Chemical and Biological Studies of Achillea setacea Herba Essential Oil—First Report on Some Antimicrobial and Antipathogenic Features
Abstract
1. Introduction
2. Results and Discussion
2.1. Physico-Chemical Characterization
2.2. Antioxidant Activity
2.3. Antimicrobial Activity
2.4. Correlation between Inhibition of Microbial Adherence and the Release of Extracellular NO
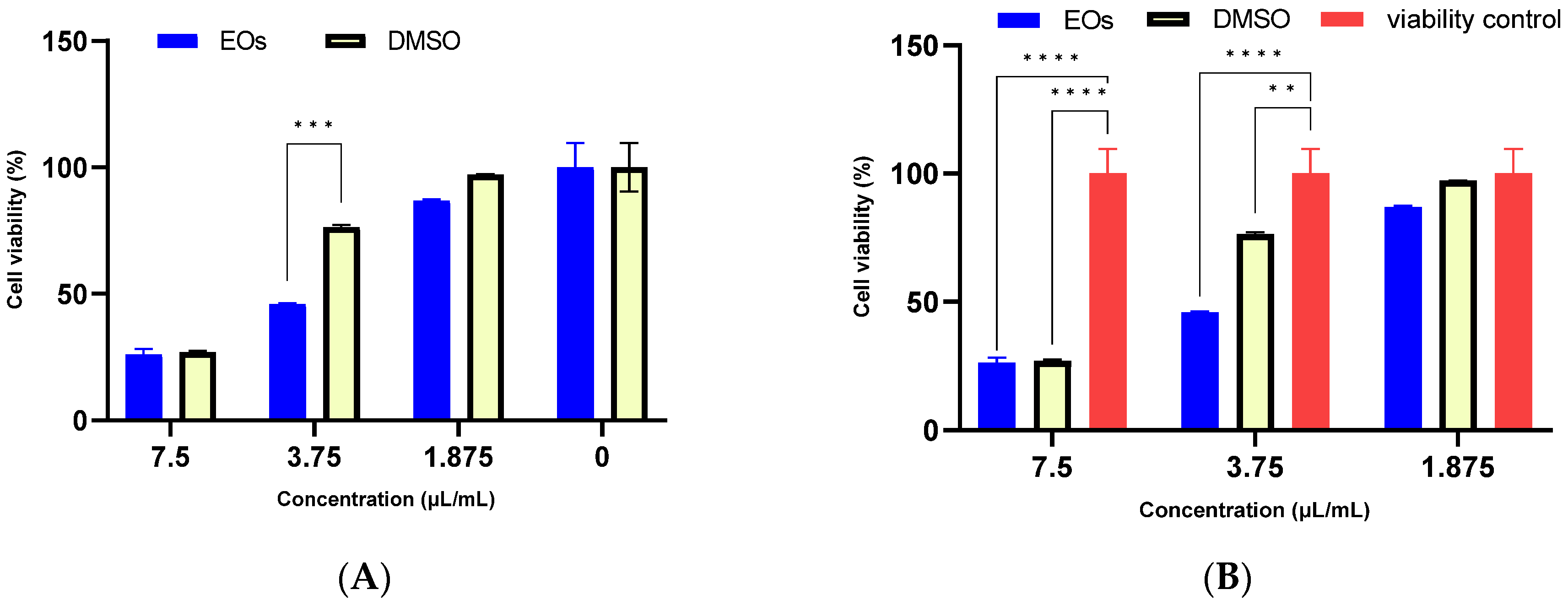
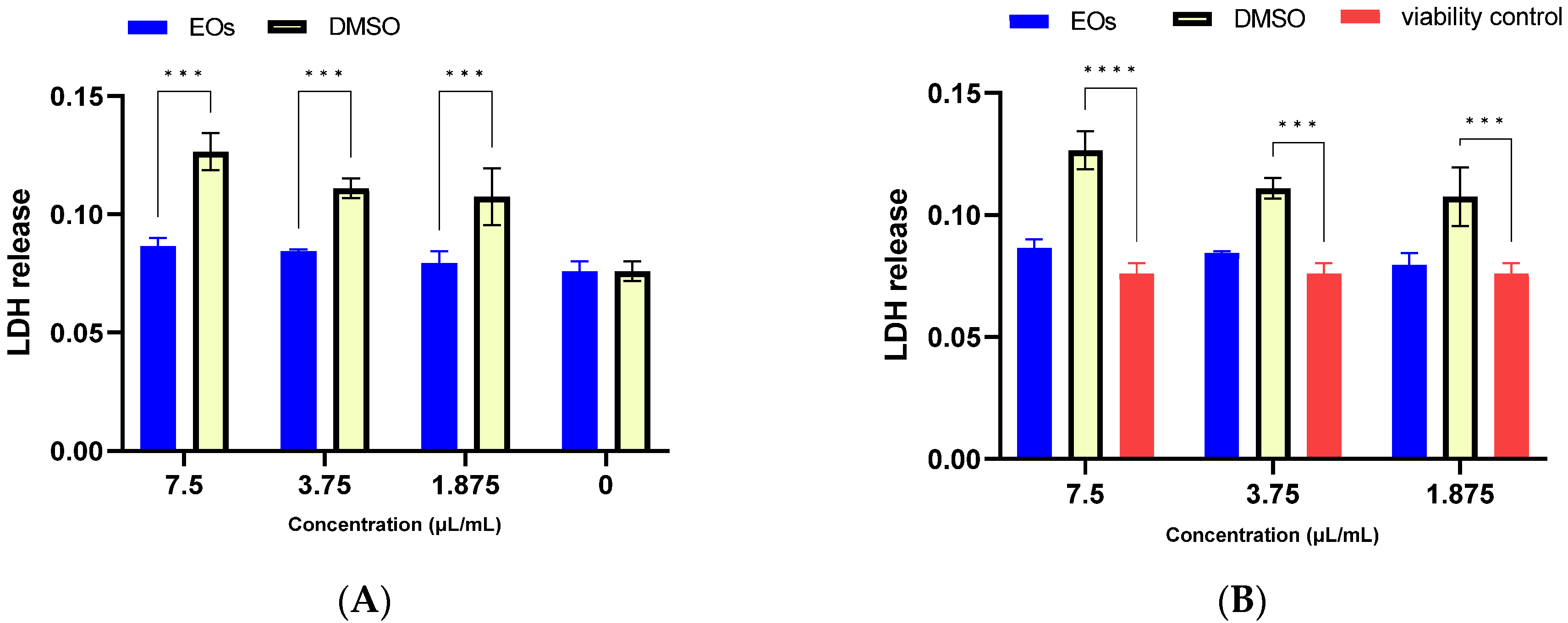
2.5. Biocompatibility of A. setacea Essential Oil
3. Materials and Methods
3.1. Plant Material
3.2. Essential Oil Isolation
3.3. Gas Chromatography-Mass Spectrometry Analysis
3.4. Antioxidant Activity
3.4.1. DPPH
3.4.2. FRAP
3.4.3. CUPRAC
3.5. Antimicrobial Activity
3.5.1. Microbial Strain
3.5.2. Qualitative Assay of Antimicrobial Activity
3.5.3. Quantitative Assay of Antibacterial Activity
3.5.4. Quantitative Assay of Antifungal Activity
3.5.5. Evaluation of NO Release
3.5.6. Microbial Adhesion Capacity to the Inert Substrate
3.6. Biocompatibility of Essential Oil
3.6.1. LDH
3.6.2. MTT
3.6.3. Selectivity Index
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Si, X.-T.; Zhang, M.-L.; Shi, Q.-W.; Kiyota, H. Chemical Constituents of the Plants in the GenusAchillea. Chem. Biodivers. 2006, 3, 1163–1180. [Google Scholar] [CrossRef] [PubMed]
- Marchart, E.; Kopp, B. Capillary Electrophoretic Separation and Quantification of Flavone-O- and C-Glycosides in Achillea setacea W. et K. J. Chromatogr. B 2003, 792, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Eisenman, S.W.; Zaurov, D.E.; Struwe, L. Medicinal Plants of Central Asia: Uzbekistan and Kyrgyzstan; Eisenman, S.W., Zaurov, D.E., Struwe, L., Eds.; Springer: New York, NY, USA, 2013; ISBN 978-1-4614-3911-0. [Google Scholar]
- Mohammadhosseini, M.; Sarker, S.D.; Akbarzadeh, A. Chemical Composition of the Essential Oils and Extracts of Achillea Species and Their Biological Activities: A Review. J. Ethnopharmacol. 2017, 199, 257–315. [Google Scholar] [CrossRef]
- Başer, K.H.C.; Hüsnü, K.; Başer, C. Essential Oils of Achillea Species of Turkey. Volatiles Essent. Oils 2016, 3, 1–14. [Google Scholar]
- Karamenderes, C.; Karabay, N.Ü.; Zeybek, U. Türkiye’nin Farkli Lokalitelerinden Toplanan Achillea setacea Waldst. Kit. Uçucu Yağinin Bileşimi ve Antimikrobiyal Aktivitesi. Ankara Univ. Eczac. Fak. Derg. 2003, 32, 113–120. [Google Scholar] [CrossRef]
- Ünlü, M.; Daferera, D.; Dönmez, E.; Polissiou, M.; Tepe, B.; Sökmen, A. Compositions and the in Vitro Antimicrobial Activities of the Essential Oils of Achillea setacea and Achillea Teretifolia (Compositae). J. Ethnopharmacol. 2002, 83, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Turkmenoglu, F.P.; Agar, O.T.; Akaydin, G.; Hayran, M.; Demirci, B. Characterization of Volatile Compounds of Eleven Achillea Species from Turkey and Biological Activities of Essential Oil and Methanol Extract of A. Hamzaoglui Arabaci & Budak. Molecules 2015, 20, 11432–11458. [Google Scholar] [CrossRef]
- Rezaei, F.; Jamei, R.; Heidari, R.; Maleki, R. Chemical Composition and Antioxidant Activity of Oil from Wild Achillea setacea and A. Vermicularis. Int. J. Food Prop. 2017, 20, 1522–1531. [Google Scholar] [CrossRef]
- Yener, I.; Yilmaz, M.A.; Olmez, O.T.; Akdeniz, M.; Tekin, F.; Hasimi, N.; Alkan, M.H.; Ozturk, M.; Ertas, A. A Detailed Biological and Chemical Investigation of Sixteen Achillea Species’ Essential Oils via Chemometric Approach. Chem. Biodivers. 2020, 17, e1900484. [Google Scholar] [CrossRef]
