Do Anti-Biofilm Antibiotics Have a Place in the Treatment of Diabetic Foot Osteomyelitis?
Abstract
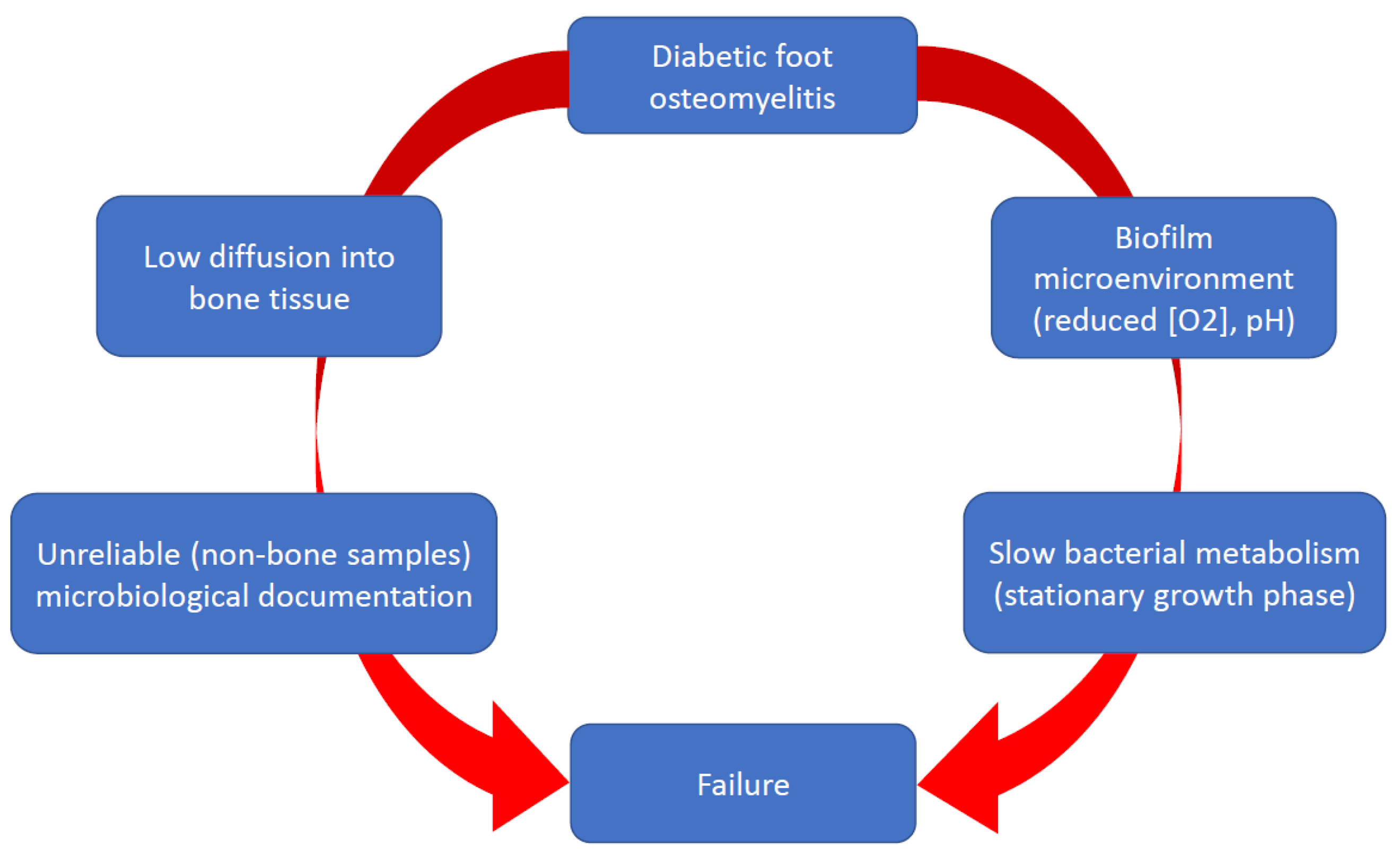
1. Introduction
2. Microbiology
3. Histology
4. Biofilm
5. Anti-Biofilm Effect of Antibiotics
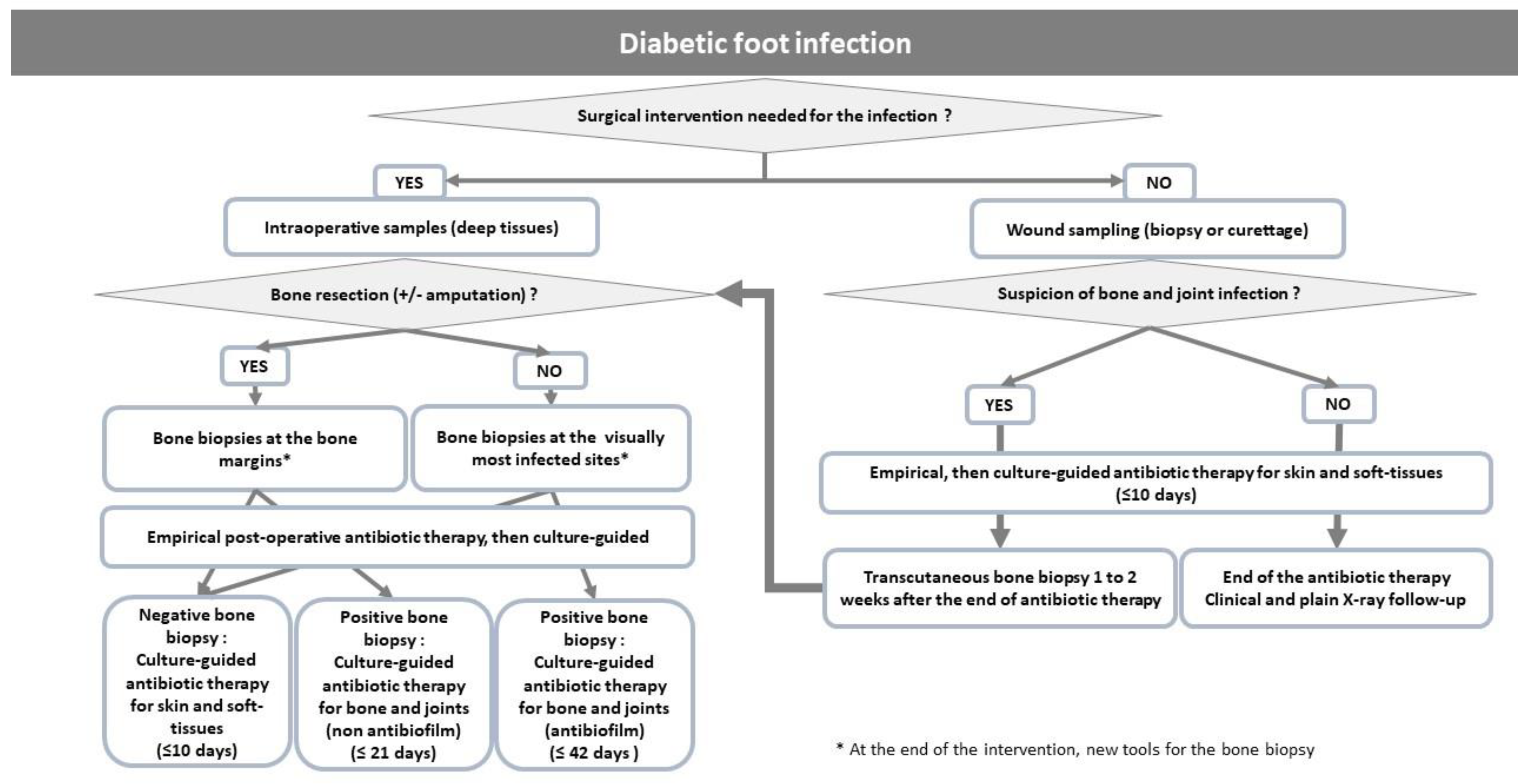
6. Antibiotic Therapy
6.1. Current Data
6.2. Rifampicin and Fluoroquinolones for the Treatment of DFO
6.3. Data Expected in the Next Future
6.4. Limitations of Rifampicin-Fluoroquinolones for the Treatment of DFO
7. New Anti-Biofilm Modalities
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lavery, L.A.; Armstrong, D.G.; Wunderlich, R.P.; Mohler, M.J.; Wendel, C.S.; Lipsky, B.A. Risk factors for foot infections in individuals with diabetes. Diabetes Care 2006, 29, 1288–1293. [Google Scholar] [CrossRef] [PubMed]
- Prompers, L.; Huijberts, M.; Apelqvist, J.; Jude, E.; Piaggesi, A.; Bakker, K.; Edmonds, M.; Holstein, P.; Jirkovska, A.; Mauricio, D.; et al. High prevalence of ischaemia, infection and serious comorbidity in patients with diabetic foot disease in Europe. Baseline results from the Eurodiale study. Diabetologia 2007, 50, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Mutluoglu, M.; Sivrioglu, A.K.; Eroglu, M.; Uzun, G.; Turhan, V.; Ay, H.; Lipsky, B.A. The implications of the presence of osteomyelitis on outcomes of infected diabetic foot wounds. Scand. J. Infect. Dis. 2013, 45, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Senneville, E.M.; Lipsky, B.A.; van Asten, S.A.V.; Peters, E.J. Diagnosing diabetic foot osteomyelitis. Diabetes Metab. Res. Revi. End. 2020, 36 (Suppl. S1), e3250. [Google Scholar] [CrossRef]
- Lavery, L.A.; Sariaya, M.; Ashry, H.; Harkless, L.B. Microbiology of osteomyelitis in diabetic foot infections. J. Foot Ankle Surg. 1995, 34, 61–64. [Google Scholar] [CrossRef]
- Wheat, J. Diagnostic strategies in osteomyelitis. Am. J. Med. 1985, 78, 218–222. [Google Scholar] [CrossRef]
- Senneville, E.; Melliez, H.; Beltrand, E.; Legout, L.; Valette, M.; Cazaubiel, M.; Cordonnier, M.; Caillaux, M.; Yazdanpanah, Y.; Mouton, Y. Culture of percutaneous bone biopsy specimens for diagnosis of diabetic foot osteomyelitis: Concordance with ulcer swab cultures. Clin. Infect. Dis. 2006, 42, 57–62. [Google Scholar] [CrossRef]
