The Antioxidant, Antibacterial and Anti-Biofilm Properties of Rapeseed Creamed Honey Enriched with Selected Plant Superfoods
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Honey and Plant Additives
3.2. Preparation of Enriched Honey Samples
3.3. Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
3.4. Antioxidant Capacity
3.5. Antibacterial Effect
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juszczak, L.; Gałkowska, D.; Ostrowska, M.; Socha, R. Antioxidant activity of honey supplemented with bee products. Nat. Prod. Res. 2016, 30, 1436–1439. [Google Scholar] [CrossRef] [PubMed]
- Juszczak, L.; Florkiewicz, A.; Socha, R.; Gałkowska, D.; Piotrowska, A. Effect of honey supplementation with bee products on quality parameters and mineral composition. Emirates J. Food Agric. 2018, 30, 990–997. [Google Scholar] [CrossRef]
- Habryka, C.; Socha, R.; Juszczak, L. The Influence of Honey Enrichment with Bee Pollen or Bee Bread on the Content of Selected Mineral Components in Multifloral Honey. Potravin. Slovak J. Food Sci. 2020, 14, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Habryka, C.; Socha, R.; Juszczak, L. The effect of enriching honey with propolis on the antioxidant activity, sensory characteristics, and quality parameters. Molecules 2020, 25, 1176. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska, A.; Newerli-Guz, J.; Szweda, P. Influence of the addition of selected spices on sensory quality and biological activity of honey. J. Food Qual. 2017, 2017, 6963904. [Google Scholar] [CrossRef]
- Novák, A.; Alexa, L.; Kovács, B.; Czipa, N. Comparative study of special honey products and herbhoneys. Acta Agrar. Debr. 2018, 74, 117–120. [Google Scholar] [CrossRef]
- Šedík, P.; Horská, E.; Ivanišová, E.; Kačániová, M.; Krasnodębski, A. Consumer behaviour of young generation in Slovakia towards cocoa-enriched honey. Potravin. Slovak J. Food Sci. 2019, 13, 18–24. [Google Scholar] [CrossRef]
- Štajner, D.; Popović, B.M.; Čanadanović-Brunet, J.; Dilas, S.; Ćetković, G. Nutritive composition and free radical scavenger activity of honey enriched with of Rosa spp. LWT—Food Sci. Technol. 2014, 55, 408–413. [Google Scholar] [CrossRef]
- Dżugan, M.; Sowa, P.; Kwaśniewska, M.; Wesołowska, M.; Czernicka, M. Physicochemical Parameters and Antioxidant Activity of Bee Honey Enriched With Herbs. Plant Foods Hum. Nutr. 2017, 72, 74–81. [Google Scholar] [CrossRef]
- Tomczyk, M.; Miłek, M.; Sidor, E.; Kapusta, I.; Litwińczuk, W.; Puchalski, C.; Dżugan, M. The effect of adding the leaves and fruits of Morus alba to rape honey on its antioxidant properties, polyphenolic profile, and amylase activity. Molecules 2020, 25, 84. [Google Scholar] [CrossRef]
- Miłek, M.; Grabek-Lejko, D.; Stȩpień, K.; Sidor, E.; Mołoń, M.; Dżugan, M. The enrichment of honey with Aronia melanocarpa fruits enhances its in vitro and in vivo antioxidant potential and intensifies its antibacterial and antiviral properties. Food Funct. 2021, 12, 8920–8931. [Google Scholar] [CrossRef] [PubMed]
- Grabek-Lejko, D.; Miłek, M.; Sidor, E.; Puchalski, C.; Dżugan, M. Antiviral and Antibacterial Effect of Honey Enriched with Rubus spp. as a Functional Food with Enhanced Antioxidant Properties. Molecules 2022, 27, 4859. [Google Scholar] [CrossRef]
- Mateescu, C.; Duta, D.; Onisei, T.; Şerbancea, F.; Utoiu, C.; Manolache, F.A.; Rascol, M.; Ionescu, V.; Popescu, C.; Dune, A. Flavored Cream Honey -A Healthy Food Choice For Consumers. Int. Symp. ISB-INMA-TECH. 2020, 2020, 236–245. [Google Scholar]
- Żebracka, A.; Winiarska-Mieczan, A.; Nowakowicz-Dębek, B.; Banach, M.; Drabik, A.; Pulit-Prociak, J.; Chmielowiec-Korzeniowska, A. Assessment of the microbiological quality and bactericidal properties of flavoured honey. Med. Weter. 2022, 78, 556–562. [Google Scholar] [CrossRef]
- TBS/AFDC; Draft Tanzania Standard Organic Fertilizer—Specification. Tanzania Bureau of Standards: Dar es Salaam, Tanzania, 2017; Volume 10, pp. 1–6.
