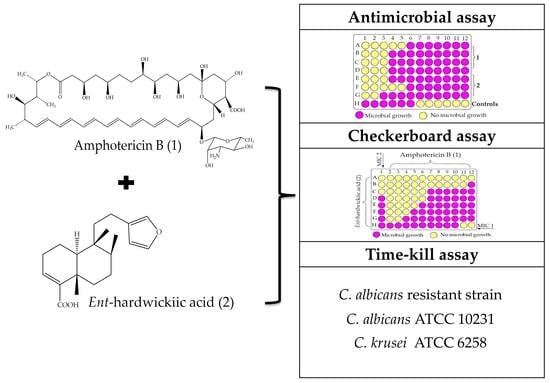
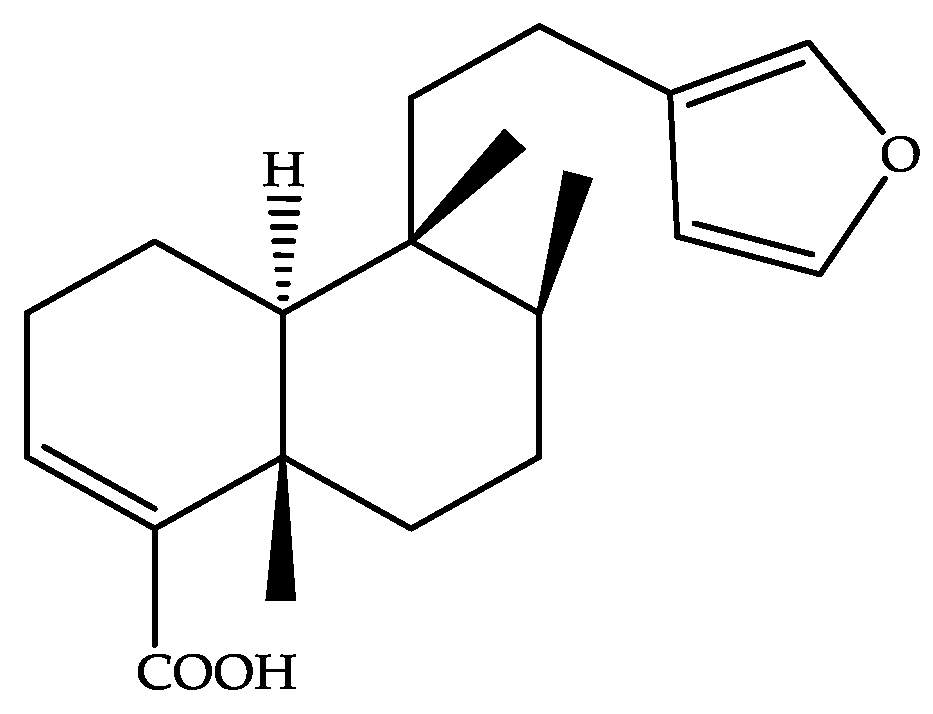
Lower Concentrations of Amphotericin B Combined with Ent-Hardwickiic Acid Are Effective against Candida Strains
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Candida Strains
4.2. Antifungal Agents
4.3. Minimum Inhibitory Concentration of Antifungal Compounds
4.4. Checkerboard Microdilution Method
4.5. Time–Kill Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Toda, M.; Williams, S.R.; Berkow, E.L.; Farley, M.M.; Harrison, L.H.; Bonner, L.; Marceaux, K.M.; Hollick, R.; Zhang, A.Y.; Schaffner, W.; et al. Population-based active surveillance for culture-confirmed candidemia—Four Sites, United States, 2012–2016. MMWR Surveill. Summ. 2019, 68, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.E.; Bicanic, T. Drug resistance and novel therapeutic approaches in invasive candidiasis. Front. Cell. Infect. Microbiol. 2021, 11, 759408. [Google Scholar] [CrossRef] [PubMed]
- Riera, F.O.; Caeiro, J.P.; Angiolini, S.C.; Vigezzi, C.; Rodriguez, E.; Icely, P.A.; Sotomayor, C.E. invasive candidiasis: Update and current challenges in the management of this mycosis in south america. Antibiotics 2022, 11, 877. [Google Scholar] [CrossRef] [PubMed]
- Parslow, B.Y.; Thornton, C.R. Continuing shifts in epidemiology and antifungal susceptibility highlight the need for improved disease management of invasive candidiasis. Microorganisms 2022, 10, 1208. [Google Scholar] [CrossRef]
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: The silent crisis. Microb. Cell 2020, 7, 143–145. [Google Scholar] [CrossRef]
- Morace, G.; Perdoni, F.; Borghi, E. Antifungal drug resistance in Candida species. J. Glob. Antimicrob. Resist. 2014, 2, 254–259. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole antifungal resistance in candida albicans and emerging non-albicans Candida species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef]
- Flowers, S.A.; Colón, B.; Whaley, S.G.; Schuler, M.A.; David Rogers, P. Contribution of clinically derived mutations in ERG11 to azole resistance in Candida albicans. Antimicrob. Agents Chemother. 2015, 59, 450–460. [Google Scholar] [CrossRef]
- Beyda, N.D.; Lewis, R.E.; Garey, K.W. Resistencia a equinocandinas en especies de candida: Mecanismos de susceptibilidad reducida y alternativas terapéuticas. Ann. Pharmacother. 2012, 46, 1086–1096. [Google Scholar] [CrossRef]
- Carolus, H.; Pierson, S.; Lagrou, K.; Van Dijck, P. Amphotericin B and other polyenes—Discovery, clinical use, mode of action and drug resistance. J. Fungi 2020, 6, 321. [Google Scholar] [CrossRef]
- Martel, C.M.; Parker, J.E.; Bader, O.; Weig, M.; Gross, U.; Warrilow, A.G.S.; Kelly, D.E.; Kelly, S.L. A clinical isolate of Candida albicans with mutations in ERG11 (encoding sterol 14α-demethylase) and ERG5 (encoding C22 desaturase) is cross resistant to azoles and amphotericin B. Antimicrob. Agents Chemother. 2010, 54, 3578–3583. [Google Scholar] [CrossRef]
