance: From molecules to population. J. Microbiol. 2019, 57, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, C.; Gao, R.; Zhang, C.; Ou-Yang, W.; Feng, Z.; Zhang, C.; Pan, X.; Huang, P.; Kong, D. Polymer Composite Sponges with Inherent Antibacterial, Hemostatic, Inflammation-Modulating and Proregenerative Performances for Methicillin-Resistant Staphylococcus aureus-Infected Wound Healing. Adv. Healthc. Mater 2021, 10, 2101247. [Google Scholar] [CrossRef]
- Hofer, U. The cost of antimicrobial resistance. Nat. Rev. Microbiol. 2019, 17, 3. [Google Scholar] [CrossRef]
- Hübner, C.; Hübner, N.-O.; Hopert, K.; Maletzki, S.; Flessa, S. Analysis of MRSA-attributed costs of hospitalized patients in Germany. Eur. J. Clin. Microbiol. 2014, 33, 1817–1822. [Google Scholar] [CrossRef]
- Filice, G.A.; Nyman, J.A.; Lexau, C.; Lees, C.H.; Bockstedt, L.A.; Como-Sabetti, K.; Lesher, L.J.; Lynfield, R. Excess costs and utilization associated with methicillin resistance for patients with Staphylococcus aureus infection. Infect. Control Hosp. Epidemiol. 2010, 31, 365–373. [Google Scholar] [CrossRef]
- Ke, Y.; Ye, L.; Zhu, P.; Zhu, Z.J.F.i.M. The clinical characteristics and microbiological investigation of pediatric burn patients with wound infections in a tertiary hospital in Ningbo, China: A ten-year retrospective study. Front. Microbiol. 2022, 13, 5238. [Google Scholar] [CrossRef]
- Pollitt, E.J.; Szkuta, P.T.; Burns, N.; Foster, S.J. Staphylococcus aureus infection dynamics. PLoS Pathog. 2018, 14, e1007112. [Google Scholar] [CrossRef]
- Serra, R.; Grande, R.; Butrico, L.; Rossi, A.; Settimio, U.F.; Caroleo, B.; Amato, B.; Gallelli, L.; De Franciscis, S. Chronic wound infections: The role of Pseudomonas aeruginosa and Staphylococcus aureus. Expert Rev. Anti-Infect. Ther. 2015, 13, 605–613. [Google Scholar] [CrossRef]
- Almeida, G.C.M.; dos Santos, M.M.; Lima, N.G.M.; Cidral, T.A.; Melo, M.C.N.; Lima, K.C. Prevalence and factors associated with wound colonization by Staphylococcus spp. and Staphylococcus aureus in hospitalized patients in inland northeastern Brazil: A cross-sectional study. BMC Infect. 2014, 14, 328. [Google Scholar]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A.; Health, P. Staphylococcus aureus biofilm: Morphology, genetics, pathogenesis and treatment strategies. Int. J. Environ. Res. 2021, 18, 7602. [Google Scholar] [CrossRef]
- Taiwo, M.; Adebayo, O. Plant essential oil: An alternative to emerging multidrug resistant pathogens. JMEN 2017, 5, 00163. [Google Scholar]
- Mishra, A.P.; Devkota, H.P.; Nigam, M.; Adetunji, C.O.; Srivastava, N.; Saklani, S.; Shukla, I.; Azmi, L.; Shariati, M.A.; Coutinho, H.D.M. Combination of essential oils in dairy products: A review of their functions and potential benefits. LWT 2020, 133, 110116. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef]
- Ni, Z.-J.; Wang, X.; Shen, Y.; Thakur, K.; Han, J.; Zhang, J.-G.; Hu, F.; Wei, Z.-J. Recent updates on the chemistry, bioactivities, mode of action, and industrial applications of plant essential oils. Trends Food Sci. Technol. 2021, 110, 78–89. [Google Scholar] [CrossRef]
