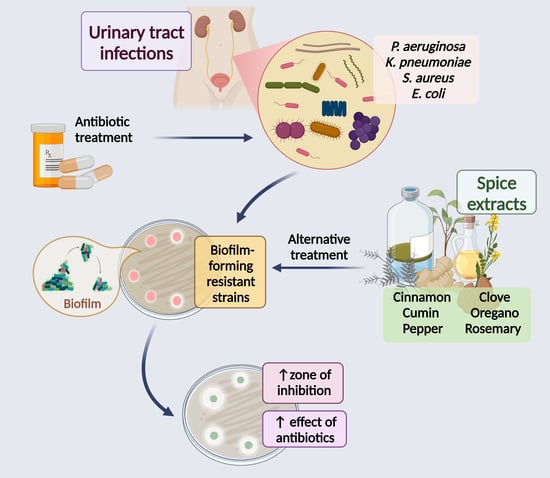
Antimicrobial Activity of Spices Popularly Used in Mexico against Urinary Tract Infections
Abstract
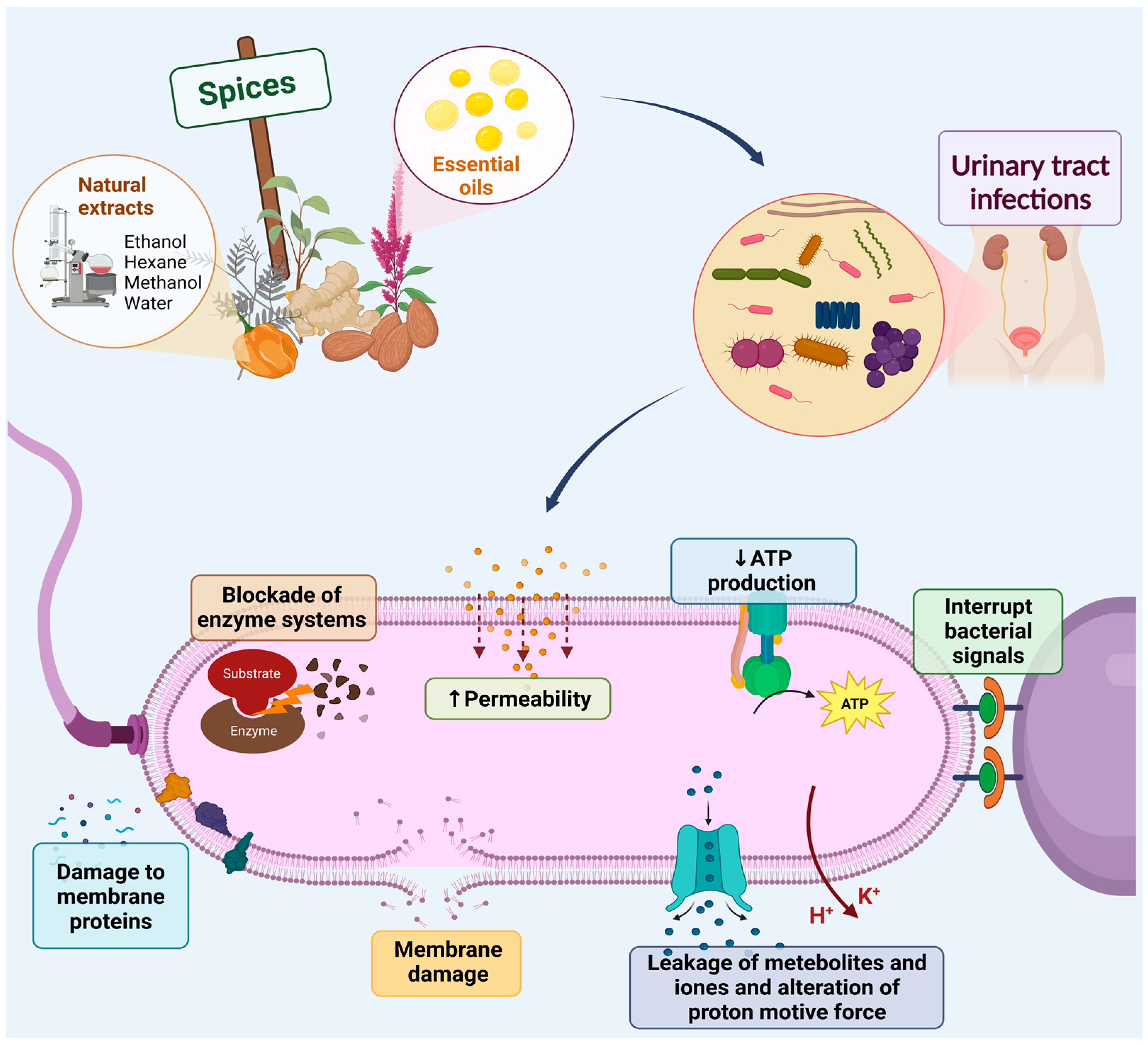
1. Introduction
2. Spices
3. Cinnamon
| Cinnamon Specie | Type of Extract | Phytochemicals | Uropathogen | MIC | MBC | Diameter of the Inhibition Zone (mm) | Ref. |
|---|---|---|---|---|---|---|---|
| C. cassia | Essential oil | trans-cinnamaldehyde, cinnamic acid, eugenol, benzaldehyde | E. coli | 26–35 mg/mL | ND | 26–38 | [57] |
| P. aeuruginosa | 12–19 mg/mL | ND | 12–19 | ||||
| P. mirabilis | 30–39 mg/mL | ND | 30–39 | ||||
| K. pneumoniae | 27–32 mg/mL | ND | 27–32 | ||||
| C. zeylanicum | Essential oil | trans-cinnamaldehyde, cinnamic acid, eugenol, benzaldehyde | P. aeuruginosa | 0.11–0.2% | 0.1125–1.8% | ND | [56] |
| NS | Essential oil | trans-cinnamaldehyde, cinnamic acid, eugenol, benzaldehyde | E. coli | 1 mg/mL | 4 mg/mL | 19.2 | [54] |
| S. aureus | 1 mg/mL | 2 mg/mL | 28.7 | ||||
| C. verum | Essential oil | trans-cinnamaldehyde, cinnamic acid, eugenol | K. pneumoniae | 0.125% | 0.25% | ND | [60] |
| C. zeylanicum | Ethanolic extract | Tannins, Flavonoids, anthraquinones, saponins | E. coli | ND | ND | 11.72 | [58] |
| K. pneumoniae | ND | ND | 25.50 | ||||
| P. aeuruginosa | ND | ND | 23.25 | ||||
| C. verum | Ethanolic extract | Tannins, Flavonoids, anthraquinones, saponins | P. aeuruginosa | 10 mg/mL | 20 mg/mL | 12.3 | [61] |
| K. pneumoniae | 20 mg/mL | 40 mg/mL | 15.3 | ||||
| S. aureus | 10 mg/mL | 20 mg/mL | 12.5 | ||||
| Dichloromethane extract | Flavonoids, anthraquinones, alkaloids, saponins | P. aeuruginosa | 20 mg/mL | 40 mg/mL | 10.0 | ||
| K. pneumoniae | 20 mg/mL | 40 mg/mL | 12.3 | ||||
| S. aureus | 5 mg/mL | 10 mg/mL | 11.5 | ||||
| Hexane extract | Tannins, alkaloids, flavonoids, anthraquinones, saponins | P. aeuruginosa | 10 mg/mL | 20 mg/mL | 10.5 | ||
| K. pneumoniae | 20 mg/mL | 20 mg/mL | 14.5 | ||||
| S. aureus | 5 mg/mL | 10 mg/mL | 15.0 |
4. Clove
| Type of Extract | Phytochemicals | Uropathogen | MIC | MBC | Diameter of the Inhibition Zone (mm) | Ref. |
|---|---|---|---|---|---|---|
| Clove oil | Eugenol, b-caryophyllene, vanillin, crategolic acid, bicornin, galotanic acid, methyl salicylate eugenin, kaempferol, ramnetin and eugenitin, oleanolic acid, stigmasterol, campesterol, and various sesquiterpenes | E. coli | 0.5 mg/mL | 0.5 mg/mL | ND | [67] |
| S. aureus | 0.5 mg/mL | 0.5 mg/mL | ND | |||
| Clove oil | Eugenol, b-caryophyllene, vanillin, crategolic acid, bicornin, galotanic acid, methyl salicylate eugenin, kaempferol, ramnetin and eugenitin, oleanolic acid, stigmasterol, campesterol, and various sesquiterpenes | A. baumanni | ND | ND | 28 | [92] |
| P. aeruginosa | ND | ND | 17 | |||
| E. faecalis | ND | ND | 25 | |||
| S. aureus | ND | ND | 20 | |||
| Clove oil | Eugenol, b-caryophyllene, vanillin, crategolic acid, bicornin, galotanic acid, methyl salicylate eugenin, kaempferol, ramnetin and eugenitin, oleanolic acid, stigmasterol, campesterol, and various sesquiterpenes | E. coli isolated from UTIs patients | 2.1 to 3.1 mg/mL | 3.1 to 4.2 mg/mL | ND | [73] |
| Antibiotic-resistant E. coli | 2.6 mg/mL | 3.7 mg/mL | ND | |||
| Clove oil | Eugenol, b-caryophyllene, vanillin, crategolic acid, bicornin, galotanic acid, methyl salicylate eugenin, kaempferol, ramnetin and eugenitin, oleanolic acid, stigmasterol, campesterol, and various sesquiterpenes | E. coli isolated from UTIs patients | 5.5 μL/mL and 0.55 μL/mL * | ND | 24.5 mm | [66] |
| K. pneumoniae isolated from UTIs patients | 5.5 μL/mL and 0.55 μL/mL * | ND | 22 mm | |||
| Ethanolic extract | Eugenol, glycosides, flavonoids, saponins, tannins, and essential oils. | S. aureus | 5 mg/mL | 10 mg/mL | 11.4 | [93] |
| P. aeruginosa | 5 mg/mL | 12.5 mg/mL | 9.2 | |||
| Ethanolic extract | Eugenol, glycosides, flavonoids, saponins, tannins, and essential oils. | E. coli | 0.39 mg/mL | 0.19 mg/mL | 17 | [68] |
| K. pneumoniae | 0.78 mg/mL | 0.39 mg/ml | 16 | |||
| Enterobacter species | 0.78 mg/mL | 0.39 mg/mL | 17 | |||
| Citrobacter Species | 0.39 mg/mL | 0.19 mg/mL | 18 | |||
| P. mirabilis | 0.39 mg/mL | 0.19 mg/mL | 19 | |||
| P. aeruginosa | 1.56 mg/mL | 0.78 mg/mL | 14 | |||
| A. baumanni | 0.78 mg/mL | 0.39 mg/mL | 18 | |||
| Ethanolic extract | Eugenol, glycosides, flavonoids, saponins, tannins, and essential oils. | P. mirabilis | ND | ND | 19.7 | [83] |
| S. epidermidis | ND | ND | 18 | |||
| K. pneumoniae | ND | ND | 12.3 | |||
| E. coli | ND | ND | 12.7 | |||
| S. aureus | ND | ND | 14.7 |
5. Cumin
6. Oregano
| Oregano Specie | Type of Extract | Phytochemicals | Uropathogen | MIC | MBC | Diameter of the Inhibition Zone (mm) | Ref. |
|---|---|---|---|---|---|---|---|
