Abstract
We report that phthalimides may be cyclized using a Mukaiyama-type aldol coupling to give variously substituted fused lactam (1,2,3,9b-tetrahydro-5H-pyrrolo[2,1-a]isoindol-5-one) systems. This novel process shows a high level of regioselectivity for o-substituted phthalimides, dictated by steric and electronic factors, but not for m-substituted phthalimides. The initial aldol adduct is prone to elimination, giving 2,3-dihydro-5H-pyrrolo[2,1-a]isoindol-5-ones, and the initial cyclisation can be conducted in such a way that aldol cyclisation-elimination is achievable in a one-pot approach. The 2,3-dihydro-5H-pyrrolo[2,1-a]isoindol-5-ones possess cross conjugation and steric effects which significantly influence the reactivity of several functional groups, but conditions suitable for epoxidation, ester hydrolysis and amide formation, and reduction, which provide for ring manipulation, were identified. Many of the derived lactam systems, and especially the eliminated systems, show low solubility, which compromises biological activity, although in some cases, antibacterial and cytotoxic activity was found, and this new class of small molecule provides a useful skeleton for further elaboration and study.
1. Introduction
The critical importance of natural products in the development of pharmaceutically active compounds has been thoroughly documented, and although popularity of this approach has waned in recent years in favour of combinatorial and rational design, there have been strong calls for its reinvigoration [1]. These calls are particularly relevant for antibacterial agents, for which there is a serious deficit of new candidates in the drug pipeline [2,3], at a time when there is considerable urgency to expand therapeutics as a result of the rapid emergence of resistant bacterial strains [4]. The challenges peculiar to antibacterial drug discovery [5,6,7,8] imply that natural products often provide biologically validated start points suitable for immediate elaboration in the quest for new pharmaceutically useful agents [9,10]. It has recently been recognised that existing strategies for the discovery of new antibacterials have not been effective [11], probably as a result at least in part of over-reliance of combinatorial approaches leading to structurally narrow libraries [12,13], and there is an urgent need for the identification of novel leads for expanding the antibacterial drug development pipeline [14,15]. The work of Waldmann [16,17] and Danishefsky [18] has reiterated the importance of natural products as a starting point for drug discovery, and our contribution to this area has been to show that chemical libraries modelled on natural products [19], including equisetin [20], reutericyclin [21], kibdelomycin [22], and streptolydigin [23], which all possess a core tetramate unit, or oxazolomycin [24] and pramanicin [25], which possess an α-hydroxypyroglutamate core, may exhibit significant antibacterial activity and provide useful opportunities for further optimisation. Critical to the success of this work has been the finding that C-acyl or C-carboxamide side chains may be introduced under mild conditions to tetramate and pyroglutamate skeletons [19] and that this leads to enhanced antibacterial activity. It would appear, therefore, that an α, α, α-tricarbonyl unit comprises, at least in part, the active pharmacophore, and this was corroborated by the finding that the core tetramate without an α, α, α-tricarbonyl unit had little intrinsic antibacterial activity [26].
The recent discovery of pyrrolizilactone [27], UCS1025A and B [28,29,30] and CJ-16264 [31], is of interest since all are comprised of a common lactam-lactone fused ring core and C-acyl decalin side chain. Studies of the biosynthesis [32], synthesis [33,34,35,36], and SAR [37] of UCS1025A suggest that the core skeleton might offer an opportunity for development, not least because of its similarity with bioactive tetramates, which are also appended with decalins [38]. Of significance is the antibacterial bioactivity of these systems, with MIC values of typically 1–15 ug/mL against Gram-positive MDR strains and some Gram-negative ones [31]. Limited SAR analysis with three CJ-16,264 stereoisomers shows MIC values of 2–16 ug/mL against MRSA, E. faecelis, and E. faecium [39]. As a result, the development of methodology for their total synthesis has attracted attention [40,41] and the total synthesis of myceliothermophins C, D, and E [42], a related structural type, has also recently been achieved. The synthesis of the azabicyclo[3.3.0]octane core provides a key background [43] and an unusual approach to the pyrrolizidine core from an 8-membered ring by transannular cyclisation has been reported [44]. Of particular interest was the elegant ring cyclisation methodology originally reported by Lambert [34] and developed later by both Hoye [35,45] and Christmann [33,46,47], since this provided rapid entry to the core lactam system from maleimides by an aldol-like ring closure, using in situ generated silyl enolates as nucleophiles. We have recently reported that this approach is suitable for substituted maleimides, and can be used to access a small library of novel pyrrolidinones [48]; of interest was their lack of antibacterial activity, but a similar phenomenon had been observed for unsubstituted tetramates [19]. We report here that the aldol cyclisation may be further extended to phthalimides, and that this gives rise to a range of functionalised systems whose biological activity has been assessed.