- Maurya, A.; Prasad, J.; Das, S.; Dwivedy, A.K. Essential oils and their application in food safety. Front. Sustain. Food Syst. 2021, 5, 653420. [Google Scholar] [CrossRef]
- Stefanakis, M.K.; Touloupakis, E.; Anastasopoulos, E.; Ghanotakis, D.; Katerinopoulos, H.E.; Makridis, P. Antibacterial activity of essential oils from plants of the genus Origanum. Food Control 2013, 34, 539–546. [Google Scholar] [CrossRef]
- Cimino, C.; Maurel, O.M.; Musumeci, T.; Bonaccorso, A.; Drago, F.; Souto, E.M.B.; Pignatello, R.; Carbone, C. Essential oils: Pharmaceutical applications and encapsulation strategies into lipid-based delivery systems. Pharmaceutics 2021, 13, 327. [Google Scholar] [CrossRef]
- Saad, N.Y.; Muller, C.D.; Lobstein, A. Major bioactivities and mechanism of action of essential oils and their components. Flavour Fragr. J. 2013, 28, 269–279. [Google Scholar] [CrossRef]
- Tzora, A.; Giannenas, I.; Karamoutsios, A.; Papaioannou, N.; Papanastasiou, D.; Bonos, E.; Skoufos, S.; Bartzanas, T.; Skoufos, I. Effects of oregano, attapulgite, benzoic acid and their blend on chicken performance, intestinal microbiology and intestinal morphology. Poult. Sci. J. 2017, 54, 218–227. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Nainu, F.; Simal-Gandara, J. Terpenes and terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. X 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Martins, M.A.; Silva, L.P.; Ferreira, O.; Schröder, B.; Coutinho, J.A.; Pinho, S.P. Terpenes solubility in water and their environmental distribution. J. Mol. Liq. 2017, 241, 996–1002. [Google Scholar] [CrossRef]
- Da Silva, B.D.; Bernardes, P.C.; Pinheiro, P.F.; Fantuzzi, E.; Roberto, C.D. Chemical composition, extraction sources and action mechanisms of essential oils: Natural preservative and limitations of use in meat products. Meat Sci. 2021, 176, 108463. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. GOST 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Rossi, C.; Chaves-López, C.; Serio, A.; Casaccia, M.; Maggio, F.; Paparella, A. Effectiveness and mechanisms of essential oils for biofilm control on food-contact surfaces: An updated review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2172–2191. [Google Scholar] [CrossRef]
- Kostoglou, D.; Protopappas, I.; Giaouris, E. Common plant-derived terpenoids present increased anti-biofilm potential against Staphylococcus bacteria compared to a quaternary ammonium biocide. Foods 2020, 9, 697. [Google Scholar] [CrossRef]
- Amiri, H. Essential oils composition and antioxidant properties of three thymus species. eCAM 2012, 2012, 728065. [Google Scholar] [CrossRef]
- Pezzani, R.; Vitalini, S.; Iriti, M. Bioactivities of Origanum vulgare L.: An update. Phytochem. Rev. 2017, 16, 1253–1268. [Google Scholar] [CrossRef]
- Bahadirli, N.P. Comparison of Chemical Composition and Antimicrobial Activity of Salvia fruticosa Mill. and S. aramiensis Rech. Fill. (Lamiaceae). J. Essent. Oil-Bear. Plants 2022, 25, 716–727. [Google Scholar] [CrossRef]