| Lippia berlandieri Schauer | Essential oil | Thymol (7.86%) and carvacrol (33.78%) | E. faecalis and E. coli | <200 mg/L | <200 mg/L | ND | [116] |
| Poliomintha longiflora | Essential oil | Thymol (23.46%) and carvacrol (18.35%) | E. faecalis and E. coli | <200 mg/L | <200 mg/L | ND | |
| O. vulgare | Essential oil | Carvacrol (68.96%) | E. coli | 0.055 µL/mL | ND | 24.5 | [66] |
| K. pneumoniae | ND | ND | 22 | ||||
| O. vulgare | Essential oil | Carvacrol (65.9%) | E. coli | 0.293–1.183 | ND | ND | [17] |
| Enterococcus | 1.183 mg/mL | ND | ND | ||||
| O. vulgare | Essential oil | Carvacrol (77.8%) | E. coli O6:H1 strain CFT073 | 0.01% | ND | ND | [8] |
| O. vulgare | Essential oil | Carvacrol (>50%) | E. coli | ND | ND | 29 | [118] |
| P. aeruginosa | ND | ND | 27 | ||||
| K. pneumoniae | ND | ND | 20 | ||||
| P. mirabilis | ND | ND | 22 | ||||
| E. aerogenes | ND | ND | 21 | ||||
| E. faecalis | ND | ND | 21 | ||||
| A. baumannii | ND | ND | 22 | ||||
| N. gonorrhoeae | ND | ND | 24 | ||||
| S. aureus | ND | ND | 26 | ||||
| S. epidermis | ND | ND | 20 | ||||
| O. vulgare | Essential oil | ND | E. coli UTIs | 0.015% | ND | ND | [117] |
| O. glandulosum | Essential oil | Thymol (33.2%), γ-terpinene (25.4%), p-cymene (16.1%), and carvacrol (13.0%) | K. pneumoniae | 5.2 mg/mL | ND | 43.5 ± 6.7 | [119] |
7. Pepper
| Pepper Specie | Type of Extract | Phytochemicals | Uropathogen | MIC | MBC | Diameter of the Inhibition Zone (mm) | Ref. |
|---|---|---|---|---|---|---|---|
| P. cubeba | Acetone extract | Flavonoids, steroids, tannins, reducing sugars, and triterpenoids | Enterococcus sp. | ND | ND | 15.2 ± 0.52 | [121] |
| P. aeruginosa | ND | ND | 15.3 ± 0.62 | ||||
| E. coli | ND | ND | 16.3 ± 0.75 | ||||
| Methanolic extract | Saponins, flavonoids, steroids, tannins, reducing sugars, cardiac glycosides, and triterpenoids | Enterococcus sp. | ND | ND | 17.6 ± 0.80 | ||
| P. aeruginosa | ND | ND | 13.2 ± 0.06 | ||||
| E. coli | ND | ND | 15.0 ± 0.30 | ||||
| Ethanolic extract | Flavonoids, steroids, tannins, reducing sugars, cardiac glycosides, and triterpenoids | Enterococcus sp. | ND | ND | 11.3 ± 0.16 | ||
| P. aeruginosa | ND | ND | 9.6 ± 0.34 | ||||
| E. coli | ND | ND | 8.5 ± 0.17 | ||||
| P. cubeba | Aqueous-ethanolic (30/70) extract | Flavonoids, alkaloids, sterols, phenols, and tannins | E. coli | ND | ND | 18 ± 0.64 | [106] |
| S. saprophyticus | ND | ND | 19 ± 0.26 | ||||
| K. pneumoni | ND | ND | 21 ± 0.51 | ||||
| P. mirabilis | ND | ND | 20 ± 0.41 | ||||
| P.longum | Aqueous extract | Alkaloids, flavonoids, triterpenes, tannins, coumarins, cardiac glycosides, anthraquinones, glycosides, saponins | P. aeruginosa | ND | ND | 19 ± 0.26 | [124] |
| S. aureus | ND | ND | 21 ± 0.51 | ||||
| E. coli | ND | ND | 20 ± 0.41 | ||||
| Methanolic extract | Alkaloids, flavonoids, triterpenes, tannins, coumarins, cardiac glycosides, anthraquinones, glycosides, saponins | P. aeruginosa | 1.875 mg/mL | ND | ND | ||
| S. aureus | 3.75 mg/mL | ND | 8–14 | ||||
| E. coli | 0.937 mg/mL | ND | ND | ||||
| P. betle | Aqueous extract | ND | S. marcescens | 16 μg/mL | ND | ND | [127] |
| P.mirabilis | 32 μg/mL | ND | ND | ||||
| P. nigrum | Methanolic extract | Capsaicin and 2- dihidrocapsaicin | S. aureus | ND | ND | 10.5 | [122] |
| E. coli | ND | ND | 18.4 | ||||
| Ethanolic extract | Gallic acid, trans-p-feruloyl-b-D-glucopyranoside, trans-p-sinapyl-b-D-glucopyranoside, quercetin 3-O-R-L-rhamnopyranoside-7-O-a-D-glucopyranosyl, quercetin 3-O-R-L-rhamnopyranoside, luteolin 6-C-a-D-glucopyranoside-8-C-R-L-arabinopyranoside, luteolin 7-O-[2-(b-D-apiofuranosyl)-b-D-glucopyranoside-8-C-R-L-arabinopyranoside, luteolin 7-O-[2-(b-D-apiofuranosyl)-4-(b-D-glucopyranosyl), kaempferol and coumarins | S. aureus | ND | ND | 20.9 | ||
| E. coli | ND | ND | 20.7 | ||||
| P. nigrum | Methanolic extract | Glycosides, terpenoids, carbohydrates, tannins and steroids | E. faecalis | 9.63 mg/mL | 4.27 mg/mL | 17 | [33] |
| S. aureus | 21.67 mg/mL | 9.63 mg/mL | 19 | ||||
| C freundii | 21.67 mg/mL | 9.63 mg/mL | 17 | ||||
| E aerogenes | 3.41 mg/mL | 1.51 mg/mL | 25 | ||||
| K pneumoniae | 4.27 mg/mL | 3.41 mg/mL | 22 | ||||
| P. mirabilis | 9.63 mg/mL | 4.27 mg/mL | 18 |
8. Rosemary
| Type of Extract | Phytochemicals | Uropathogen | MIC | MBC | Diameter of the Inhibition Zone (mm) | Ref. |
|---|---|---|---|---|---|---|
| Ethanolic extract | Rosmarinic acid, rosmanol, geniposide | S. saprophyticus | 130 mg/mL | 130 mg/mL | ND | [140] |
| S. epidermidis | 70 mg/mL | >400 mg/mL | ND | |||
| E. faecalis | 100 mg/mL | 300 mg/mL | ND | |||
| Essential oil | 1,8-cineole (46.4%), camphor (11.4%), a-pinene (11%), b-pinene (9.2%), camphene (5.02%) | E. coli | 18.25–19.75 mL/mL | ND | ND | [73] |
