Medical-Grade Honey Enhances the Healing of Caesarean Section Wounds and Is Similarly Effective to Antibiotics Combined with Povidone-Iodine in the Prevention of Infections—A Prospective Cohort Study
Abstract
1. Introduction
2. Results
2.1. MGH Is as Effective as Antibiotics Combined with Povidone-Iodine in Controlling Postoperative Complications and Infections
2.2. The Type of Complication Varied between the Study Groups
2.3. Several Predisposing Factors Increase the Risk of Complications after a CS
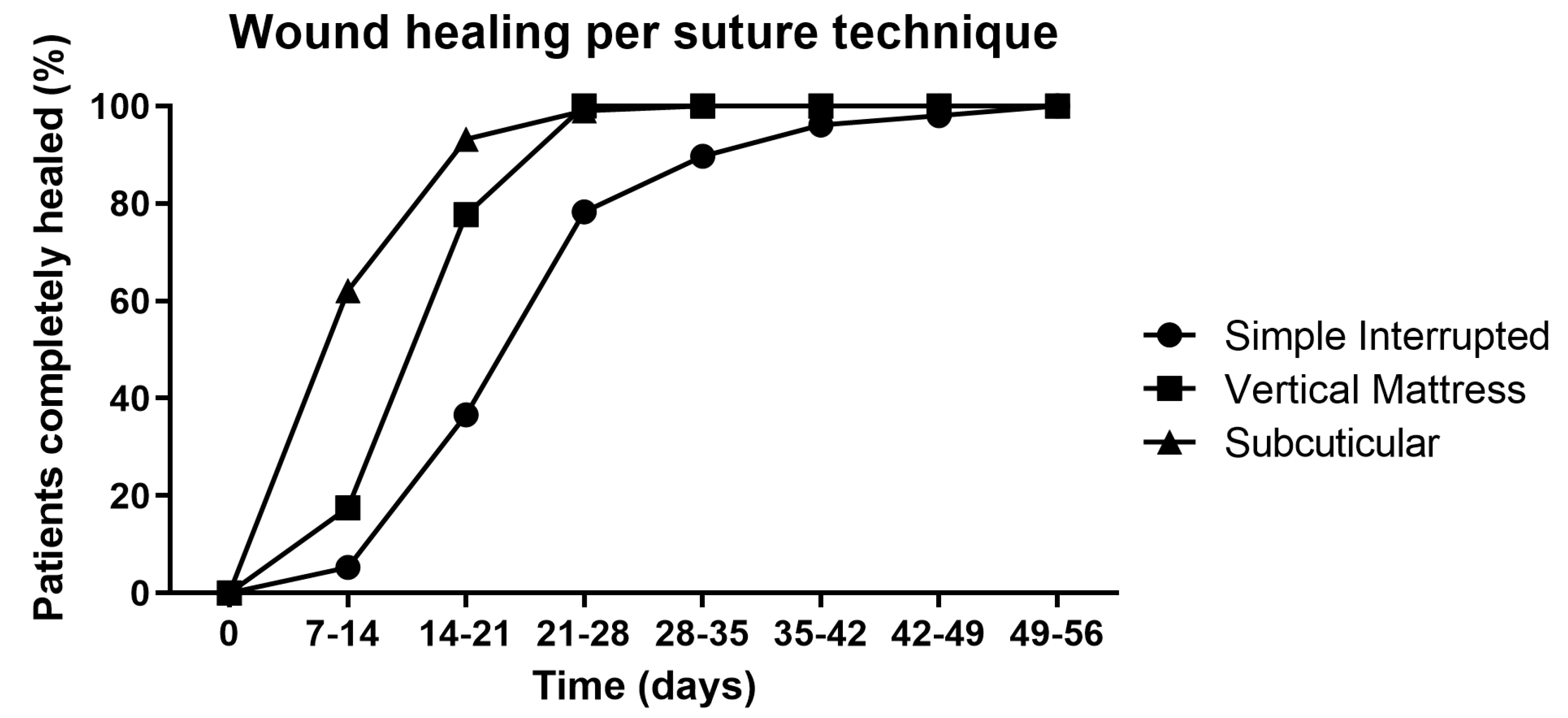
2.4. The Application of MGH for Postoperative Wound Care Leads to Faster Wound Healing
3. Discussion
4. Materials and Methods
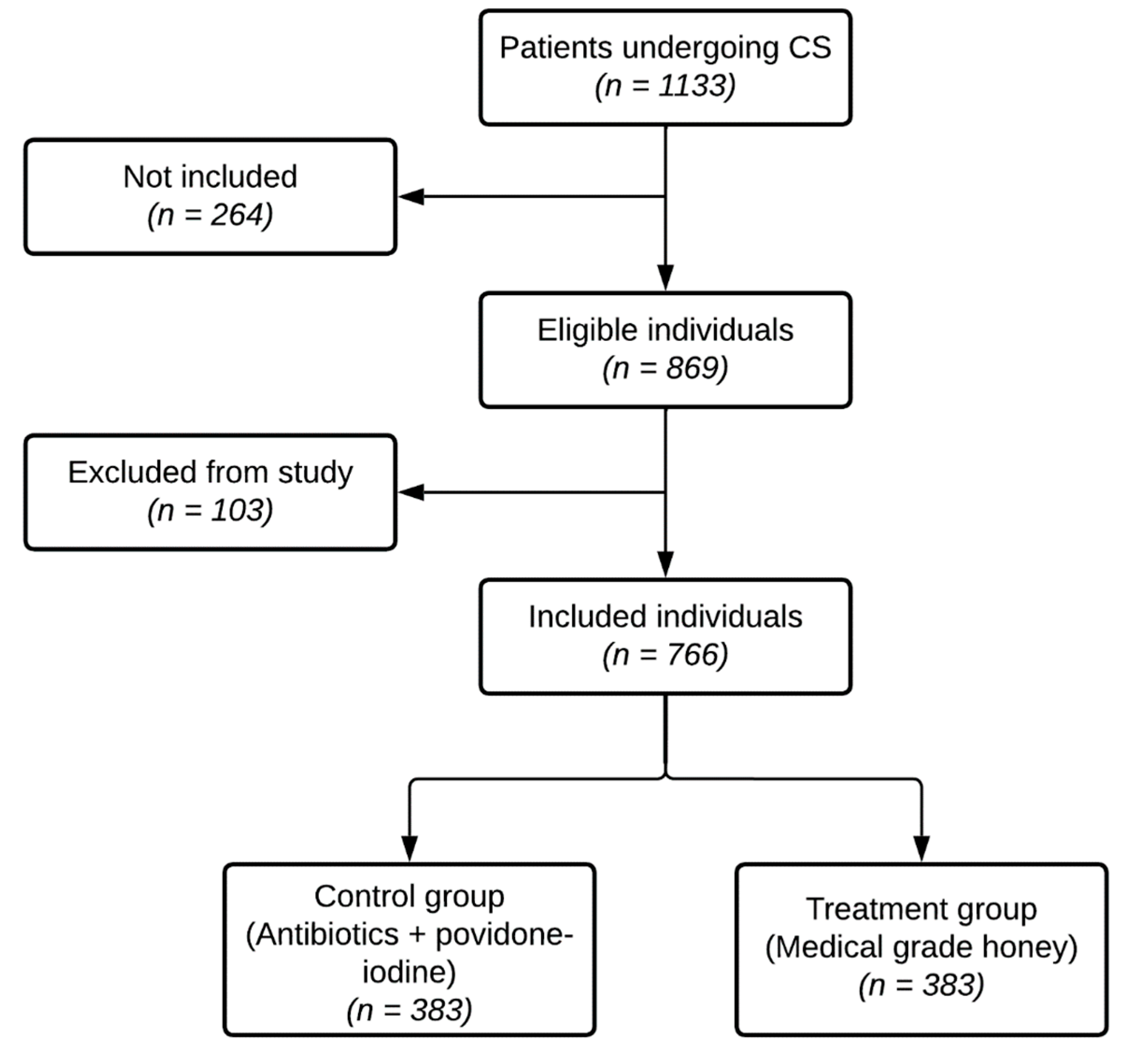
4.1. Study Population, and (Non-)Inclusion and Exclusion Criteria
4.2. Ethical Statement
4.3. Treatment Protocol and Outcome Measures
4.4. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duley, L. The global impact of pre-eclampsia and eclampsia. Semin. Perinatol. 2009, 33, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Todman, D. A history of caesarean section: From ancient world to the modern era. Aust. N. Z. J. Obstet. Gynaecol. 2007, 47, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Antoine, C.; Young, B.K. Cesarean section one hundred years 1920–2020: The Good, the Bad and the Ugly. J. Perinat. Med. 2020, 49, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Betran, A.P.; Ye, J.; Moller, A.B.; Souza, J.P.; Zhang, J. Trends and projections of caesarean section rates: Global and regional estimates. BMJ Glob. Health 2021, 6, e005671. [Google Scholar] [CrossRef] [PubMed]
- Boatin, A.A.; Schlotheuber, A.; Betran, A.P.; Moller, A.B.; Barros, A.J.D.; Boerma, T.; Torloni, M.R.; Victora, C.G.; Hosseinpoor, A.R. Within country inequalities in caesarean section rates: Observational study of 72 low and middle income countries. BMJ 2018, 360, k55. [Google Scholar] [CrossRef] [PubMed]
- Geleto, A.; Chojenta, C.; Taddele, T.; Loxton, D. Association between maternal mortality and caesarean section in Ethiopia: A national cross-sectional study. BMC Pregnancy Childbirth 2020, 20, 588. [Google Scholar] [CrossRef] [PubMed]
- Sobhy, S.; Arroyo-Manzano, D.; Murugesu, N.; Karthikeyan, G.; Kumar, V.; Kaur, I.; Fernandez, E.; Gundabattula, S.R.; Betran, A.P.; Khan, K.; et al. Maternal and perinatal mortality and complications associated with caesarean section in low-income and middle-income countries: A systematic review and meta-analysis. Lancet 2019, 393, 1973–1982. [Google Scholar] [CrossRef]
- Deneux-Tharaux, C.; Carmona, E.; Bouvier-Colle, M.H.; Breart, G. Postpartum maternal mortality and cesarean delivery. Obstet. Gynecol. 2006, 108, 541–548. [Google Scholar] [CrossRef]
- Gomaa, K.; Abdelraheim, A.R.; El Gelany, S.; Khalifa, E.M.; Yousef, A.M.; Hassan, H. Incidence, risk factors and management of post cesarean section surgical site infection (SSI) in a tertiary hospital in Egypt: A five year retrospective study. BMC Pregnancy Childbirth 2021, 21, 634. [Google Scholar] [CrossRef]
- Adane, F.; Mulu, A.; Seyoum, G.; Gebrie, A.; Lake, A. Prevalence and root causes of surgical site infection among women undergoing caesarean section in Ethiopia: A systematic review and meta-analysis. Patient Saf. Surg. 2019, 13, 34. [Google Scholar] [CrossRef]