- Karousou, R.; Vokou, D.; Kokkini, S. Variation of Salvia fruticosa essential oils on the island of Crete (Greece). Bot. Acta 1998, 111, 250–254. [Google Scholar] [CrossRef]
- Senatore, F.; Napolitano, F.; Ozcan, M. Composition and antibacterial activity of the essential oil from Crithmum maritimum L.(Apiaceae) growing wild in Turkey. Flavour Fragr. J. 2000, 15, 186–189. [Google Scholar] [CrossRef]
- Battisti, M.A.; Caon, T.; de Campos, A.M. A short review on the antimicrobial micro-and nanoparticles loaded with Melaleuca alternifolia essential oil. J. Drug Deliv. Sci. Technol. 2021, 63, 102283. [Google Scholar] [CrossRef]
- Aljeldah, M.M. Antioxidant and antimicrobial potencies of chemically-profiled essential oil from asteriscus graveolens against clinically-important pathogenic microbial strains. Molecules 2022, 27, 3539. [Google Scholar] [CrossRef]
- Jaradat, N. Phytochemical profile and in vitro antioxidant, antimicrobial, vital physiological enzymes inhibitory and cytotoxic effects of artemisia jordanica leaves essential oil from Palestine. Molecules 2021, 26, 2831. [Google Scholar] [CrossRef]
- Chávez-González, M.; Rodríguez-Herrera, R.; Aguilar, C. Essential oils: A natural alternative to combat antibiotics resistance. In Antibiotic Resistance. Mechanisms and New Antimicrobial Approaches; Rai, K.K.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 227–237. [Google Scholar]
- Martin, I.; Sawatzky, P.; Liu, G.; Mulvey, M. STIs and sexual health awareness month: Antimicrobial resistance to Neisseria gonorrhoeae in Canada: 2009–2013. CCDR 2015, 41, 35. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. JPA 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zain, K.J.; Brad, B.A.; Al Kurdi, S.B.; Jumaa, M.M.G.; Alkhouli, M. Evaluation of Antimicrobial Activity of β-tricalcium Phosphate/Calcium Sulfate Mixed-up with Gentamicin: In-Vitro Study. Int. J. Dent. Oral Sci. 2021, 8, 4753–4757. [Google Scholar]
- Kulshreshtha, G.; Critchley, A.; Rathgeber, B.; Stratton, G.; Banskota, A.H.; Hafting, J.; Prithiviraj, B. Antimicrobial effects of selected, cultivated red seaweeds and their components in combination with tetracycline, against poultry pathogen Salmonella enteritidis. J. Mar. Sci. Eng. 2020, 8, 511. [Google Scholar] [CrossRef]
- Tsekoura, E.; Helling, A.; Wall, J.; Bayon, Y.; Zeugolis, D. Battling bacterial infection with hexamethylene diisocyanate cross-linked and Cefaclor-loaded collagen scaffolds. Biomed. Mater. 2017, 12, 035013. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive review of antimicrobial activities of plant flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Chokejaroenrat, C.; Sakulthaew, C.; Satchasataporn, K.; Snow, D.D.; Ali, T.E.; Assiri, M.A.; Watcharenwong, A.; Imman, S.; Suriyachai, N.; Kreetachat, T. Enrofloxacin and Sulfamethoxazole Sorption on Carbonized Leonardite: Kinetics, Isotherms, Influential Effects, and Antibacterial Activity toward S. aureus ATCC 25923. Antibiotics 2022, 11, 1261. [Google Scholar] [CrossRef]