| Essential oil | 1,8-cineole, camphor, a-pinene, b-pinene, camphene | E. coli | 10 mg/mL | ND | ND | [137] |
| Essential oil | 1,8-cineole (17.16%), a-pinene (16.95%), verbenone (15.78%), camphor (8.08%) | S. aureus | 0.06–0.16 mg/mL | 0.06–0.16 mg/mL | 7–9.6 | [142] |
| K. pneumoniae | 0.06–0.16 mg/mL | 0.06–0.16 mg/mL | 7–9.6 | |||
| P. vulgaris | 0.06–0.16 mg/mL | 0.06–0.16 mg/mL | 7–9.6 | |||
| Methanolic extract | Caffeic acid, borneol, limonene, camphor | E. coli | 64 mg/mL | ND | 5.48 | [138] |
| Methanolic extract | Caffeic acid, borneol, limonene, camphor | E. coli | 5 mg/mL | 10 mg/mL | ND | [139] |
9. Concluding Remarks and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, X.; Chen, H.; Zheng, Y.; Qu, S.; Wang, H.; Yi, F. Disease burden and long-term trends of urinary tract infections: A worldwide report. Front. Public Health 2022, 10, 888205. [Google Scholar] [CrossRef]
- Ballesteros-Monrreal, M.G.; Arenas-Hernández, M.M.P.; Barrios-Villa, E.; Juarez, J.; Álvarez-Ainza, M.L.; Taboada, P.; De la Rosa-López, R.; Bolado-Martínez, E.; Valencia, D. Bacterial Morphotypes as Important Trait for Uropathogenic E. coli Diagnostic; a Virulence-Phenotype-Phylogeny Study. Microorganisms 2021, 9, 2381. [Google Scholar] [CrossRef] [PubMed]
- Bien, J.; Sokolova, O.; Bozko, P. Role of Uropathogenic Escherichia coli Virulence Factors in Development of Urinary Tract Infection and Kidney Damage. Int. J. Nephrol. 2012, 2012, 681473. [Google Scholar] [CrossRef]
- Asadi Karam, M.R.; Habibi, M.; Bouzari, S. Urinary tract infection: Pathogenicity, antibiotic resistance and development of effective vaccines against Uropathogenic Escherichia coli. Mol. Immunol. 2019, 108, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Naseer, F. Antibacterial Activity of Medicinal Plants (Clove, Cinnamon, Garlic) Extracts and their Combined Effect with Antibiotics in Urinary Tract Infection Caused by Escherichia coli. Int. J. Pharm. Pharmacol. 2018, 2, 128. [Google Scholar] [CrossRef]
- Kim, A.; Ahn, J.H.; Choi, W.S.; Park, H.K.; Kim, S.; Paick, S.H.; Kim, H.G. What is the Cause of Recurrent Urinary Tract Infection? Contemporary Microscopic Concepts of Pathophysiology. Int. Neurourol. J. 2021, 25, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Murray, B.O.; Flores, C.; Williams, C.; Flusberg, D.A.; Marr, E.E.; Kwiatkowska, K.M.; Charest, J.L.; Isenberg, B.C.; Rohn, J.L. Recurrent Urinary Tract Infection: A Mystery in Search of Better Model Systems. Front. Cell. Infect. Microbiol. 2021, 11, 691210. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, Y.-G.; Lee, J. Carvacrol-rich oregano oil and thymol-rich thyme red oil inhibit biofilm formation and the virulence of uropathogenic Escherichia coli. J. Appl. Microbiol. 2017, 123, 1420–1428. [Google Scholar] [CrossRef]
- Lucas, M.; Macías, J.; Cañarte, J. Perfil de sensibilidad a antimicrobianos como principal criterio para la selección del tratamiento de infecciones del tracto urinario. Revisión Sistemática. Rev. Kasmera 2021, 49, 1–11. [Google Scholar] [CrossRef]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Kaur, R.; Kaur, R. Symptoms, risk factors, diagnosis and treatment of urinary tract infections. Postgrad. Med. J. 2021, 97, 803–812. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Barrajón-Catalán, E.; Herranz-López, M.; Micol, V. Antibacterial plant compounds, extracts and essential oils: An updated review on their effects and putative mechanisms of action. Phytomedicine 2021, 90, 153626. [Google Scholar] [CrossRef] [PubMed]
- Christaki, S.; Moschakis, T.; Kyriakoudi, A.; Biliaderis, C.G.; Mourtzinos, I. Recent advances in plant essential oils and extracts: Delivery systems and potential uses as preservatives and antioxidants in cheese. Trends Food Sci. Technol. 2021, 116, 264–278. [Google Scholar] [CrossRef]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Jalil, B.; Abdel-Tawab, M.; Echeverria, J.; Kulić, Ž.; McGaw, L.J.; Pezzuto, J.M.; Potterat, O.; Wang, J.-B. Best Practice in the chemical characterisation of extracts used in pharmacological and toxicological research—The ConPhyMP—Guidelines12. Front. Pharmacol. 2022, 13, 953205. [Google Scholar] [CrossRef]
- Sakkas, H.; Papadopoulou, C. Antimicrobial Activity of Basil, Oregano, and Thyme Essential Oils. J. Microbiol. Biotechnol. 2017, 27, 429–438. [Google Scholar] [CrossRef]
- Ebani, V.; Nardoni, S.; Bertelloni, F.; Pistelli, L.; Mancianti, F. Antimicrobial Activity of Five Essential Oils against Bacteria and Fungi Responsible for Urinary Tract Infections. Molecules 2018, 23, 1668. [Google Scholar] [CrossRef]
- FDA CPG Sec 525.750 Spices—Definitions. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/cpg-sec-525750-spices-definitions (accessed on 23 December 2022).