- Ahmad, S.; Joseph, L.; Parker, J.E.; Asadzadeh, M.; Kelly, S.L.; Meis, J.F.; Khan, Z. ERG6 and ERG2 are major targets conferring reduced susceptibility to amphotericin B in clinical candida glabrata isolates in kuwait. Antimicrob. Agents Chemother. 2019, 63, e01900–e01918. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Carvalhaes, C.G.; Devries, S.; Rhomberg, P.R.; Castanheira, M. Impact of COVID-19 on the antifungal susceptibility profiles of isolates collected in a global surveillance program that monitors invasive fungal infections. Med. Mycol. 2022, 60, myac028. [Google Scholar] [CrossRef]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef]
- Perez-De Los Santos, F.J.; Garcia-Ortega, L.F.; Robledo-Marquez, K.; Guzman-Moreno, J.; Riego-Ruiz, L. Transcriptome analysis unveils GLN3 role in amino acids assimilation and fluconazole resistance in Candida glabrata. J. Microbiol. Biotechnol. 2021, 31, 659–666. [Google Scholar] [CrossRef]
- Khalifa, H.O.; Arai, T.; Majima, H.; Watanabe, A.; Kamei, K. Genetic basis of azole and echinocandin resistance in clinical Candida glabrata in japan. Antimicrob. Agents Chemother. 2020, 64, e00783-20. [Google Scholar] [CrossRef]
- Segrelles-Calvo, G.; de S Araújo, G.R.; Llopis-Pastor, E.; Carrillo, J.; Hernández-Hernández, M.; Rey, L.; Melean, N.R.; Escribano, I.; Antón, E.; Zamarro, C.; et al. Candida spp. co-Infection in COVID-19 patients with severe pneumonia: Prevalence study and associated risk factors. Respir. Med. 2021, 188, 106619. [Google Scholar] [CrossRef]
- Altinkaya Çavuş, M.; Sav, H. Opportunistic Candida infections in critical COVID-19 patients. Polish J. Microbiol. 2022, 71, 411–419. [Google Scholar] [CrossRef]
- Frías-De-león, M.G.; Pinto-Almazán, R.; Hernández-Castro, R.; García-Salazar, E.; Meza-Meneses, P.; Rodríguez-Cerdeira, C.; Arenas, R.; Conde-Cuevas, E.; Acosta-Altamirano, G.; Martínez-Herrera, E. Epidemiology of systemic mycoses in the COVID-19 pandemic. J. Fungi 2021, 7, 556. [Google Scholar] [CrossRef]
- Seagle, E.E.; Jackson, B.R.; Lockhart, S.R.; Georgacopoulos, O.; Nunnally, N.S.; Roland, J.; Barter, D.M.; Johnston, H.L.; Czaja, C.A.; Kayalioglu, H.; et al. The landscape of candidemia during the coronavirus disease 2019 (COVID-19) pandemic. Clin. Infect. Dis. 2022, 9, 802–811. [Google Scholar] [CrossRef]
- Hoenigl, M.; Seidel, D.; Sprute, R.; Cunha, C.; Oliverio, M.; Goldman, G.H.; Ibrahim, A.S.; Carvalho, A. COVID-19-associated fungal infections. Nat. Microbiol. 2022, 7, 1127–1140. [Google Scholar] [CrossRef] [PubMed]
- Posteraro, B.; De Angelis, G.; Menchinelli, G.; D’inzeo, T.; Fiori, B.; De Maio, F.; Cortazzo, V.; Sanguinetti, M.; Spanu, T. Risk factors for mortality in adult COVID-19 patients who develop bloodstream infections mostly caused by antimicrobial-resistant organisms: Analysis at a large teaching hospital in Italy. J. Clin. Med. 2021, 10, 1752. [Google Scholar] [CrossRef] [PubMed]
- Morton, C.O.; Griffiths, J.S.; Loeffler, J.; Orr, S.; White, P.L. Defective antifungal immunity in patients with COVID-19. Front. Immunol. 2022, 13, 1080822. [Google Scholar] [CrossRef] [PubMed]