- Das, K.; Tiwari, R.; Shrivastava, D. Techniques for evaluation of medicinal plant products as antimicrobial agent: Current methods and future trends. J. Med. Plant Res. 2010, 4, 104–111. [Google Scholar]
- Houta, O.; Akrout, A.; Najja, H.; Neffati, M.; Amri, H.J.J.o.E.O.B.P. Chemical composition, antioxidant and antimicrobial activities of essential oil from Crithmum maritimum cultivated in Tunisia. J. Essent. Oil Bear. Plants 2015, 18, 1459–1466. [Google Scholar] [CrossRef]
- De Lima Marques, J.; Volcão, L.M.; Funck, G.D.; Kroning, I.S.; da Silva, W.P.; Fiorentini, Â.M.; Ribeiro, G.A. Antimicrobial activity of essential oils of Origanum vulgare L. and Origanum majorana L. against Staphylococcus aureus isolated from poultry meat. Ind. Crops Prod. 2015, 77, 444–450. [Google Scholar] [CrossRef]
- Chan, W.-K.; Tan, L.T.-H.; Chan, K.-G.; Lee, L.-H.; Goh, B.-H. Nerolidol: A sesquiterpene alcohol with multi-faceted pharmacological and biological activities. Molecules 2016, 21, 529. [Google Scholar] [CrossRef]
- Kunduhoglu, B.; Pilatin, S.; Caliskan, F. Antimicrobial screening of some medicinal plants collected from Eskisehir, Turkey. Fresenius Environ. Bull. 2011, 20, 945–952. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-fifth Informational Supplement—CLSI Document M100-S25; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing: Twenty-second Informational Supplement—CLSI Document M100-S22; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard—Ninth Edition—CLSI Document M07-A9; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- Kulaksiz, B.; Sevda, E.; Üstündağ-Okur, N.; Saltan-İşcan, G. Investigation of antimicrobial activities of some herbs containing essential oils and their mouthwash formulations. Turk. J. Pharm. Sci. 2018, 15, 370. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Varoni, E.M.; Iriti, M.; Martorell, M.; Setzer, W.N.; del Mar Contreras, M.; Salehi, B.; Soltani-Nejad, A.; Rajabi, S.; Tajbakhsh, M. Carvacrol and human health: A comprehensive review. Phytother. Res. 2018, 32, 1675–1687. [Google Scholar] [CrossRef]
- Di Pasqua, R.; Betts, G.; Hoskins, N.; Edwards, M.; Ercolini, D.; Mauriello, G. Membrane toxicity of antimicrobial compounds from essential oils. J. Agric. Food Chem. 2007, 55, 4863–4870. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.-L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.; von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Kachur, K.; Suntres, Z. The antibacterial properties of phenolic isomers, carvacrol and thymol. Crit. Rev. Food Sci. Nutr. 2020, 60, 3042–3053. [Google Scholar] [CrossRef]
- Juven, B.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Churklam, W.; Chaturongakul, S.; Ngamwongsatit, B.; Aunpad, R. The mechanisms of action of carvacrol and its synergism with nisin against Listeria monocytogenes on sliced bologna sausage. Food Control 2020, 108, 106864. [Google Scholar] [CrossRef]
- Rattanachaikunsopon, P.; Phumkhachorn, P. Assessment of factors influencing antimicrobial activity of carvacrol and cymene against Vibrio cholerae in food. JBB 2010, 110, 614–619. [Google Scholar] [CrossRef]