- Vázquez-Fresno, R.; Rosana, A.R.R.; Sajed, T.; Onookome-Okome, T.; Wishart, N.A.; Wishart, D.S. Herbs and Spices- Biomarkers of Intake Based on Human Intervention Studies—A Systematic Review. Genes Nutr. 2019, 14, 18. [Google Scholar] [CrossRef]
- Peter, K.V.; Shylaja, M.R. Introduction to herbs and spices: Definitions, trade and applications. In Handbook of Herbs and Spices; Elsevier: Amsterdam, The Netherlands, 2012; pp. 1–24. [Google Scholar]
- De La Torre, J.E.; Gassara, F.; Kouassi, A.P.; Brar, S.K.; Belkacemi, K. Spice use in food: Properties and benefits. Crit. Rev. Food Sci. Nutr. 2017, 57, 1078–1088. [Google Scholar] [CrossRef]
- Mercado-Mercado, G.; Carrillo, L.d.l.R.; Wall-Medrano, A.; Díaz, J.A.L.; Álvarez-Parrilla, E. Compuestos polifenólicos y capacidad antioxidante de especias típicas consumidas en México. Nutr. Hosp. 2013, 28, 36–46. [Google Scholar] [CrossRef]
- OEC Spices. Available online: https://oec.world/es/profile/hs/spices (accessed on 24 December 2022).
- Sobel, J. Investigation of Multistate Foodborne Disease Outbreaks. Public Health Rep. 2002, 117, 8–19. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Lim, J.; Henry, C.J. Spices in the management of diabetes mellitus. Food Chem. 2017, 217, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, S.; Pandey, M.M.; Kumar Singh Rawat, A. Spices: Therapeutic Potential in Cardiovascular Health. Curr. Pharm. Des. 2017, 23, 989–998. [Google Scholar] [CrossRef] [PubMed]
- Rubió, L.; Motilva, M.-J.; Romero, M.-P. Recent Advances in Biologically Active Compounds in Herbs and Spices: A Review of the Most Effective Antioxidant and Anti-Inflammatory Active Principles. Crit. Rev. Food Sci. Nutr. 2013, 53, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Satheeshkumar, N.; Vijayan, R.S.K.; Lingesh, A.; Santhikumar, S.; Vishnuvardhan, C. Spices: Potential therapeutics for Alzheimer’s disease. In The Benefits of Natural Products for Neurodegenerative Diseases; Essa, M., Akbar, M., Guillemin, G., Eds.; Springer Cham: Midtown Manhattan, NY, USA, 2016; pp. 57–78. [Google Scholar]
- Anderson, C.A.; Cobb, L.K.; Miller, E.R.; Woodward, M.; Hottenstein, A.; Chang, A.R.; Mongraw-Chaffin, M.; White, K.; Charleston, J.; Tanaka, T.; et al. Effects of a behavioral intervention that emphasizes spices and herbs on adherence to recommended sodium intake: Results of the SPICE randomized clinical trial. Am. J. Clin. Nutr. 2015, 102, 671–679. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef]
- Calucci, L.; Pinzino, C.; Zandomeneghi, M.; Capocchi, A.; Ghiringhelli, S.; Saviozzi, F.; Tozzi, S.; Galleschi, L. Effects of γ-Irradiation on the Free Radical and Antioxidant Contents in Nine Aromatic Herbs and Spices. J. Agric. Food Chem. 2003, 51, 927–934. [Google Scholar] [CrossRef]
- Mickymaray, S.; Al Aboody, M.S. In Vitro Antioxidant and Bactericidal Efficacy of 15 Common Spices: Novel Therapeutics for Urinary Tract Infections? Medicina 2019, 55, 289. [Google Scholar] [CrossRef]
- Rath, S.; Padhy, R.N. Monitoring in vitro antibacterial efficacy of 26 Indian spices against multidrug resistant urinary tract infecting bacteria. Integr. Med. Res. 2014, 3, 133–141. [Google Scholar] [CrossRef]
- Opara, E.; Chohan, M. Culinary Herbs and Spices: Their Bioactive Properties, the Contribution of Polyphenols and the Challenges in Deducing Their True Health Benefits. Int. J. Mol. Sci. 2014, 15, 19183–19202. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Bashir, R.; Naeem, N.; Waheed, A.; Sultan, N. Advantageous Impact of Spices in Controlling Urinary Tract Infections. Eur. J. Med. Health Sci. 2021, 3, 1–5. [Google Scholar] [CrossRef]
- Cadena, B.R. Cocina Mexicana: Las Especias Más Usadas. Available online: https://aprende.com/blog/gastronomia/comida-mexicana/especias-cocina-mexicana/ (accessed on 25 December 2022).
- Iturriaga, J. La Cocina Mexicana: Patrimonio Cultural de la Humanidad. Available online: https://go.gale.com/ps/i.do?id=GALE%7CA296952025&sid=googleScholar&v=2.1&it=r&linkaccess=abs&issn=14023357&p=IFME&sw=w&userGroupName=anon~fd3aadd5 (accessed on 25 December 2022).
- Nabavi, S.; Di Lorenzo, A.; Izadi, M.; Sobarzo-Sánchez, E.; Daglia, M.; Nabavi, S. Antibacterial Effects of Cinnamon: From Farm to Food, Cosmetic and Pharmaceutical Industries. Nutrients 2015, 7, 7729–7748. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef]
- Rasool, N.; Saeed, Z.; Pervaiz, M.; Ali, F.; Younas, U.; Bashir, R.; Bukhari, S.M.; Mahmood Khan, R.R.; Jelani, S.; Sikandar, R. Evaluation of essential oil extracted from ginger, cinnamon and lemon for therapeutic and biological activities. Biocatal. Agric. Biotechnol. 2022, 44, 102470. [Google Scholar] [CrossRef]
- Mbaveng, A.T.; Kuete, V. Cinnamon species. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 385–395. [Google Scholar]
- Rao, P.V.; Gan, S.H. Cinnamon: A Multifaceted Medicinal Plant. Evid. -Based Complement. Altern. Med. 2014, 2014, 642942. [Google Scholar] [CrossRef]
- Błaszczyk, N.; Rosiak, A.; Kałużna-Czaplińska, J. The Potential Role of Cinnamon in Human Health. Forests 2021, 12, 648. [Google Scholar] [CrossRef]
- Kowalska, J.; Tyburski, J.; Matysiak, K.; Jakubowska, M.; Łukaszyk, J.; Krzymińska, J. Cinnamon as a Useful Preventive Substance for the Care of Human and Plant Health. Molecules 2021, 26, 5299. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Rao, L.J.M. Chemistry, Biogenesis, and Biological Activities of Cinnamomum zeylanicum. Crit. Rev. Food Sci. Nutr. 2011, 51, 547–562. [Google Scholar] [CrossRef]
- Hussain, S.; Rahman, R.; Mushtaq, A.; Zerey-Belaskri, A. El Clove: A review of a precious species with multiple uses. Int. J. Chem. Biochem. Sci. 2017, 11, 129–133. [Google Scholar]
- Yun, J.-W.; You, J.-R.; Kim, Y.-S.; Kim, S.-H.; Cho, E.-Y.; Yoon, J.-H.; Kwon, E.; Jang, J.-J.; Park, J.-S.; Kim, H.-C.; et al. In vitro and in vivo safety studies of cinnamon extract (Cinnamomum cassia) on general and genetic toxicology. Regul. Toxicol. Pharmacol. 2018, 95, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.-T.; Tung, T.-H.; Jiesisibieke, Z.L.; Chien, C.-W.; Liu, W.-Y. Safety of Cinnamon: An Umbrella Review of Meta-Analyses and Systematic Reviews of Randomized Clinical Trials. Front. Pharmacol. 2022, 12, 790901. [Google Scholar] [CrossRef] [PubMed]
- Modi, P.I.; Parikh, J.K.; Desai, M.A. Sonohydrodistillation: Innovative approach for isolation of essential oil from the bark of cinnamon. Ind. Crops Prod. 2019, 142, 111838. [Google Scholar] [CrossRef]
- Kumar, V.; Marković, T.; Emerald, M.; Dey, A. Herbs: Composition and dietary importance. In Encyclopedia of Food and Health; Elsevier: Amsterdam, The Netherlands, 2016; pp. 332–337. [Google Scholar]
- Pereira, W.A.; Pereira, C.D.S.; Assunção, R.G.; da Silva, I.S.C.; Rego, F.S.; Alves, L.S.R.; Santos, J.S.; Nogueira, F.J.R.; Zagmignan, A.; Thomsen, T.T.; et al. New Insights into the Antimicrobial Action of Cinnamaldehyde towards Escherichia coli and Its Effects on Intestinal Colonization of Mice. Biomolecules 2021, 11, 302. [Google Scholar] [CrossRef] [PubMed]
- Waty, S.; Suryanto, D. Yurnaliza Antibacterial activity of cinnamon ethanol extract (cinnamomum burmannii) and its application as a mouthwash to inhibit streptococcus growth. IOP Conf. Ser. Earth Environ. Sci. 2018, 130, 012049. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, X.; Wang, Y.; Jiang, P.; Quek, S. Antibacterial activity and mechanism of cinnamon essential oil against Escherichia coli and Staphylococcus aureus. Food Control 2016, 59, 282–289. [Google Scholar] [CrossRef]