- Lesens, O.; Desbiez, F.; Vidal, M.; Robin, F.; Descamps, S.; Beytout, J.; Laurichesse, H.; Tauveron, I. Culture of per-wound bone specimens: A simplified approach for the medical management of diabetic foot osteomyelitis. Clin. Microbiol. Infect. 2011, 17, 285–291. [Google Scholar] [CrossRef]
- Widatalla, A.H.; Mahadi, S.E.; Shawer, M.A.; Mahmoud, S.M.; Abdelmageed, A.E.; Ahmed, M.E. Diabetic foot infections with osteomyelitis: Efficacy of combined surgical and medical treatment. Diabet. Foot Ankle 2012, 3, 18809. [Google Scholar] [CrossRef]
- Van Asten, S.A.; La Fontaine, J.; Peters, E.J.; Bhavan, K.; Kim, P.J.; Lavery, L.A. The microbiome of diabetic foot osteomyelitis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 293–298. [Google Scholar] [CrossRef]
- Johani, K.; Fritz, B.G.; Bjarnsholt, T.; Lipsky, B.A.; Jensen, S.O.; Yang, M.; Dean, A.; Hu, H.; Vickery, K.; Malone, M. Understanding the microbiome of diabetic foot osteomyelitis: Insights from molecular and microscopic approaches. Clin. Microbiol. Infect. 2019, 25, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Pouget, C.; Dunyach-Remy, C.; Magnan, C.; Pantel, A.; Sotto, A.; Lavigne, J.P. Polymicrobial Biofilm Organization of Staphylococcus aureus and Pseudomonas aeruginosa in a Chronic Wound Environment. Int. J. Mol. Sci. 2022, 23, 10761. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, G.; Gonschorek, O.; Hofmann, G.O.; Bührenn, V. Stabilisierungsverfahren bei Osteomyelitis. Osteosyn. Intern. 1997, 5, 226–231. [Google Scholar]
- Chantelau, E.; Wolf, A.; Ozdemir, S.; Hachmöller, A.; Ramp, U. Bone histomorphology may be unremarkable in diabetes mellitus. Med. Klin. (Munich) 2007, 102, 429–433. [Google Scholar] [CrossRef]
- Aragón-Sánchez, F.J.; Cabrera-Galván, J.J.; Quintana-Marrero, Y.; Hernández-Herrero, M.J.; Lázaro-Martínez, J.L.; García-Morales, E.; Beneit-Montesinos, J.V.; Armstrong, D.G. Outcomes of surgical treatment of diabetic foot osteomyelitis: A series of 185 patients with histopathological confirmation of bone involvement. Diabetologia 2008, 51, 1962–1970. [Google Scholar] [CrossRef] [PubMed]
- Lebeaux, D.; Chauhan, A.; Rendueles, O.; Beloin, C. From in vitro to in vivo models of bacterial biofilm-related infections. Pathogens 2013, 2, 288–356. [Google Scholar] [CrossRef]
- Tuchscherr, L.; Medina, E.; Hussain, M.; Volker, W.; Heitmann, V.; Niemann, S.; Holzinger, D.; Roth, J.; Proctor, R.A.; Becker, K.; et al. Staphylococcus aureus phenotype switching: An effective bacterial strategy to escape host immune response and establish a chronic infection. EMBO Mol. Med. 2011, 3, 129–141. [Google Scholar] [CrossRef]