- Buleandra, M.; Moldovan, Z.; Badea, I.A.; David, I.G.; Popa, D.E.; Oprea, E.; Caglar, T.A.T.; Basaga, S.H. Comparative Assessment of the Volatile Profile, Antioxidant Capacity and Cytotoxic Potential of Different Preparation of Millefolli Herba Samples. Rev. Chim. 2020, 71, 69–78. [Google Scholar] [CrossRef]
- Sánchez, P.; Vélez-del-Burgo, A.; Suñén, E.; Martínez, J.; Postigo, I. Fungal Allergen and Mold Allergy Diagnosis: Role and Relevance of Alternaria Alternata Alt a 1 Protein Family. J. Fungi 2022, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Alen Coutinho, I.; Lopes, M.; Lima, F.; Ventura, C.; Rabadão, E.; Alfaro, T.; da Cunha, J.S.; Regateiro, F.S. Concomitant Allergic Bronchopulmonary Aspergillosis and Eosinophilic Granulomatosis with Polyangiitis after Aspergillus Niger Infection. Pulmonology 2022, 28, 231–234. [Google Scholar] [CrossRef]
- Sandoval-Denis, M.; Gené, J.; Sutton, D.A.; Wiederhold, N.P.; Cano-Lira, J.F.; Guarro, J. New Species of Cladosporium Associated with Human and Animal Infections. Persoonia-Mol. Phylogeny Evol. Fungi 2016, 36, 281–298. [Google Scholar] [CrossRef]
- Lianou, D.T.; Skoulakis, A.; Michael, C.K.; Katsarou, E.I.; Chatzopoulos, D.C.; Solomakos, N.; Tsilipounidaki, K.; Florou, Z.; Cripps, P.J.; Katsafadou, A.I.; et al. Isolation of Listeria Ivanovii from Bulk-Tank Milk of Sheep and Goat Farms—From Clinical Work to Bioinformatics Studies: Prevalence, Association with Milk Quality, Antibiotic Susceptibility, Predictors, Whole Genome Sequence and Phylogenetic Relationships. Biology 2022, 11, 871. [Google Scholar] [CrossRef] [PubMed]
- Matto, C.; D’Alessandro, B.; Mota, M.I.; Braga, V.; Buschiazzo, A.; Gianneechini, E.; Varela, G.; Rivero, R. Listeria Innocua Isolated from Diseased Ruminants Harbour Minor Virulence Genes of L. Monocytogenes. Vet. Med. Sci. 2022, 8, 735–740. [Google Scholar] [CrossRef]
- Nisa, I.; Qasim, M.; Yasin, N.; Ullah, R.; Ali, A. Shigella Flexneri: An Emerging Pathogen. Folia Microbiol. 2020, 65, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torrado, R.; Querol, A. Opportunistic Strains of Saccharomyces Cerevisiae: A Potential Risk Sold in Food Products. Front. Microbiol. 2016, 6, 01522. [Google Scholar] [CrossRef]
- Kumar, K.; Askari, F.; Sahu, M.; Kaur, R. Candida Glabrata: A Lot More Than Meets the Eye. Microorganisms 2019, 7, 39. [Google Scholar] [CrossRef]
- Prodan, I. Achillea. In Flora României; Săvulescu, T., Ed.; Editura Academiei Române: Bucureşti, Romania, 1964; Volume 9, p. 390. [Google Scholar]
- Ciocârlan, V. Flora Ilustrată a României: Pteridophyta et Spermatophyta; Ceres: Bucureşti, Romania, 2009; p. 798. ISBN 978-973-40-0817-9. [Google Scholar]
- Sârbu, I.; Ștefan, N.; Oprea, A. Plante Vasculare Din România–Determinator Ilustrat de Teren; Victor B Victor: Bucuresti, Romania, 2013; p. 802. ISBN 9786068149080. [Google Scholar]
- Héthelyi, É.; Dános, B.; Tétényi, P.; Héthelyi; Dános, B.; Tétényi, P. Phytochemical Studies on the Essential Oils of Species Belonging to TheAchillea Genus by Gas Chromatography/Mass Spectrometry. Biol. Mass Spectrom. 1989, 18, 629–636. [Google Scholar] [CrossRef]
- Zitterl-Eglseer, K.; Jurenitsch, J.; Korhammer, S.; Haslinger, E.; Sosa, S.; Loggia, R.; Kubelka, W.; Franz, C. Entzündungshemmende Sesquiterpenlactone von Achillea setacea. Planta Med. 1991, 57, 444–446. [Google Scholar] [CrossRef]
- Todorova, M.; Vogler, B.; Tsankova, E. Terpenoids from Achillea setacea. Z. Naturforsch. C 2000, 55, 840–842. [Google Scholar] [CrossRef]