- Thiyagarajan, S.; John, S. Antimicrobial activity of Cinnamomum zeylanicum aqueous extract against bacteria and fungi responsible for urinary tract infection. Int. J. Health Allied Sci. 2020, 9, 229. [Google Scholar] [CrossRef]
- Utchariyakiat, I.; Surassmo, S.; Jaturanpinyo, M.; Khuntayaporn, P.; Chomnawang, M.T. Efficacy of cinnamon bark oil and cinnamaldehyde on anti-multidrug resistant Pseudomonas aeruginosa and the synergistic effects in combination with other antimicrobial agents. BMC Complement. Altern. Med. 2016, 16, 158. [Google Scholar] [CrossRef]
- Zenati, F.; Benbelaid, F.; Khadir, A.; Bellahsene, C.; Bendahou, M. Antimicrobial effects of three essential oils on multidrug resistant bacteria responsible for urinary infections. J. Appl. Pharm. Sci. 2014, 4, 015–018. [Google Scholar] [CrossRef]
- Dhore, M.R.; Jha, A.R. Antimicrobial activity of Allium cepa and Cinnamomum zeylanicum against common bacteria causing urinary tract infections: In vitro study. Int. J. Basic Clin. Pharmacol. 2019, 8, 1185. [Google Scholar] [CrossRef]
- Narayanan, A.; Muyyarikkandy, M.S.; Mooyottu, S.; Venkitanarayanan, K.; Amalaradjou, M.A.R. Oral supplementation of trans -cinnamaldehyde reduces uropathogenic Escherichia coli colonization in a mouse model. Lett. Appl. Microbiol. 2017, 64, 192–197. [Google Scholar] [CrossRef] [PubMed]
- Rafeeq, H.; Sharba, Z. Study the Effect of Cinnamon and Tea Tree Oils on Biofilm Formation of Klebsiella Pneumoniae. J. Appl. Sci. Nanotechnol. 2022, 2, 16–26. [Google Scholar] [CrossRef]
- Idris, F.Z.; Habibu, U.A. In-vitro antibacterial activity of cinnamon bark extracts on clinical multi-drug resistant (mdr) Staphylococcus aureus, Klebsiella pneumoniae and Pseudomonas aeruginosa isolates. Bayero J. Pure Appl. Sci. 2021, 14, 38–44. [Google Scholar] [CrossRef]
- Cock, E.; Cheesman, M. Plants of the genus syzygium (Myrtaceae): A review on ethnobotany, medicinal properties, and phytochemistry. In Bioactive Compounds of Medicinal Plants; Goyal, M.R., Ed.; Apple Academic Press: Palm Bay, FL, USA, 2018; p. 50. [Google Scholar]
- Ayushi; Khan, U.A.; Danish, S.M.; Mohammad; Parveen, U. A review on biological and therapeutic uses of Syzygium aromaticum Linn. (Clove): Based on phyto-chemistry and pharmacological evidences. Int. J. Bot. Stud. 2020, 5, 33–39. [Google Scholar]
- Shan, B.; Cai, Y.Z.; Sun, M.; Corke, H. Antioxidant Capacity of 26 Spice Extracts and Characterization of Their Phenolic Constituents. J. Agric. Food Chem. 2005, 53, 7749–7759. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Compound Summary for CID 3314, Eugenol. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Eugenol (accessed on 1 December 2022).
- Băicuș, A.; Mattuzzi, F.C.; Paraschiv, A.M.; Dinu, R.-S.; Dumitrescu, M.C.; Marinescu, A.A.; Ionescu, D.; Dragos, D. Antibacterial Activity of Clove, Oregano, Thyme, Eucalyptus, and Tea Tree Essential Oils against Escherichia coli and Klebsiella pneumoniae strains. Rev. Rom. Med. Lab. 2022, 30, 327–338. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, C.; Lin, L. The specific antibacterial activity of liposome-encapsulated Clove oil and its application in tofu. Food Control 2015, 56, 128–134. [Google Scholar] [CrossRef]
- Faujdar, S.; Bisht, D.; Sharma, A. Antibacterial activity of Syzygium aromaticum (clove) against uropathogens producing ESBL, MBL, and AmpC beta-lactamase: Are we close to getting a new antibacterial agent? J. Fam. Med. Prim. Care 2020, 9, 180. [Google Scholar] [CrossRef]
- Mytle, N.; Anderson, G.L.; Doyle, M.P.; Smith, M.A. Antimicrobial activity of clove (Syzgium aromaticum) oil in inhibiting Listeria monocytogenes on chicken frankfurters. Food Control 2006, 17, 102–107. [Google Scholar] [CrossRef]
- Nuñez, L.; D’Aquino, M. Microbicide activity of clove essential oil (Eugenia caryophyllata). Braz. J. Microbiol. 2012, 43, 1255–1260. [Google Scholar] [CrossRef]
- Guan, W.; Li, S.; Yan, R.; Tang, S.; Quan, C. Comparison of essential oils of clove buds extracted with supercritical carbon dioxide and other three traditional extraction methods. Food Chem. 2007, 101, 1558–1564. [Google Scholar] [CrossRef]
- Hatami, T.; Johner, J.C.F.; Zabot, G.L.; Meireles, M.A.A. Supercritical fluid extraction assisted by cold pressing from clove buds: Extraction performance, volatile oil composition, and economic evaluation. J. Supercrit. Fluids 2019, 144, 39–47. [Google Scholar] [CrossRef]
- Dąbrowski, M.; Sienkiewicz, M.; Zielińska-Bliźniewska, H.; Dąbrowska, M.; Seredyńska, M.; Kochan, E. Antibiotic resistance among Escherichia coli urinary isolates and their susceptibility to clove essential oil. Ann. Univ. Mariae Curie-Sklodowska Sect. C Biol. 2018, 71, 41. [Google Scholar] [CrossRef]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Roy, S.; Chaurvedi, P.; Chowdhary, A. Evaluation of antiviral activity of essential oil of Trachyspermum Ammi against Japanese encephalitis virus. Pharmacogn. Res. 2015, 7, 263. [Google Scholar] [CrossRef] [PubMed]
- Swamy, M.K.; Akhtar, M.S.; Sinniah, U.R. Antimicrobial Properties of Plant Essential Oils against Human Pathogens and Their Mode of Action: An Updated Review. Evidence-Based Complement. Altern. Med. 2016, 2016, 3012462. [Google Scholar] [CrossRef]
- Goñi, P.; López, P.; Sánchez, C.; Gómez-Lus, R.; Becerril, R.; Nerín, C. Antimicrobial activity in the vapour phase of a combination of cinnamon and clove essential oils. Food Chem. 2009, 116, 982–989. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef]
- Rhayour, K.; Bouchikhi, T.; Tantaoui-Elaraki, A.; Sendide, K.; Remmal, A. The Mechanism of Bactericidal Action of Oregano and Clove Essential Oils and of Their Phenolic Major Components on Escherichia coli and Bacillus subtilis. J. Essent. Oil Res. 2003, 15, 356–362. [Google Scholar] [CrossRef]
- Valle, D.L.; Cabrera, E.C.; Puzon, J.J.M.; Rivera, W.L. Antimicrobial Activities of Methanol, Ethanol and Supercritical CO2 Extracts of Philippine Piper betle L. on Clinical Isolates of Gram Positive and Gram Negative Bacteria with Transferable Multiple Drug Resistance. PLoS ONE 2016, 11, e0146349. [Google Scholar] [CrossRef]
- Mahmoud, A.M.; El-Baky, R.M.A.; Ahmed, A.B.F.; Fadl, G. Antibacterial Activity of Essential Oils and in Combination with Some Standard Antimicrobials against Different Pathogens Isolated from Some ClinicalSpecimens. Am. J. Microbiol. Res. 2016, 4, 16–25. [Google Scholar] [CrossRef]
- Rakshit, M.; Ramalingam, C. Screening and Comparision of Antibacterial Activity of Indian Spices. J. Exp. Sci. 2010, 1, 33–36. [Google Scholar]
- Rosarior, V.L.; Lim, P.S.; Wong, W.K.; Yue, C.S.; Yam, H.C.; Tan, S.-A. Antioxidant-rich Clove Extract, A Strong Antimicrobial Agent against Urinary Tract Infections-causing Bacteria in vitro. Trop. Life Sci. Res. 2021, 32, 45–63. [Google Scholar] [CrossRef] [PubMed]
- Code of Federal Regulations. Chapter I. Food and Drug Administration. Subchapter E. Animal drugs, feeds, and related products. In Food and Drugs; 2015; p. 21 CFR 582.20. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=582.20 (accessed on 1 December 2022).