- Cristani, M.; D’Arrigo, M.; Mandalari, G.; Castelli, F.; Sarpietro, M.G.; Micieli, D.; Venuti, V.; Bisignano, G.; Saija, A.; Trombetta, D. Interaction of four monoterpenes contained in essential oils with model membranes: Implications for their antibacterial activity. J. Agric. Food Chem. 2007, 55, 6300–6308. [Google Scholar] [CrossRef] [PubMed]
- Shahabi, N.; Tajik, H.; Moradi, M.; Forough, M.; Ezati, P. Physical, antimicrobial and antibiofilm properties of Zataria multiflora Boiss essential oil nanoemulsion. Int. J. Food Sci. Technol. 2017, 52, 1645–1652. [Google Scholar] [CrossRef]
- Maloupa, E.; Krigas, N. The Balkan Botanic Garden of Kroussia, Northern Greece. Sibbaldia Int. J. Bot. Gard. Hortic. 2008, 6, 9–27. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Burton, D.; Parra, F.; López, J.; Muñoz, P.; Escobar, H.; Parra, C. Antioxidant and antibacterial capacities of Origanum vulgare L. essential oil from the arid Andean Region of Chile and its chemical characterization by GC-MS. Metabolites 2020, 10, 414. [Google Scholar] [CrossRef]
- Sarrou, E.; Tsivelika, N.; Chatzopoulou, P.; Tsakalidis, G.; Menexes, G.; Mavromatis, A. Conventional breeding of Greek oregano (Origanum vulgare ssp. hirtum) and development of improved cultivars for yield potential and essential oil quality. Euphytica 2017, 213, 104. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of essential oil components by gas chromatography/mass spectrometry. In Quadrupole Mass Spectroscopy; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456. [Google Scholar]
- Fotou, K.; Tzora, A.; Voidarou, C.; Alexopoulos, A.; Plessas, S.; Avgeris, I.; Bezirtzoglou, E.; Akrida-Demertzi, K.; Demertzis, P. Isolation of microbial pathogens of subclinical mastitis from raw sheep’s milk of Epirus (Greece) and their role in its hygiene. Anaerobe 2011, 17, 315–319. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Disk Susceptibility Tests: Approved Standard—CLSI Document M02-A11; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2015. [Google Scholar]
- CLSI. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: Approved Standard—Eighth Edition—CLSI Document M07-A7; Clinical & Laboratory Standards Institute: Wayne, PA, USA, 2006. [Google Scholar]
- Stepanović, S.; Vuković, D.; Dakić, I.; Savić, B.; Švabić-Vlahović, M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Borges, S.; Silva, J.; Teixeira, P. Survival and biofilm formation by Group B streptococci in simulated vaginal fluid at different pHs. ALJMAO 2012, 101, 677–682. [Google Scholar] [CrossRef]
| Thymus sibthorpii | Origanum vulgare | Salvia fruticosa | Crithmum maritimum | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Compound | RI | RIL | % | Compound | RI | RIL | % | Compound | RI | RIL | % | Compound | RI | RIL | % |
| Carvacrol | 1309 | 1298 | 52.62 | Carvacrol | 1309 | 1298 | 78.72 | 1,8-cineol | 1036 | 1033 | 39.70 | β-phellandrene | 1034 | 1031 | 28.01 |
| p-cymene | 1029 | 1026 | 18.75 | p-cymene | 1029 | 1026 | 8.19 | Camphor | 1150 | 1143 | 12.39 | Sabinene | 975 | 976 | 20.96 |