- Lemberkovics, É.; Kéry, Á.; Kakasy, A.; Szoke, É.; Simándi, B. Effect of Extraction Methods on the Composition of Essential Oils. Acta Hortic. 2004, 597, 49–56. [Google Scholar] [CrossRef]
- Muhammad, S.; Abdul Khalil, H.P.S.; Abd Hamid, S.; Danish, M.; Marwan, M.; Yunardi, Y.; Abdullah, C.K.; Faisal, M.; Yahya, E.B. Characterization of Bioactive Compounds from Patchouli Extracted via Supercritical Carbon Dioxide (SC-CO2) Extraction. Molecules 2022, 27, 6025. [Google Scholar] [CrossRef]
- Apak, R.; Özyürek, M.; Güçlü, K.; Çapanoğlu, E. Antioxidant Activity/Capacity Measurement. 1. Classification, Physicochemical Principles, Mechanisms, and Electron Transfer (ET)-Based Assays. J. Agric. Food Chem. 2016, 64, 997–1027. [Google Scholar] [CrossRef] [PubMed]
- Kordali, S.; Cakir, A.; Mavi, A.; Kilic, H.; Yildirim, A. Screening of Chemical Composition and Antifungal and Antioxidant Activities of the Essential Oils from Three Turkish Artemisia Species. J. Agric. Food Chem. 2005, 53, 1408–1416. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Mikallou, M.; Petropoulos, S.; Tzortzakis, N. Profiling of Essential Oils Components and Polyphenols for Their Antioxidant Activity of Medicinal and Aromatic Plants Grown in Different Environmental Conditions. Agronomy 2020, 10, 727. [Google Scholar] [CrossRef]
- Xu, G.; Guo, J.; Sun, C. Eucalyptol Ameliorates Early Brain Injury after Subarachnoid Haemorrhage via Antioxidant and Anti-Inflammatory Effects in a Rat Model. Pharm. Biol. 2021, 59, 112–118. [Google Scholar] [CrossRef]
- Drikvandi, P.; Bahramikia, S.; Alirezaei, M. Modulation of the Antioxidant Defense System in Liver, Kidney, and Pancreas Tissues of Alloxan-induced Diabetic Rats by Camphor. J. Food Biochem. 2020, 44, e13527. [Google Scholar] [CrossRef] [PubMed]
- Capuzzo, A.; Occhipinti, A.; Maffei, M.E. Antioxidant and Radical Scavenging Activities of Chamazulene. Nat. Prod. Res. 2014, 28, 2321–2323. [Google Scholar] [CrossRef]
- Aati, H.; El-Gamal, A.; Kayser, O. Chemical Composition and Biological Activity of the Essential Oil from the Root of Jatropha Pelargoniifolia Courb. Native to Saudi Arabia. Saudi Pharm. J. 2019, 27, 88–95. [Google Scholar] [CrossRef]
- Karakaya, S.; Yilmaz, S.V.; Özdemir, Ö.; Koca, M.; Pınar, N.M.; Demirci, B.; Yıldırım, K.; Sytar, O.; Turkez, H.; Baser, K.H.C. A Caryophyllene Oxide and Other Potential Anticholinesterase and Anticancer Agent in Salvia verticillata Subsp. Amasiaca (Freyn & Bornm.) Bornm. (Lamiaceae). J. Essent. Oil Res. 2020, 32, 512–525. [Google Scholar] [CrossRef]
- Nogueira Neto, J.D.; de Almeida, A.A.C.; da Silva Oliveira, J.; dos Santos, P.S.; de Sousa, D.P.; de Freitas, R.M. Antioxidant Effects of Nerolidol in Mice Hippocampus After Open Field Test. Neurochem. Res. 2013, 38, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, S.Y.; Hong, C.Y.; Gwak, K.S.; Park, M.J.; Smith, D.; Choi, I.G. Whitening and Antioxidant Activities of Bornyl Acetate and Nezukol Fractionated from Cryptomeria Japonica Essential Oil. Int. J. Cosmet. Sci. 2013, 35, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Aydin, E.; Türkez, H.; Geyikoğlu, F. Antioxidative, Anticancer and Genotoxic Properties of α-Pinene on N2a Neuroblastoma Cells. Biologia 2013, 68, 1004–1009. [Google Scholar] [CrossRef]
