Influence of Skin Commensals on Therapeutic Outcomes of Surgically Debrided Diabetic Foot Infections—A Large Retrospective Comparative Study
Abstract
1. Introduction
2. Results
2.1. Study Population and Infections
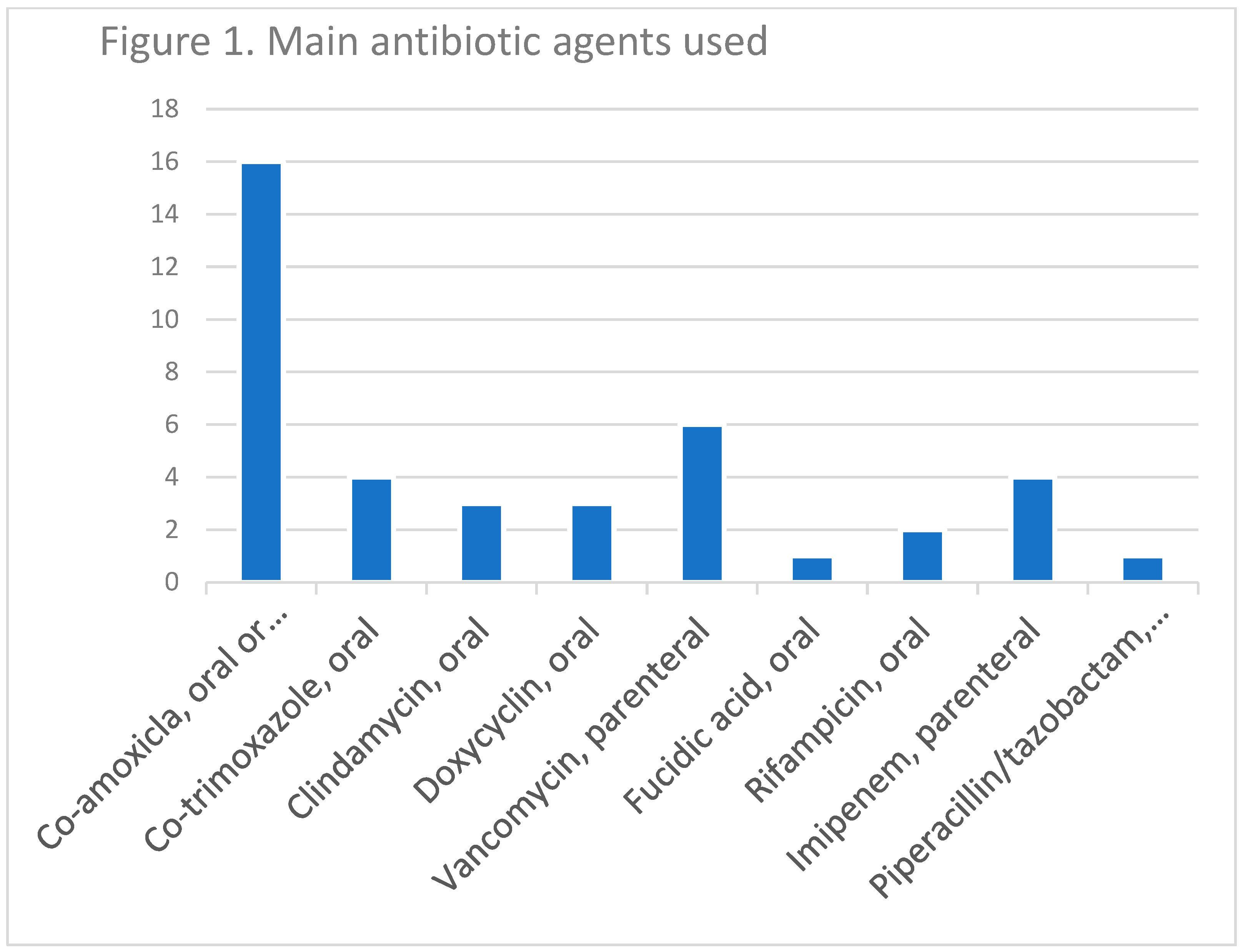
2.2. Therapy and Outcomes
3. Discussion
4. Conclusions
5. Methods
Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Uçkay, I.; Gariani, K.; Pataky, Z.; Lipsky, B.A. Diabetic foot infections: State-of-the-art. Diabetes Obes. Metab. 2014, 16, 305–316. [Google Scholar] [CrossRef] [PubMed]
- Zenelaj, B.; Bouvet, C.; Lipsky, B.A.; Uçkay, I. Do diabetic foot infections with methicillin-resistant Staphylococcus aureus differ from those with other pathogens? Int. J. Low. Extrem. Wounds 2014, 13, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Seghrouchni, K.; van Delden, C.; Dominguez, D.; Benkabouche, M.; Bernard, L.; Assal, M.; Hoffmeyer, P.; Uçkay, I. Remission after treatment of osteoarticular infections due to Pseudomonas aeruginosa versus Staphylococcus aureus: A case-controlled study. Int. Orthop. 2012, 36, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Henig, O.; Pogue, J.M.; Martin, E.; Hayat, U.; Ja’ara, M.; Kilgore, P.E.; Cha, R.; Dhar, S.; Kaye, K.S. The Impact of Multidrug-Resistant Organisms on Outcomes in Patients with Diabetic Foot Infections. Open. Forum. Infect. Dis. 2020, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Lebowitz, D.; Kressmann, B.; von Dach, E.; Lipsky, B.A.; Gariani, K. Pseudomonal Diabetic Foot Infections: Vive la Différence? Mayo. Clin. Proc. Innov. Qual. Outcomes 2022, 6, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Charles, P.G.; Uçkay, I.; Kressmann, B.; Emonet, S.; Lipsky, B.A. The role of anaerobes in diabetic foot infections. Anaerobe 2015, 34, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Malone, M.; Mayer, D.; Salisbury, A.M.; Schultz, G. Role of anaerobes in polymicrobial communities and biofilms complicating diabetic foot ulcers. Int. Wound J. 2018, 15, 776–782. [Google Scholar] [CrossRef]