- Sowa, P.; Tarapatskyy, M.; Puchalski, C.; Jarecki, W.; Dżugan, M. A novel honey-based product enriched with coumarin from Melilotus flowers. J. Food Meas. Charact. 2019, 13, 1748–1754. [Google Scholar] [CrossRef]
- Đorđević, S.; Nedić, N.; Pavlović, A.; Milojković-Opsenica, D.; Tešić, Ž.; Gašić, U. Honey with added value—Enriched with rutin and quercetin from Sophora flower. J. Herb. Med. 2022, 34, 100580. [Google Scholar] [CrossRef]
- Gašić, U.M.; Milojković-Opsenica, D.M.; Tešić, Ž.L. Polyphenols as possible markers of botanical origin of honey. J. AOAC Int. 2017, 100, 852–861. [Google Scholar] [CrossRef]
- Cianciosi, D.; Forbes-Hernández, T.Y.; Afrin, S.; Gasparrini, M.; Reboredo-Rodriguez, P.; Manna, P.P.; Zhang, J.; Lamas, L.B.; Flórez, S.M.; Toyos, P.A.; et al. Phenolic compounds in honey and their associated health benefits: A review. Molecules 2018, 23, 2322. [Google Scholar] [CrossRef]
- Kwakman, P.H.S.; Zaat, S.A.J. Antibacterial Components of Honey. IUBMB Life 2012, 64, 48–55. [Google Scholar] [CrossRef]
- Gopalan, A.; Reuben, S.C.; Ahmed, S.; Darvesh, A.S.; Hohmann, J.; Bishayee, A. The health benefits of blackcurrants. Food Funct. 2012, 3, 795–809. [Google Scholar] [CrossRef]
- Hoshyar, R.; Mahboob, Z.; Zarban, A. The antioxidant and chemical properties of Berberis vulgaris and its cytotoxic effect on human breast carcinoma cells. Cytotechnology 2016, 68, 1207–1213. [Google Scholar] [CrossRef] [PubMed]
- Winther, K.; Campbell-Tofte, J.; Vinther Hansen, A.S. Bioactive ingredients of rose hips (Rosa canina L) with special reference to antioxidative and anti-inflammatory properties: In vitro studies. Bot. Targets Ther. 2016, 6, 11–23. [Google Scholar] [CrossRef]
- Shin, D.; Chae, K.S.; Choi, H.R.; Lee, S.J.; Gim, S.W.; Kwon, G.T.; Lee, H.T.; Song, Y.C.; Kim, K.J.; Kong, H.S.; et al. Bioactive and pharmacokinetic characteristics of pre-matured black raspberry, Rubus occidentalis. Ital. J. Food Sci. 2018, 30, 428–439. [Google Scholar]