| Thymoquinone | 1247 | 1249 | 6.71 | γ-terpinene | 1061 | 1062 | 2.11 | β-thujone | 1116 | 1114 | 7.54 | γ-terpinene | 1061 | 1062 | 18.69 |
| β-caryophyllene | 1413 | 1418 | 3.70 | Myrcene | 991 | 991 | 1.64 | α-pinene | 936 | 939 | 7.03 | 1,8-cineol | 1036 | 1033 | 9.53 |
| Thymol | 1295 | 1290 | 2.15 | β-caryophyllene | 1413 | 1418 | 1.27 | α-terpinyl acetate | 1345 | 1346 | 6.72 | Thymol methyl ether | 1236 | 1235 | 4.07 |
| Carvacrol methyl ether | 1242 | 1244 | 1.98 | α-terpinene | 1020 | 1018 | 1.01 | p-cymene | 1029 | 1026 | 4.31 | cis-β-ocimene | 1040 | 1040 | 3.68 |
| cis-sabinene hydrate | 1062 | 1065 | 1.85 | α-pinene | 936 | 939 | 0.98 | Camphene | 953 | 953 | 4.11 | p-cymene | 1029 | 1026 | 3.55 |
| β-bisabolene | 1507 | 1509 | 1.74 | cis-sabinene hydrate | 1062 | 1065 | 0.62 | 3-octanone | 988 | 986 | 3.26 | Terpinen-4-ol | 1183 | 1177 | 2.66 |
| Thymohydroquinone | 1558 | 1553 | 1.36 | Terpinen-4-ol | 1183 | 1177 | 0.55 | β-pinene | 980 | 980 | 2.35 | α-pinene | 936 | 939 | 2.42 |
| Caryophyllene oxide | 1593 | 1581 | 1.03 | α-thujene | 929 | 931 | 0.48 | Limonene | 1032 | 1031 | 2.27 | α-terpinene | 1020 | 1018 | 1.64 |
| α-thujene | 929 | 931 | 0.86 | Borneol | 1175 | 1165 | 0.42 | α-terpineol | 1187 | 1189 | 2.00 | Myrcene | 991 | 991 | 1.44 |
| α-terpinene | 1020 | 1018 | 0.74 | 1-octen-3-ol | 985 | 978 | 0.38 | α-thujone | 1105 | 1102 | 1.27 | α-terpinolene | 1086 | 1088 | 0.91 |
| 1,8-cineol | 1036 | 1033 | 0.57 | α-humulene | 1452 | 1452 | 0.30 | Borneol | 1175 | 1165 | 0.80 | α-thujene | 929 | 931 | 0.48 |
| α-humulene | 1452 | 1452 | 0.42 | Thymol | 1295 | 1290 | 0.28 | β-caryophyllene | 1420 | 1418 | 0.74 | α-phellandrene | 1008 | 1005 | 0.44 |
| α-pinene | 936 | 939 | 0.36 | Limonene | 1032 | 1031 | 0.27 | Terpinen-4-ol | 1183 | 1177 | 0.64 | trans-β-ocimene | 1050 | 1050 | 0.24 |
| trans-sabinene hydrate | 1103 | 1098 | 0.32 | Camphene | 953 | 953 | 0.25 | Linalyl acetate | 1257 | 1257 | 0.52 | Allo-ocimene | 1132 | 1129 | 0.23 |
| Terpinen-4-ol | 1183 | 1177 | 0.29 | Caryophyllene oxide | 1593 | 1581 | 0.24 | δ-terpineol | 1161 | 1162 | 0.47 | β-pinene | 980 | 980 | 0.20 |
| Limonene | 1032 | 1031 | 0.27 | β-phellandrene | 1034 | 1031 | 0.23 | trans-pinocamphone | 1159 | 1160 | 0.32 | Bicyclogermacrene | 1492 | 1494 | 0.14 |
| 1-octen-3-ol | 985 | 978 | 0.22 | α-phellandrene | 1008 | 1005 | 0.18 | Linalool | 1104 | 1098 | 0.31 | cis-2-p-menthen-1-ol | 1120 | 1117 | 0.11 |
| β-pinene | 980 | 980 | 0.17 | β-pinene | 980 | 980 | 0.16 | Caryophyllene oxide | 1593 | 1581 | 0.18 | α-terpineol | 1187 | 1189 | 0.08 |
| β-phellandrene | 1034 | 1031 | 0.16 | α-terpinolene | 1086 | 1088 | 0.15 | Viridiflorol | 1590 | 1590 | 0.18 | β-caryophyllene | 1420 | 1418 | 0.08 |
| trans-β-farnesene | 1456 | 1458 | 0.12 | δ-cadinene | 1517 | 1524 | 0.13 | Tricyclene | 925 | 926 | 0.13 | Camphene | 953 | 953 | 0.07 |
| Germacrene D | 1478 | 1480 | 0.11 | δ-3-carene | 1010 | 1011 | 0.10 | α-thujene | 929 | 931 | 0.13 | cis-sabinene hydrate | 1062 | 1065 | 0.07 |