- Baudoux, F.; Neut, C.; Beltrand, E.; Lancelevee, J.; Lebrun, C.; Lemoux, O.; Vambergue, A.; Dubreuil, L.; Fontaine, P.; Senneville, E. O44 Facteurs de pathogénicité dans l’ostéite du pied diabétique: Étude de la charge bactérienne et de la capacité à former un biofilm. Diabetes Metab. 2012, 38 (Suppl. S2), 11. [Google Scholar] [CrossRef]
- Malone, M.; Fritz, B.G.; Vickery, K.; Schwarzer, S.; Sharma, V.; Biggs, N.; Radzieta, M.; Jeffries, T.T.; Dickson, H.G.; Jensen, S.O.; et al. Analysis of proximal bone margins in diabetic foot osteomyelitis by conventional culture, DNA sequencing, and microscopy. APMIS 2019, 127, 660–670. [Google Scholar] [CrossRef]
- Zimmerli, W.; Sendi, P. Orthopaedic biofilm infections. APMIS 2017, 125, 353–364. [Google Scholar] [CrossRef]
- Espíndola, R.; Vella, V.; Benito, N.; Mur, I.; Tedeschi, S.; Zamparini, E.; Hendriks, J.G.E.; Sorlí, L.; Murillo, O.; Soldevila, L.; et al. Rates and Predictors of Treatment Failure in Staphylococcus aureus Prosthetic Joint Infections According to Different Management Strategies: A Multinational Cohort Study-The ARTHR-IS Study Group. Infect. Dis. Ther. 2022, 11, 2177–2203. [Google Scholar] [CrossRef]
- Oliva, A.; Stefani, S.; Venditti, M.; Di Domenico, E.G. Biofilm-Related Infections in Gram-Positive Bacteria and the Potential Role of the Long-Acting Agent Dalbavancin. Front. Microbiol. 2021, 12, 749685. [Google Scholar] [CrossRef] [PubMed]
- Senneville, E.; Joulie, D.; Legout, L.; Valette, M.; Dezèque, H.; Beltrand, E.; Roselé, B.; d’Escrivan, T.; Loïez, C.; Caillaux, M.; et al. Outcome and predictors of treatment failure in total hip/knee prosthetic joint infections due to Staphylococcus aureus. Clin. Infect. Dis. 2011, 53, 334–340. [Google Scholar] [CrossRef]
- Lora-Tamayo, J.; Murillo, O.; Iribarren, J.A.; Soriano, A.; Sánchez-Somolinos, M.; Baraia-Etxaburu, J.M.; Rico, A.; Palomino, J.; Rodríguez-Pardo, D.; Horcajada, J.P.; et al. A large multicenter study of methicillin-susceptible and methicillin-resistant Staphylococcus aureus prosthetic joint infections managed with implant retention. Clin. Infect. Dis. 2013, 56, 182–194. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Pardo, D.; Pigrau, C.; Lora-Tamayo, J.; Soriano, A.; del Toro, M.D.; Cobo, J.; Palomino, J.; Euba, G.; Riera, M.; Sánchez-Somolinos, M.; et al. Gram-negative prosthetic joint infection: Outcome of a debridement, antibiotics and implant retention approach. A large multicentre study. Clin. Microbiol. Infect. 2014, 20, O911–O919. [Google Scholar] [CrossRef]