- Criste, A.; Urcan, A.C.; Bunea, A.; Furtuna, F.R.P.; Olah, N.K.; Madden, R.H.; Corcionivoschi, N. Phytochemical composition and biological activity of berries and leaves from four romanian sea buckthorn (Hippophae rhamnoides L.) varieties. Molecules 2020, 25, 1170. [Google Scholar] [CrossRef]
- El-Sohaimy, S.A.; Shehata, M.G.; Mathur, A.; Darwish, A.G.; Abd El-Aziz, N.M.; Gauba, P.; Upadhyay, P. Nutritional Evaluation of Sea Buckthorn “Hippophae rhamnoides” Berries and the Pharmaceutical Potential of the Fermented Juice. Fermentation 2022, 8, 391. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G.P. Bioactive properties of Sambucus nigra L. As a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Santos, P.; Badim, H.; Salvador, Â.C.; Silvestre, A.J.D.; Santos, S.A.O.; Rocha, S.M.; Sousa, A.M.; Pereira, M.O.; Wilson, C.P.; Rocha, C.M.R.; et al. Chemical characterization of Sambucus nigra L. Flowers aqueous extract and its biological implications. Biomolecules 2021, 11, 1222. [Google Scholar] [CrossRef]
- Michel, T.; Destandau, E.; Le Floch, G.; Lucchesi, M.E.; Elfakir, C. Antimicrobial, antioxidant and phytochemical investigations of sea buckthorn (Hippophaë rhamnoides L.) leaf, stem, root and seed. Food Chem. 2012, 131, 754–760. [Google Scholar] [CrossRef]
- Raudone, L.; Puzerytė, V.; Vilkickyte, G.; Niekyte, A.; Lanauskas, J.; Viskelis, J.; Viskelis, P. Sea buckthorn leaf powders: The impact of cultivar and drying mode on antioxidant, phytochemical, and chromatic profile of valuable resource. Molecules 2021, 26, 4765. [Google Scholar] [CrossRef] [PubMed]
- Wilczyńska, A. Phenolic content and antioxidant activity of different types of polish honey—A short report. Polish J. Food Nutr. Sci. 2010, 60, 309–313. [Google Scholar]
- Dżugan, M.; Tomczyk, M.; Sowa, P.; Grabek-Lejko, D. Antioxidant activity as biomarker of honey variety. Molecules 2018, 23, 2069. [Google Scholar] [CrossRef] [PubMed]