- Quintans-Júnior, L.; Moreira, J.C.F.; Pasquali, M.A.B.; Rabie, S.M.S.; Pires, A.S.; Schröder, R.; Rabelo, T.K.; Santos, J.P.A.; Lima, P.S.S.; Cavalcanti, S.C.H.; et al. Antinociceptive Activity and Redox Profile of the Monoterpenes (+)-Camphene, p -Cymene, and Geranyl Acetate in Experimental Models. ISRN Toxicol. 2013, 2013, 459530. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.-G.; Shibamoto, T. Antioxidant Activities of Volatile Components Isolated FromEucalyptus Species. J. Sci. Food Agric. 2001, 81, 1573–1579. [Google Scholar] [CrossRef]
- Yammine, J.; Chihib, N.-E.; Gharsallaoui, A.; Dumas, E.; Ismail, A.; Karam, L. Essential Oils and Their Active Components Applied as: Free, Encapsulated and in Hurdle Technology to Fight Microbial Contaminations. A Review. Heliyon 2022, 8, e12472. [Google Scholar] [CrossRef]
- Beicu, R.; Alexa, E.; Obiștioiu, D.; Cocan, I.; Imbrea, F.; Pop, G.; Circioban, D.; Moisa, C.; Lupitu, A.; Copolovici, L.; et al. Antimicrobial Potential and Phytochemical Profile of Wild and Cultivated Populations of Thyme (Thymus Sp.) Growing in Western Romania. Plants 2021, 10, 1833. [Google Scholar] [CrossRef]
- Morcia, C.; Malnati, M.; Terzi, V. In Vitro Antifungal Activity of Terpinen-4-Ol, Eugenol, Carvone, 1,8-Cineole (Eucalyptol) and Thymol against Mycotoxigenic Plant Pathogens. Food Addit. Contam. Part A 2011, 29, 415–422. [Google Scholar] [CrossRef]
- Kotan, R.; Kordali, S.; Cakir, A. Screening of Antibacterial Activities of Twenty-One Oxygenated Monoterpenes. Z. Naturforsch. C 2007, 62, 507–513. [Google Scholar] [CrossRef]
- Luo, L.; Li, G.; Luan, D.; Yuan, Q.; Wei, Y.; Wang, X. Antibacterial Adhesion of Borneol-Based Polymer via Surface Chiral Stereochemistry. ACS Appl. Mater. Interfaces 2014, 6, 19371–19377. [Google Scholar] [CrossRef] [PubMed]
- Fadli, M.; Bolla, J.-M.; Mezrioui, N.-E.; Pagès, J.-M.; Hassani, L. First Evidence of Antibacterial and Synergistic Effects of Thymus Riatarum Essential Oil with Conventional Antibiotics. Ind. Crop. Prod. 2014, 61, 370–376. [Google Scholar] [CrossRef]
- Haiyan, L.; Chongxin, X.; Xiao, Z.; Ying, L.; Xianjin, L. Antibacterial Effect of Limonene on Food-Borne Pathogens. J. Zhejiang Univ. (Agric. Life Sci.) 2016, 42, 306–312. [Google Scholar]
- Leite, A.M.; de Lima, E.O.; de Souza, E.L.; de Diniz, M.F.F.M.; Trajano, V.N.; deMedeiros, I.A. Inhibitory Effect of Beta-Pinene, Alpha-Pinene and Eugenol on the Growth of Potential Infectious Endocarditis Causing Gram-Positive Bacteria. Rev. Bras. Ciênc. Farm. 2007, 43, 121–126. [Google Scholar] [CrossRef]
- Moro, I.J.; Gondo, G.D.G.A.; Pierri, E.G.; Pietro, R.C.L.R.; Soares, C.P.; de Sousa, D.P.; dos Santos, A.G. Evaluation of Antimicrobial, Cytotoxic and Chemopreventive Activities of Carvone and Its Derivatives. Braz. J. Pharm. Sci. 2018, 53, e00076. [Google Scholar] [CrossRef]
- Catherine, A.A.; Deepika, H.; Negi, P.S. Antibacterial Activity of Eugenol and Peppermint Oil in Model Food Systems. J. Essent. Oil Res. 2012, 24, 481–486. [Google Scholar] [CrossRef]
- da Silva, J.K.R.; Andrade, E.H.A.; Guimarães, E.F.; Maia, J.G.S. Essential Oil Composition, Antioxidant Capacity and Antifungal Activity of Piper Divaricatum. Nat. Prod. Commun. 2010, 5, 477–480. [Google Scholar] [CrossRef] [PubMed]
- Castro, J.C.; Endo, E.H.; de Souza, M.R.; Zanqueta, E.B.; Polonio, J.C.; Pamphile, J.A.; Ueda-Nakamura, T.; Nakamura, C.V.; Dias Filho, B.P.; de Abreu Filho, B.A. Bioactivity of Essential Oils in the Control of Alternaria Alternata in Dragon Fruit (Hylocereus Undatus Haw.). Ind. Crop. Prod. 2017, 97, 101–109. [Google Scholar] [CrossRef]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory Activity of Yarrow Essential Oil on Listeria Planktonic Cells and Biofilms. Food Control. 2013, 29, 125–130. [Google Scholar] [CrossRef]