- Berrios-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Corbett, G.A.; O’Shea, E.; Nazir, S.F.; Hanniffy, R.; Chawke, G.; Rothwell, A.; Gilsenan, F.; MacIntyre, A.; Meenan, A.M.; O’Sullivan, N.; et al. Reducing Caesarean Section Surgical Site Infection (SSI) by 50%: A Collaborative Approach. J. Healthc. Qual. 2021, 43, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Zejnullahu, V.A.; Isjanovska, R.; Sejfija, Z.; Zejnullahu, V.A. Surgical site infections after cesarean sections at the University Clinical Center of Kosovo: Rates, microbiological profile and risk factors. BMC Infect. Dis. 2019, 19, 752. [Google Scholar] [CrossRef]
- Alnajjar, M.S.; Alashker, D.A. Surgical site infections following caesarean sections at Emirati teaching hospital: Incidence and implicated factors. Sci. Rep. 2020, 10, 18702. [Google Scholar] [CrossRef]
- Saeed, K.B.; Greene, R.A.; Corcoran, P.; O’Neill, S.M. Incidence of surgical site infection following caesarean section: A systematic review and meta-analysis protocol. BMJ Open 2017, 7, e013037. [Google Scholar] [CrossRef] [PubMed]
- Mekonnen, A.G.; Mittiku, Y.M. Surgical site infection and its association with rupture of membrane following cesarean section in Africa: A systematic review and meta-analysis of published studies. Matern. Health Neonatol. Perinatol. 2021, 7, 2. [Google Scholar] [CrossRef]
- Tan, P.C.; Rohani, E.; Lim, M.; Win, S.T.; Omar, S.Z. A randomised trial of caesarean wound coverage: Exposed versus dressed. BJOG 2020, 127, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Kawakita, T.; Landy, H.J. Surgical site infections after cesarean delivery: Epidemiology, prevention and treatment. Matern. Health Neonatol. Perinatol. 2017, 3, 12. [Google Scholar] [CrossRef]
- Goharshenasan, P.; Amini, S.; Atria, A.; Abtahi, H.; Khorasani, G. Topical Application of Honey on Surgical Wounds: A Randomized Clinical Trial. Forsch. Komplementmed. 2016, 23, 12–15. [Google Scholar] [CrossRef]
- Holubova, A.; Chlupacova, L.; Cetlova, L.; Cremers, N.A.J.; Pokorna, A. Medical-Grade Honey as an Alternative Treatment for Antibiotics in Non-Healing Wounds-A Prospective Case Series. Antibiotics 2021, 10, 918. [Google Scholar] [CrossRef]
- Smaropoulos, E.; Cremers, N.A.J. The pro-healing effects of medical grade honey supported by a pediatric case series. Complement. Ther. Med. 2019, 45, 14–18. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Defining the standards for medical grade honey. J. Apic. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Naik, P.P.; Mossialos, D.; Wijk, B.V.; Novakova, P.; Wagener, F.; Cremers, N.A.J. Medical-Grade Honey Outperforms Conventional Treatments for Healing Cold Sores-A Clinical Study. Pharmaceuticals 2021, 14, 1246. [Google Scholar] [CrossRef] [PubMed]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another Alternative in the Fight against Antibiotic-Resistant Bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Oryan, A.; Alemzadeh, E.; Moshiri, A. Biological properties and therapeutic activities of honey in wound healing: A narrative review and meta-analysis. J. Tissue Viability 2016, 25, 98–118. [Google Scholar] [CrossRef]
- Cremers, N.A. Something old, something new: Does medical grade honey target multidrug resistance? J. Wound Care 2021, 30, 160–161. [Google Scholar] [CrossRef]
- Gustafsson, K.; Tatz, A.J.; Slavin, R.A.; Sutton, G.A.; Dahan, R.; Abu Ahmad, W.; Kelmer, G. Intra-incisional medical grade honey decreases the prevalence of incisional infection in horses undergoing colic surgery: A prospective randomised controlled study. Equine Vet. J. 2021, 53, 1112–1118. [Google Scholar] [CrossRef]
- Mandel, H.H.; Sutton, G.A.; Abu, E.; Kelmer, G. Intralesional application of medical grade honey improves healing of surgically treated lacerations in horses. Equine Vet. J. 2020, 52, 41–45. [Google Scholar] [CrossRef]