- Dżugan, M.; Grabek-Lejko, D.; Sidor, E.; Tomczyk, M. The impact of ultrasound decrystallization on enzymatic, antioxidant and antibacterial properties of honey. Innov. Food Sci. Emerg. Technol. 2021, 71, 102709. [Google Scholar] [CrossRef]
- Ma, X.; Yang, W.; Kallio, H.; Yang, B. Health promoting properties and sensory characteristics of phytochemicals in berries and leaves of sea buckthorn (Hippophaë rhamnoides). Crit. Rev. Food Sci. Nutr. 2022, 62, 3798–3816. [Google Scholar] [CrossRef] [PubMed]
- Dossett, M.; Lee, J.; Finn, C.E. Characterization of a novel anthocyanin profile in wild black raspberry mutants: An opportunity for studying the genetic control of pigment and color. J. Funct. Foods 2011, 3, 207–214. [Google Scholar] [CrossRef]
- Georgieva, S.; Angelov, G.; Boyadzhieva, S. Concentration of vitamin C and antioxidant activity of rosehip extracts. J. Chem. Technol. Metall. 2014, 49, 451–454. [Google Scholar]
- Sandulach, E.; Macari, A.; Cojocari, D.; Balan, G.; Popa, S.; Turculet, N.; Ghendov-Mosanu, A.; Sturza, R. Antimicrobial properties of sea buckthorn grown in the Republic of Moldova. J. Eng. Sci. 2022, 29, 164–175. [Google Scholar] [CrossRef]
- Yue, X.-F.; Shang, X.; Zhang, Z.-J.; Zhang, Y.-N. Phytochemical composition and antibacterial activity of the essential oils from different parts of sea buckthorn (Hippophae rhamnoides L.). J. Food Drug Anal. 2016, 25, 327–332. [Google Scholar] [CrossRef]
- Christaki, E. Hippophae rhamnoides L.(Sea Buckthorn): A potential source of nutraceuticals. Food Public Health 2012, 2, 69–72. [Google Scholar] [CrossRef]
- Cisowska, A.; Wojnicz, D.; Hendrich, A.B. Anthocyanins as antimicrobial agents of natural plant origin. Nat. Prod. Commun. 2011, 6, 149–156. [Google Scholar] [CrossRef]