| δ-cadinene | 1517 | 1524 | 0.11 | trans-β-farnesene | 1456 | 1458 | 0.10 | Aromadendrene | 1434 | 1419 | 0.11 | Caryophyllene oxide | 1593 | 1581 | 0.02 |
| Borneol | 1175 | 1165 | 0.07 | β-bisabolene | 1507 | 1509 | 0.10 | Viridiflorene | 1491 | 1493 | 0.08 | ||||
| Camphene | 953 | 953 | 0.06 | Germacrene D | 1478 | 1480 | 0.08 | cis-sabinene hydrate | 1062 | 1065 | 0.07 | ||||
| δ-3-carene | 1010 | 1011 | 0.05 | 1,8-cineol | 1036 | 1033 | 0.07 | α-terpinene | 1020 | 1018 | 0.06 | ||||
| Spathulenol | 1580 | 1576 | 0.05 | 1-octen-3-ol | 985 | 978 | 0.05 | ||||||||
| γ-terpinene | 1061 | 1062 | 0.05 | ||||||||||||
| β-bisabolene | 1507 | 1509 | 0.05 | ||||||||||||
| Treatment | Disk Content | Methicillin-Sensitive S. aureus | Methicillin-Resistant S. aureus | S. aureus ATCC 29213 | |||
|---|---|---|---|---|---|---|---|
| Zone Diameter (mm) | MIC (mg/mL) | Zone Diameter (mm) | MIC (mg/mL) | Zone Diameter (mm) | MIC (mg/mL) | ||
| Thymus sibthorpii | 5% | 13.968 ± 0.679 c,d | 0.091 | 15.527 ± 0.698 b | 0.091 | 32.415 ± 1.992 g | 0.091 |
| 20% | 61.645 ± 1.923 k | 68.970 ± 4.667 d | 60.908 ± 0.298 h | ||||
| 50% | 70.765 ± 6.283 l | 68.983 ± 2.340 d | 61.380 ± 0.490 h | ||||
| 100% | 78.913 ± 2.897 m | 70.128 ± 5.797 d | 69.353 ± 2.581 i | ||||
| Origanum vulgare | 5% | 7.039 ± 0.388 a,b | 0.182 | 6.310 ± 0.046 a | 0.091 | 6.000 ± 0.000 a | 0.091 |
| 20% | 17.811 ± 0.342 d,e | 14.005 ± 0.260 b | 11.137 ± 0.093 c | ||||
| 50% | 24.960 ± 0.149 f,g | 22.778 ± 0.293 c | 17.122 ± 0.171 d | ||||
| 100% | 25.089 ± 0.253 f,g | 23.569 ± 0.318 c | 17.552 ± 0.080 d | ||||
| Salvia fruticosa | 5% | 6.000 ± 0.000 a | 2.853 | 6.000 ± 0.000 a | 2.853 | 6.000 ± 0.000 a | 2.853 |
| 20% | 13.643 ± 0.494 c,d | 6.000 ± 0.000 a | 6.000 ± 0.000 a | ||||
| 50% | 14.289 ± 0.534 c,d | 8.213 ± 0.249 a | 7.149 ± 0.103 a,b | ||||
| 100% | 17.464 ± 0.253 d,e | 11.184 ± 0.209 a,b | 9.399 ± 0.148 b,c | ||||
| Crithmum maritimum | 5% | 6.000 ± 0.000 a | 5.644 | 6.000 ± 0.000 a | 5.644 | 6.000 ± 0.000 a | 5.644 |
| 20% | 6.000 ± 0.000 a | 6.000 ± 0.000 a | 6.000 ± 0.000 a | ||||
| 50% | 9.407 ± 0.138 a,b,c | 6.471 ± 0.066 a | 7.011 ± 0.164 a | ||||
| 100% | 11.128 ± 0.201 b,c | 7.689 ± 0.236 a | 7.527 ± 0.133 a,b | ||||
| Gentamicin | 10 µg | 30.348 ± 0.149 h | 0.00025 | 22.914 ± 0.134 c | 0.0005 | 20.948 ± 0.022 e | 0.00025 |
| Tetracycline | 30 µg | 41.125 ± 0.220 j | 0.002 | 10.426 ± 0.187 a,b | 0.032 | 26.897 ± 0.188 f | 0.001 |
| Cefaclor | 30 µg | 28.120 ± 0.052 g,h | 0.002 | 6.693 ± 0.097 a | 0.016 | 20.826 ± 0.048 e | 0.002 |
| Penicillin | 10 units | 22.355 ± 0.129 e | ND | 8.476 ± 0.038 a | ND | 18.719 ± 0.113 d,e | ND |
| Enrofloxacin | 5 µg | 36.118 ± 0.091 i | ND | 25.059 ± 0.091 c | ND | 24.690 ± 0.132 f | ND |
| Treatment | Concentration | Methicillin-Sensitive S. aureus | Methicillin-Resistant S. aureus | S. aureus ATCC 29213 |
|---|---|---|---|---|
| Thymus sibthorpii | x4 MIC | 95.134 ± 0.053 c | 95.817 ± 0.097 c | 93.528 ± 0.073 b |
| x2 MIC | 95.293 ± 0.053 c | 95.817 ± 0.097 c | 93.577 ± 0.042 b | |
| MIC | 95.275 ± 0.061 c | 95.786 ± 0.081 c | 93.359 ± 0.042 b | |
