Abstract
Truncal acne is common, and the psychosocial burden may be underestimated as patients most often complain of facial acne. The Acne Symptom and Impact Scale (ASIS) is a 17-item patient-reported outcome (PRO) measure designed to assess the signs and impacts of acne vulgaris. ASIS has previously been validated in a prospective, non-interventional study as a reliable PRO instrument for facial acne. In a pilot study, ASIS, and an additional 10 new questions that focused on the concerns of patients (ASIS-C), were given to 10 patients with moderate-to-severe truncal acne vulgaris who received 3 months of monotherapy with oral sarecycline, a narrow-spectrum tetracycline-class antibiotic. ASIS-C questionnaires were also given to 10 acne-free control subjects. Average ASIS-C answers decreased by 4% for Signs, 15% for Impact, and 16% for Concerns in the 10 patients, with greater decreases of 5% for Signs, 20% for Impact, and 19% for Concerns in the 60% of patients whose truncal acne was clear or almost clear after 12 weeks of sarecycline treatment. In this study, sarecycline was effective in reducing the psychosocial burden associated with truncal acne based on the ASIS-C PRO measures.
1. Introduction
Acne vulgaris is a common chronic inflammatory condition that affects adolescents and adults. Most current literature on the pathophysiology and treatment of acne primarily focuses on facial acne. While facial acne is the most common presenting patient complaint, truncal acne may be underestimated [1]. Since the severity of truncal acne does not correlate with the severity of facial acne [2], the psychosocial burden and physical pain and bleeding of truncal acne may also be underestimated [3]. In a study of 696 patients across 5 U.S. cities, >3% of patients presented with only truncal lesions, while ~50% presented with truncal and facial acne [4]. Of the cohort with both truncal and facial acne, ~25% of these patients did not voluntarily mention truncal acne, but clinical examination detected truncal acne [4]. While the majority presented with mild to moderate truncal acne, more than 75% were interested in truncal acne treatment [4].
The Acne Symptom and Impact Scale (ASIS) is a 17-item patient-reported outcome measure (PROM) designed to assess the signs and impacts of acne vulgaris [5]. ASIS has previously been validated in a prospective, non-interventional study as a reliable PROM for facial acne [6]; however, ASIS has not been utilized in evaluating the psychosocial impact of truncal acne. Additionally, 10 new items have been proposed to be added to the ASIS questionnaire to address the patient’s concerns. These items have not yet been validated. We refer to this questionnaire as ASIS-C. The aim of this study is to investigate if there is a role for ASIS-C in the evaluation of the impact of truncal acne.
Sarecycline is a narrow-spectrum tetracycline-class antibiotic approved in October 2018 by the Food and Drug Administration (FDA) for the treatment of moderate-to-severe acne vulgaris in patients 9 years of age and older [7]. Statistically significant improvement was observed with sarecycline in truncal acne, with improvement at week 3 on the back and improvement at week 6 on the chest, based on investigator global assessment (IGA) scores in the pivotal phase 3 trials [8]. Ten patients with moderate-to-severe truncal acne vulgaris, the ASIS questionnaire at baseline, and after 12 weeks of monotherapy with oral sarecycline 1.5 mg/kg/day. We hypothesized that the psychosocial impact of truncal acne may be assessed by measuring ASIS before and after truncal acne treatment.
2. Materials and Methods
2.1. Study Design
This pilot study is a phase 4, open-label prospective case series conducted at a single dermatology center in the United States. We gave 10 patients with moderate (baseline IGA 3) to severe (baseline IGA 4) truncal acne vulgaris the ASIS questionnaire, along with the new Concerns questions, at baseline and after 12 weeks of monotherapy with oral sarecycline 1.5 mg/kg/day.
2.2. Inlcusion Criteria
To be included in this study, patients had to be 9 to 45 years, weigh 33 to 136 kg, and have achieved a score of 3 (moderate) or 4 (severe) on the Investigator’s Global Assessment (IGA) scale for inflammatory lesions of acne of the trunk. An IGA score of 3 is defined as some-to-many non-inflammatory lesions and possibly some inflammatory lesions, but no more than one small nodular lesion [9]. IGA 4 (severe) is defined as some-to-many non-inflammatory and inflammatory lesions, but no more than a few nodular lesions [9].