- Lebowitz, D.; Kressmann, B.; Gjoni, S.; Zenelaj, B.; Grosgurin, O.; Marti, C.; Zingg, M.; Uçkay, I. Clinical features of anaerobic orthopaedic infections. Infect. Dis. 2017, 49, 137–140. [Google Scholar] [CrossRef]
- Gariani, K.; Pham, T.T.; Kressmann, B.; Jornayvaz, F.R.; Gastaldi, G.; Stafylakis, D.; Philippe, J.; Lipsky, B.A.; Uçkay, I. Three Weeks Versus Six Weeks of Antibiotic Therapy for Diabetic Foot Osteomyelitis: A Prospective, Randomized, Noninferiority Pilot Trial. Clin. Infect. Dis. 2021, 73, 1539–1545. [Google Scholar] [CrossRef]
- Pham, T.T.; Gariani, K.; Richard, J.C.; Kressmann, B.; Jornayvaz, F.R.; Philippe, J.; Lipsky, B.A.; Uçkay, I. Moderate to Severe Soft Tissue Diabetic Foot Infections: A Randomized, Controlled, Pilot Trial of Post-debridement Antibiotic Treatment for 10 versus 20 days. Ann. Surg. 2022, 276, 233–238. [Google Scholar] [CrossRef]
- Tae, K.K.; Armstrong, D.G. Microbiology and Antimicrobial Therapy for Diabetic Foot Infections. Infect. Chemother. 2018, 50, 11–20. [Google Scholar]
- Sadeghpour Heravi, F.; Zakrzewski, M.; Vickery, K.; Armstrong, D.G.; Hu, H. Bacterial Diversity of Diabetic Foot Ulcers: Current Status and Future Prospectives. J. Clin. Med. 2019, 8, 1935. [Google Scholar] [CrossRef] [PubMed]
- Lipsky, B.A.; Senneville, E.; Abbas, Z.A.; Aragón-Sánchez, J.; Diggle, M.; Embil, J.M.; Kono, S.; Lavery, L.A.; Malone, M.; van Asten, S.A.; et al. IWGDF Guideline on the Diagnosis and Treatment of Foot Infection in Persons with Diabetes. Available online: www.iwgdfguidelines.org (accessed on 19 December 2022).