- Fierascu, I.; Ungureanu, C.; Avramescu, S.M.; Fierascu, R.C.; Ortan, A.; Soare, L.C.; Paunescu, A. In Vitro Antioxidant and Antifungal Properties of Achillea Millefolium L. Rom. Biotechnol. Lett. 2015, 20, 10626–10636. [Google Scholar]
- Khan, F.A.; Khan, N.M.; Ahmad, S.; Nasruddin; Aziz, R.; Ullah, I.; Almehmadi, M.; Allahyani, M.; Alsaiari, A.A.; Aljuaid, A. Phytochemical Profiling, Antioxidant, Antimicrobial and Cholinesterase Inhibitory Effects of Essential Oils Isolated from the Leaves of Artemisia Scoparia and Artemisia Absinthium. Pharmaceuticals 2022, 15, 1221. [Google Scholar] [CrossRef]
- Polatoğlu, K.; Demirci, B.; Demirci, F.; Gören, N.; Başer, K.H.C. Biological Activity and Essential Oil Composition of Two New Tanacetum Chiliophyllum (Fisch. & Mey.) Schultz Bip. Var. Chiliophyllum Chemotypes from Turkey. Ind. Crop. Prod. 2012, 39, 97–105. [Google Scholar] [CrossRef]
- Mssillou, I.; Agour, A.; Allali, A.; Saghrouchni, H.; Bourhia, M.; El Moussaoui, A.; Salamatullah, A.M.; Alzahrani, A.; Aboul-Soud, M.A.M.; Giesy, J.P.; et al. Antioxidant, Antimicrobial, and Insecticidal Properties of a Chemically Characterized Essential Oil from the Leaves of Dittrichia Viscosa L. Molecules 2022, 27, 2282. [Google Scholar] [CrossRef]
- Gilmore, B.F.; Flynn, P.B.; O’Brien, S.; Hickok, N.; Freeman, T.; Bourke, P. Cold Plasmas for Biofilm Control: Opportunities and Challenges. Trends Biotechnol. 2018, 36, 627–638. [Google Scholar] [CrossRef] [PubMed]
- Arce Miranda, J.E.; Sotomayor, C.E.; Albesa, I.; Paraje, M.G. Oxidative and Nitrosative Stress in Staphylococcus Aureus Biofilm. FEMS Microbiol. Lett. 2011, 315, 23–29. [Google Scholar] [CrossRef]
- Barraud, N.; Hassett, D.J.; Hwang, S.-H.; Rice, S.A.; Kjelleberg, S.; Webb, J.S. Involvement of Nitric Oxide in Biofilm Dispersal of Pseudomonas Aeruginosa. J. Bacteriol. 2006, 188, 7344–7353. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.C.; Vázquez-Torres, A. Reactive Nitrogen Species in Host–Bacterial Interactions. Curr. Opin. Immunol. 2019, 60, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Arasimowicz-Jelonek, M.; Floryszak-Wieczorek, J. Nitric Oxide in the Offensive Strategy of Fungal and Oomycete Plant Pathogens. Front. Plant Sci. 2016, 7, 252. [Google Scholar] [CrossRef]
- Mayrs, E.B.C. British Pharmacopoeia Appendix 9; Medicines & Healthcare products Regulatory Agency: London, UK, 2017; Volume IV. [Google Scholar]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; ISBN 9781932633214. [Google Scholar]
- Ahmed, A.F.; Attia, F.A.K.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant Activity and Total Phenolic Content of Essential Oils and Extracts of Sweet Basil (Ocimum basilicum L.) Plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC Assays for Estimating Antioxidant Activity from Guava Fruit Extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L. Essential Oils as Antioxidants: Their Evaluation by DPPH, ABTS, FRAP, CUPRAC, and β-Carotene Bleaching Methods. Mon. Für Chem.-Chem. Mon. 2016, 147, 2083–2091. [Google Scholar] [CrossRef]
- Bagla, V.P.; McGaw, L.J.; Elgorashi, E.E.; Eloff, J.N. Antimicrobial Activity, Toxicity and Selectivity Index of Two Biflavonoids and a Flavone Isolated from Podocarpus Henkelii (Podocarpaceae) Leaves. BMC Complement. Altern. Med. 2014, 14, 383. [Google Scholar] [CrossRef] [PubMed]