2. Results and Discussion
Substituted phthalic anhydrides 1a–d (Scheme 1) and 2a–e (Scheme 2) and γ-aminobutyric acid (GABA) were condensed by heating without solvent to 170 °C for 6 h, during which the molten mixture slowly turned to a straw yellow colour, using the previously reported procedure [49,50,51,52,53], and successfully gave a range of substituted systems in excellent yields. Upon completion of the reaction, the cooled solid mass was dissolved in dichloromethane and washed using 0.5 N HCl, giving the desired products 3a–d and 4a–e in excellent yields (Scheme 1 and Scheme 2, and Table 1). Esterification of acids 3a–d and 4a–e to their corresponding methyl esters 5a–d and 6a–e using thionyl chloride and MeOH at rt over 16 h gave the products in quantitative yields in many cases (Table 1); however, this was ineffective for 4b due to its unexpectedly low solubility, and synthesis of 6b required direct condensation of methyl γ-aminobutanoate hydrochloride with the anhydride in toluene with DIPEA under reflux for 16 h, giving the desired product 6b (quantitative yield), the structure of which was confirmed by single crystal X-ray diffraction (Figure S1, Supporting Information (SI)) [54,55,56,57]. Protection of the free hydroxyl group of hydroxyphthalimide 5d as the OBn and OMe ethers 5e and 5f was achieved using standard procedures in excellent yields. Benzylation of 3a using thionyl chloride/benzyl alcohol gave benzyl ester 7 in up to 60% yield (Scheme 1) and conversion to anilide 7b and 8-amidoquinoline 7c using the appropriate amine was similarly possible.
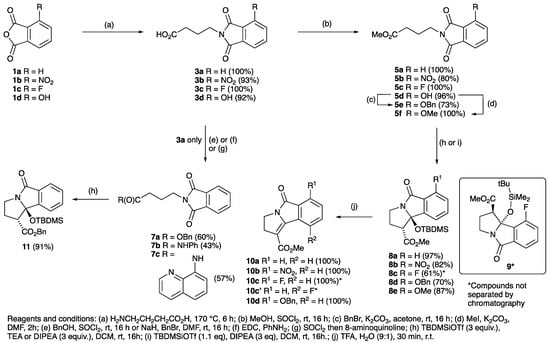
Scheme 1.
Synthesis and ring closure of substituted phthalimides.
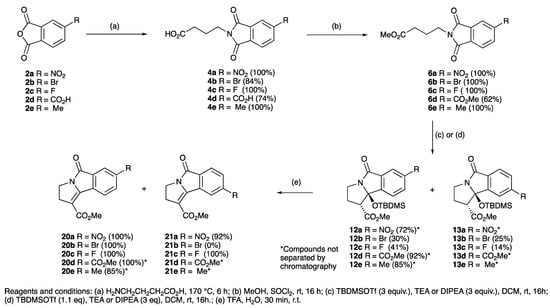
Scheme 2.
Synthesis and ring closure of substituted phthalimides.
With the required phthalimides in hand, 5a was treated with N,N-diisopropylethylamine (DIPEA) and t-butyldimethylsilyl triflate (TBDMSOTf) according to a modification of the literature’s procedure [34], and purification using flash column chromatography afforded the silyl containing tricyclic pyrrolizidinone 8a with a good yield of 97% (Scheme 1 and Table 1). This material was readily characterised by standard spectroscopic techniques; of interest were the non-equivalent silyl dimethyl groups that had shifted upfield to −0.08 and −0.51 ppm due to the anisotropy of the adjacent aromatic ring, consistent with ring closure. The stereochemistry was confirmed by a combination of one and single crystal X-ray diffraction (Figure S1, ESI) [54,55,56,57]; the trans-relationship of the methyl ester and silyloxy moiety were evident, placing the methyl ester into a pseudoaxial position, and with one of the silyl methyl groups located over the aromatic ring, accounting for the shielding observed in the NMR spectrum. While the structure of 8a was further confirmed by LRMS and HRMS, with the major mass ions being 362 [MH+] and 384 [MNa+] as expected, importantly these signals were accompanied by a mass ion of 132 less than the desired product at 230 [MH+]; this was consistent with in situ desilyloxylation giving 10a. In fact, the cyclisation of 5a was found to be unreliable, instead often giving 10a directly and quantitively by in situ elimination. Synthesis of similar tetrahydro-1H-pyrrolo[2,1-a]isoindoles [58,59,60] and their unsaturated systems [61,62,63] has been reported. In order to understand the progress of this reaction, varying equivalents of TBDMSOTf were used with phthalimides 5a,b and it was found that while the formation of the products 8a,b was achievable in high yields with 1.1 equivalents of TBDMSOTf, nearly quantitative direct conversion of 8a to unsaturated pyrrolizidinones 10a,b could be achieved using 2.0 equivalents of TBDMSOTf (Table S1, ESI).