- Shirvani, F.; Fattahi, A. Pulmonary candidiasis associated with COVID-19: Evaluation of causative agents and their antifungal susceptibility patterns. Tanaffos 2021, 20, 29–35. [Google Scholar]
- Ghosh, A.; Sarkar, A.; Paul, P.; Patel, P. The rise in cases of mucormycosis, candidiasis and aspergillosis amidst COVID-19. Fungal Biol. Rev. 2021, 38, 67–91. [Google Scholar] [CrossRef]
- Lemke, A.; Kiderlen, A.F.; Kayser, O. Amphotericin B. Appl. Microbiol. Biotechnol. 2005, 68, 151–162. [Google Scholar] [CrossRef]
- Rovina, N.; Koukaki, E.; Romanou, V.; Ampelioti, S.; Loverdos, K.; Chantziara, V.; Koutsoukou, A.; Dimopoulos, G. Fungal infections in critically ill COVID-19 patients: Inevitabile malum. J. Clin. Med. 2022, 11, 2017. [Google Scholar] [CrossRef]
- Bhatt, K.; Agolli, A.; Patel, M.H.; Garimella, R.; Devi, M.; Garcia, E.; Amin, H.; Domingue, C.; Del Castillo, R.G.; Sanchez-Gonzalez. High mortality co-infections of COVID-19 patients: Mucormycosis and other fungal infections. Discoveries 2021, 9, e126. [Google Scholar] [CrossRef]
- Benitez, L.L.; Carver, P.L. Adverse effects associated with long-term administration of azole antifungal agents. Drugs 2019, 79, 833–853. [Google Scholar] [CrossRef]
- Balcerek, M.I.; Stewart, A.G.; Chapman, P.; Lazarus, S. Reducing the off-target endocrinologic adverse effects of azole antifungals—Can it be done? Int. J. Antimicrob. Agents 2022, 59, 106587. [Google Scholar] [CrossRef]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on current status of echinocandins use. Antibiotics 2020, 9, 227. [Google Scholar] [CrossRef]
- Keating, G.M.; Figgitt, D.P. Caspofungin: A review of its use in oesophageal candidiasis, invasive candidiasis and invasive aspergillosis. Drugs 2003, 63, 2235–2263. [Google Scholar] [CrossRef]
- Carmona, E.M.; Limper, A.H. Overview of treatment approaches for fungal infections. Clin. Chest Med. 2017, 38, 393–402. [Google Scholar] [CrossRef]
- Chang, C.C.; Slavin, M.A.; Chen, S.C.A. New developments and directions in the clinical application of the echinocandins. Arch. Toxicol. 2017, 91, 1613–1621. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Chakraborti, S.; Ramakrishnan, G.; Srinivasan, N. In silico modeling of fda-approved drugs for discovery of anticandida agents: A drug-repurposing approach. In In Silico Drug Design: Repurposing Techniques and Methodologies; Kunal, R., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 463–526. [Google Scholar]
- Aldholmi, M.; Marchand, P.; Ourliac-Garnier, I.; Le Pape, P.; Ganesan, A. A decade of antifungal leads from natural products: 2010–2019. Pharmaceuticals 2019, 12, 182. [Google Scholar] [CrossRef]
- Zida, A.; Bamba, S.; Yacouba, A.; Ouedraogo-Traore, R.; Guiguemdé, R.T. Substances naturelles actives sur Candida albicans, sources de nouveaux médicaments antifongiques: Revue de la littérature. J. Mycol. Med. 2017, 27, 1–19. [Google Scholar] [CrossRef]