- van Riel, S.; Lardenoije, C.; Oudhuis, G.J.; Cremers, N.A.J. Treating (Recurrent) Vulvovaginal Candidiasis with Medical-Grade Honey-Concepts and Practical Considerations. J. Fungi 2021, 7, 664. [Google Scholar] [CrossRef]
- Naik, P.P.; Chrysostomou, D.; Cinteza, M.; Pokorna, A.; Cremers, N.A. When time does not heal all wounds-the use of medical grade honey in wound healing: A case series. J. Wound Care 2022, 31, 548–558. [Google Scholar] [CrossRef]
- Nair, H.K.R.; Tatavilis, N.; Pospisilova, I.; Kucerova, J.; Cremers, N.A.J. Medical-Grade Honey Kills Antibiotic-Resistant Bacteria and Prevents Amputation in Diabetics with Infected Ulcers: A Prospective Case Series. Antibiotics 2020, 9, 529. [Google Scholar] [CrossRef] [PubMed]
- Pleeging, C.C.F.; Wagener, F.; de Rooster, H.; Cremers, N.A.J. Revolutionizing non-conventional wound healing using honey by simultaneously targeting multiple molecular mechanisms. Drug Resist. Updat 2022, 62, 100834. [Google Scholar] [CrossRef] [PubMed]
- Ayukekbong, J.A.; Ntemgwa, M.; Atabe, A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control 2017, 6, 47. [Google Scholar] [CrossRef] [PubMed]
- Velin, L.; Umutesi, G.; Riviello, R.; Muwanguzi, M.; Bebell, L.M.; Yankurije, M.; Faktor, K.; Nkurunziza, T.; Rukundo, G.; de Dieu Gatete, J.; et al. Surgical Site Infections and Antimicrobial Resistance After Cesarean Section Delivery in Rural Rwanda. Ann. Glob. Health 2021, 87, 77. [Google Scholar] [CrossRef]
- van Wattum, J.J.; Leferink, T.M.; Wilffert, B.; Ter Horst, P.G.J. Antibiotics and lactation: An overview of relative infant doses and a systematic assessment of clinical studies. Basic Clin. Pharmacol. Toxicol. 2019, 124, 5–17. [Google Scholar] [CrossRef]
- Thomas, S.P.; Denizer, E.; Zuffa, S.; Best, B.M.; Bode, L.; Chambers, C.D.; Dorrestein, P.C.; Liu, G.Y.; Momper, J.D.; Nizet, V.; et al. Transfer of antibiotics and their metabolites in human milk: Implications for infant health and microbiota. Pharmacotherapy 2022. online ahead of print. [Google Scholar] [CrossRef]
- Regmi, A.; Ojha, N.; Singh, M.; Ghimire, A.; Kharel, N. Risk Factors Associated with Surgical Site Infection following Cesarean Section in Tertiary Care Hospital, Nepal. Int. J. Reprod. Med. 2022, 2022, 4442453. [Google Scholar] [CrossRef]
- Opoien, H.K.; Valbo, A.; Grinde-Andersen, A.; Walberg, M. Post-cesarean surgical site infections according to CDC standards: Rates and risk factors. A prospective cohort study. Acta Obstet. Gynecol. Scand. 2007, 86, 1097–1102. [Google Scholar] [CrossRef]
- Cho, M.; Kang, J.; Kim, I.K.; Lee, K.Y.; Sohn, S.K. Underweight body mass index as a predictive factor for surgical site infections after laparoscopic appendectomy. Yonsei Med. J. 2014, 55, 1611–1616. [Google Scholar] [CrossRef]
- Dobner, J.; Kaser, S. Body mass index and the risk of infection—From underweight to obesity. Clin. Microbiol. Infect. 2018, 24, 24–28. [Google Scholar] [CrossRef]
- Lawrence, S.; Malacova, E.; Reutens, D.; Sturgess, D.J. Increased maternal body mass index is associated with prolonged anaesthetic and surgical times for caesarean delivery but is partially offset by clinician seniority and established epidural analgesia. Aust. N. Z. J. Obstet. Gynaecol. 2021, 61, 394–402. [Google Scholar] [CrossRef]
- Bracken, O.; Langhe, R. Evaluation of maternal and perinatal outcomes in pregnancy with high BMI. Ir. J. Med. Sci. 2021, 190, 1439–1444. [Google Scholar] [CrossRef]
- Pergialiotis, V.; Prodromidou, A.; Perrea, D.N.; Doumouchtsis, S.K. The impact of subcutaneous tissue suturing at caesarean section on wound complications: A meta-analysis. BJOG 2017, 124, 1018–1025. [Google Scholar] [CrossRef]