Application of these cyclisation conditions to phthalimides 5b–f successfully gave cyclised products 8b–e in good to excellent yield (Scheme 1 and Scheme 2 and Table 1). TLC and 1H NMR spectroscopic analysis indicated formation of only a single regioisomer, except for 5c which gave an isomeric mixture of 8c also containing 9 (ratio 7:1). Structural assignment was confirmed in the case of 8b,c, 9 and 12a by single crystal X-ray diffraction (Figure S1, ESI) [54,55,56,57]. The mode of cyclisation appeared to be dictated by the sterically bulky substituents on the aromatic ring, but in the case of 5c was biased by both the small size and electronegativity of the fluorine substituent which also gave the alternative isomer 9. Substituted phthalimides 6a–e were subjected to the same ring-closing conditions, giving good to excellent yields of products 12a–e and 13a–e (Scheme 1 and Scheme 2, and Table 1), usually as an approximately equal mixture of isomers, which proved to be difficult to separate by flash column chromatography, and arising by ring closure onto either phthalimide carbonyl group. While the cyclisations using methyl esters were very high yielding and reliable reactions, of interest was whether reactions of substrates with bulkier esters would be as effective; benzyl ester variant 7a in fact cyclised to 11 with an excellent yield of 91% using the standard conditions (1.1 eq TBDMSOTf, 3 eq DIPEA) fully diastereoselectively, as the trans- isomer (Scheme 1), although both anilide 7b and quinoline 7c did not.
The solventless phthalimide synthesis proved to be very effective with phthalic anhydride and glutamic acid, giving the desired diacid product 14a in 60% yield (Scheme 3) [64,65,66]. However, this material was not easily soluble, but L-glutamic acid 5-methyl ester along with phthalic anhydride gave the desired and much more soluble product 14b in 72% yield after heating at 175 °C for 6 h; this reaction remained effective on a multigram scale. In addition, L-glutamic dimethyl ester hydrochloride and phthalic anhydride under the same conditions gave the product 14c in a yield of 30%; the same product could be prepared by esterification of diacid 14a and with a similar yield (38%). This approach was similarly suitable for L-glutamic acid 5-benzyl ester, which gave the product 14d with a yield of 38% [67,68]. The most effective method for the monomethyl esterification of benzyl glutamate 14e used MeI, Cs2CO3, DMF, which gave the desired product 14f in 40% yield. This approach could also be used for imide formation with phthalic anhydride and 2-aminophenylacetic acid via solventless conditions to give 15a, followed by esterification which gave the desired ester 15b with a yield of 38% (Scheme 4); however, a better alternative proved to be direct condensation of the methyl ester of aminophenylacetic acid to give 15b and in quantitative yield. Nitrile 18, was also readily available, prepared as shown (Scheme 4).
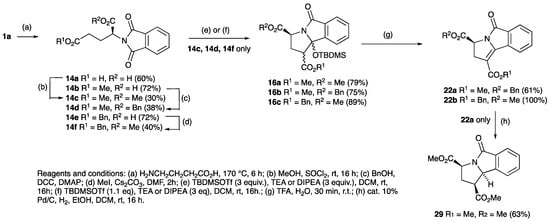
Scheme 3.
Synthesis and ring closure of glutamyl substituted phthalimides.