- De Sousa, I.P.; Teixeira, M.V.S.; Furtado, N.A.J.C. An overview of biotransformation and toxicity of diterpenes. Molecules 2018, 23, 1387. [Google Scholar] [CrossRef]
- De Sousa, I.P.; Ferreira, A.G.; Crotti, A.E.M.; Alves dos Santos, R.; Kiermaier, J.; Kraus, B.; Heilmann, J.; Furtado, N.A.J.C. New antifungal ent-labdane diterpenes against Candida glabrata produced by microbial transformation of ent-polyalthic acid. Bioorg. Chem. 2020, 95, 103560. [Google Scholar] [CrossRef]
- Saha, P.; Rahman, F.I.; Hussain, F.; Rahman, S.M.A.; Rahman, M.M. Antimicrobial diterpenes: Recent development from natural sources. Front. Pharmacol. 2022, 12, 820312. [Google Scholar] [CrossRef]
- Da Silva, J.J.M.; Crevelin, E.J.; Carneiro, L.J.; Rogez, H.; Veneziani, R.C.S.; Ambrósio, S.R.; Beraldo Moraes, L.A.; Bastos, J.K. Development of a validated ultra-high-performance liquid chromatography tandem mass spectrometry method for determination of acid diterpenes in Copaifera oleoresins. J. Chromatogr. A 2017, 1515, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Símaro, G.V.; Lemos, M.; Da Silva, J.J.M.; Ribeiro, V.P.; Arruda, C.; Schneider, A.H.; De Souza, W.W.C.; Carneiro, L.J.; Mariano, R.L.; Ambrósio, S.R.; et al. Antinociceptive and anti-inflammatory activities of Copaifera pubiflora Benth oleoresin and its major metabolite ent-hardwickiic acid. J. Ethnopharmacol. 2021, 271, 113883. [Google Scholar] [CrossRef] [PubMed]
- Abrão, F.; Silva, T.S.; Moura, C.L.; Ambrósio, S.R.; Veneziani, R.C.S.; de Paiva, R.E.F.; Bastos, J.K.; Martins, C.H.G. Oleoresins and naturally occurring compounds of Copaifera genus as antibacterial and antivirulence agents against periodontal pathogens. Sci. Rep. 2021, 11, 4953. [Google Scholar] [CrossRef] [PubMed]
- Crentsil, J.A.; Yamthe, L.R.T.; Anibea, B.Z.; Broni, E.; Kwofie, S.K.; Tetteh, J.K.A.; Osei-Safo, D. Leishmanicidal potential of hardwickiic acid isolated from Croton sylvaticus. Front. Pharmacol. 2020, 11, 753. [Google Scholar] [CrossRef] [PubMed]
- Lama, R.; Zhong, B.; Kulman, D.G.; Su, B. Bioassay guided identification of small chaperone proteins α-crystallin and HSP-27 inhibitors from copaiba oil. Phytochem. Lett. 2014, 10, 65–75. [Google Scholar] [CrossRef]
- Teixeira, M.V.S.; Fernandes, L.M.; De Paula, V.S.; Ferreira, A.G.; Furtado, N.A.J.C. Ent-hardwickiic acid from C. pubiflora and its microbial metabolites are more potent than fluconazole in vitro against Candida glabrata. Lett. Appl. Microbiol. 2022, 74, 622–629. [Google Scholar] [CrossRef]
- Oliveira, L.C.; Porto, T.S.; Junior, A.H.C.; Santos, M.F.C.; Ramos, H.P.; Braun, G.H.; de Lima Paula, L.A.; Bastos, J.K.; Furtado, N.A.J.C.; Parreira, R.L.T.; et al. Schistosomicidal activity of kaurane, labdane and clerodane-type diterpenes obtained by fungal transformation. Process Biochem. 2020, 98, 34–40. [Google Scholar] [CrossRef]