2.3. Exclusion Criteria
Individuals were excluded if they had a dermatologic condition, any chronic illness interfering with study evaluations, any allergy or resistance to tetracyclines, drug-induced acne, hormonal contraceptive initiation, systemic retinoids, systemic corticosteroids, androgens, or anti-androgens within 12 weeks prior to sarecycline initiation.
2.4. Control Patients
Ten acne-free control patients were recruited from a local university. A survey was sent out to university students to identify subjects that approximately matched the ages of the treatment subjects.
2.5. Methods
The patients treated with sarecycline and the control patients. The validated ASIS questionnaire was divided into Signs and Impact domains. The contents of the ASIS-C questionnaire are shown in Table 1. The patients were evaluated by a single dermatologist at a single dermatology center in the United States. Ten patients with moderate-to-severe acne were given the ASIS-C questionnaire (Table 1). In addition, ten acne-free subjects were given the questionnaire for comparison and matched by approximate age ranges.

Table 1.
ASIS-C Questionnaire.
2.6. Data Analysis
This is a qualitative study. In order to analyze the results of the ASIS questionnaire, the answers for each question were assigned a number 1 to 5. For each patient, the number was averaged per question, then averaged in respective categories of Signs, Impact, and Concerns. Average baseline ASIS-C scores were compared with ASIS-C scores after 12 weeks of treatment in all 10 patients as well as with ASIS-C scores in the patients that reached objective truncal or facial IGA success, defined as a >2-point decrease from baseline and IGA scores of clear (0) or almost clear (1).
Scores of the baseline answers of the 10 patients with moderate-to-severe acne were compared to the answers of the 10 acne-free control subjects to assess the % difference in the psychosocial difference between patients with moderate-to-severe acne and age-matched subjects with no acne. Comparisons in the % difference with the control subset were also made between all patients at week 12 and the patients who achieved IGA success at week 12, respectively.
3. Results
3.1. Patient Characteristics
Ten patients participated in this study, with 5 males (50%) and 5 females (50%). Patient demographics are represented in Table 2. The patient’s average age was 16.3, with a range of 13–22. While all 10 patients had facial acne, an IGA score of 3 or 4 for facial acne was not an inclusion criterion. Sixty percent of patients achieved IGA success of the trunk after 12 weeks of sarecycline monotherapy, and 80% of patients achieved IGA success of the trunk or face.

Table 2.
Patient Demographics for Sarecycline Arm and Control Arm.
3.2. Control Characteristics
Ten acne-free control patients were analyzed in this pilot study, with 7 females (70%) and 3 males (30%). Age ranges are represented in Table 2.
3.3. Baseline ASIS-C Scores
At baseline in the 10 patients with moderate-to-severe truncal acne, the average ASIS-C score for the Signs domain was 2.7, for the Impact domain 2.85, and for the Concerns domain 2.56. In comparison, the control acne-free subjects had average scores of 1.52 for the Signs domain, 1.53 for the Impact domain, and 1.42 for the Concerns domain. Average Baseline scores of patients with truncal acne were higher than control acne-free subjects by 44% for Signs, 46% for Impact, and 45% for Concerns.
3.4. ASIS-C Scores after 12 Weeks of Sarecycline Therapy
After 12 weeks of sarecycline monotherapy, average ASIS-C scores decreased from baseline ASIS-C scores by 4% in the Signs domain from 2.7 at baseline to 2.6, by 15% in the Impact domain from 2.9 to 2.4, and by 16% in the Concerns domain from 2.6 to 2.2. Average ASIS-C scores in the 60% of patients achieving IGA success decreased from baseline ASIS-C scores by 5% in the Signs domain from 2.6 to 2.5, by 20% in the Impact domain from 2.8 to 2.2, and by 19% in the Concerns domain from 2.4 to 2.0.
3.5. Percent Improvement in Sarecycline Responders
When comparing ASIS-C scores from patients achieving IGA success to control acne-free subjects, there were differences of 42% for Signs, 34% for Impact, and 29% for Concerns (Figure 1).
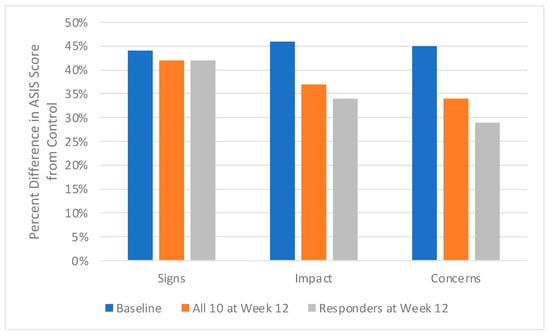
Figure 1.