- John, A.K.; Baldoni, D.; Haschke, M.; Rentsch, K.; Schaerli, P.; Zimmerli, W.; Trampuz, A. Efficacy of daptomycin in implant-associated infection due to methicillin-resistant Staphylococcus aureus: Importance of combination with rifampin. Antimicrob. Agents Chemother. 2009, 53, 2719–2724. [Google Scholar] [CrossRef] [PubMed]
- Valour, F.; Trouillet-Assant, S.; Riffard, N.; Tasse, J.; Flammier, S.; Rasigade, J.P.; Chidiac, C.; Vandenesch, F.; Ferry, T.; Laurent, F. Antimicrobial activity against intraosteoblastic Staphylococcus aureus. Antimicrob. Agents Chemother. 2015, 59, 2029–2036. [Google Scholar] [CrossRef] [PubMed]
- Parra-Ruiz, J.; Bravo-Molina, A.; Pena-Monje, A.; Hernandez-Quero, J. Activity of linezolid and high-dose Daptomycin, alone or in combination, in an in vitro model of Staphylococcus aureus biofilm. J. Antimicrob. Chemother. 2012, 67, 2682–2685. [Google Scholar] [CrossRef]
- Meeker, D.G.; Beenken, K.E.; Mills, W.B.; Loughran, A.J.; Spencer, H.J.; Lynn, W.B.; Smeltzer, M.S. Evaluation of Antibiotics Active against Methicillin-Resistant Staphylococcus aureus Based on Activity in an Established Biofilm. Antimicrob. Agents Chemother. 2016, 60, 5688–5694. [Google Scholar] [CrossRef]
- Peters, E.J.; Lipsky, B.A.; Berendt, A.R.; Embil, J.M.; Lavery, L.A.; Senneville, E.; Urbančič-Rovan, V.; Bakker, K.; Jeffcoate, W.J. A systematic review of the effectiveness of interventions in the management of infection in the diabetic foot. Diab. Metabol. Res. Rev. 2012, 28 (Suppl. S1), 142–162. [Google Scholar] [CrossRef]
- Landersdorfer, C.B.; Bulitta, J.B.; Kinzig, M.; Holzgrabe, U.; Sörgel, F. Penetration of antibacterials into bone: Pharmacokinetic, pharmacodynamic and bioanalytical considerations. Clin. Pharmacokinet. 2009, 48, 89–124. [Google Scholar] [CrossRef] [PubMed]
- Senneville, E.; Lombart, A.; Beltrand, E.; Valette, M.; Legout, L.; Cazaubiel, M.; Yazdanpanah, Y.; Fontaine, P. Outcome of diabetic foot osteomyelitis treated nonsurgically: A retrospective cohort study. Diabetes Care 2008, 31, 637–642. [Google Scholar] [CrossRef]