- Kuś, P.M.; Szweda, P.; Jerković, I.; Tuberoso, C.I.G. Activity of Polish unifloral honeys against pathogenic bacteria and its correlation with colour, phenolic content, antioxidant capacity and other parameters. Lett. Appl. Microbiol. 2016, 62, 269–276. [Google Scholar] [CrossRef]
- Gondil, V.S.; Kalaiyarasan, T.; Bharti, V.K.; Chhibber, S. Antibiofilm potential of Seabuckthorn silver nanoparticles (SBT@AgNPs) against Pseudomonas aeruginosa. 3 Biotech 2019, 9, 402. [Google Scholar] [CrossRef]
- Ivanišová, E.; Blašková, M.; Terentjeva, M.; Grygorieva, O.; Vergun, O.; Brindza, J.; Kačániová, M. Biological properties of sea buckthorn (Hippophae rhamnoides L.) derived products. Acta Sci. Pol. Technol. Aliment. 2020, 19, 195–205. [Google Scholar] [CrossRef]
- Kim, S.K.; Kim, H.; Kim, S.A.; Park, H.K. Anti-Inflammatory and Anti-Superbacterial Activity of Polyphenols Isolated from Black Raspberry. Korean J. Physiol. Pharmacol. 2013, 17, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.P.; Extremina, C.; Fonseca, A.F.; Sousa, J.C. Effect of subinhibitory concentration of piperacillin/tazobactam on Pseudomonas aeruginosa. J. Med. Microbiol. 2004, 53, 903–910. [Google Scholar] [CrossRef] [PubMed]
- Pletz, M.W.R.; McGee, L.; Van Beneden, C.A.; Petit, S.; Bardsley, M.; Barlow, M.; Klugman, K.P. Fluoroquinolone resistance in invasive Streptococcus pyogenes isolates due to spontaneous mutation and horizontal gene transfer. Antimicrob. Agents Chemother. 2006, 50, 943–948. [Google Scholar] [CrossRef]
- Hassett, D.J.; Elkins, J.G.; Ma, J.F.; Mcdermott, T.R. Pseudomonas aeruginosa biofilm sensitivity to biocides: Use of hydrogen peroxide as model antimicrobial agent for examining resistance mechanisms. Methods Enzymol. 1999, 310, 599–608. [Google Scholar] [CrossRef] [PubMed]
- Wolter, J.M.; McCormack, J.G. The effect of subinhibitory concentrations of antibiotics on adherence of pseudomonas aeruginosa to cystic fibrosis (CF) and non-CF-affected tracheal epithelial cells. J. Infect. 1998, 37, 217–223. [Google Scholar] [CrossRef] [PubMed]