- Guglielminotti, J.; Landau, R.; Li, G. Adverse Events and Factors Associated with Potentially Avoidable Use of General Anesthesia in Cesarean Deliveries. Anesthesiology 2019, 130, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Blumenfeld, Y.J.; El-Sayed, Y.Y.; Lyell, D.J.; Nelson, L.M.; Butwick, A.J. Risk Factors for Prolonged Postpartum Length of Stay Following Cesarean Delivery. Am. J. Perinatol. 2015, 32, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Chen, B.P.; Soleas, I.M.; Ferko, N.C.; Cameron, C.G.; Hinoul, P. Prolonged Operative Duration Increases Risk of Surgical Site Infections: A Systematic Review. Surg. Infect. 2017, 18, 722–735. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, H.; Westerbos, S.J.; Ubbink, D.T. Benefit and harm of iodine in wound care: A systematic review. J. Hosp. Infect. 2010, 76, 191–199. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, Z.; Su, F.; Zhang, T. Comparison of topical honey and povidone iodine-based dressings for wound healing: A systematic review and meta-analysis. J. Wound Care 2021, 30, S28–S36. [Google Scholar] [CrossRef]
- Abdelhafeez, A.T.; Rushdee, S.; Khalaf, M.R. Topical honey application for post-caesarean section wound infection and gapping. Al-Azhar Assiut Med. J. 2003, 1, 114–121. [Google Scholar]
- Shirvani, M.A.; Nikpour, M.; Azadbakht, M.; Banihosseini, S.Z.; Zanjani, R. The effect of honey gel on cesarean incision pain: A triple blind clinical trial. Afr. J. Pharm. Pharmacol. 2013, 7, 19–24. [Google Scholar] [CrossRef]
- Nikpour, M.; Shirvani, M.A.; Azadbakht, M.; Zanjani, R.; Mousavi, E. The effect of honey gel on abdominal wound healing in cesarean section: A triple blind randomized clinical trial. Oman Med. J. 2014, 29, 255–259. [Google Scholar] [CrossRef]
- Heidari, T.; Roozbahani, N.; Farahani, L.A.; Attarha, M.; Torkestani, N.A.; Jamilian, M.; Bekhradi, R. Does Iranian Astragalus gossypinus honey assist in healing caesarean wounds and scars? Eur. J. Integr. Med. 2013, 5, 226–233. [Google Scholar] [CrossRef]
- Dryden, M.; Goddard, C.; Madadi, A.; Heard, M.; Saeed, K.; Cooke, J. Using antimicrobial surgihoneyto prevent caesarean wound infection. Br. J. Midwifery 2014, 22, 111–115. [Google Scholar] [CrossRef]
- Pleeging, C.C.F.; Coenye, T.; Mossialos, D.; de Rooster, H.; Chrysostomou, D.; Wagener, F.; Cremers, N.A.J. Synergistic Antimicrobial Activity of Supplemented Medical-Grade Honey against Pseudomonas aeruginosa Biofilm Formation and Eradication. Antibiotics 2020, 9, 866. [Google Scholar] [CrossRef] [PubMed]
- Cremers, N.; Belas, A.; Santos Costa, S.; Couto, I.; de Rooster, H.; Pomba, C. In vitro antimicrobial efficacy of two medical grade honey formulations against common high-risk meticillin-resistant staphylococci and Pseudomonas spp. pathogens. Vet. Dermatol. 2020, 31, 90–96. [Google Scholar] [CrossRef]
- de Groot, T.; Janssen, T.; Faro, D.; Cremers, N.A.J.; Chowdhary, A.; Meis, J.F. Antifungal Activity of a Medical-Grade Honey Formulation against Candida auris. J. Fungi 2021, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Smaropoulos, E.; Cremers, N.A.J. Medical-Grade Honey for the Treatment of Extravasation-Induced Injuries in Preterm Neonates: A Case Series. Adv. Neonatal Care 2021, 21, 122–132. [Google Scholar] [CrossRef]
- Sacko, M. Les infections associées aux soins dans le service de gynécologie et obstétrique du CHU Gabriel Touré [Thèse de Médecine]. Mali Med. 2017, 35, 43–49. [Google Scholar]