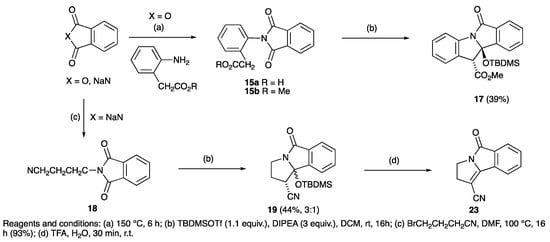
Scheme 4.
Synthesis and ring closure of substituted phthalimides.
Cyclisation of these analogues was examined using the conditions optimised above. The L-dimethyl ester glutamic acid appended phthalimide 14c cyclised in excellent yield of 79% to give 16a as a diastereomeric mixture (Scheme 3); one of these was successfully crystallised and the structure for the major one determined by single crystal X-ray diffraction (Figure S1, ESI). This clearly shows that the two methyl esters are cis-related, with all substituents in a pseudoaxial-like arrangement [54,55,56,57]. The cyclisation of 14d under the same conditions gave a single diastereomer of 16b, most likely due to the greater steric hindrance of the two substituents, the structure of which was confirmed by single crystal X-ray diffraction (Figure S1, ESI) [54,55,56,57]. Benzyl glutamate 14f was subjected to TBDMSOTf mediated cyclisations, but gave poor yields of 16c of around 35% when using 1.1 eq of TBDMSOTf, although this improved to much higher yields (89%) with 3.0 eq of TBDMSOTf, as a mixture of inseparable diastereomers (d.r of 1:0.6), the major of which was assumed to have the same stereochemistry as 16a, based on comparison to established NMR spectroscopic data. When compound 15b (Scheme 4) was subjected to standard cyclisation conditions, product 17 was successfully obtained as a single stereoisomer in 39% yield. Of interest is that nitrile 18 also readily cyclised to the analogous product 19 as a mixture of diastereomers; a related material has previously been reported by photocyclisation [69].
Since TFA elimination reactions had been previously reported on silyloxyethers [60,70,71,72,73], similar reactions were then carried on 8a–d and 12a–e, 13a–e, giving quantitative conversions to the eliminated products 10a–d, 20a–e, 21a–e, 22a–b (Scheme 1, Scheme 2, Scheme 3 and Scheme 4). When the diastereomeric mixture of 19 was stirred in TFA/H2O (9:1) for 30 min, only the trans- isomer reacted, leaving the cis-isomer unconverted, consistent with a fast antiperiplanar elimination; the structure of the unsaturated product 23 was confirmed by single crystal X-ray diffraction (Scheme 4 and Figure S2, (SI)) [54,55,56,57]. Moreover, it was found that the eliminated cyclised products could also be obtained directly by using TBDMSOTf (2 equiv.) for the ring closure reaction of both phthalimides 5a,b,c,e and 14c,f adducts and in excellent yield (Scheme 5).
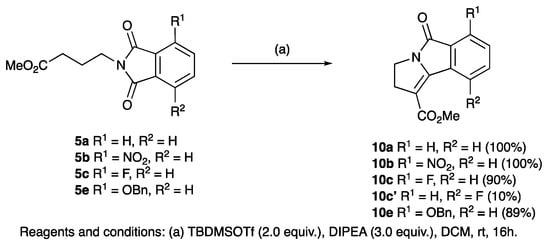
Scheme 5.
One-pot ring closure and elimination of substituted phthalimides.
Hoye described the mechanism of this ring-closing process as an intramolecular Mukaiyama-like addition in which formation of a silyl ketene acetal is followed by addition to one of the imide carbonyls via in situ silyl activation [45], and Christmann proposed the intermediacy of a bis-silylketene acetal formed in situ from the starting carboxylic acid [46]. Although the Mukaiyama aldol addition [74] is very well known [75,76,77,78,79,80], Mukaiyama-type additions to imides are not; however, a one-pot approach, in which silyl ketene acetals are intermediates, has been described for addition to imines [81]. We propose a similar mechanism for a Mukaiyama-imide aldol addition involving the formation of the silyl ketene acetal followed either by coordination of the imide carbonyl giving a 5,6-bicyclic transition state that undergoes aldol addition (Route A), or cyclisation involving separate imide activation by a second molecule of TBDMSOTf (Route B) (Figure 1).
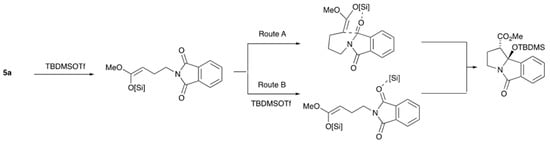
Figure 1.