- Drago, L.; De Vecchi, E.; Mombelli, B.; Nicola, L.; Valli, M.; Gismondo, M.R. Activity of levofloxacin and ciprofloxacin against urinary pathogens. J. Antimicrob. Chemother. 2001, 48, 37–45. [Google Scholar] [CrossRef]
- Miłek, M.; Ciszkowicz, E.; Tomczyk, M.; Sidor, E.; Zaguła, G.; Lecka-szlachta, K.; Pasternakiewicz, A.; Dżugan, M. The Study of Chemical Profile and Antioxidant Properties of Poplar-Type Polish Propolis Considering Local Flora Diversity in Relation to Antibacterial and Anticancer Activities in Human Breast Cancer Cells. Molecules 2022, 27, 725. [Google Scholar] [CrossRef]
- Bocian, A.; Ciszkowicz, E.; Hus, K.K.; Buczkowicz, J.; Lecka-Szlachta, K.; Pietrowska, M.; Petrilla, V.; Petrillova, M.; Legáth, Ľ.; Legáth, J. Antimicrobial activity of protein fraction from Naja ashei venom against Staphylococcus epidermidis. Molecules 2020, 25, 293. [Google Scholar] [CrossRef]
- Zapała, L.; Kosińska-Pezda, M.; Byczyński, Ł.; Zapała, W.; Maciołek, U.; Woźnicka, E.; Ciszkowicz, E.; Lecka-Szlachta, K. Green synthesis of niflumic acid complexes with some transition metal ions (Mn(II), Fe(III), Co(II), Ni(II), Cu(II) and Zn(II)). Spectroscopic, thermoanalytical and antibacterial studies. Thermochim. Acta 2021, 696, 178814. [Google Scholar] [CrossRef]
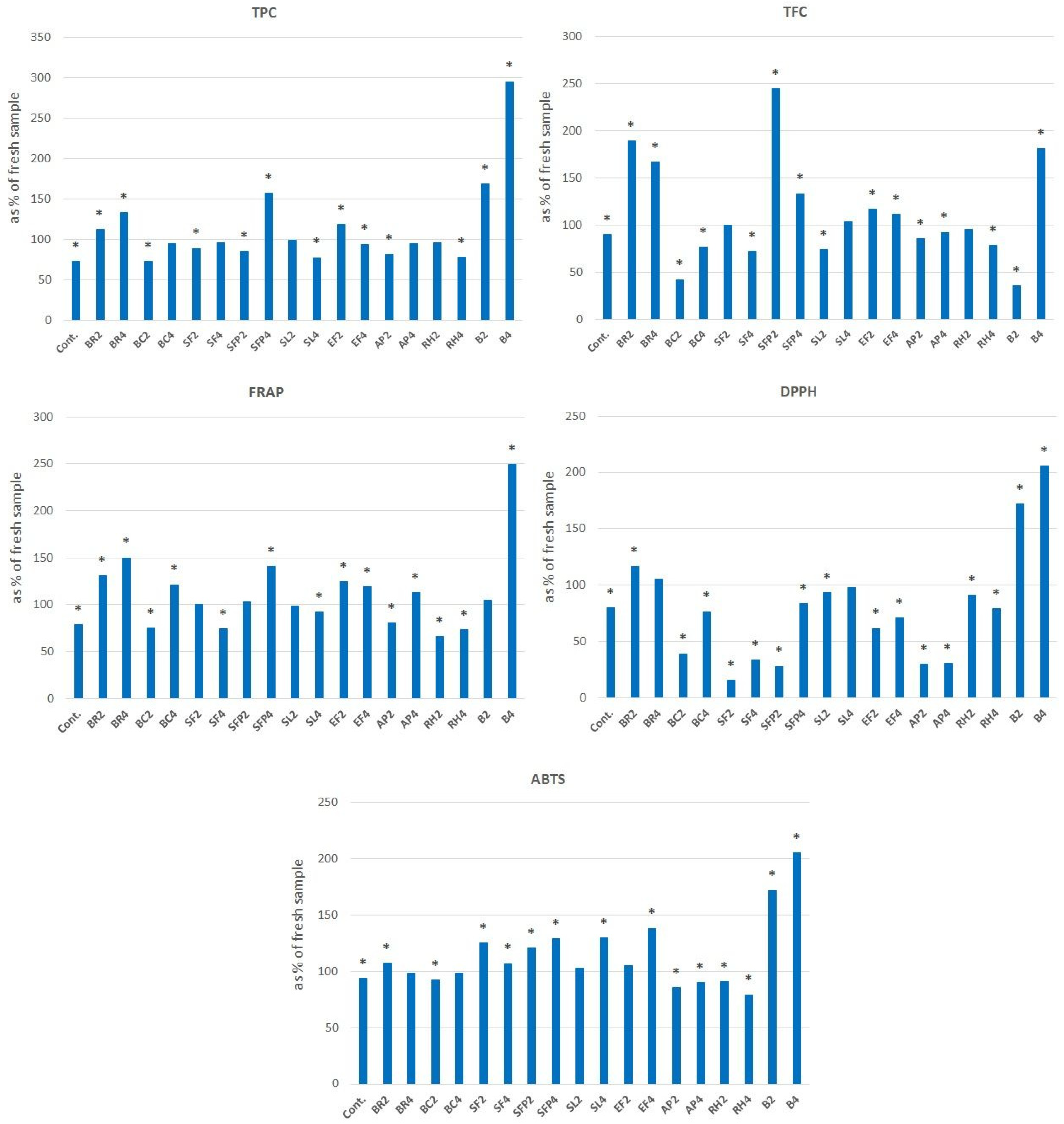
| Honey Sample | TPC [mg GAE/100 g] | TFC [mg QE/100 g] | FRAP [μmol TE/100 g] | DPPH [μmol TE/100 g] | ABTS [μmol TE/100 g] |
|---|---|---|---|---|---|
| Control rapeseed honey | 35.56 ± 0.21 | 9.16 ± 0.43 | 59.36 ± 0.31 | 52.66 ± 5.82 | 298.63 ± 2.15 |
| Black raspberry (2%) | 78.27 ± 2.10 * | 15.27 ± 0.00 | 301.61 ± 12.32 * | 191.03 ± 5.81 * | 825.23 ± 26.87 * |
| Black raspberry (4%) | 150.45 ± 3.58 * | 52.67 ± 0.65 * | 679.68 ± 2.28 * | 412.81 ± 2.12 * | 1091.94 ± 0.00 * |
| Blackcurrant (2%) | 82.44 ± 0.84 * | 24.28 ± 3.67 | 280.97 ± 15.05 * | 156.25 ± 1.06 * | 646.66 ± 51.58 * |