- Schwartz, D.; Lellouch, J. Explanatory and pragmatic attitudes in therapeutical trials. J. Chronic Dis. 1967, 20, 637–648. [Google Scholar] [CrossRef]
- Chauveaux, D. Preventing surgical-site infections: Measures other than antibiotics. Orthop. Traumatol. Surg. Res. 2015, 101, S77–S83. [Google Scholar] [CrossRef]
- Tikkanen, M. Etiology, clinical manifestations, and prediction of placental abruption. Acta Obstet. Gynecol. Scand. 2010, 89, 732–740. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Subgroup | Control Group (Antibiotics + Povidone-Iodine) n (%) | Treatment Group (MGH) n (%) |
|---|---|---|---|
| Age | ≤19 | 69 (18.0) | 78 (20.4) |
| 20–34 | 239 (62.4) | 236 (61.6) | |
| ≥35 | 75 (19.6) | 69 (18.0) | |
| Gravidity | Primigravida (1) | 105 (27.4) | 102 (26.6) |
| Paucigravida (2 or 3) | 114 (29.8) | 124 (32.4) | |
| Multigravida (4 or 5) | 88 (23.0) | 93 (24.3) | |
| Grand multigravida (>5) | 76 (19.8) | 64 (16.7) | |
| Parity | Nullipara (0) | 112 (29.2) | 106 (27.7) |
| Primipara (1) | 65 (17.0) | 66 (17.2) | |
| Paucipara (2 or 3) | 111 (29.0) | 130 (33.9) | |
| Multipara (4 or 5) | 76 (19.8) | 47 (12.3) | |
| Grand multipara (>5) | 19 (5.0) | 34 (8.9) | |
| BMI | ≤18.5 | 5 (1.3) | 6 (1.6) |
| 18.5–25 | 163 (42.5) | 164 (42.8) | |
| 25–30 | 142 (37.1) | 131 (34.2) | |
| 30–35 | 48 (12.5) | 51 (13.3) | |
| 35–40 | 19 (5.0) | 21 (5.5) | |
| >40 | 6 (1.6) | 10 (2.6) | |
| Education | Primary | 100 (26.1) | 78 (20.4) |
| level | Secondary | 68 (17.8) | 79 (20.6) |
| Tertiary | 20 (5.2) | 24 (6.3) | |
| No education | 188 (49.1) | 187 (48.8) | |
| Religious | 7 (1.8) | 15 (3.9) | |
| Occupation | Housewife | 278 (72.6) | 267 (69.7) |
| Store employee | 47 (12.3) | 46 (12.0) | |
| Government | 37 (9.7) | 44 (11.5) | |
| Student | 19 (5.0) | 24 (6.3) | |
| Other | 2 (0.5) | 2 (0.5) |
| Control Group (Antibiotics + Povidone-Iodine) n (%) | Treatment Group (MGH) n (%) | Total n (%) | ||
|---|---|---|---|---|
| Postoperative | Yes | 74 (19.3) | 72 (18.8) | 146 (19.1) |
| complications | No | 309 (80.7) | 311 (81.2) | 620 (80.9) |
| Total | 383 (100) | 383 (100) | 766 (100) | |
| Surgical site | Yes | 61 (15.9) | 55 (14.4) | 116 (15.1) |
| infections | No | 322 (84.1) | 328 (85.6) | 650 (84.9) |
| Total | 383 (100) | 383 (100) | 766 (100) |
| Control Group (Antibiotics + Povidone-Iodine) | Treatment Group (MGH) | ||||||
|---|---|---|---|---|---|---|---|
| Complication | RR (IC 95%) within Group | Complication | RR (IC 95%) within Group | RR (IC 95%) between Groups | |||
| Type of Complication | Yes n (%) | No n (%) | Yes n (%) | No n (%) | |||
| Pain at the surgical site | 74 (19.3) | 309 (80.7) | - | 72 (18.8) | 311 (81.2) | - | 0.97 [0.73–1.30] |
| Superficial pus discharge | 31 (41.9) | 43 (58.1) | 3.88 * [1.91–7.86] | 49 (68.1) | 23 (31.9) | 6.30 * [3.21–12.34] | 1.62 * [1.19–2.22] |
| Deep pus discharge | 30 (40.5) | 44 (59.5) | 3.75 * [1.84–7.63] | 6 (8.3) | 66 (91.7) | 0.77 [0.28–2.11] | 0.21 * [0.09–0.46] |
| Crust formation | 8 (10.8) | 66 (89.2) | Reference | 0 (0) | 72 (100) | 0.06 [0.00–1.00] | 0.06 [0.00–1.03] |
| Presence of wound exudate | 0 (0) | 74 (100) | 0.06 [0.00–1.00] | 7 (9.7) | 65 (90.3) | 0.90 [0.34–2.35] | 15.41 [0.90–264.98] |
| Peritonitis | 2 (2.7) | 72 (97.3) | 0.25 [0. 05–1.14] | 1 (1.4) | 71 (98.6) | 0.13 [0.02–1.00] | 0.51 [0.048–5.54] |
| Endometritis | 0 (0) | 74 (100) | 0.06 [0.00–1.00] | 1 (1.4) | 71 (98.6) | 0.13 [0.02–1.00] | 3.08 [0.13–74.44] |
| Wound bleeding | 5 (6.8) | 69 (93.2) | 0.63 [0.21–1.82] | 13 (18.1) | 59 (81.9) | 1.67 [0.74–3.79] | 2.67 * [1.00–7.11] |