Possible mechanism for aldol ring closure.
With the pyrrolidinones in hand, of interest was an examination of their further reactivity; it was expected that this might not be straightforward, since low solubility was found for many compounds, especially the planar derivatives such as 10, 20 and 21. Additionally, the high level of cross-conjugation along with significant steric effects in these densely functionalised systems was expected to significantly modify their chemical behaviour. Functionalisation of the (electron deficient) carbon-carbon double bond of the unsaturated system of cyclised adducts, for which there was some precedent literature [82], was examined [83] using 35% aqueous hydrogen peroxide in the presence of 4-methyl morpholine. The unsubstituted variant 10a proved to be unreactive under these conditions, although when dissolved in dichloromethane with mCPBA and left stirring for 16 h at room temperature, α-ketoester 24 was obtained in low yield (Scheme 6). Such a product would be expected to arise by initial epoxidation of the double bond, followed by a further attack by mCPBA leading to a ring opening. However, it was found that if this reaction was conducted in the presence of calcium carbonate, successful epoxidation was achieved, giving 25. This approach proved not to be successful for 10b, since 1.2 equivalents of mCPBA gave not the expected epoxide but adduct 26 (Scheme 6), whose structure was confirmed by careful NMR spectroscopic analysis. Catalytic hydrogenation gave highly efficient conversion of lactams 10a–d, 20a–e, 21a–e to lactams 27a–j, in a reaction in which the strong yellow colour of the starting material was fully discharged, consistent with the removal of the extended conjugation (Table 2 and Scheme 7). In the case of the nitro derivatives 10b, 20a, and 21a, concomitant reduction to the amine derivatives 27b–d occurred. The structures of 27b and 27f were confirmed by single crystal X-ray diffraction (Figure S2, ESI) [54,55,56,57]. Reduction of 10d also involved hydrogenolysis and afforded phenol 27h. Glutamate derivatives 22a,b were subjected to the same hydrogenation conditions and gave cis-dimethyl esters 29a,b (Scheme 3), whose stereochemistry was shown from nOe analysis.
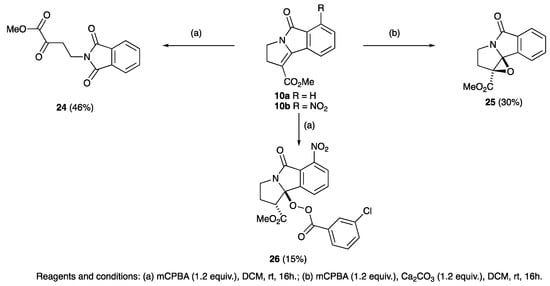
Scheme 6.
Elaboration of 2,3-dihydro-5H-pyrrolo[2,1-a]isoindol-5-ones.

Table 2.
Reduction of unsaturated bicyclic lactams 10a–d, 20a–e, 21a–e.
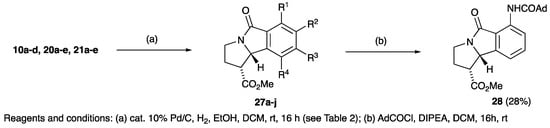
Scheme 7.
Reduction of 2,3-dihydro-5H-pyrrolo[2,1-a]isoindol-5-ones.
With 27b in hand, conversion to corresponding amide 28 using 1-adamantanecarbonyl chloride (Scheme 8) was made, as this group had given some of the highest levels of antibacterial activity seen for tetramates [19]; although this reaction proceeded successfully, the yield was low (28%), and this most likely arose by the combination of an electronically and sterically deactivated amine with a hindered acid chloride.
Scheme 8.
Elaboration of lactam systems.