Acne Patients Before and After Oral Sarecycline Treatment.
4. Discussion
Numerous studies have been performed in order to evaluate the impact of acne on health-related quality of life (HRQoL), but few have focused on the impact of truncal acne [10,11,12,13,14]. Psychosocial impacts include depression, low self-esteem, and suicidal ideation. In a cross-sectional, questionnaire-based study of 3775 participants, 14% reported having substantial acne (a lot and very much). Among those with “very much acne“ as compared to those with “no/little acne”, suicidal ideation was twice as frequently reported among girls and three times more frequently reported among boys [13].
Another qualitative study of 60 patients with facial and truncal acne and acne scars investigated the psychosocial burden through personification. The dominant theme that emerged included characterizations of acne as an intruder and an unwanted companion responsible for poor self-esteem and emotional impairment. It was demonstrated that the continuous burden of active acne starting from adolescence continues into adulthood besides clinically active lesions with scarring [14]. In a multi-country, population-based survey, it was demonstrated that facial and truncal acne together had a greater impact on HRQoL than facial acne alone. Importantly, more severe truncal acne was associated with an adverse impact on HRQoL irrespective of the severity of facial acne. This study utilized the dermatology life quality index (DLQI) and the Comprehensive Acne Quality of Life (CompAQ) [3].
PRO measures are an important tool in recognizing the patient’s perspective in the treatment of their acne. A recent systematic review from 2011 to 2021 evaluated how often PROs are utilized in the study of acne and rosacea. In this review of 206 randomized clinical trials (RCTs), 53% of rosacea and acne trials included at least 1 PRO. Only 7% of the RCTs analyzed included a PROM as a primary outcome [15]. This demonstrates that PROMs might be underutilized in the study of acne.
ASIS was developed to provide a reliable and valid PROM that satisfied FDA criteria. The development process consisted of a literature review, concept elicitation interviews, item generation, and cognitive interviews. A literature review of other acne-specific PROMs revealed that the Acne Quality of Life Index (Acne-QOLI), Acne Quality of Life scale (AQOL), Acne-Specific Quality of Life Questionnaire (Acne-QoL), and the Dermatology-Specific Quality of Life Instrument for Acne (DSQL-Acne) were not in accordance with the FDA patient-reported outcome guidance. The framework was originally derived from the literature review and then refined by the concept of elicitation interviews. A 15-item draft questionnaire was created, which was finalized as a 17-item questionnaire following feedback from the cognitive interviews [5]. The questionnaire was divided into Signs and Impact domains to evaluate how the patients viewed their facial acne and how their acne impacted them, respectively. The Impact domain consists of two subscales, Emotional and Social.
Recently, 10 new questions were proposed for the Impact domain of ASIS that focus on patient Concerns (ASIS-C) and were included in our study. Answers to these questions were compared to the validated 17 questions in the original ASIS. In our reported study, the Concerns domain improved by 16% in all 10 patients and 19% in the patients who achieved IGA success. These results validate the 10 additional questions on Concern. Larger studies are needed for further validation of these additional questions.
Patient feedback was incorporated at each step of the development process of ASIS as the developers aimed to focus on patient-centered research. Items that patients identified as important during development were specific terminologies, such as the use of terms blackheads, whiteheads, scars, redness, dark marks, and overall acne. Other PROMs developed prior to ASIS did not include this. Similarly, symptoms addressed in existing PROMs such as pain, burning sensation, and soreness/tenderness were not identified as important by patients and, thus, not included in the ASIS. It is important to note that ASIS was designed to target patients with mild to moderate acne. Patients with severe cystic acne, acne conglobata, or any active or developing nodules were excluded from development [5].
Additionally, the developers chose different recall periods for the Signs and Impacts domain. For Signs, the recall period of “right now” was used to emphasize the symptoms that the patient is currently experiencing. For Impact, a recall period of 7 days was used to allow the patient time to process the impact of their current acne while still minimizing the time that a patient would have to rely on their memory [5]. Further, ASIS has been validated as a reliable PROM for facial acne. One hundred and fifty subjects completed baseline and follow-up assessments. Psychometric evaluation was performed using both traditional FDA psychometrics and new psychometric methods, such as the Rasch Measurement Theory (RMT). Both the Signs and Impact domains fulfilled traditional criteria and mostly satisfied Rasch criteria [6].