| x1/2 MIC | 95.364 ± 0.081 c | 95.364 ± 0.609 c | 93.577 ± 0.042 b | |
| Origanum vulgare | x4 MIC | 94.306 ± 0.239 c | 95.786 ± 0.047 c | 93.528 ± 0.126 b |
| x2 MIC | 95.170 ± 0.214 c | 95.770 ± 0.054 c | 93.455 ± 0.073 b | |
| MIC | 95.205 ± 0.110 c | 95.708 ± 0.027 c | 93.334 ± 0.183 b | |
| x1/2 MIC | 94.993 ± 0.152 c | 93.772 ± 1.001 c | 93.068 ± 0.373 b | |
| Salvia fruticosa | x2 MIC | 94.253 ± 0.583 c | 95.380 ± 0.311 c | 93.140 ± 0.414 b |
| MIC | 94.905 ± 0.186 c | 95.427 ± 0.027 c | 93.189 ± 0.168 b | |
| x1/2 MIC | 85.985 ± 12.555 c | 95.068 ± 0.241 c | 93.262 ± 0.374 b | |
| Crithmum maritimum | x2 MIC | 95.081 ± 0.242 c | 95.583 ± 0.241 c | 93.043 ± 0.484 b |
| MIC | 94.658 ± 0.692 c | 95.551 ± 0.124 c | 93.031 ± 0.364 b | |
| x1/2 MIC | 83.799 ± 7.710 c | 95.349 ± 0.216 c | 91.521 ± 1.505 b | |
| Gentamicin | x4 MIC | 95.275 ± 0.162 c | 95.458 ± 0.540 c | 93.261 ± 0.183 b |
| x2 MIC | 58.342 ± 13.212 b | 81.598 ± 1.935 b | 48.201 ± 16.185 a | |
| MIC | 32.198 ± 20.528 a | 77.899 ± 2.234 b | 57.994 ± 10.493 a | |
| x1/2 MIC | 43.957 ± 20.026 a,b | 69.860 ± 7.767 a | 58.745 ± 16.368 a | |
| Tetracycline | x4 MIC | 95.240 ± 0.242 c | 95.833 ± 0.000 c | 93.552 ± 0.042 b |
| x2 MIC | 95.187 ± 0.092 c | 95.754 ± 0.118 c | 93.504 ± 0.042 b | |
| MIC | 95.169 ± 0.061 c | 95.848 ± 0.071 c | 93.528 ± 0.192 b | |
| x1/2 MIC | 95.223 ± 0.170 c | 95.520 ± 0.450 c | 93.553 ± 0.151 b | |
| Cefaclor | x4 MIC | 95.152 ± 0.061 c | 95.630 ± 0.275 c | 93.407 ± 0.210 b |
| x2 MIC | 95.117 ± 0.200 c | 95.567 ± 0.282 c | 93.189 ± 0.294 b | |
| MIC | 95.205 ± 0.110 c | 95.770 ± 0.177 c | 92.462 ± 0.965 b | |
| x1/2 MIC | 92.508 ± 4.779 c | 95.817 ± 0.135 c | 83.106 ± 3.567 b | |
| Standard Error | 1.973 | 0.738 | 1.483 | |
| ANOVA p-value | <0.001 | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ersanli, C.; Tzora, A.; Skoufos, I.; Fotou, K.; Maloupa, E.; Grigoriadou, K.; Voidarou, C.; Zeugolis, D.I. The Assessment of Antimicrobial and Anti-Biofilm Activity of Essential Oils against Staphylococcus aureus Strains. Antibiotics 2023, 12, 384. https://doi.org/10.3390/antibiotics12020384
Ersanli C, Tzora A, Skoufos I, Fotou K, Maloupa E, Grigoriadou K, Voidarou C, Zeugolis DI. The Assessment of Antimicrobial and Anti-Biofilm Activity of Essential Oils against Staphylococcus aureus Strains. Antibiotics. 2023; 12(2):384. https://doi.org/10.3390/antibiotics12020384
Chicago/Turabian StyleErsanli, Caglar, Athina Tzora, Ioannis Skoufos, Konstantina Fotou, Eleni Maloupa, Katerina Grigoriadou, Chrysoula (Chrysa) Voidarou, and Dimitrios I. Zeugolis. 2023. "The Assessment of Antimicrobial and Anti-Biofilm Activity of Essential Oils against Staphylococcus aureus Strains" Antibiotics 12, no. 2: 384. https://doi.org/10.3390/antibiotics12020384
APA StyleErsanli, C., Tzora, A., Skoufos, I., Fotou, K., Maloupa, E., Grigoriadou, K., Voidarou, C., & Zeugolis, D. I. (2023). The Assessment of Antimicrobial and Anti-Biofilm Activity of Essential Oils against Staphylococcus aureus Strains. Antibiotics, 12(2), 384. https://doi.org/10.3390/antibiotics12020384