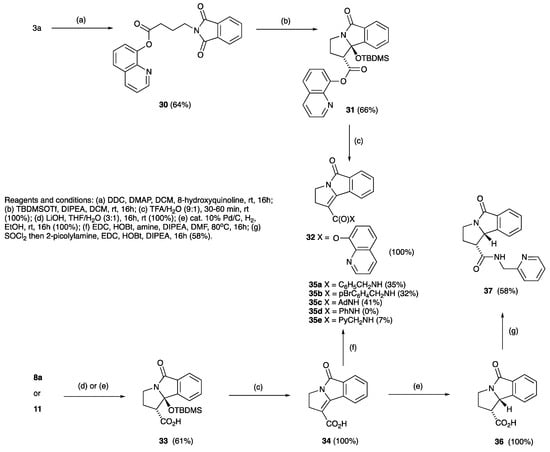
Of interest was whether this approach might be able to be adjusted to allow the ready introduction of ring substituents on the core skeleton, including C-H functionalisation, since related systems had been shown to be amenable to such manipulation [84]. Since the use of 8-aminoquinoline as a directing group for C-H activation is now well-known [85,86,87], 8-hydroxyquinoline ester 30 was prepared via N,N’-dicyclohexylcarbodiimide coupling with 8-hydroxyquinoline (64%), and although this could be effectively cyclised to 31 under standard conditions, in subsequent reactions 31 did not undergo remote C-H arylation. However, 31 when treated with TFA:H2O afforded the desired unsaturated system 32 quantitatively (Scheme 8). While ester hydrolysis of 8a and 11 was found to be straightforward, giving acids 33 and 34, the attempted DCC/DMAP coupling of 33 with 8-aminoquinoline proved to be unsuccessful, giving only the rearranged N-acylurea intermediate; 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) along with 1-hydroxybenzotriazole (HOBt) gave a similar outcome. It was also found that 10a could be hydrolysed directly under basic conditions in excellent yield to give 33 directly, and that this, when treated with TFA, water, and methanol, gave acid 34 in quantitative yield (Scheme 8). With 34 in hand, amide formation was examined (Scheme 8) but the products 35a–c could be obtained only in modest yield, and aniline was completely unreactive. This likely reflects the unusual electronic character of the extended conjugated push-pull system in the starting material. Alternatively, 36 could be obtained by direct reduction of acid 34 or by hydrolysis of 27a in good yield (Scheme 8) and conversion to the picolyl amide 37 under a variety of conditions gave a modest yield of product.
3. Bioassays
The compounds were tested using a primary 96-well plate screening assay against MRSA (Gram+) and E. coli (Gram−) bacterial strains and MIC values and along with the calculated molecular weights, ClogP, tPSA along with HBD and HBA (Table S2, ESI). The only systems showing activity were 12e/13e, 31, and 32. This probably reflects the high level of hydrophobicity of the silyloxy ethers and the particularly low solubility of the unsaturated systems, even though their cheminformatic descriptors are broadly desirable. This outcome suggests that some fragments might be suitable for further elaboration to identify better antibacterial activity. Some compounds were also tested for cytotoxic activity against four different cell lines: HeLa, HEK 293, CaCo, and MDCK (Table S3 (SI)). Nearly all the compounds that were tested showed some weak activity, but 35c was found to be moderately active against HeLa and HEK 293 with lesser activity against CaCo and MDCK. Once again, the low solubility of these compounds under assay conditions is likely to be an important limitation of this compound class.
4. Materials and Methods
Full experimental details are provided in the Supplementary Materials File S1.
5. Conclusions
We have shown that phthalimides may be effectively cyclized using a Mukaiyama-type aldol coupling leading to variously substituted fused lactam (1,2,3,9b-tetrahydro-5H-pyrrolo[2,1-a]isoindol-5-one) systems. This novel process shows a high level of regioselectivity for o-substituted phthalimides, dictated by steric and electronic factors, but not for m-substituted phthalimides. The initial aldol adduct is prone to elimination, and the cyclisation can be conducted in such a way that aldol cyclisation-elimination is achievable in one pot. The eliminated skeletal systems (2,3-dihydro-5H-pyrrolo[2,1-a]isoindol-5-one) possess cross-conjugation and steric effects which significantly influence the reactivity of several functional groups, but conditions suitable for epoxidation, ester hydrolysis and amide formation, and reduction, were identified. Many of the derived lactam systems, and especially the eliminated systems, show low solubility, which compromises biological activity, although in some cases, antibacterial and cytotoxic activity was found and this new class of small molecule provides a useful skeleton for further elaboration and study. We have earlier shown that a core bicyclic tetramate displays no intrinsic antibacterial activity [26], but that this can be restored after appropriate heterocyclic ring substitution [19]. The work herein shows that the core tetrahydro-5H-pyrrolo[2,1-a]isoindol-5-one system is now synthetically readily available, and further investigation is needed to develop the understanding of both its medicinal chemistry and biological activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics12010009/s1. File S1: Supporting Information (SI). References [88,89,90,91,92,93,94,95,96] occur only in the supplementary materials.