- Tone, A.; Nguyen, S.; Devemy, F.; Topolinski, H.; Valette, M.; Cazaubiel, M.; Fayard, A.; Beltrand, É.; Lemaire, C.; Senneville, É. Six-week versus twelve-week antibiotic therapy for nonsurgically treated diabetic foot osteomyelitis: A multicenter open-label controlled randomized study. Diabetes Care 2015, 38, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Embil, J.M.; Rose, G.; Trepman, E.; Math, M.C.; Duerksen, F.; Simonsen, J.N.; Nicolle, L.E. Oral antimicrobial therapy for diabetic foot osteomyelitis. Foot. Ankle Int. 2006, 27, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Valabhji, J.; Oliver, N.; Samarasinghe, D.; Mali, T.; Gibbs, R.G.; Gedroyc, W.M. Conservative management of diabetic forefoot ulceration complicated by underlying osteomyelitis: The benefits of magnetic resonance imaging. Diabet. Med. 2009, 26, 1127–1134. [Google Scholar] [CrossRef]
- Acharya, S.; Soliman, M.; Egun, A.; Rajbhandari, S.M. Conservative management of diabetic foot osteomyelitis. Diabetes Res. Clin. Pract. 2013, 101, e18–e20. [Google Scholar] [CrossRef]
- Lazaro-Martinez, J.L.; Aragon-Sanchez, J.; Garcia-Morales, E. Antibiotics versus conservative surgery for treating diabetic foot osteomyelitis: A randomized comparative trial. Diabetes Care 2014, 37, 789–795. [Google Scholar] [CrossRef]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef]
- Baciewicz, A.M.; Chrisman, C.R.; Finch, C.K.; Self, T.H. Update on rifampin and rifabutin drug interactions. Am. Med. Sci. 2008, 335, 126–136. [Google Scholar] [CrossRef]
- Ribera, E.; Pou, L.; Fernandez-Sola, A.; Campos, F.; Lopez, R.M.; Ocaña, I.; Ruiz, I.; Pahissa, A. Rifampin reduces concentrations of trimethoprim and sulfamethoxazole in serum in human immunodeficiency virus-infected patients. Antimicrob. Agents Chemother. 2001, 45, 3238–3241. [Google Scholar] [CrossRef]
- Zeller, V.; Dzeing-Ella, A.; Kitzis, M.D.; Ziza, J.M.; Mamoudy, P.; Desplaces, N. Continuous clindamycin infusion, an innovative approach to treating bone and joint infections. Antimicrob. Agents Chemother. 2010, 54, 88–92. [Google Scholar] [CrossRef]