- Carneiro, L.J.; Tasso, T.O.; Santos, M.F.C.; Goulart, M.O.; dos Santos, R.A.; Bastos, J.K.; da Silva, J.J.M.; Crotti, A.E.M.; Parreira, R.L.T.; Orenha, R.P.; et al. Copaifera Multijuga, Copaifera Pubiflora and Copaifera Trapezifolia oleoresins: Chemical characterization and in vitro cytotoxic potential against tumoral cell lines. J. Braz. Chem. Soc. 2020, 31, 1679–1689. [Google Scholar] [CrossRef]
- Canela, H.M.S.; Cardoso, B.; Vitali, L.H.; Coelho, H.C.; Martinez, R.; da Silva Ferreira, M.E. Prevalence, virulence factors and antifungal susceptibility of Candida spp. isolated from bloodstream infections in a tertiary care hospital in Brazil. Mycoses 2018, 61, 11–21. [Google Scholar] [CrossRef]
- Van Daele, R.; Spriet, I.; Wauters, J.; Maertens, J.; Mercier, T.; Van Hecke, S.; Brüggemann, R. Antifungal drugs: What brings the future? Med. Mycol. 2019, 57, S328–S343. [Google Scholar] [CrossRef]
- Barry, A.L.; Pfaller, M.A.; Brown, S.D.; Espinel-Ingroff, A.; Ghannoum, M.A.; Knapp, C.; Rennie, R.P.; Rex, J.H.; Rinaldi, M.G. Quality control limits for broth microdilution susceptibility tests of ten antifungal agents. J. Clin. Microbiol. 2000, 38, 3457–3459. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Performance Standards for Antifungal Susceptibility Testing of Yeasts, 1st ed.; CLSI supplement M60; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2017. [Google Scholar]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of amphotericin B: An overview of the main antifungal agent used to treat invasive fungal infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Rossi, S.A.; De Oliveira, H.C.; Agreda-mellon, D.; Zaragoza, O. Identification of off-patent drugs that show synergism with amphotericin B or that present antifungal action against. Antimicrob. Agents Chemother. 2020, 64, e01921-19. [Google Scholar] [CrossRef]
- Laniado-Laborín, R.; Cabrales-Vargas, M.N. Amphotericin B: Side effects and toxicity. Rev. Iberoam. Micol. 2009, 26, 223–227. [Google Scholar] [CrossRef]
- Legrand, M.; Bell, S.; Forni, L.; Joannidis, M.; Koyner, J.L.; Liu, K.; Cantaluppi, V. Pathophysiology of COVID-19-associated acute kidney injury. Nat. Rev. Nephrol. 2021, 17, 751–764. [Google Scholar] [CrossRef] [PubMed]
- Sabaghian, T.; Kharazmi, A.B.; Ansari, A.; Omidi, F.; Kazemi, S.N.; Hajikhani, B.; Vaziri-Harami, R.; Tajbakhsh, A.; Omidi, S.; Haddadi, S.; et al. COVID-19 and acute kidney injury: A systematic review. Front. Med. 2022, 9, 705908. [Google Scholar] [CrossRef] [PubMed]
- Cheng, F.; Kovács, I.A.; Barabási, A.L. Network-based prediction of drug combinations. Nat. Commun. 2019, 10, 1197. [Google Scholar] [CrossRef] [PubMed]
- Cantón, E.; Pemán, J.; Gobernado, M.; Viudes, A.; Espinel-Ingroff, A. Patterns of amphotericin B killing kinetics against seven Candida species. Antimicrob. Agents Chemother. 2004, 48, 2477–2482. [Google Scholar] [CrossRef]
- Tollemar, J.; Klingspor, L.; Ringdén, O. Liposomal amphotericin B (ambisome) for fungal infections in immunocompromised adults and children. Clin. Microbiol. Infect. 2001, 7, 68–79. [Google Scholar] [CrossRef]