- Asong, J.A.; Amoo, S.O.; McGaw, L.J.; Nkadimeng, S.M.; Aremu, A.O.; Otang-Mbeng, W. Antimicrobial Activity, Antioxidant Potential, Cytotoxicity and Phytochemical Profiling of Four Plants Locally Used against Skin Diseases. Plants 2019, 8, 350. [Google Scholar] [CrossRef] [PubMed]
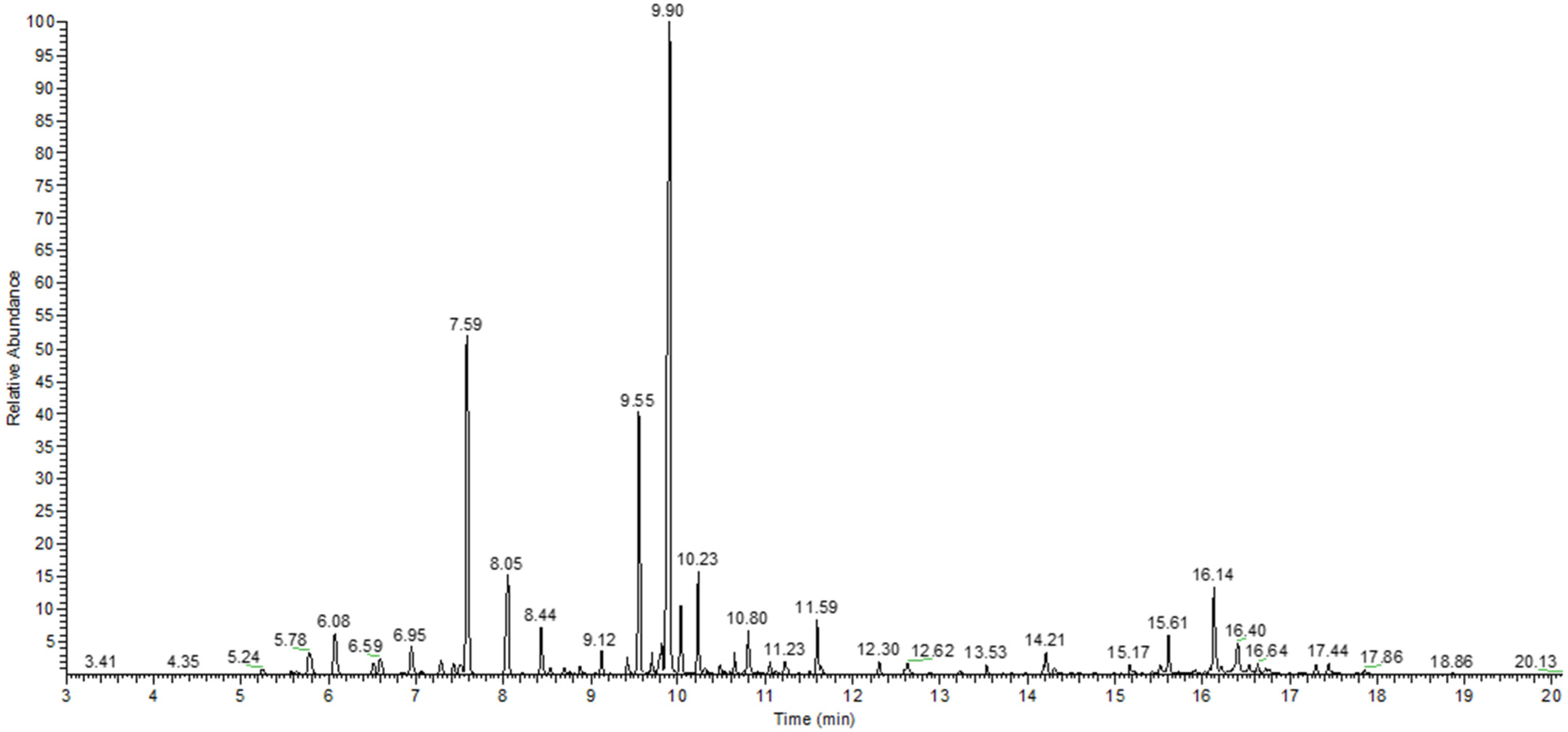
| No. | Compound Name | Compound Classes | RI [l] | Relative Area (%) |
|---|---|---|---|---|
| 1 | Santolina triene | MH [a] | 915 | 0.35 |
| 2 | Tricyclene | MH [a] | 928 | 0.15 |
| 3 | α-Thujene | MH [a] | 932 | 0.17 |
| 4 | α-Pinene | MH [a] | 940 | 1.40 |
| 5 | Camphene | MH [a] | 956 | 2.45 |
| 6 | Sabinene (β-Thujene) | MH [a] | 983 | 0.58 |
| 7 | β-Pinene | MH [a] | 985 | 0.89 |
| 8 | 2,3-Dehydro-1,8-cineole | ME [c] | 998 | 0.19 |
| 9 | Yomogi alcohol | MA [b] | 1006 | 1.31 |
| 10 | α-Phellandrene | MH [a] | 1013 | 0.17 |
| 11 | α -Terpinene | MH [a] | 1025 | 0.69 |
| 12 | p-Cymene | MH [a] | 1033 | 0.49 |
| 13 | Limonene | MH [a] | 1038 | 0.46 |
| 14 | Eucalyptol (1,8-Cineole) | ME [c] | 1042 | 14.94 |
| 15 | Artemisia ketone | MK [e] | 1067 | 4.70 |
| 16 | cis-Sabinene hydrate | MA [b] | 1078 | 0.07 |
| 17 | Artemisia alcohol | MA [b] | 1094 | 1.73 |
| 18 | α-Terpinolene | MH [b] | 1097 | 0.28 |
| 19 | trans-Sabinene hydrate | MA [b] | 1111 | 0.09 |
| 20 | α-Thujone | MK [e] | 1117 | 0.30 |
| 21 | cis-p-Menth-2-en-1-ol | MA [b] | 1132 | 0.85 |
| 22 | trans-p-Menth-2-en-1-ol | MA [b] | 1151 | 0.71 |
| 23 | Camphor | MK [e] | 1160 | 10.13 |
| 24 | cis-Chrysanthenol | MA [c] | 1169 | 0.76 |
| 25 | Lavandulol | MA [b] | 1177 | 0.45 |
| 26 | Borneol | MA [b] | 1181 | 32.97 |
| 27 | Terpinen-4-ol | MA [b] | 1191 | 2.36 |
| 28 | α-Terpineol | MA [b] | 1204 | 3.23 |
| 29 | cis-Piperitol | MA [b] | 1220 | 0.15 |
| 30 | cis-Carveol | MA [b] | 1232 | 0.64 |
| 31 | trans-Crysanthenyl acetate | MAE [d] | 1242 | 1.62 |
| 32 | Carvone | MK [e] | 1259 | 0.38 |
| 33 | Geraniol | MA [b] | 1264 | 0.14 |
| 34 | Piperitone | MK [e] | 1271 | 0.46 |
| 35 | cis-Chrysanthenyl acetate | MAE [d] | 1273 | 0.24 |
| 36 | Bornyl acetate | MAE [d] | 1296 | 1.49 |
| 37 | trans-Carvyl acetate | MAE [d] | 1348 | 0.43 |
| 38 | Eugenol | PM [f] | 1372 | 0.29 |
| 39 | cis-Jasmone | CK [g] | 1417 | 0.16 |
| 40 | β-caryophyllene | SH [h] | 1441 | 0.36 |
| 41 | α-Humulene | SH [h] | 1476 | 0.10 |
| 42 | γ-Muurolene | SH [h] | 1494 | 0.25 |
| 43 | Germacrene D | SH [h] | 1503 | 0.26 |
| 44 | Viridiflorene (Ledene) | SH [h] | 1509 | 0.11 |
| 45 | δ-Cadinene | SH [h] | 1542 | 0.12 |
| 46 | trans-β-Nerolidol | SA [i] | 1575 | 0.28 |
| 47 | Spathulenol | SA [i] | 1604 | 0.41 |
| 48 | Caryophyllene oxide | SE [j] | 1612 | 1.33 |
| 49 | γ-Eudesmol | SA [i] | 1659 | 3.23 |
| 50 | α-Eudesmol | SA [i] | 1682 | 1.46 |
| 51 | α-Bisabolol | SA [i] | 1704 | 0.31 |
| 52 | Chamazulene | AH [k] | 1756 | 0.35 |
| Total | 97.43 | |||
| Monoterpenes hydrocarbons | 8.07 | |||
| Monoterpenes alcohols and esters | 49.23 | |||
| Monoterpenes ketones | 15.97 | |||
| Sesquiterpenes hydrocarbons | 1.19 | |||
| Sesquiterpenes alcohols and ethers | 6.75 |
| DPPH (IC50, mg/mL) | FRAP (µM TE/mg) | CUPRAC (µM TE/mg) | |
|---|---|---|---|
| A. setacea essential oil | 12.38 ± 0.63 | 14.70 ± 0.99 | 6.17 ± 0.68 |
| BHT | 0.64 ± 0.07 | 823.46 ± 14.69 | 988.03 ± 11.35 |
| p-value | <0.0001 | <0.0001 | <0.0001 |
| IDZ * (mm) | MIC (µL/mL) | MMC (µL/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| A. setacea EO | DMSO | A. setacea EO | DMSO | p-Value | A. setacea EO | DMSO | ||