Few comparative studies evaluating the validity of PROMs have been performed. One study evaluating depression, anxiety, and life quality in acne patients treated with topical therapy versus isotretinoin used the DLQI, the Hospital anxiety and depression (HAD) scale, and the Beck Depression Inventory (BDI). Significant results were only found when comparing DLQI scores [16]. In a systematic review of 21 PROMs, only the CompAQ and the Acne-Q met the standards to be recommended for use in acne clinical studies. This study evaluated 10 acne-specific PROMs, 6 dermatology-specific PROMs, and 5 generic PROMs using the Consensus-Based Standards for the Selection of Health Measurement Instruments (COSMIN) criteria [17].
The 10 acne-specific PROMs included the Acne Disability Index (ADI), Acne Impact on Adult Daily Life (AI-ADL), ASIS, Acne-Q, AQOL, Acne-QoL, Acne-QOLI, Assessment of the Psychological and Social Effects of Acne (APSEA), Cardiff Acne Disability Index (CADI), and CompAQ. While CompAQ and the Acne-Q were the only PROMs to meet the COSMIN criteria in this study for content validity, it was found that all the PROMs studied were lacking a degree of content validity or other measurements. ASIS and ASIS-C should now be considered for measuring acne-associated HRQoL if additional evaluation of content validity is performed. ASIS was graded as moderate on Structural Validity, Internal Consistency, Reliability, and Construct Validity. Acne-Q was graded as low on Reliability and Construct Validity. The 6 dermatology-specific PROMs assessed included the DLQI, Children’s Dermatology Life Quality Index (CDLQI), Dermatology-Specific Quality of Life (DSQL), Oily Skin Self-Assessment Scale (OSSAS), Oily Skin Impact Scale (OSIS), and Skindex-29. The 5 generic PROMs analyzed were the UK Sickness Profile, EuroQol 5-Dimension, Short Form-36 (SF-36), Patient Benefit Index (PBI), and Patient-Reported Outcomes Measurement Information System–Anxiety. Each of the dermatology-specific and the general PROMs were graded as very low overall on content validity and may not be the most accurate tools to use in the assessment of acne. Overall, the PROMs remain difficult to assess given their qualitative nature. A major limitation of the study is that only what the individual PROM studies reported could be assessed. It is possible that all items in the COSMIN risk of bias checklists might not have been addressed due to limits on publication space or focus on other criteria [17].
Yoon et al. analyzed the content and phrasing of acne-specific PROMs with the conclusion that the PROMs have notable differences in the covered content, and PROM selection could potentially be guided depending on the clinical context. For example, if the primary focus is on psychological well-being, Acne-Q could be the most appropriate tool [18]. ASIS and ASIS-C are broader measures that encompass both the physical and emotional symptoms of facial acne, also considering the readability and length of the questionnaire [5].
Effective treatments for truncal acne are necessary to begin relieving the associated emotional impact. In line with PROMs, Auffret et al. developed the Truncal Acne Severity Scale (TRASS) as a patient-centered approach to validate treatment [19]. Seventeen current topical treatments for truncal acne include dapsone, azelaic acid, benzoyl peroxide, tretinoin, trifarotene, and clindamycin [20,21,22,23,24,25,26]. Systemic treatments include oral isotretinoin and tetracycline antibiotics [27,28]. Since few studies are designed to include truncal acne as an inclusion criterion, there is little objective data regarding the efficacy of these treatments [1]. Much less data exists on how these treatments affect the psychosocial aspect of truncal acne. One study of pooled data from 2 phase 3 trials on the efficacy and tolerability of dapsone 7.5% gel included ASIS as an endpoint and identified significant improvement across ASIS domains [29].
Sarecycline is a narrow-spectrum tetracycline-class antibiotic approved by the Food and Drug Administration (FDA) for moderate-to-severe acne vulgaris in patients 9 years of age and older [7]. Sarecycline demonstrates potent anti-inflammatory activity and Gram-positive activity against Cutibacterium acnes, a bacteria implicated in the pathogenesis of acne. Additionally, sarecycline has shown reduced activity against Gram-negative bacteria commonly found in the gut which may contribute to the favorable side effect profile and the low potential for antibiotic resistance [7]. Improvement in truncal acne with daily sarecycline treatment was observed in investigator global assessment (IGA) scores in phase 3 clinical trials, however, truncal acne was not an inclusion criterion [8,30]. While sarecycline has been reported as efficacious in the treatment of truncal acne, little data exists on the efficacy of sarecycline in relieving the psychosocial burden of truncal acne.