| Blackcurrant (4%) | 159.82 ± 0.43 * | 30.69 ± 0.65 * | 589.36 ± 15.97 * | 362.69 ± 6.48 * | 1101.82 ± 1.08 * |
| Sea buckthorn—fruit (2%) | 46.87 ± 0.21 | 34.50 ± 0.86 * | 102.58 ± 17.34 * | 111.00 ± 2.64 * | 373.86 ± 56.96 |
| Sea buckthorn—fruit (4%) | 105.21 ± 3.16 * | 99.77 ± 2.59 * | 370.00 ± 14.14 * | 168.22 ± 5.29 * | 792.55 ± 19.34 * |
| Sea buckthorn—fruit powder (2%) | 56.40 ± 0.63 | 10.69 ± 1.73 | 131.94 ± 7.76 * | 97.16 ± 3.17 * | 417.93 ± 22.57 * |
| Sea buckthorn—fruit powder (4%) | 82.89 ± 3.31 * | 54.81 ± 1.08 * | 275.48 ± 10.95 * | 161.86 ± 7.93 * | 750.24 ± 12.90 * |
| Sea buckthorn—leaf (2%) | 192.41 ± 4.00 * | 29.62 ± 0.43 * | 1043.22 ± 33.76 * | 562.03 ± 47.07 * | 1057.75 ± 37.61 * |
| Sea buckthorn—leaf (4%) | 616.82 ± 5.26 * | 76.64 ± 2.16 * | 3543.23 ± 62.04 * | 1852.72 ± 13.22 * | 2200.60 ± 2.15 * |
| Elderberry—flower (2%) | 92.41 ± 0.63 * | 51.45 ± 3.26 * | 273.87 ± 8.67 * | 191.78 ± 2.64 * | 775.07 ± 9.67 * |
| Elderberry—flower (4%) | 211.31 ± 1.26 * | 82.75 ± 2.16 * | 638.71 ± 5.47 * | 470.02 ± 4.76 * | 1711.24 ± 6.45 * |
| Apple powder (2%) | 40.77 ± 2.53 | 11.76 ± 0.22 | 81.29 ± 1.82 * | 61.63 ± 1.59 | 349.54 ± 5.37 |
| Apple powder (4%) | 59.08 ± 7.78 | 17.41 ± 0.00 | 133.87 ± 19.61 * | 112.12 ± 2.12 * | 501.52 ± 18.27 * |
| Rose hip (2%) | 165.00 ± 7.01 * | 23.52 ± 2.06 * | 851.04 ± 4.80 * | 288.40 ± 6.36 * | 1374.43 ± 44.41 * |
| Rose hip (4%) | 306.11 ± 6.96 * | 43.64 ± 4.59 * | 1242.12 ± 45.00 * | 360.46 ± 7.54 * | 2051.75 ± 82.23 * |
| Barberry (2%) | 79.08 ± 1.34 | 16.63 ± 1.89 | 309.01 ± 15.20 * | 141.95 ± 4.24 * | 404.71 ± 12.67 * |
| Barberry (4%) | 143.20 ± 1.52 * | 25.32 ± 3.06 | 600.13 ± 10.01 * | 265.90 ± 23.26 * | 523.74 ± 18.84 * |
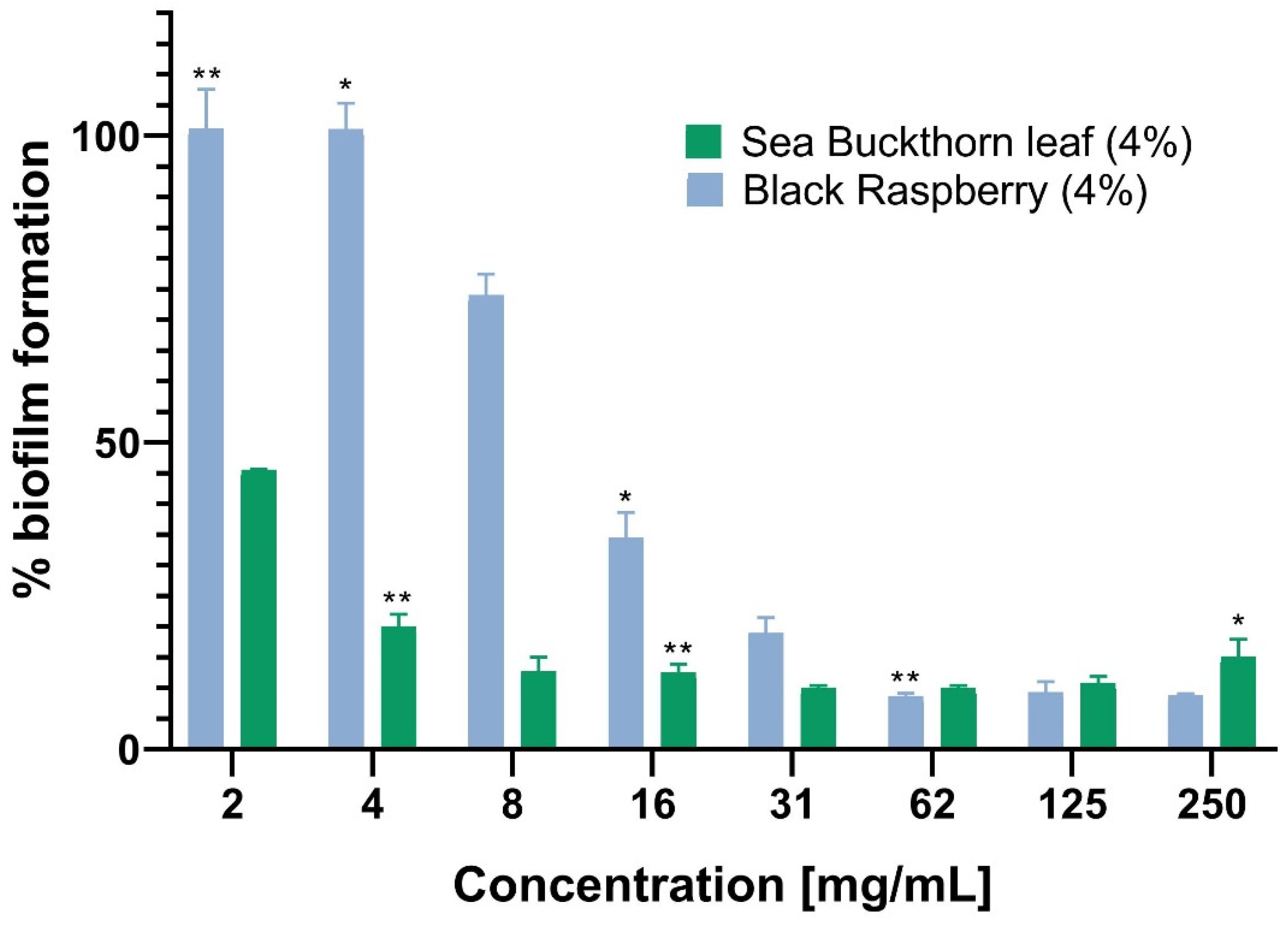
| Tested Sample | MIC [mg/mL] * | |||
|---|---|---|---|---|
| Escherichia coli ATCC 10536 | Pseudomonas aeruginosa ATCC 15442 | Staphylococcus aureus ATCC 6538 | Klebsiella pneumoniae ATCC 13883 | |