- Shalaby, S.; El-Din, M.; Abo-Donia, S.; Mettwally, M.; Attia, Z. Toxicological effects of essential oils from Eucalyptus Eucalyptus globules and clove Eugenia caryophyllus on albino rats. Polish J. Environ. Stud. 2011, 20, 429–434. [Google Scholar]
- Vijayasteltar, L.; Nair, G.G.; Maliakel, B.; Kuttan, R.; Krishnakumar, I.M. Safety assessment of a standardized polyphenolic extract of clove buds: Subchronic toxicity and mutagenicity studies. Toxicol. Rep. 2016, 3, 439–449. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; de Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef]
- Hartnoll, G.; Moore, D.; Douek, D. Near fatal ingestion of oil of cloves. Arch. Dis. Child. 1993, 69, 392–393. [Google Scholar] [CrossRef]
- Slameňová, D.; Horváthová, E.; Wsólová, L.; Šramková, M.; Navarová, J. Investigation of anti-oxidative, cytotoxic, DNA-damaging and DNA-protective effects of plant volatiles eugenol and borneol in human-derived HepG2, Caco-2 and VH10 cell lines. Mutat. Res. Toxicol. Environ. Mutagen. 2009, 677, 46–52. [Google Scholar] [CrossRef]
- Doleželová, P.; Mácová, S.; Plhalová, L.; Pištěková, V.; Svobodová, Z. The acute toxicity of clove oil to fish Danio rerio and Poecilia reticulata. Acta Vet. Brno 2011, 80, 305–308. [Google Scholar] [CrossRef]
- FAO/WHO. Evaluation of Certain Food Additives and Contaminants: Twenty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives [Meeting Held in Rome from 19 to 28 April 1982]; WHO: Geneva, Switzerland, 1982.
- Abdullah, B.H.; Hatem, S.F.; Jumaa, W. A Comparative Study of the Antibacterial Activity of Clove and Rosemary Essential Oils on Multidrug Resistant Bacteria. Pharm. Biosci. J. 2015, 3, 18–22. [Google Scholar] [CrossRef]
- Mostafa, A.A.; Al-Askar, A.A.; Almaary, K.S.; Dawoud, T.M.; Sholkamy, E.N.; Bakri, M.M. Antimicrobial activity of some plant extracts against bacterial strains causing food poisoning diseases. Saudi J. Biol. Sci. 2018, 25, 361–366. [Google Scholar] [CrossRef]
- National Center for Biotechnology Information PubChem Taxonomy Summary for TaxonomyBob 52462, Cuminum Cyminum. Available online: https://pubchem.ncbi.nlm.nih.gov/taxonomy/Cuminum%20cyminum (accessed on 19 December 2022).
- Singh, R.P.; Gangadharappa, H.V.; Mruthunjaya, K. Cuminum cyminum—A Popular Spice: An Updated Review. Pharmacogn. J. 2017, 9, 292–301. [Google Scholar] [CrossRef]
- Siow, H.-L.; Gan, C.-Y. Extraction, identification, and structure–activity relationship of antioxidative and α-amylase inhibitory peptides from cumin seeds (Cuminum cyminum). J. Funct. Foods 2016, 22, 1–12. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S.; Kumar, S.; Narashiman, B. A review on traditional uses, phytochemistry, pharmacology, and clinical research of dietary spice Cuminum cyminum L. Phyther. Res. 2021, 35, 5007–5030. [Google Scholar] [CrossRef]
- Rajput, R.P.S.; Paramakrishnan, N.; Gangadharappa, H.V. Cumin (Cuminum cyminum L.) Seed. In Oilseeds: Health Attributes and Food Applications; Springer Singapore: Singapore, 2021; pp. 507–516. [Google Scholar]
- Animal Gourmet Comino, la Semilla del Sabor Virreinal. Available online: https://www.animalgourmet.com/2015/04/17/50saboresmexicanos-comino-la-semilla-del-sabor-virreinal/#:~:text=En%20M%C3%A9xico%20fue%20popularizada%20por,su%20sabor%20picante%20y%20acre (accessed on 19 December 2022).
- EI-kani, M.H.; Golmohammad, F.; Mirza, M.; Rowshanzamir, S. Extraction of volatile oil from cumin (Cuminum cyminum L.) With superheated water. J. Food Process Eng. 2007, 30, 255–266. [Google Scholar] [CrossRef]
- Iacobellis, N.S.; Lo Cantore, P.; Capasso, F.; Senatore, F. Antibacterial Activity of Cuminum cyminum L. and Carum carvi L. Essential Oils. J. Agric. Food Chem. 2005, 53, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Bokaeian, M.; Shiri, Y.; Bazi, S.; Saeidi, S.; Sahi, Z. Antibacterial activities of Cuminum cyminum Linn. Essential Oil Against Multi-Drug resistant Escherichia coli. Int. J. Infect. 2014, 1, e18739. [Google Scholar] [CrossRef]
- Sattari, M.; Bigdeli, M.; Derakhshan, S. Effect of cumin (Cuminum cyminum) seed essential oil on biofilm formation and plasmid Integrity of Klebsiella pneumoniae. Pharmacogn. Mag. 2010, 6, 57. [Google Scholar] [CrossRef]
- Saee, Y.; Dadashi, M.; Eslami, G.; Goudarzi, H.; Taheri, S.; Fallah, F. Evaluation of Antimicrobial Activity of Cuminum cyminum Essential Oil and Extract against Bacterial Strains Isolated from Patients with Symptomatic Urinary Tract Infection. Nov. Biomed. 2016, 4, 147–152. [Google Scholar]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, antioxidant and antimicrobial potential of nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef]
- Rehman, J.U.; Iqbal, A.; Mahmood, A.; Asif, H.M.; Mohiuddin, E.; Akram, M. Phytochemical analysis, antioxidant and antibacterial potential of some selected medicinal plants traditionally utilized for the management of urinary tract infection. Pak. J. Pharm. Sci. 2021, 34, 1056–1062. [Google Scholar] [CrossRef]
- Prakash, E.; Gupta, D.K. Cytotoxic Activity of Ethanolic Extract of Cuminum cyminum Linn against Seven Human Cancer Cell Line. Univers. J. Agric. Res. 2014, 2, 27–30. [Google Scholar] [CrossRef]
- Niu, C.; Gilbert, E.S. Colorimetric Method for Identifying Plant Essential Oil Components That Affect Biofilm Formation and Structure. Appl. Environ. Microbiol. 2004, 70, 6951–6956. [Google Scholar] [CrossRef] [PubMed]
- Gutmann, L.; Williamson, R.; Moreau, N.; Kitzis, M.-D.; Collatz, E.; Acar, J.F.; Goldstein, F.W. Cross-Resistance to Nalidixic Acid, Trimethoprim, and Chloramphenicol Associated with Alterations in Outer Membrane Proteins of Klebsiella, Enterobacter, and Serratia. J. Infect. Dis. 1985, 151, 501–507. [Google Scholar] [CrossRef] [PubMed]
- Johri, R. Cuminum cyminum and Carum carvi: An update. Pharmacogn. Rev. 2011, 5, 63. [Google Scholar] [CrossRef] [PubMed]