In this study, 60% of the patients treated with sarecycline achieved IGA success of the trunk, and 80% achieved IGA success of the trunk or face. Additionally, this study demonstrated success in improving scores in Signs, Impact, and Concern with sarecycline treatment in comparison with the control acne-free subjects.
It is of note that, since ASIS was developed for facial acne, the exact wording in the questions in the Signs domain may need to be modified by deleting the word “facial” in the questions in order for the questionnaire to be utilized more optimally for truncal acne studies. The inclusion of the word “facial” may have skewed the answers in the Signs domain for this study on truncal acne and diminished the true impact of the Signs domain and may explain the low impact on the Signs domain with sarecycline treatment. In order to reliably evaluate truncal acne, the word “face” should be edited to “skin”, for example.
In this study, a large % difference is observed in the ASIS-C scores at baseline in the truncal acne patients compared to the control acne-free subjects; this illustrates the large psychosocial burden of truncal acne at baseline (Figure 1). After 12 weeks, the % difference in the ASIS-C scores from the control acne-free subjects decreases, even more so in those whose truncal acne cleared with sarecycline monotherapy; this suggests that ASIS-C scores, especially the Impact domain and the newly introduced Concern domain, may reflect the improvement in the psychosocial burden of acne.
This pilot study is limited by the small sample size of 10 patients. A small sample size such as this could potentially lead to a type II error in which no real difference exists between the ASIS-C scores before and after treatment. The aim of this pilot study was to determine if there is a role for ASIS-C in the evaluation of the psychosocial burden of truncal acne. It was observed that the average answers decreased in each domain after 12 weeks of treatment for sarecycline. Despite the small sample size of this pilot study, our data suggest a need for further investigation of ASIS-C as a reliable PROM in truncal acne.
5. Conclusions
Truncal acne is often unappreciated but carries a significant psychosocial burden, as the results in this study indicate.
In this pilot study, the average ASIS answers decreased by 4% for the Signs domain, 15% for the Impact domain, and 16% in the Concerns domain after 12 weeks of treatment with sarecycline. ASIS and ASIS-C may be important PRO tools in evaluating psychosocial burden as well as improvement with successful acne treatment and would benefit from further validation in additional larger facial and truncal acne studies.
Author Contributions
Conceptualization, A.Y.M.; Methodology, A.Y.M. and S.A.M.; Validation, A.Y.M. and S.A.M.; Formal Analysis, A.Y.M., K.H., S.A.M., L.M. and I.Z.; Investigation, A.Y.M., S.A.M., L.M. and I.Z.; Resources, A.Y.M., S.A.M. and L.M.; Data Curation, A.Y.M., K.H., L.M., S.A.M. and I.Z.; Writing—Original Draft Preparation, K.H.; Writing—Review and Editing, A.Y.M.; Visualization, A.Y.M., K.H. and L.M.; Supervision, A.Y.M.; Project Administration, A.Y.M.; Funding Acquisition, A.Y.M. All authors have read and agreed to the published version of the manuscript.
Funding
Arlington Research Center received Investigator Initiated Study funds from Almirall (US) for portions of this study.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Institutional Review Board (or Ethics Committee) of Advarra #00049785.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
A.Y.M. has received honoraria or research funds from Almirall, Galderma, and Parexel. K.H., S.A.M., L.M. and I.Z. have no conflict of interest.