| MIC ** | MV% # | MV% # | ||||||
| S. aureus | 13.00 ± 1.00 | - | 7.50 | 1.62 ± 0.28 | 55.45 ± 2.53 | 0.001116 | 7.50 | >30.00 |
| B. cereus | 20.00 ± 2.00 | - | 3.75 | 0.71 ± 0.29 | 82.57 ± 2.76 | 0.000574 | 3.75 | >30.00 |
| L. inoccua | NA ## | - | 3.75 | 3.57 ± 0.33 | 80.46 ± 3.95 | 0.001326 | 15.00 | >30.00 |
| L. ivanovii | NA | - | 1.88 | 0.59 ± 0.42 | 102.04 ± 2.28 | 0.000261 | 1.88 | >30.00 |
| E. coli | 8.33 ± 0.58 | - | 7.50 | 23.97 ± 0.76 | 88.59 ± 2.53 | 0.000835 | 7.50 | >30.00 |
| S. flexneris | 8.00 ± 2.00 | - | 30.00 | >30 | 12.55 ± 8.46 | - | 30.00 | >30.00 |
| S. enterica | 6.33 ± 0.58 | - | 15.00 | 36.75 ± 2.03 | 49.23 ± 1.45 | 0.019399 | 30.00 | >30.00 |
| C. glabrata | 10.00 ± 1.00 | - | 3.75 | 4.40 ± 0.93 | 80.57 ± 1.87 | 0.000376 | 3.75 | >30.00 |
| S. cerevisiae | 13.33 ± 0.58 | - | 1.88 | 6.19 ± 0.34 | 46.00 ± 7.74 | 0.350835 | 3.75 | >30.00 |
| A. niger | 8.00 ± 1.00 | - | 0.78 | 7.32 ± 3.45 | 82.93 ± 0.00 | 0.001039 | 12.50 | >12.50 |
| R. nigricans | 6.33 ± 0.58 | - | 1.56 | 32.89 ± 1.86 | 68.42 ± 7.44 | 0.022511 | >12.50 | >12.50 |
| A. alternata | 7.66 ± 0.58 | - | 1.56 | 0.00 ± 0.40 | 114.61 ± 7.15 | 0.001946 | >12.50 | >12.50 |
| C. cladosporioides | 9.33 ± 0.16 | - | 3.125 | 7.69 ± 4.35 | 70.77 ± 1.09 | 0.002517 | >12.50 | >12.50 |
| EO | DMSO | p-Value | EO | DMSO | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| MIC/2 | MA (%) * | MA (%) * | MIC/4 | MA (%) * | MA (%) * | |||
| S. aureus | 3.75 | 34.38 ± 12.64 | 60.84 ± 2.30 | 0.100432 | 1.88 | 78.57 ± 11.78 | 65.06 ± 2.27 | 0.252256 |
| B. cereus | 1.88 | 14.19 ± 1.76 | 68.73 ± 3.71 | 0.002822 | 0.94 | 12.60 ± 2.43 | 39.93 ± 3.14 | 0.010389 |
| L. inoccua | 1.88 | 1.87 ± 0.64 | 54.57 ± 0.83 | 0.000198 | 0.94 | 1.92 ± 0.70 | 58.70 ± 2.33 | 0.000917 |
| L. ivanovii | 0.94 | 7.08 ± 0.24 | 78.80 ± 2.16 | 0.000459 | <0.94 | - | - | - |
| E. coli | 3.75 | 101.76 ± 2.15 | 134.88 ± 14.75 | 0.088098 | 1.88 | 104.18 ± 5.69 | 150.39 ± 21.55 | 0.099298 |
| S. enterica | 7.50 | 13.02 ± 3.66 | 25.99 ± 2.02 | 0.048218 | 3.75 | 11.52 ± 0.27 | 27.20 ± 3.73 | 0.027284 |
| C. glabrata | 1.88 | 0.18 ± 0.25 | 70.22 ± 2.72 | 0.000760 | 0.94 | 22.64 ± 0.97 | 127.84 ± 2.02 | 0.000227 |
| C. cladosporioides | 1.56 | 32.81 ± 4.42 | 105.88 ± 13.87 | 0.687280 | 0.78 | 40.63 ± 2.21 | 86.27 ± 11.09 | 0.029350 |
| R. nigricans | 0.78 | 23.64 ± 1.29 | 58.18 ± 1.29 | 0.001392 | 0.39 | 25.45 ± 0.00 | 49.09 ± 2.57 | 0.005858 |
| A. niger | 0.39 | 87.5 ± 13.26 | 229.69 ± 15.47 | 0.010111 | 0.20 | 120.21 ± 2.21 | 223.42 ± 12.31 | 0.007262 |
| A. alternata | 0.78 | 118.39 ± 13.00 | 133.33 ± 17.88 | 0.440060 | 0.39 | 145.98 ± 16.26 | 112.64 ± 1.63 | 0.102062 |
| EO | DMSO | p-Value | EO | DMSO | p-Value | |||
|---|---|---|---|---|---|---|---|---|
| MIC/2 | ENOC * | ENOC * | MIC/4 | ENOC * | ENOC * | |||
| S. aureus | 3.75 | 1.82 ± 0.14 | 0.39 ± 0.14 | <0.000001 | 1.88 | 0.18 ± 0.14 | 1.41 ± 0.14 | <0.00001 |
| L. inoccua | 1.88 | 4.63 ± 0.43 | 3.55 ± 0.58 | <0.0001 | 0.94 | 5.49 ± 0.43 | 3.25 ± 0.14 | <0.000001 |
| L. ivanovii | 0.94 | 2.14 ± 0.14 | NA ** | <0.000001 | 0.47 | 0.92 ± 0.14 | 0.51 ± 0.14 | >0.05 |
| S. flexneri | 15.00 | NA | 0.82 ± 0.14 | <0.001 | 7.50 | 0.82 ± 0.14 | 0.41 ± 0.14 | >0.05 |
| E. coli | 3.75 | 2.43 ± 0.14 | 1.20 ± 0.43 | <0.00001 | 1.88 | 1.10 ± 0.29 | 1.00 ± 0.14 | >0.05 |
| S. enterica | 7.50 | 1.10 ± 0.00 | 0.59 ± 0.14 | <0.05 | 3.75 | 1.41 ± 0.14 | 0.69 ± 0.00 | <0.01 |
| C. glabrata | 1.88 | 3.47 ± 0.14 | 0.41 ± 0.14 | <0.000001 | 0.94 | 0.41 ± 0.14 | 0.10 ± 0.00 | <0.001 |