Author Contributions
Conceptualization, M.G.M. and L.T.I.; methodology K.E.C., M.G., A.P., D.P., M.G.M. and L.T.I.; formal analysis, K.E.C., M.G., A.P., D.P., M.G.M. and L.T.I.; writing—original draft preparation, M.G.M.; writing—review and editing, K.E.C., M.G.M. and L.T.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Acknowledgments
We are grateful for the award of beamtime to the Block Allocation Group award (MT20876) used to collect the Single Crystal Synchrotron X-ray diffraction data on the I19 beamline at Diamond Light Source.
Conflicts of Interest
The authors declare no conflict of interest.
References and Notes
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D. 10 × ’20 Progress—Development of new drugs active against gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Cooper, M.A. Antibiotics in the clinical pipeline in 2011. J. Antibiot. 2011, 64, 413–425. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- Singh, S.B. Confronting the challenges of discovery of novel antibacterial agents. Biorg. Med. Chem. Lett. 2014, 24, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Dick, T.; Young, D. How antibacterials really work: Impact on drug discovery. Future Microbiol. 2011, 6, 603–604. [Google Scholar] [CrossRef]
- Gwynn, M.N.; Portnoy, A.; Rittenhouse, S.F.; Payne, D.J. Challenges of antibacterial discovery revisited. Ann. N. Y. Acad. Sci. 2010, 1213, 5–19. [Google Scholar] [CrossRef]
- Walsh, C.T.; Wencewicz, T.A. Prospects for new antibiotics: A molecule-centered perspective. J. Antibiot. 2014, 67, 7–22. [Google Scholar] [CrossRef]
- Wencewicz, T.A. New antibiotics from Nature’s chemical inventory. Bioorg. Med. Chem. Lett. 2016, 24, 6227–6252. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.P.; Chang, Y.-T. Chemical genetics. Chem. Rev. 2006, 106, 2476–2530. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.; Mossialos, E. Stoking the antibiotic pipeline. Br. Med. J. 2010, 340, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- So, A.D.; Gupta, N.; Cars, O. Tackling antibiotic resistance. Br. Med. J. 2010, 340, 1091–1092. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Waldmann, H. Protein structure similarity clustering and natural product structure as guiding principles in drug discovery. Drug Discov. Today 2005, 10, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Dekker, F.J.; Waldmann, H. Design of compound libraries based on natural product scaffolds and protein structure similarity clustering (PSSC). Mol. BioSyst. 2005, 1, 36–45. [Google Scholar] [CrossRef]
- Danishefsky, S. On the potential of natural products in the discovery of pharma leads: A case for reassessment. Nat. Prod. Rep. 2010, 27, 1114–1116. [Google Scholar] [CrossRef]
- Khan, M.K.; Wang, D.; Moloney, M.G. Functionalised Nitrogen Heterocycles and the Search for New Antibacterials and Bioactives. Synthesis 2020, 52, 1602–1616. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Goetz, M.A.; Dombrowski, A.W.; Polishook, J.D.; Hazuda, D.J. Equisetin and a novel opposite stereochemical homolog phomasetin, two fungal metabolites as inhibitors of HIV-1 integrase. Tetrahedron Lett. 1998, 39, 2243–2246. [Google Scholar] [CrossRef]
- Ganzle, M.G. Reutericyclin: Biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol. 2004, 64, 326–332. [Google Scholar] [CrossRef]