References
- Del Rosso, J.Q.; Stein-Gold, L.; Lynde, C.; Tanghetti, E.; Alexis, A.F. Truncal Acne: A Neglected Entity. J. Drugs Dermatol. JDD 2019, 18, 205–1208. [Google Scholar] [PubMed]
- Bunick, C.; Keri, J.; Tanaka, S.; Furey, N.; Damiani, G.; Johnson, J.; Grada, A. Antibacterial Mechanisms and Efficacy of Sarecycline in Animal Models of Infection and Inflammation. Antibiotics 2021, 10, 439. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Beissert, S.; Cook-Bolden, F.; Chavda, R.; Harper, J.; Hebert, A.; Lain, E.; Layton, A.; Rocha, M.; Weiss, J.; et al. Impact of facial and truncal acne on quality of life: A multi-country population-based survey. JAAD Int. 2021, 3, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Del Rosso, J.Q.; Bikowski, J.B.; Baum, E.; Smith, J.; Hawkes, S.; Benes, V.; Bhatia, N. A closer look at truncal acne vulgaris: Prevalence, severity, and clinical significance. J. Drugs Dermatol. JDD 2007, 6, 597–600. [Google Scholar] [PubMed]
- Alexis, A.; Daniels, S.R.; Johnson, N.; Pompilus, F.; Burgess, S.M.; Harper, J.C. Development of a new patient-reported outcome measure for facial acne: The Acne Symptom and Impact Scale (ASIS). J. Drugs Dermatol. 2014, 13, 333–340. [Google Scholar]
- Hudgens, S.; Harper, J.C.; Daniels, S.R.; Banderas, B.; Varon, S.; Alexis, A.F. Validation of a New Patient-Reported Outcome Measure for Facial Acne: The Acne Symptom and Impact Scale (ASIS). J. Drugs Dermatol. 2015, 14, 552–559. [Google Scholar]
- Zhanel, G.; Critchley, I.; Lin, L.-Y.; Alvandi, N. Microbiological profile of sarecycline, a novel targeted spectrum tetracycline for the treatment of acne vulgaris. Antimicrob. Agents Chemother. 2019, 63, e01297-18. [Google Scholar] [CrossRef]
- Moore, A.; Green, L.J.; Bruce, S.; Sadick, N.; Tschen, E.; Werschler, P.; Cook-Bolden, F.E.; Dhawan, S.S.; Forsha, D.; Gold, M.H.; et al. Once-daily oral sarecycline 1.5 mg/kg/day is effective for moderate to severe acne vulgaris: Results from two identically designed, phase 3, randomized, double-blind clinical trials. J. Drugs Dermatol. 2018, 17, 987–996. [Google Scholar] [CrossRef]
- Del Rosso, J.Q.; Kircik, L.; Tanghetti, E. Management of Truncal Acne Vulgaris with Topical Dapsone 7.5% Gel. J. Clin. Aesthetic Dermatol. 2018, 11, 45–50. [Google Scholar]
- Yazici, K.; Baz, K.; Yazici, A.E.; Kokturk, A.; Tot, S.; Demirseren, D.D.; Buturak, V. Disease-specific quality of life is associated with anxiety and depression in patients with acne. J. Eur. Acad. Dermatol. Venereol. 2004, 18, 435–439. [Google Scholar] [CrossRef]
- Dunn, L.K.; O’Neill, J.L.; Feldman, S.R. Acne in adolescents: Quality of life, selfesteem, mood, and psychological disorders. Dermatol. Online J. 2011, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Vallerand, I.; Lewinson, R.; Parsons, L.; Lowerison, M.; Frolkis, A.; Kaplan, G.; Barnabe, C.; Bulloch, A.; Patten, S. Risk of depression among patients with acne in the UK: A population-based cohort study. Br. J. Dermatol. 2018, 178, e194–e195. [Google Scholar] [CrossRef] [PubMed]
- Halvorsen, J.A.; Stern, R.S.; Dalgard, F.; Thoresen, M.; Bjertness, E.; Lien, L. Suicidal ideation, mental health problems, and social impairment are increased in adolescents with acne: A population-based study. J. Investig. Dermatol. 2011, 131, 363–370. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Chavda, R.; Leclerc, M.; Dréno, B. Projective Personification Approach to the Experience of People with Acne and Acne Scarring-Expressing the Unspoken. JAMA Dermatol. 2022, 158, 1005–1012. [Google Scholar] [CrossRef] [PubMed]
- Ly, S.; Miller, J.; Tong, L.; Blake, L.; Mostaghimi, A.; Barbieri, J.S. Use of Patient-Reported Outcomes in Acne Vulgaris and Rosacea Clinical Trials from 2011 to 2021: A Systematic Review. JAMA Dermatol. 2022, 158, 1419–1428. [Google Scholar] [CrossRef]