| S. cerevisiae | 0.94 | 0.41 ± 0.00 | NA | >0.05 | 0.47 | 0.20 ± 0.00 | 0.31 ± 0.14 | >0.05 |
| C. cladosporioides | 1.56 | 2.68 ± 0.07 | 1.92 ± 0.14 | <0.01 | 0.78 | 2.68 ± 0.07 | 2.07 ± 0.21 | <0.01 |
| R. nigricans | 0.78 | 2.73 ± 0.07 | 1.97 ± 0.14 | <0.01 | 0.39 | 2.78 ± 0.14 | 2.07 ± 0.29 | <0.05 |
| A. niger | 0.39 | 3.64 ± 0.14 | 2.32 ± 0.14 | <0.001 | 0.20 | 2.07 ± 0.07 | 0.61 ± 0.14 | <0.0001 |
| A. alternata | 0.78 | 2.89 ± 0.64 | 2.02 ± 0.64 | >0.05 | 0.39 | 1.87 ± 0.36 | 2.12 ± 0.07 | >0.05 |
| Strains | R2 | p-Value | Significance (α = 0.05) |
|---|---|---|---|
| S. aureus | 0.6246 | 0.0228 | Yes |
| L. inoccua | 0.8790 | 0.0437 | Yes |
| L. ivanovii | 0.6036 | 0.0578 | No |
| E. coli | 0.3825 | 0.2079 | No |
| S. enterica | 0.9022 | 0.0152 | Yes |
| C. glabrata | 0.4970 | 0.1183 | No |
| C. cladosporioides | 0.9861 | 0.0041 | Yes |
| R. nigricans | 0.9772 | 0.0060 | Yes |
| A. niger | 0.5044 | 0.0677 | No |
| A. alternata | 0.3558 | 0.0561 | No |
| SI (IC50/MIC) | ||||||
|---|---|---|---|---|---|---|
| A. setacea EO | DMSO | |||||
| MIC | SI | Interpretation | MIC | SI | Interpretation | |
| S. aureus | 7.50 | 0.624 | *MTEC | >60.00 | - | *MTEC |
| B. cereus | 3.75 | 1.248 | **MTMC | 30.00 | 0.191 | *MTEC |
| L. inoccua | 3.75 | 1.248 | **MTMC | >60.00 | - | *MTEC |
| L. ivanovii | 1.88 | 2.489 | **MTMC | 60.00 | 0.095 | *MTEC |
| E. coli | 7.50 | 0.624 | *MTEC | >60.00 | - | *MTEC |
| S. flexneris | 30.00 | 0.156 | *MTEC | 30.00 | 0.191 | *MTEC |
| S. enterica | 15.00 | 0.312 | *MTEC | 60.00 | 0.095 | *MTEC |
| C. glabrata | 3.75 | 1.248 | **MTMC | 30.00 | 0.191 | *MTEC |
| S. cerevisiae | 1.88 | 2.489 | **MTMC | 15.00 | 0.381 | *MTEC |
| A. niger | 0.78 | 6.000 | **MTMC | 6.25 | 0.915 | *MTEC |
| R. nigricans | 1.56 | 3.000 | **MTMC | 12.50 | 0.458 | *MTEC |
| A. alternata | 1.56 | 3.000 | **MTMC | >25.00 | - | *MTEC |
| C. cladosporioides | 3.125 | 1.498 | **MTMC | 6.25 | 0.915 | *MTEC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinas, I.C.; Oprea, E.; Gaboreanu, D.M.; Gradisteanu Pircalabioru, G.; Buleandra, M.; Nagoda, E.; Badea, I.A.; Chifiriuc, M.C. Chemical and Biological Studies of Achillea setacea Herba Essential Oil—First Report on Some Antimicrobial and Antipathogenic Features. Antibiotics 2023, 12, 371. https://doi.org/10.3390/antibiotics12020371
Marinas IC, Oprea E, Gaboreanu DM, Gradisteanu Pircalabioru G, Buleandra M, Nagoda E, Badea IA, Chifiriuc MC. Chemical and Biological Studies of Achillea setacea Herba Essential Oil—First Report on Some Antimicrobial and Antipathogenic Features. Antibiotics. 2023; 12(2):371. https://doi.org/10.3390/antibiotics12020371
Chicago/Turabian StyleMarinas, Ioana Cristina, Eliza Oprea, Diana Madalina Gaboreanu, Gratiela Gradisteanu Pircalabioru, Mihaela Buleandra, Eugenia Nagoda, Irinel Adriana Badea, and Mariana Carmen Chifiriuc. 2023. "Chemical and Biological Studies of Achillea setacea Herba Essential Oil—First Report on Some Antimicrobial and Antipathogenic Features" Antibiotics 12, no. 2: 371. https://doi.org/10.3390/antibiotics12020371
APA StyleMarinas, I. C., Oprea, E., Gaboreanu, D. M., Gradisteanu Pircalabioru, G., Buleandra, M., Nagoda, E., Badea, I. A., & Chifiriuc, M. C. (2023). Chemical and Biological Studies of Achillea setacea Herba Essential Oil—First Report on Some Antimicrobial and Antipathogenic Features. Antibiotics, 12(2), 371. https://doi.org/10.3390/antibiotics12020371