- Zhanel, G.G.; Saunders, D.G.; Hoban, D.J.; Karlowsky, J.A. Influence of human serum on antifungal pharmacodynamics with Candida albicans. Antimicrob. Agents Chemother. 2001, 45, 2018–2022. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Santos, A.G.; Tininis, A.G.; Costa, P.M.; Cavalheiro, A.J.; Bolzani, V.S.; Moraes, M.O.; Costa-Lotufo, L.V.; Montenegro, R.C.; Pessoa, C. Casearin X exhibits cytotoxic effects in leukemia cells triggered by apoptosis. Chem. Biol. Interact. 2010, 188, 497–504. [Google Scholar] [CrossRef]
- Damasceno, J.P.L.; Da Rosa, H.S.; De Araújo, L.S.; Furtado, N.A.J.C. Andrographis paniculata formulations: Impact on diterpene lactone oral bioavailability. Eur. J. Drug Metab. Pharmacokinet. 2022, 47, 19–30. [Google Scholar] [CrossRef]
- Sousa, F.; Ferreira, D.; Reis, S.; Costa, P. Current insights on antifungal therapy: Novel nanotechnology approaches for drug delivery systems and new drugs from natural sources. Pharmaceuticals 2020, 13, 248. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Orhan, I.E.; Banach, M.; Rollinger, J.M.; Barreca, D.; Weckwerth, W.; Bauer, R.; Bayer, E.A.; et al. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Nim, S.; Mónico, A.; Rawal, M.K.; Duarte, N.; Prasad, R.; Di Pietro, A.; Ferreira, M.J.U. Overcoming multidrug resistance in Candida albicans: Macrocyclic diterpenes from Euphorbia species as potent inhibitors of drug efflux pumps. Planta Med. 2016, 82, 1180–1185. [Google Scholar] [CrossRef]
- Moraes, T.D.; Leandro, L.F.; Santiago, M.B.; Silva, L.O.; Bianchi, T.C.; Veneziani, R.C.S.; Ambrósio, S.R.; Ramos, S.B.; Bastos, J.K.; Martins, C.H. Assessment of the antibacterial, antivirulence, and action mechanism of Copaifera pubiflora oleoresin and isolated compounds against oral bacteria. Biomed. Pharmacother. 2020, 129, 110467. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; CLSI standard M27; Clinical and Laboratory Standards Institute: Wayne, NJ, USA, 2017. [Google Scholar]
- Raber, H.F.; Sejfijaj, J.; Kissmann, A.K.; Wittgens, A.; Gonzalez-Garcia, M.; Alba, A.; Vázquez, A.A.; Vicente, F.E.M.; Erviti, J.P.; Kubiczek, D.; et al. Antimicrobial peptides pom-1 and pom-2 from Pomacea poeyana are active against Candida auris, C. parapsilosis and C. albicans biofilms. Pathogens 2021, 10, 496. [Google Scholar] [CrossRef]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and simplified method for drug combination studies by checkerboard assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- Saracino, I.M.; Foschi, C.; Pavoni, M.; Spigarelli, R.; Valerii, M.C.; Spisni, E. Antifungal activity of natural compounds vc. Candida spp.: A mixture of cinnamaldehyde and eugenol show promising in vitro results. Antibiotics 2022, 11, 73. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute (CLSI). Methods for Determining Bactericidal Activity of Antimicrobial Agents; CLSI document M26-A; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 1999. [Google Scholar]

| Strains | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans Resistant Strain | C. albicans ATCC 10231 | C. krusei ATCC 6258 | ||||||||||