- Gandelman, K.; Zhu, T.; Fahmi, O.A.; Glue, P.; Lian, K.; Obach, R.S.; Damle, B. Unexpected effect of rifampin on the pharmacokinetics of linezolid: In silico and in vitro approaches to explain its mechanism. J. Clin. Pharmacol. 2011, 51, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Guillaume, M.; Garraffo, R.; Bensalem, M.; Janssen, C.; Bland, S.; Gaillat, J.; Bru, J.P. Pharmacokinetic and dynamic study of levofloxacin and rifampicin in bone and joint infections. Med. Mal. Infect. 2012, 42, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Temple, M.E.; Nahata, M.C. Interaction between ciprofloxacin and rifampicin. Ann. Pharmacother. 1999, 33, 868–870. [Google Scholar] [CrossRef]
- Tornero, E.; Morata, L.; Martínez-Pastor, J.C.; Angulo, S.; Combalia, A.; Bori, G.; García-Ramiro, S.; Bosch, J.; Mensa, J.; Soriano, A. Importance of selection and duration of antibiotic regimen in prosthetic joint infections treated with debridement and implant retention. J. Antimicrob. Chemother. 2016, 71, 1395–1401. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, W.; Widmer, A.F.; Blatter, M.; Frei, R.; Ochsner, P.E. Role of rifampin for treatment of orthopedic implant-related staphylococcal infections: A randomized controlled trial. Foreign-Body Infection(FBI) Study Group. JAMA 1998, 279, 1537–1541. [Google Scholar] [CrossRef]
- Puhto, A.P.; Puhto, T.; Niinimäki, T.; Ohtonen, P.; Leppilahti, J.; Syrjälä, H. Predictors of treatment outcome in prosthetic joint infections treated with prosthesis retention. Int. Orthop. 2015, 39, 1785–1791. [Google Scholar] [CrossRef]
- Kaye, K.S.; Fraimow, H.S.; Abrutyn, E. Pathogens resistant to antimicrobial agents: Epidemiology, molecular mechanisms, and clinical management. Infect. Dis. Clin. N. Am. 2004, 18, 467–511. [Google Scholar] [CrossRef]
- Legout, L.; Senneville, E.; Stern, R.; Yazdanpanah, Y.; Savage, C.; Roussel-Delvalez, M.; Rosele, B.; Migaud, H.; Mouton, Y. Treatment of bone and joint infections caused by Gram-negative bacilli with a cefepime-fluoroquinolone combination. Clin. Microbiol. Inf. 2006, 12, 1030–1033. [Google Scholar] [CrossRef]
- Senneville, E.; Yazdanpanah, Y.; Cazaubiel, M.; Cordonnier, M.; Valette, M.; Beltrand, E.; Khazarjian, A.; Maulin, L.; Alfandari, S.; Caillaux, M.; et al. Rifampicin-ofloxacin oral regimen for the treatment of mild to moderate diabetic foot osteomyelitis. J. Antimicrob. Chemother. 2001, 48, 927–930. [Google Scholar] [CrossRef]
- Wilson, B.M.; Bessesen, M.T.; Doros, G.; Brown, S.T.; Saade, E.; Hermos, J.; Perez, F.; Skalweit, M.; Spellberg, B.; Bonomo, R.A. Adjunctive Rifampin Therapy For Diabetic Foot Osteomyelitis in the Veterans Health Administration. JAMA Netw. Open 2019, 2, e1916003. [Google Scholar] [CrossRef] [PubMed]