- Uçkay, I.; Pires, D.; Agostinho, A.; Guanziroli, N.; Öztürk, M.; Bartolone, P.; Tscholl, P.; Betz, M.; Pittet, D. Enterococci in orthopaedic infections: Who is at risk getting infected? J. Infect. 2017, 75, 309–314. [Google Scholar] [CrossRef] [PubMed]
- van Asten, S.A.; La Fontaine, J.; Peters, E.J.G.; Bhavan, K.; Kim, P.J.; Lavery, L.A. The microbiome of diabetic foot osteomyelitis. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Citron, D.M.; Goldstein, E.J.C.; Merriam, C.V.; Lipsky, B.A.; Abramson, M.A. Bacteriology of moderate-to-severe diabetic foot infections and in vitro activity of antimicrobial agents. J. Clin. Microbiol. 2007, 45, 2819–2828. [Google Scholar] [CrossRef] [PubMed]
- Mohamad, M.; Uçkay, I.; Hannouche, D.; Miozzari, H. Particularities of Staphylococcus lugdunensis in orthopaedic infections. Infect. Dis. 2018, 50, 223–225. [Google Scholar] [CrossRef]
- Uçkay, I.; Harbarth, S.; Ferry, T.; Lübbeke, A.; Emonet, S.; Hoffmeyer, P.; Pittet, D. Meticillin resistance in orthopaedic coagulase-negative staphylococcal infections. J. Hosp. Infect. 2011, 79, 248–253. [Google Scholar] [CrossRef]
- Kalt, F.; Schulthess, B.; Sidler, F.; Herren, S.; Fucentese, S.F.; Zingg, P.O.; Berli, M.; Zinkernagel, A.S.; Zbinden, R.; Achermann, Y. Corynebacterium Species Rarely Cause Orthopedic Infections. J. Clin. Microbiol. 2018, 56, 1200–1218. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Agostinho, A.; Landelle, C.; Coppens, E.; Cunningham, G.; Pittet, D. Incidence of Propionibacterium acnes infection in orthopedic and trauma surgery. Antimicrob. Resist. Infect. Control 2015, 4, 28. [Google Scholar] [CrossRef]
- Coenye, T.; Spittaels, K.J.; Achermann, Y. The role of biofilm formation in the pathogenesis and antimicrobial susceptibility of Cutibacterium acnes. Biofilm 2021, 4, 100063. [Google Scholar] [CrossRef]
- Hinchliffe, R.J.; Brownrigg, J.R.; Apelqvist, J.; Boyko, E.J.; Fitridge, R.; Mills, J.L.; Reekers, J.; Shearman, C.P.; Zierler, R.E.; Schaper, N.C. IWGDF guidance on the diagnosis, prognosis and management of peripheral artery disease in patients with foot ulcers in diabetes. Diabetes Metab. Res. Rev. 2016, 32, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.T.; Wetzel, O.; Gariani, K.; Kressmann, B.; Jornayvaz, F.R.; Lipsky, B.A.; Uçkay, İ. Is routine measurement of the serum C-reactive protein level helpful during antibiotic therapy for diabetic foot infection? Diabetes Obes. Metab. 2021, 23, 637–641. [Google Scholar] [CrossRef] [PubMed]
- Gariani, K.; Lebowitz, D.; Kressmann, B.; von Dach, E.; Sendi, P.; Waibel, F.; Berli, M.; Huber, T.; Lipsky, B.A.; Uçkay, I. Oral amoxicillin-clavulanate for treating diabetic foot infections. Diabetes. Obes. Metab. 2019, 21, 1483–1486. [Google Scholar] [CrossRef]
- Kalan, L.R.; Meisel, J.S.; Loesche, M.A.; Horwinski, J.; Soaita, I.; Chen, X.; Uberoi, A.; Gardner, S.E.; Grice, E.A. Strain- and Species-Level Variation in the Microbiome of Diabetic Wounds Is Associated with Clinical Outcomes and Therapeutic Efficacy. Cell Host Microbe 2019, 2, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Jneida, J.; Lavigne, J.P.; La Scolaa, B.; Cassira, N. The diabetic foot microbiota: A review. Hum. Microbiome J. 2017, 5, 1–6. [Google Scholar] [CrossRef]
- Lai, Y.; Di Nardo, A.; Nakatsuji, T.; Leichtle, A.; Yang, Y.; Cogen, A.L.; Wu, Z.R.; Hooper, L.V.; Schmidt, R.R.; von Aulock, S.; et al. Commensal bacteria regulate Toll-like receptor 3-dependent inflammation after skin injury. Nat. Med. 2009, 15, 1377–1382. [Google Scholar] [CrossRef]
- Kadamb Patel, B.K.; Patel, K.H.; Huang, R.Y.; Chuen Neng Lee, C.; Moochhala, S.M. The Gut-Skin Microbiota Axis and Its Role in Diabetic Wound Healing—A Review Based on Current Literature. Int. J. Mol. Sci. 2022, 23, 2375. [Google Scholar] [CrossRef] [PubMed]