- Kaymak, Y.; Taner, E.; Taner, Y. Comparison of depression, anxiety and life quality in acne vulgaris patients who were treated with either isotretinoin or topical agents. Int. J. Dermatol. 2009, 48, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, Z.H.; Thiboutot, D.; Homsi, H.A.; Perez-Chada, L.M.; Barbieri, J.S. Patient-Reported Outcome Measures for Health-Related Quality of Life in Patients with Acne Vulgaris: A Systematic Review of Measure Development and Measurement Properties. JAMA Dermatol. 2022, 158, 900–911. [Google Scholar] [CrossRef]
- Yoon, J.; Homsi, H.A.; Barbieri, J.S. Analysis of Content and Phrasing of Health-Related Quality-of-Life Patient-Reported Outcome Measures Used in Patients with Acne. JAMA. Dermatol. 2022, 158, 1072–1073. [Google Scholar] [CrossRef]
- Auffret, N.; Nguyen, J.M.; Leccia, M.T.; Claudel, J.P.; Dréno, B. TRASS: A global approach to assess the severity of truncal acne. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 897–904. [Google Scholar] [CrossRef]
- Hoffman, L.K.; Del Rosso, J.Q.; Kircik, L.H. The Efficacy and Safety of Azelaic Acid 15% Foam in the Treatment of Truncal Acne Vulgaris. J. Drugs Dermatol. JDD 2017, 16, 534–538. [Google Scholar]
- St. Surin-Lord, S.; Miller, J. Topical Treatment of Truncal Acne with Tretinoin Lotion 0.05% and Azelaic Acid Foam. Case Rep. Dermatol. Med. 2020, 2020, e5217567. [Google Scholar] [CrossRef]
- Bikowski, J. A Review of the Safety and Efficacy of Benzoyl Peroxide (5.3%) Emollient Foam in the Management of Truncal Acne Vulgaris. J. Clin. Aesthetic Dermatol. 2010, 3, 26–29. [Google Scholar]
- Leyden, J.J.; Del Rosso, J.Q. The effect of benzoyl peroxide 9.8% emollient foam on reduction of Propionibacterium acnes on the back using a short contact therapy approach. J. Drugs Dermatol. JDD 2012, 11, 830–833. [Google Scholar] [PubMed]
- Jarratt, M.; Werner, C.P.; Alió Saenz, A.B. Tazarotene foam versus tazarotene gel: A randomized relative bioavailability study in acne vulgaris. Clin. Drug Investig. 2013, 33, 283–289. [Google Scholar] [CrossRef]
- Tan, J.; Thiboutot, D.; Popp, G.; Gooderham, M.; Lynde, C.; Del Rosso, J.; Weiss, J.; Blume-Peytavi, U.; Weglovska, J.; Johnson, S.; et al. Randomized phase 3 evaluation of trifarotene 50 μg/g cream treatment of moderate facial and truncal acne. J. Am. Acad. Dermatol. 2019, 80, 1691–1699. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Fowler, J.; Kemény, L.; Draelos, Z.; Cook-Bolden, F.; Dirschka, T.; Eichenfield, L.; Graeber, M.; Ahmad, F.; Saenz, A.A.; et al. Long-term safety and efficacy of trifarotene 50 μg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 166–173. [Google Scholar] [CrossRef]
- Cunliffe, W.J.; Glass, D.; Goode, K.; Stables, G.I.; Boorman, G.C. A double-blind investigation of the potential systemic absorption of isotretinoin, when combined with chemical sunscreens, following topical application to patients with widespread acne of the face and trunk. Acta Derm. Venereol. 2001, 81, 14–17. [Google Scholar] [CrossRef]
- Del Rosso, J.Q. Truncal acne vulgaris: The relative roles of topical and systemic antibiotic therapy. J. Drugs Dermatol. JDD 2007, 6, 148–151. [Google Scholar]
- Taylor, S.C.; Cook-Bolden, F.E.; McMichael, A.; Downie, J.B.; Rodriguez, D.A.; Alexis, A.F.; Callender, V.D.; Alvandi, N. Efficacy, Safety, and Tolerability of Topical Dapsone Gel, 7.5% for Treatment of Acne Vulgaris by Fitzpatrick Skin Phototype. J. Drugs Dermatol. 2018, 17, 160–167. [Google Scholar]
- Del Rosso, J.Q.; Baldwin, H.; Harper, J.C.; Zeichner, J.; Obagi, S.; Graber, E.; Jimenez, X.; Vicente, F.H.; Grada, A. Management of Truncal Acne with Oral Sarecycline: Pooled Results from Two Phase-3 Clinical Trials. J. Drugs Dermatol. JDD 2021, 20, 634–640. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).