- Bessesen, M.T.; Doros, G.; Henrie, A.M.; Harrington, K.M.; Hermos, J.A.; Bonomo, R.A.; Ferguson, R.E.; Huang, G.D.; Brown, S.T. A multicenter randomized placebo controlled trial of rifampin to reduce pedal amputations for osteomyelitis in veterans with diabetes (VA INTREPID). BMC Infect. Dis. 2020, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Thill, P.; Robineau, O.; Roosen, G.; Patoz, P.; Gachet, B.; Lafon-Desmurs, B.; Tetart, M.; Nadji, S.; Senneville, E.; Blondiaux, N. Rifabutin versus rifampicin bactericidal and anti-biofilm activities against clinical strains of Staphylococcus spp. Isolated from bone and joint infections. J. Antimicrob. Chemother. 2022, 77, 1036–1040. [Google Scholar] [CrossRef]
- Li, H.K.; Rombach, I.; Zambellas, R.; Walker, A.S.; McNally, M.A.; Atkins, B.L.; Lipsky, B.A.; Hughes, H.C.; Bose, D.; Kümin, M.; et al. OVIVA Trial Collaborators Oral versus intravenous antibiotics for bone and joint infection. N. Engl. J. Med. 2019, 380, 425–436. [Google Scholar] [CrossRef] [PubMed]
- Pitman, S.K.; Hoang, U.T.P.; Wi, C.H.; Alsheikh, M.; Hiner, D.A.; Percival, K.M. Revisiting Oral Fluoroquinolone and Multivalent Cation Drug-Drug Interactions: Are They Still Relevant? Antibiotics 2019, 8, 108. [Google Scholar] [CrossRef] [PubMed]
- Fish, R.; Kutter, E.; Bryan, D.; Wheat, G.; Kuhl, S. Resolving digital staphylococcal osteomyelitis using bacteriophage—A case report. Antibiotics 2018, 7, 87. [Google Scholar] [CrossRef]
- Dutta, P.; Das, S. Mammalian antimicrobial peptides: Promising therapeutic targets against infection and chronic inflammation. Curr. Top. Med. Chem. 2016, 16, 99–129. [Google Scholar] [CrossRef]
- Pouget, C.; Dunyach-Remy, C.; Pantel, A.; Schuldiner, S.; Sotto, A.; Lavigne, J.P. Biofilms in Diabetic Foot Ulcers: Significance and Clinical Relevance. Microorganisms 2020, 8, 1580. [Google Scholar] [CrossRef]
| Antibiotic | Diffusion into the Biofilm Matrix | Activity against Bacterial Cells in the Stationary Phase | Activity Assessed in Tissue Cage Model and/or Human Study |
|---|---|---|---|
| Fluoroquinolones | Yes | Yes | Yes |
| Rifampicin | Yes | Yes | Yes |
| Minocycline | Yes | Yes | No |
| Fosfomycin | Yes | Yes | No |
| Daptomycin | Yes | Yes | No |
| Linezolid | Yes | Reduced | No |
| Dalbavancin | Reduced | Reduced | No |