| MIC 1 Alone (µg/mL): 16 | MIC 2 Alone (µg/mL): 12.5 | MIC 1 Alone (µg/mL): 8 | MIC 2 Alone (µg/mL): 6.25 | MIC 1 Alone (µg/mL): 8 | MIC 2 Alone (µg/mL): 3.12 | |||||||
| MIC Combinated 1 (µg/mL) | MIC Combinated 2 (µg/mL) | Ʃ FIC | Interaction Type * | MIC Combinated 2 (µg/mL) | Ʃ FIC | Interaction Type * | MIC Combinated 2 (µg/mL) | Ʃ FIC | Interaction Type * | |||
| 16 | 1.56 | 1.12 | Indifferent | 0.39 | 2.06 | Indifferent | 0.195 | 2.06 | Indifferent | |||
| 8 | 1.56 | 0.62 | Additive | 0.39 | 1.06 | Indifferent | 0.195 | 1.06 | Indifferent | |||
| 4 | 3.12 | 0.50 | Synergism | 0.39 | 0.56 | Additive | 0.195 | 0.56 | Additive | |||
| 2 | 6.25 | 0.62 | Additive | 0.39 | 0.31 | Synergism | 0.195 | 0.31 | Synergism | |||
| 1 | 6.25 | 0.56 | Additive | 0.78 | 0.24 | Synergism | 0.39 | 0.25 | Synergism | |||
| 0.5 | 12.5 | 1.03 | Indifferent | 0.78 | 0.18 | Synergism | 0.39 | 0.18 | Synergism | |||
| 0.25 | 12.5 | 1.01 | Indifferent | 1.56 | 0.28 | Synergism | 0.39 | 0.15 | Synergism | |||
| 0.125 | 12.5 | 1.00 | Additive | 3.12 | 0.50 | Synergism | 0.39 | 0.14 | Synergism | |||
| 0.062 | 25 | 2.00 | Indifferent | 3.12 | 0.50 | Synergism | 0.78 | 0.25 | Synergism | |||
| 0.031 | 25 | 2.00 | Indifferent | 3.12 | 0.50 | Synergism | 0.78 | 0.24 | Synergism | |||
| 0.0156 | 25 | 2.00 | Indifferent | 6.25 | 1.00 | Additive | 0.78 | 0.25 | Synergism | |||
| Strains | ||||||
|---|---|---|---|---|---|---|
| C. Albicans Resistant Strain | C. albicans ATCC 10231 | C. krusei ATCC 6258 | ||||
| Amphotericin B (1) and Ent-Hardwickiic Acid (2) (µg/mL) | ||||||
| Selected Combinations | 1 | 2 | 1 | 2 | 1 | 2 |
| 1° | 0.25 | 12.5 | 0.031 | 3.12 | 0.0156 | 0.78 |
| 2° | 0.5 | 12.5 | 0.125 | 3.12 | 0.031 | 0.78 |
| 3° | 1 | 6.25 | 0.25 | 1.56 | 0.062 | 0.78 |
| 4° | 4 | 3.12 | 0.5 | 0.78 | 0.25 | 0.39 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, M.V.S.; Aldana-Mejía, J.A.; da Silva Ferreira, M.E.; Furtado, N.A.J.C. Lower Concentrations of Amphotericin B Combined with Ent-Hardwickiic Acid Are Effective against Candida Strains. Antibiotics 2023, 12, 509. https://doi.org/10.3390/antibiotics12030509
Teixeira MVS, Aldana-Mejía JA, da Silva Ferreira ME, Furtado NAJC. Lower Concentrations of Amphotericin B Combined with Ent-Hardwickiic Acid Are Effective against Candida Strains. Antibiotics. 2023; 12(3):509. https://doi.org/10.3390/antibiotics12030509
Chicago/Turabian StyleTeixeira, Maria V. Sousa, Jennyfer A. Aldana-Mejía, Márcia E. da Silva Ferreira, and Niege A. J. Cardoso Furtado. 2023. "Lower Concentrations of Amphotericin B Combined with Ent-Hardwickiic Acid Are Effective against Candida Strains" Antibiotics 12, no. 3: 509. https://doi.org/10.3390/antibiotics12030509
APA StyleTeixeira, M. V. S., Aldana-Mejía, J. A., da Silva Ferreira, M. E., & Furtado, N. A. J. C. (2023). Lower Concentrations of Amphotericin B Combined with Ent-Hardwickiic Acid Are Effective against Candida Strains. Antibiotics, 12(3), 509. https://doi.org/10.3390/antibiotics12030509