| Control Group (Antibiotics + Povidone-Iodine) | Treatment Group (MGH) | ||||||
|---|---|---|---|---|---|---|---|
| Complication | RR (IC 95%) within Group | Complication | RR (IC 95%) within Group | ||||
| Yes (%) | No (%) | Yes (%) | No (%) | ||||
| Fever between Day 0–30 | Yes | 5 (71.4) | 2 (28.6) | 10.44 * [2.07–52.76] | 1 (33.3) | 2 (66.7) | 2.16 [0.20–23.49] |
| No | 69 (18.4) | 307 (81.6) | 71 (18.7) | 309 (81.3) | |||
| Control Group (Antibiotics + Povidone-Iodine) | Treatment Group (MGH) | |||||||
|---|---|---|---|---|---|---|---|---|
| Complication | RR (IC 95%) within Group | Complication | RR (IC 95%) within Group | RR (IC 95%) between Groups | ||||
| Yes (%) | No (%) | Yes (%) | No (%) | |||||
| Age (years) | ≤19 | 13 (18.8) | 56 (81.2) | 0.87 [0.50–1.49] | 8 (10.3) | 70 (89.7) | 0.47 * [0.23–0.95] | 0.54 [0.24–1.23] |
| 20–34 | 52 (21.8) | 187 (78.2) | Reference | 53 (16.5) | 183 (77.5) | 1.03 [0.74–1.45] | 1.03 [0.74–1.45] | |
| ≥ 35 | 9 (12.0) | 66 (88.0) | 0.55 [0.29–1.07] | 11 (15.9) | 58 (84.1) | 0.73 [0.41–1.33] | 1.33 [0.59–3.01] | |
| Gravidity | Primigravida (1) | 27 (25.7) | 78 (74.3) | 1.40 [0.84–2.31] | 17 (16.7) | 85 (83.3) | 0.90 [0.51–1.62] | 0.65 [0.38–1.11] |
| Paucigravida (2 or 3) | 21 (18.4) | 93 (81.6) | Reference | 20 (16.1) | 104 (83.9) | 0.88 [0.50–1.53] | 0.88 [0.50–1.53] | |
| Multigravida (4 or 5) | 15 (17.0) | 73 (83.0) | 0.93 [0.51–1.69] | 18 (19.4) | 75 (80.6) | 1.05 [0.60–1.85] | 1.14 [0.61–2.11] | |
| Grand multigravida (>5) | 11 (14.5) | 65 (85.5) | 0.79 [0.40–1.53] | 17 (26.6) | 47 (73.4) | 1.44 [0.82–2.53] | 1.84 [0.93–3.63] | |
| Parity | Nullipara (0) | 27 (24.1) | 85 (75.9) | Reference | 17 (16.0) | 89 (84.0) | 0.67 [0.39–1.15] | 0.67 [0.39–1.15] |
| Primipara (1) | 11 (16.9) | 54 (83.1) | 0.70 [0.37–1.32] | 10 (15.2) | 56 (84.8) | 0.63 [0.33–1.21] | 0.90 [0.41–1.96] | |
| Paucipara (2 or 3) | 26 (23.4) | 85 (76.6) | 0.97 [0.61–1.56] | 26 (20.0) | 104 (80.0) | 0.83 [0.52–1.33] | 0.85 [0.41–1.96] | |
| Multipara (4 or 5) | 9 (11.8) | 67 (88.2) | 0.49 [0.24–0.99] | 11 (23.4) | 36 (76.6) | 0.97 [0.53–1.79] | 1.98 [0.89–4.41] | |
| Grand multipara (>5) | 1 (5.3) | 18 (94.7) | 0.21 [0.03–1.51] | 8 (23.5) | 26 (76.5) | 0.98 [0.49–1.95] | 4.47 [0.60–33.09] | |
| BMI (kg/m2) | ≤18.5 | 3 (60.0) | 2 (40.0) | 3.15 * [1.44–6.90] | 1 (16.7) | 5 (83.3) | 0.88 [0.14–5.39] | 0.28 [0.04–1.91] |
| 18.5–25 | 31 (19.0) | 132 (81.0) | Reference | 21 (12.8) | 143 (87.2) | 0.67 [0.40–1.12] | 0.67 [0.40–1.12 | |
| 25–30 | 25 (17.6) | 117 (82.4) | 0.93 [0.57–1.49] | 33 (25.2) | 98 (74.8) | 1.32 [0.86–2.04] | 1.43 [0.90–2.27] | |
| 30–35 | 9 (18.8) | 39 (81.2) | 0.99 [0.51–1.92] | 11 (21.6) | 40 (78.4) | 1.13 [0.62–2.09] | 1.15 [0.52–2.53] | |
| 35–40 | 3 (15.8) | 16 (84.2) | 0.83 [0.28–2.46] | 3 (14.3) | 18 (85.7) | 0.75 [0.25–2.24] | 0.90 [0.21–3.96] | |
| > 40 | 3 (50.0) | 3 (50.0) | 2.63* [1.11–6.22] | 3 (30.0) | 7 (70.0) | 1.58 [0.58–4.28] | 0.60 [0.17–2.07] | |
| Education level | Primary | 19 (19.0) | 81 (81.0) | 1.02 [0.62–1.69] | 17 (21.8) | 61 (78.2) | 1.17 [0.70–1.96] | 1.15 [0.64–2.06] |
| Secondary | 19 (27.9) | 49 (72.1) | 1.50 [0.92–2.44] | 15 (19.0) | 64 (81.0) | 1.02 [0.59–1.76] | 0.68 [0.38–1.23] | |
| Tertiary | 1 (5.0) | 19 (95.0) | 0.27 [0.04–1.86] | 3 (12.5) | 21 (87.5) | 0.76 [0.22–2.02] | 2.50 [0.28–22.21] | |
| No education | 35 (18.6) | 153 (81.4) | Reference | 35 (18.7) | 152 (81.3) | 1.01 [0.66–1.53] | 1.01 [0.66–1.53] | |
| Religious | 0 (0) | 7 (100.0) | 0.33 [0.02–4.95] | 2 (13.3) | 13 (86.7) | 0.72 [0.19–2.69] | 2.50 [0.14–46.14] | |