- Chacón-Vargas, K.F.; Sánchez-Torres, L.E.; Chávez-González, M.L.; Adame-Gallegos, J.R.; Nevárez-Moorillón, G.V. Mexican Oregano (Lippia berlandieri Schauer and Poliomintha longiflora Gray) Essential Oils Induce Cell Death by Apoptosis in Leishmania (Leishmania) mexicana Promastigotes. Molecules 2022, 27, 5183. [Google Scholar] [CrossRef]
- Bautista-Hernández, I.; Aguilar, C.N.; Martínez-Ávila, G.C.G.; Torres-León, C.; Ilina, A.; Flores-Gallegos, A.C.; Kumar Verma, D.; Chávez-González, M.L. Mexican Oregano (Lippia graveolens Kunth) as Source of Bioactive Compounds: A Review. Molecules 2021, 26, 5156. [Google Scholar] [CrossRef]
- García-Pérez, E.; Francisco Castro-Álvarez, F.; Alejandra Gutiérrez-Uribe, J.; García-Lara, S. Revisión de la producción, composición fitoquímica y propiedades nutracéuticas del orégano mexicano* Revision of the production, phytochemical composition, and nutraceutical properties of Mexican oregano. Rev. Mex. Cienc. Agrícolas 2012, 3, 339–353. [Google Scholar]
- Soltani, S.; Shakeri, A.; Iranshahi, M.; Boozari, M. A review of the phytochemistry and antimicrobial properties of origanum vulgare l. And subspecies. Iran. J. Pharm. Res. 2021, 20, 268–285. [Google Scholar] [CrossRef]
- Fimbres-García, J.O.; Flores-Sauceda, M.; Othon-Díaz, E.D.; García-Galaz, A.; Tapia-Rodríguez, M.R.; Silva-Espinoza, B.A.; Ayala-Zavala, J.F. Facing Resistant Bacteria with Plant Essential Oils: Reviewing the Oregano Case. Antibiotics 2022, 11, 1777. [Google Scholar] [CrossRef]
- Zapién-Chavarría, K.A.; Plascencia-Terrazas, A.; Venegas-Ortega, M.G.; Varillas-Torres, M.; Rivera-Chavira, B.E.; Adame-Gallegos, J.R.; González-Rangel, M.O.; Nevárez-Moorillón, G.V. Susceptibility of Multidrug-Resistant and Biofilm-Forming Uropathogens to Mexican Oregano Essential Oil. Antibiotics 2019, 8, 186. [Google Scholar] [CrossRef] [PubMed]
- Xiao, S.; Cui, P.; Shi, W.; Zhang, Y. Identification of essential oils with activity against stationary phase Staphylococcus aureus. BMC Complement. Med. Ther. 2020, 20, 99. [Google Scholar] [CrossRef] [PubMed]
- Hadi Alkhafaji, R.T.; Jayashankar, M. Physicochemical Properties and Inhibitory Effects of Oregano Oil against Uropathogenic. Pharmacogn. Res. 2022, 14, 328–332. [Google Scholar] [CrossRef]
- Benbrahim, C.; Barka, M.S.; Basile, A.; Maresca, V.; Flamini, G.; Sorbo, S.; Carraturo, F.; Notariale, R.; Piscopo, M.; Khadir, A.; et al. Chemical Composition and Biological Activities of Oregano and Lavender Essential Oils. Appl. Sci. 2021, 11, 5688. [Google Scholar] [CrossRef]
- Drissi, B.; Mahdi, I.; Yassir, M.; Ben Bakrim, W.; Bouissane, L.; Sobeh, M. Cubeb (Piper cubeba L.f.): A comprehensive review of its botany, phytochemistry, traditional uses, and pharmacological properties. Front. Nutr. 2022, 9, 2836. [Google Scholar] [CrossRef]
- Akshita, C.; Vijay, B.V.; Praveen, D. Evaluation of phytochemical screening and antimicrobial efficacy of Mesua Ferrea and Piper cubeba fruit extracts against multidrug resistant bacteria. Pharmacophore 2020, 11, 15–20. [Google Scholar]
- Al-Shahwany, A.W. Alkaloids and Phenolic Compound Activity of Piper Nigrum against Some Human Pathogenic Bacteria. Biomed. Biotechnol. 2014, 2, 20–28. [Google Scholar] [CrossRef]
- Subbu Lakshmi, S.; Chelladurai, G.; Suresh, B. In vitro studies on medicinal plants used against bacterial diabetic foot ulcer (BDFU) and urinary tract infected (UTI) causing pathogens. J. Parasit. Dis. 2016, 40, 667–673. [Google Scholar] [CrossRef]
- Chaudhary, V.; Rk, R.; Chaudhary, N.; Sharma, G. Bio-control of multiple drug-resistant uropathogens using medicinal plant extracts. Asian J. Pharm. Clin. Res. 2019, 12, 371–376. [Google Scholar] [CrossRef]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59, S210–S243. [Google Scholar] [CrossRef]
- Kadosh, Y.; Muthuraman, S.; Yaniv, K.; Baruch, Y.; Gopas, J.; Kushmaro, A.; Kumar, R.S. Quorum Sensing and NF-κB Inhibition of Synthetic Coumaperine Derivatives from Piper nigrum. Molecules 2021, 26, 2293. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, R.; Vigneshwari, L.; Rajavel, T.; Durgadevi, R.; Kannappan, A.; Balamurugan, K.; Pandima Devi, K.; Veera Ravi, A. Biogenic synthesis of silver nanoparticles using Piper betle aqueous extract and evaluation of its anti-quorum sensing and antibiofilm potential against uropathogens with cytotoxic effects: An in vitro and in vivo approach. Environ. Sci. Pollut. Res. 2018, 25, 10538–10554. [Google Scholar] [CrossRef]
- Karadağ, A.E.; Demirci, B.; Çaşkurlu, A.; Demirci, F.; Okur, M.E.; Orak, D.; Sipahi, H.; Başer, K.H.C. In vitro antibacterial, antioxidant, anti-inflammatory and analgesic evaluation of Rosmarinus officinalis L. flower extract fractions. South African J. Bot. 2019, 125, 214–220. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Bravo-Díaz, L.; Ayala-Gómez, A. Rosmarinus officinalis L. (Rosemary): An Ancient Plant with Uses in Personal Healthcare and Cosmetics. Cosmetics 2020, 7, 77. [Google Scholar] [CrossRef]
- Younes, M.; Aggett, P.; Aguilar, F.; Crebelli, R.; Dusemund, B.; Filipič, M.; Frutos, M.J.; Galtier, P.; Gott, D.; Gundert-Remy, U.; et al. Refined exposure assessment of extracts of rosemary (E 392) from its use as food additive. EFSA J. 2018, 16, e05373. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, J.R.; Camargo, S.E.A.; de Oliveira, L.D. Rosmarinus officinalis L. (rosemary) as therapeutic and prophylactic agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Senanayake, S.P.J.N. Rosemary extract as a natural source of bioactive compounds. J. Food Bioact. 2018, 2, 51–57. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef]
- Kloy, A.; Ahmad, J.; Yusuf, U.; Muhammad, M. Antibacterial Properties of Rosemary (Rosmarinus officinalis). South Asian Res. J. Pharm. Sci. 2020, 02, 4–7. [Google Scholar] [CrossRef]
- Li, L.; Li, Z.-W.; Yin, Z.-Q.; Wei, Q.; Jia, R.-Y.; Zhou, L.-J.; Xu, J.; Song, X.; Zhou, Y.; Du, Y.-H.; et al. Antibacterial activity of leaf essential oil and its constituents from Cinnamomum longepaniculatum. Int. J. Clin. Exp. Med. 2014, 7, 1721–1727. [Google Scholar]