| Vancomycin | Severely reduced | Not known | No |
| Aminoglycosides | Reduced | Reduced | No |
| Author (Ref) | N° Patients/Episodes of DFO | Remission (%) | Antibiotic Regimens |
|---|---|---|---|
| Lessens, 2011 [8] | 68 medical management (MM): 36 surgical management (SM): 32 | MM: 55 SM: 45 | amoxicillin-clavulanic acid, ciprofloxacin, cotrimoxazole, rifampicin-fluoroquinolone/clindamycin |
| Senneville, 2008 [32] | 50 | 64 | fluoroquinolone-rifampicin, fluoroquinolone-pristinamycin, fluoroquinolone plus 3rd or 4th generation cephalosporin |
| Tone, 2015 [33] | 40 | 65 | gram-positive cocci: rifampicin plus (levofloxacin, cotrimoxazole, doxycycline, or linezolid) gram-negative bacilli: levofloxacin or ciprofloxacin plus (cefotaxime, ceftriaxone, or cefepime) for the first 2 weeks of treatment, then levofloxacin or ciprofloxacin monotherapy |
| Embil, 2006 [34] | 94/117 | 80.5 | metronidazole, ciprofloxacin, cotrimoxazole, clindamycin, cephalexin amoxicillin +/− clavulanic acid |
| Valabhji, 2009 [35] | 47/53 | 75 | amoxicillin-clavulanic acid, clindamycin-ciprofloxacin, rifampicin-doxycyline |
| Acharya, 2013 [36] | 130 | 66.9 | flucloxacillin-fusidic acid, ciprofloxacin-clindamycin |
| Lazaro-Martinez, 2014 [37] | 46 medical management (MM): 24 surgical management (SM): 22 | MM: 79.1 SM: 68.2 | amoxicillin-clavulanic acid, ciprofloxacin, cotrimoxazole |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senneville, E.; Gachet, B.; Blondiaux, N.; Robineau, O. Do Anti-Biofilm Antibiotics Have a Place in the Treatment of Diabetic Foot Osteomyelitis? Antibiotics 2023, 12, 317. https://doi.org/10.3390/antibiotics12020317
Senneville E, Gachet B, Blondiaux N, Robineau O. Do Anti-Biofilm Antibiotics Have a Place in the Treatment of Diabetic Foot Osteomyelitis? Antibiotics. 2023; 12(2):317. https://doi.org/10.3390/antibiotics12020317
Chicago/Turabian StyleSenneville, Eric, Benoit Gachet, Nicolas Blondiaux, and Olivier Robineau. 2023. "Do Anti-Biofilm Antibiotics Have a Place in the Treatment of Diabetic Foot Osteomyelitis?" Antibiotics 12, no. 2: 317. https://doi.org/10.3390/antibiotics12020317
APA StyleSenneville, E., Gachet, B., Blondiaux, N., & Robineau, O. (2023). Do Anti-Biofilm Antibiotics Have a Place in the Treatment of Diabetic Foot Osteomyelitis? Antibiotics, 12(2), 317. https://doi.org/10.3390/antibiotics12020317