| Occupation | Housewife | 55 (19.8) | 223 (80.2) | Reference | 52 (19.5) | 215 (80.5) | 0.98 [0.7–1.38] | 0.98 [0.70–1.38] |
| Store employee | 9 (19.1) | 38 (80.9) | 0.97 [0.51–1.82] | 11 (23.9) | 35 (76.1) | 1.21 [0.69–2.13] | 1.25 [0.57–2.73] | |
| Government | 4 (10.8) | 33 (89.2) | 0.55 [0.21–1.42] | 7 (15.9) | 37 (84.1) | 0.80 [0.39–1.65] | 1.47 [0.47–4.64] | |
| Student | 6 (31.6) | 13 (68.4) | 1.60 [0.79–3.22] | 1 (4.2) | 23 (95.8) | 0.21 [0.03–1.46] | 0.13 [0.02–1.004] | |
| Other | 0 (0) | 2 (100) | 0.84 [0.07–10.64] | 1 (50) | 1 (50) | 2.53 [0.62–10.31] | 3.00 [0.19–47.97] | |
| Control Group (Antibiotics + Povidone-Iodine) | Treatment Group (MGH) | |||||||
|---|---|---|---|---|---|---|---|---|
| Complication | RR (IC 95%) within Group | Complication | RR (IC 95%) within Group | RR (IC 95%) between Groups | ||||
| Yes (%) | No (%) | Yes (%) | No (%) | |||||
| Admission to the hospital | On own initiative | 13 (14.0) | 80 (86.0) | 0.66 [0.38–1.15] | 17 (22.1) | 60 (77.9) | 1.05 [0.65–1.69] | 1.58 [0.82–3.04] |
| Referral | 61 (21.0) | 229 (79.0) | Reference | 55 (18.0) | 251 (82.0) | 0.85 [0.62–1.19] | 0.85 [0.62–1.18] | |
| Duration of hospitalization (in days) | ≤5 | 58 (21.1) | 217 (78.9) | Reference | 47 (17.0) | 229 (83.0) | 0.81 [0.57–1.14] | 0.81 [0.57–1.14] |
| 5 d–10 d | 11 (11.1) | 88 (88.9) | 0.53 * [0.29–0.96] | 24 (23.5) | 78 (76.5) | 1.12 [0.73–1.69] | 2.12 * [1.10–4.09] | |
| 10 d–15 d | 5 (55.6) | 4 (44.4) | 2.63 * [1.41–4.93] | 1 (20.0) | 4 (80.0) | 0.95 [0.16–5.56] | 0.36 [0.06–2.28] | |
| Presence of membranes during CS | Intact | 58 (19.4) | 241 (80.6) | Reference | 61 (19.4) | 254 (80.6) | 1.00 [0.72–1.38] | 1.00 [0.72–1.38] |
| Not intact | 16 (19.0) | 68 (81.0) | 0.98 [0.60–1.62] | 11 (16.2) | 57 (83.8) | 0.83 [0.46–1.50] | 0.85 [0.42–1.71] | |
| Delay between rupture of membranes (in hours) | ≤24 h | 15 (21.4) | 55 (78.6) | Reference | 11 (16.4) | 56 (83.6) | 0.77 [0.38–1.55] | 0.77 [0.38–1.55] |
| 24 h–72 h | 1 (16.7) | 5 (83.3) | 0.78 [0.12–4.92] | 0 (0) | 1 (100.0) | 1.15 [0.10–13.15] | 1.17 [0.07–18.96] | |
| 72 h–100 h | 0 (0) | 5 (100.0) | 0.38 [0.03–5.62] | 0 (0) | 0 (0) | 2.29 [0.31–17.07] | 6.00 [0.22–162.54] | |
| ≥120 h | 0 (0) | 1 (100.0) | 1.15 [0.10–13.15] | 0 (0) | 0 (0) | 2.29 [0.31–17.07] | 2.00 [0.09–44.35] | |
| Anesthesia | General | 37 (50) | 100 (32.4) | 1.78 * [1.19–2.67] | 32 (25.0) | 96 (75.0) | 1.65 * [1.08–2.51] | 0.93 [0.62–1.39] |
| Spinal | 37 (17.0) | 207 (83.0) | Reference | 39 (15.6) | 211 (84.4) | 1.03 [0.68–1.56] | 1.03 [0.68–1.56] | |
| Epidural | 0 (0.0) | 2 (100.0) | 1.09 [0.09–13.91] | 1 (20.0) | 4 (80.0) | 1.32 [0.22–7.81] | 1.50 [0.08–26.86] | |
| Duration of CS | 15–30 min | 15 (16.7) | 75 (83.3) | 0.91 [0.53–1.57] | 12 (12.5) | 84 (87.5) | 0.68 [0.37–1.24] | 0.75 [0.37–1.51] |
| 30–45 min | 39 (18.3) | 174 (81.7) | Reference | 43 (19.3) | 180 (80.7) | 1.05 [0.71–1.56] | 1.05 [0.71–1.56] | |
| 45–60 min | 15 (23.4) | 49 (76.6) | 1.28 [0.76–2.17] | 16 (35.6) | 29 (64.4) | 1.94 * [1.20–3.15] | 1.52 [0.84–2.74] | |
| ≥60 min | 5 (31.3) | 11 (68.7) | 1.71 [0.78–3.72] | 1 (5.3) | 18 (94.7) | 0.29 [0.4–1.98] | 0.17 [0.02–1.30] | |
| Suture technique | Simple interrupted | 70 (21.5) | 256 (78.5) | Reference | 60 (20.2) | 237 (79.8) | 0.94 [0.69–1.28] | 0.94 [0.69–1.28] |
| Vertical mattress | 0 (0) | 24 (100) | 0.09 [0.01–1.45] | 2 (12.5) | 14 (87.5) | 0.58 [0.16–2.16] | 7.35 [0.38–143.79] | |
| Subcuticular | 4 (12.1) | 29 (87.9) | 0.56 [0.22–1.45] | 10 (14.3) | 60 (85.7) | 0.66 [0.36–1.22] | 1.18 [0.40–3.48] | |