- Lagha, R.; Ben Abdallah, F.; AL-Sarhan, B.; Al-Sodany, Y. Antibacterial and Biofilm Inhibitory Activity of Medicinal Plant Essential Oils Against Escherichia coli Isolated from UTI Patients. Molecules 2019, 24, 1161. [Google Scholar] [CrossRef] [PubMed]
- Abdulhasan, G.A. Synergism effect of rosemary essential oil and some antibiotic against Escherichia coli isolated from clinical samples. IOSR J. Pharm. Biol. Sci. 2017, 12, 39–42. [Google Scholar] [CrossRef]
- Amirian, F.; Kazemi Pour, N.; Khoshroo, S.M.R.; Sayadi, A.; Karmostaji, A.; Mousavi, S.M. Synergistic Effect and Antibacterial Activities of Extracts of Salvia and Rosemary Officinalis Against Escherichia coli Isolated from Clinical Urinary Tract Infection. Ann. Mil. Health Sci. Res. 2017, 15, e80148. [Google Scholar] [CrossRef]
- Beigomi, M.; Biabangard, A.; Rohani, R. Evaluation of antimicrobial effects of Rosemary and Withania somnifera methanol extract prepared by ultrasound waveform on Escherichia coli biofilm isolated from urinary tract infection. Micro Env. 2021, 1, 17–25. [Google Scholar] [CrossRef]
- Petrolini, F.V.B.; Lucarini, R.; de Souza, M.G.M.; Pires, R.H.; Cunha, W.R.; Martins, C.H.G. Evaluation of the antibacterial potential of Petroselinum crispum and Rosmarinus officinalis against bacteria that cause urinary tract infections. Braz. J. Microbiol. 2013, 44, 829–834. [Google Scholar] [CrossRef] [PubMed]
- Sienkiewicz, M.; Łysakowska, M.; Pastuszka, M.; Bienias, W.; Kowalczyk, E. The Potential of Use Basil and Rosemary Essential Oils as Effective Antibacterial Agents. Molecules 2013, 18, 9334–9351. [Google Scholar] [CrossRef]
- Al Zuhairi, J.J.M.J.; Jookar Kashi, F.; Rahimi-Moghaddam, A.; Yazdani, M. Antioxidant, cytotoxic and antibacterial activity of Rosmarinus officinalis L. essential oil against bacteria isolated from urinary tract infection. Eur. J. Integr. Med. 2020, 38, 101192. [Google Scholar] [CrossRef]
- Wawrysiuk, S.; Naber, K.; Rechberger, T.; Miotla, P. Prevention and treatment of uncomplicated lower urinary tract infections in the era of increasing antimicrobial resistance—Non-antibiotic approaches: A systemic review. Arch. Gynecol. Obstet. 2019, 300, 821–828. [Google Scholar] [CrossRef]
- Sabadash, M.; Shulyak, A. Canephron® N in the treatment of recurrent cystitis in women of child-bearing Age: A randomised controlled study. Clin. Phytoscience 2017, 3, 9. [Google Scholar] [CrossRef]
- Rechberger, E.; Rechberger, T.; Wawrysiuk, S.; Miotla, P.; Kulik- Rechberger, B.; Kuszka, A.; Wróbel, A. A Randomized Clinical Trial to Evaluate the Effect of Canephron N in Comparison to Ciprofloxacin in the Prevention of Postoperative Lower Urinary Tract Infections after Midurethral Sling Surgery. J. Clin. Med. 2020, 9, 3391. [Google Scholar] [CrossRef]
| Type of Extract | Phytochemicals | Uropathogen | MIC | MBC | Diameter of the Inhibition Zone (mm) | Ref. |
|---|---|---|---|---|---|---|
| Essential oil | Cuminaldehyde, α-thujene, α,b-pinene, p-cymene, g-terpinene, cumin oils | K. pneumoniae | 0.8–3.5 μg/mL | ND | ND | [103] |
| Essential oil | Cuminaldehyde, α-thujene, α,b-pinene, p-cymene, g-terpinene, cumin oils | E. coli | 10–50 ppm and 100–250 ppm * | ND | ND | [102] |
| Essential oil | Cuminaldehyde, α-thujene, α,b-pinene, p-cymene, g-terpinene, cumin oils | E. coli | 0.25 mg/mL | 0.5 mg/mL | 23 | [104] |
| K. pneumoniae | 0.25 mg/mL | 0.5 mg/mL | 22 | |||
| p. aeruginosa | 0.25 mg/mL | 0.5 mg/mL | 20 | |||
| S. agalactiae | 0.25 mg/mL | 0.5 mg/mL | 21 | |||
| group A streptococci | 0.015 mg/mL | 0.03 mg/mL | 20 | |||
| E. faecalis | 0.125 mg/mL | 0.25 mg/mL | 20 | |||
| S.epidermidis | 0.25 mg/mL | 0.5 mg/mL | 10 | |||
| S. aureus | ND | ND | 7 | |||
| S. saprophyticus | 0.25 mg/mL | 0.5 mg/mL | 20 | |||
| Essential oil | Cuminaldehyde, α-thujene, α,b-pinene, p-cymene, g-terpinene, cumin oils | S. aureus | 1161 μg/mL | ND | 45 | [81] |
| P. aeruginosa | 84.97 μg/mL | ND | 8 | |||
| K. pneumoniae | 204.87 μg/mL | ND | 12 | |||
| E. coli | 7.219 μg/mL | ND | 52 | |||
| Ethanolic extract | ND | E. coli | 0.125 mg/mL | 0.25 mg/mL | 22 | [104] |
| K. pneumoniae | 0.125 mg/mL | 0.25 mg/mL | 22 | |||
| P. aeruginosa | 0.25 mg/mL | 0.5 mg/mL | 20 | |||
| S. agalactiae | ND | ND | 7 | |||
| group A streptococci | 0.125 mg/mL | 0.25 mg/mL | 23 | |||
| E. faecalis | 0.125 mg/mL | 0.25 mg/mL | 23 | |||
| S. epidermidis | 0.125 mg/mL | 0.25 mg/mL | 25 | |||
| S. aureus | 0.125 mg/mL | 0.25 mg/mL | 20 | |||
| S. saprophyticus | 0.25 mg/mL | 0.5 mg/mL | 23 | |||
| Aqueous-ethanolic (30/70) extract | Carbohydrates, flavonoids, protein, alkaloids, phenols | E. coli | ND | ND | 26 | [106] |
| K. pneumonia | ND | ND | 22 | |||
| S. saprophyticus | ND | ND | 25 | |||
| P. mirabilis | ND | ND | 21.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ortega-Lozano, A.J.; Hernández-Cruz, E.Y.; Gómez-Sierra, T.; Pedraza-Chaverri, J. Antimicrobial Activity of Spices Popularly Used in Mexico against Urinary Tract Infections. Antibiotics 2023, 12, 325. https://doi.org/10.3390/antibiotics12020325
Ortega-Lozano AJ, Hernández-Cruz EY, Gómez-Sierra T, Pedraza-Chaverri J. Antimicrobial Activity of Spices Popularly Used in Mexico against Urinary Tract Infections. Antibiotics. 2023; 12(2):325. https://doi.org/10.3390/antibiotics12020325
Chicago/Turabian StyleOrtega-Lozano, Ariadna Jazmín, Estefani Yaquelin Hernández-Cruz, Tania Gómez-Sierra, and José Pedraza-Chaverri. 2023. "Antimicrobial Activity of Spices Popularly Used in Mexico against Urinary Tract Infections" Antibiotics 12, no. 2: 325. https://doi.org/10.3390/antibiotics12020325
APA StyleOrtega-Lozano, A. J., Hernández-Cruz, E. Y., Gómez-Sierra, T., & Pedraza-Chaverri, J. (2023). Antimicrobial Activity of Spices Popularly Used in Mexico against Urinary Tract Infections. Antibiotics, 12(2), 325. https://doi.org/10.3390/antibiotics12020325