| Control rapeseed honey | NA | NA | NA | NA |
| Black raspberry (4%) | NA | NA | 250 (17.85% v/v) | 250 (17.85% v/v) |
| Blackcurrant (4%) | NA | NA | 250 (17.85% v/v) | 250 (17.85% v/v) |
| Sea buckthorn—fruit (4%) | NA | NA | NA | NA |
| Sea buckthorn—fruit powder (4%) | NA | NA | NA | NA |
| Sea buckthorn—leaf (4%) | NA | 250 (17.85% v/v) | 125 (8.93% v/v) | 125 (8.93% v/v) |
| Elderberry—flower (4%) | NA | NA | NA | NA |
| Apple powder (4%) | NA | NA | NA | NA |
| Rose hip (4%) | NA | NA | NA | 250 |
| Barberry (4%) | NA | NA | NA | NA |
| Chloramphenicol | 3.9 × 10−3 | 0.25 | 7.8 × 10−3 | 7.8 × 10−3 |
| Gentamicin | 1.9 × 10−3 | 1.9 | 0.97 × 10−3 | 0.2 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miłek, M.; Ciszkowicz, E.; Sidor, E.; Hęclik, J.; Lecka-Szlachta, K.; Dżugan, M. The Antioxidant, Antibacterial and Anti-Biofilm Properties of Rapeseed Creamed Honey Enriched with Selected Plant Superfoods. Antibiotics 2023, 12, 235. https://doi.org/10.3390/antibiotics12020235
Miłek M, Ciszkowicz E, Sidor E, Hęclik J, Lecka-Szlachta K, Dżugan M. The Antioxidant, Antibacterial and Anti-Biofilm Properties of Rapeseed Creamed Honey Enriched with Selected Plant Superfoods. Antibiotics. 2023; 12(2):235. https://doi.org/10.3390/antibiotics12020235
Chicago/Turabian StyleMiłek, Michał, Ewa Ciszkowicz, Ewelina Sidor, Joanna Hęclik, Katarzyna Lecka-Szlachta, and Małgorzata Dżugan. 2023. "The Antioxidant, Antibacterial and Anti-Biofilm Properties of Rapeseed Creamed Honey Enriched with Selected Plant Superfoods" Antibiotics 12, no. 2: 235. https://doi.org/10.3390/antibiotics12020235
APA StyleMiłek, M., Ciszkowicz, E., Sidor, E., Hęclik, J., Lecka-Szlachta, K., & Dżugan, M. (2023). The Antioxidant, Antibacterial and Anti-Biofilm Properties of Rapeseed Creamed Honey Enriched with Selected Plant Superfoods. Antibiotics, 12(2), 235. https://doi.org/10.3390/antibiotics12020235