| Duration of Wound Healing (Days) | Control Group (Antibiotics + Povidone-Iodine) | Treatment Group (MGH) | ||||
|---|---|---|---|---|---|---|
| Complication | RR (IC 95%) within Group | Complication | RR (IC 95%) within Group | |||
| Yes n (%) | No n (%) | Yes n (%) | No n (%) | |||
| 7–14 | 0 (0) | 26 (100.0) | 0.24 [0.01–4.11] | 2 (2.6) | 76 (97.4) | 0.35 [0.07–1.71] |
| 14–21 | 6 (7.2) | 77 (92.8) | Reference | 15 (8.9) | 153 (91.1) | 1.23 [0.50–3.07] |
| 21–28 | 27 (14.9) | 154 (85.1) | 2.06 [0.88–4.81] | 28 (29.8) | 66 (70.2) | 4.12 * [1.80–9.46] |
| 28–35 | 12 (26.1) | 34 (73.9) | 3.61 * [1.45–8.98] | 11 (42.3) | 15 (57.7) | 5.85 * [2.40–14.28] |
| 35–42 | 12 (40.0) | 18 (60.0) | 5.53 * [2.28–13.43] | 10 (100.0) | 0 (0) | 13.83 * [6.40–29.90] |
| 42–49 | 9 (100.0) | 0 (0) | 13.83 * [6.40–29.90] | 2 (66.7) | 1 (33.3) | 9.22 * [3.04–28.01] |
| 49–56 | 8 (100.0) | 0 (0) | 13.83 * [6.40–29.90] | 4 (100.0) | 0 (0) | 13.83 * [6.40–29.90] |
| Duration of Wound Healing in Days | Control Group (Antibiotics + Povidone-Iodine) | Treatment Group (MGH) | ||||
|---|---|---|---|---|---|---|
| Simple Interrupted Suture n (%) | Vertical Mattress Suture n (%) | Subcuticular Suture n (%) | Simple Interrupted Suture n (%) | Vertical Mattress Suture n (%) | Subcuticular Suture n (%) | |
| 7–14 | 15 (45.5) | 3 (42.9) | 8 (12.5) | 18 (54.5) | 4 (57.1) | 56 (87.5) |
| 14–21 | 47 (24.1) | 12 (50.0) | 24 (75.0) | 148 (75.9) | 12 (50.0) | 8 (25.0) |
| 21–28 | 172 (66.2) | 9 (100.0) | 0 (0) | 88 (33.8) | 0 (0) | 6 (100.0) |
| 28–35 | 45 (63.4) | 0 (0) | 1 (100.0) | 26 (36.6) | 0 (0) | 0 (0) |
| 35–42 | 30 (75.0) | 0 (0) | 0 (0) | 10 (25.0) | 0 (0) | 0 (0) |
| 42–49 | 9 (75.0) | 0 (0) | 0 (0) | 3 (25.0) | 0 (0) | 0 (0) |
| 49–56 | 8 (66.7) | 0 (0) | 0 (0) | 4 (33.0) | 0 (0) | 0 (0) |
| Total | 326 (85.1) | 24 (6.3) | 33 (8.6) | 297 (77.5) | 16 (4.2) | 70 (18.3) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bocoum, A.; Riel, S.J.J.M.v.; Traoré, S.O.; Ngo Oum II, E.F.; Traoré, Y.; Thera, A.T.; Fané, S.; Dembele, B.T.; Cremers, N.A.J. Medical-Grade Honey Enhances the Healing of Caesarean Section Wounds and Is Similarly Effective to Antibiotics Combined with Povidone-Iodine in the Prevention of Infections—A Prospective Cohort Study. Antibiotics 2023, 12, 92. https://doi.org/10.3390/antibiotics12010092
Bocoum A, Riel SJJMv, Traoré SO, Ngo Oum II EF, Traoré Y, Thera AT, Fané S, Dembele BT, Cremers NAJ. Medical-Grade Honey Enhances the Healing of Caesarean Section Wounds and Is Similarly Effective to Antibiotics Combined with Povidone-Iodine in the Prevention of Infections—A Prospective Cohort Study. Antibiotics. 2023; 12(1):92. https://doi.org/10.3390/antibiotics12010092
Chicago/Turabian StyleBocoum, Amadou, Senna J. J. M. van Riel, Soumana Oumar Traoré, Elisabeth Florine Ngo Oum II, Youssouf Traoré, Augustin Tioukani Thera, Seydou Fané, Bakary Tientigui Dembele, and Niels A. J. Cremers. 2023. "Medical-Grade Honey Enhances the Healing of Caesarean Section Wounds and Is Similarly Effective to Antibiotics Combined with Povidone-Iodine in the Prevention of Infections—A Prospective Cohort Study" Antibiotics 12, no. 1: 92. https://doi.org/10.3390/antibiotics12010092
APA StyleBocoum, A., Riel, S. J. J. M. v., Traoré, S. O., Ngo Oum II, E. F., Traoré, Y., Thera, A. T., Fané, S., Dembele, B. T., & Cremers, N. A. J. (2023). Medical-Grade Honey Enhances the Healing of Caesarean Section Wounds and Is Similarly Effective to Antibiotics Combined with Povidone-Iodine in the Prevention of Infections—A Prospective Cohort Study. Antibiotics, 12(1), 92. https://doi.org/10.3390/antibiotics12010092