- Bouvet, C.; Gjoni, S.; Zenelaj, B.; Lipsky, B.A.; Hakko, E.; Uçkay, I. Staphylococcus aureus soft tissue infection may increase the risk of subsequent staphylococcal soft tissue infections. Int. J. Infect. Dis. 2017, 60, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Waibel, F.; Berli, M.; Catanzaro, S.; Sairanen, K.; Schöni, M.; Böni, T.; Burkhard, J.; Holy, D.; Huber, T.; Bertram, M.; et al. Optimization of the antibiotic management of diabetic foot infections: Protocol for two randomized controlled trials. Trials 2020, 21, 54. [Google Scholar] [CrossRef] [PubMed]
- Uçkay, I.; Kressmann, B.; Malacarne, S.; Toumanova, A.; Jaafar, J.; Lew, D.; Lipsky, B.A. A randomized, controlled study to investigate the efficacy and safety of a topical gentamicin-collagen sponge in combination with systemic antibiotic therapy in diabetic patients with a moderate or severe foot ulcer infection. BMC. Infect. Dis. 2018, 18, 361. [Google Scholar] [CrossRef] [PubMed]
| Pathogenic Bacteria Only | Skin Commensals + | ||
|---|---|---|---|
| Factor | n = 641 | p Value * | n = 54 |
| Median age (years) | 80 | 0.26 | 83 |
| Osteomyelitis | 251 (39%) | 0.62 | 23 (43%) |
| Bacteremia associated with diabetic foot infection | 71 (11%) | 0.21 | 3 (6%) |
| Median C-reactive protein level on admission | 105 mg/L | 0.01 | 25 mg/L |
| Median number of surgical debridement | 1 | 0.18 | 1 |
| Median duration of antibiotic treatment | 21 days | 0.71 | 30 days |
| Median duration of parenteral therapy | 6 days | 0.88 | 6 days |
| Hyperbaric oxygen therapy | 73 (11%) | 0.19 | 3 (6%) |
| Clinical failures (after end of therapy) | 153 (24%) | 0.22 | 9 (17%) |
| Microbiological recurrence (with same pathogens) | 111 (17%) | 0.24 | 6 (11%) |
| Outcome “Clinical Failure” | Univariate | Multivariate | Multivariate | Univariate | “Microbiological Recurrence” |
|---|---|---|---|---|---|
| Age | 1.0, 1.0–1.0 | 1.0, 0.9–1.0 | 1.0, 0.9–1.1 | 1.0, 1.0–1.0 | Age |
| Number of surgical debridement | 0.7, 0.6–0.8 | 1.2, 0.8–1.8 | 2.2, 0.7–6.7 | 1.1, 0.9–1.3 | Number of surgical debridement |
| Total duration of antibiotic therapy | 1.0, 1.0–1.0 | 1.0, 1.0–1.0 | 1.0, 1.0–1.0 | 1.0, 1.0–1.0 | Total duration of antibiotic therapy |
| Initial serum C-reactive protein level | 1.0, 1.0–1.0 | 1.0, 1.0–1.0 | 1.0, 1.0–1.0 | 1.0, 1.0–1.0 | Initial serum C-reactive protein level |
| Bacteremia | 0.6, 0.3–1.1 | 0.5, 0.1–2.8 | 1.8, 0.3–3.3 | 1.4, 0.7–2.6 | Bacteremia |
| Osteomyelitis | 0.8, 0.6–1.1 | 0.8, 0.3–2.1 | 1.2, 0.3–4.3 | 0.9, 0.8–1.4 | Osteomyelitis |
| Infection due to skin commensals | 0.6, 0.3–1.3 | 0.4, 0.1–3.8 | 0.5, 0.1–4.2 | 0.6, 0.2–1.4 | Infection due to skin commensals |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uçkay, I.; Lebowitz, D.; Kressmann, B.; Lipsky, B.A.; Gariani, K. Influence of Skin Commensals on Therapeutic Outcomes of Surgically Debrided Diabetic Foot Infections—A Large Retrospective Comparative Study. Antibiotics 2023, 12, 316. https://doi.org/10.3390/antibiotics12020316
Uçkay I, Lebowitz D, Kressmann B, Lipsky BA, Gariani K. Influence of Skin Commensals on Therapeutic Outcomes of Surgically Debrided Diabetic Foot Infections—A Large Retrospective Comparative Study. Antibiotics. 2023; 12(2):316. https://doi.org/10.3390/antibiotics12020316
Chicago/Turabian StyleUçkay, Ilker, Dan Lebowitz, Benjamin Kressmann, Benjamin A. Lipsky, and Karim Gariani. 2023. "Influence of Skin Commensals on Therapeutic Outcomes of Surgically Debrided Diabetic Foot Infections—A Large Retrospective Comparative Study" Antibiotics 12, no. 2: 316. https://doi.org/10.3390/antibiotics12020316
APA StyleUçkay, I., Lebowitz, D., Kressmann, B., Lipsky, B. A., & Gariani, K. (2023). Influence of Skin Commensals on Therapeutic Outcomes of Surgically Debrided Diabetic Foot Infections—A Large Retrospective Comparative Study. Antibiotics, 12(2), 316. https://doi.org/10.3390/antibiotics12020316