- Phillips, J.W.; Goetz, M.; Smith, S.; Zink, D.; Polishook, J.; Onishi, R.; Salowe, S.; Wiltsie, J.; Allocco, J.; Sigmund, J.; et al. Discovery of kibdelomycin, a potent new class of bacterial type II topoisomerase inhibitor by chemical-genetic profiling in Staphylococcus aureus. Chem. Biol. 2011, 18, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Tuske, S.; Sarafianos, S.G.; Wang, X.; Hudson, B.; Sineva, E.; Mukhopadhyay, J.; Birktoft, J.J.; Leroy, O.; Ismail, S.; Clark, A.D.; et al. Inhibition of Bacterial RNA Polymerase by Streptolydigin: Stabilization of a Straight-Bridge-Helix Active-Center Conformation. Cell 2005, 122, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.G.; Trippier, P.C.; Yaqoob, M.; Wang, Z. The Oxazolomycins: A Structurally Novel Class of Bioactive Compounds. Curr. Drug Discov. Technol. 2004, 1, 181–199. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Helms, G.L.; Bolessa, E.A.; Wilson, K.E.; Giacobbe, R.A.; Tkacz, J.S.; Bills, G.F.; Liesch, J.M.; Zink, D.L.; Curotto, J.E.; et al. Pramanicin, a novel antimicrobial agent from a fungal fermentation. Tetrahedron 1994, 50, 1675–1686. [Google Scholar] [CrossRef]
- Jeong, Y.-C.; Moloney, M.G. Tetramic Acids as Scaffolds: Synthesis, Tautomeric and Antibacterial Behaviour. Synlett 2009, 2487–2491. [Google Scholar] [CrossRef]
- Nogawa, T.; Kawatani, M.; Uramoto, M.; Okano, A.; Aono, H.; Futamura, Y.; Koshino, H.; Takahashi, S.; Osada, H. Pyrrolizilactone, a new pyrrolizidinone metabolite produced by a fungus. J. Antibiot. 2013, 66, 621–623. [Google Scholar] [CrossRef]
- Nakai, R.; Ishida, H.; Asai, A.; Ogawa, H.; Yamamoto, Y.; Kawasaki, H.; Akinaga, S.; Mizukami, T.; Yamashita, Y. Telomerase inhibitors identified by a forward chemical genetics approach using a yeast strain with shortened telomere length. Chem. Biol. 2006, 13, 183–190. [Google Scholar] [CrossRef]
- Agatsuma, T.; Akama, T.; Nara, S. Matsumiya, S.; Nakai, R.; Ogawa, H.; Otaki, S.; Ikeda, S.-I.; Saitoh, Y.; Kanda, Y. UCS1025A and B, new antitumor antibiotics from the fungus Acremonium species. Org. Lett. 2002, 4, 4387–4390. [Google Scholar] [CrossRef]
- Nakai, R.; Ogawa, H.; Asai, A.; Ando, K.; Agaisuma, T.; Maisumiya, S.; Akinaga, S.; Yamashita, Y.; Mizukami, T. UCS1025A, a Novel Antibiotic Produced by Acremonium sp. J. Antibiot. 2000, 53, 294–296. [Google Scholar] [CrossRef]
- Sugie, Y.; Hirai, H.; Kachi-Tonai, H.; Kim, Y.-J.; Kojima, Y.; Shiomi, Y.; Sugiura, A.; Suzuki, Y.; Yoshikawa, N.; Brennan, L.; et al. New Pyrrolizidinone Antibiotics CJ-16, 264 and CJ-16, 367. J. Antibiot. 2001, 54, 917–925. [Google Scholar] [CrossRef]
- Li, L.; Tang, M.C.; Tang, S.; Gao, S.; Soliman, S.; Hang, L.; Xu, W.; Ye, T.; Watanabe, K.; Tang, Y. Genome Mining and Assembly-Line Biosynthesis of the UCS1025A Pyrrolizidinone Family of Fungal Alkaloids. J. Am. Chem. Soc. 2018, 140, 2067–2071. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, R.M.; Fröhlich, R.; Christmann, M. Efficient Synthesis and Resolution of Pyrrolizidines. Angew. Chem. Int. Ed. 2007, 46, 2883–2886. [Google Scholar] [CrossRef] [PubMed]
- Lambert, T.H.; Danishefsky, S.J. Total Synthesis of UCS1025A. J. Am. Chem. Soc. 2006, 128, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Dvornikovs, V. Comparative Diels−Alder Reactivities within a Family of Valence Bond Isomers: A Biomimetic Total Synthesis of (±)-UCS1025A. J. Am. Chem. Soc. 2006, 128, 2550–2551. [Google Scholar] [CrossRef]
- Nozaki, K.; Oshima, K.; Utimoto, K. Trialkylborane as an initiator and terminator of free radical reactions. Facile routes to boron enolates via α-carbonyl radicals and aldol reaction of boron enolates. Bull. Chem. Soc. Jpn. 1991, 64, 403–409. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pulukuri, K.K.; Rigol, S.; Buchman, M.; Shah, A.A.; Cen, N.; McCurry, M.D.; Beabout, K.; Shamoo, Y. Enantioselective Total Synthesis of Antibiotic CJ-16,264, Synthesis and Biological Evaluation of Designed Analogues, and Discovery of Highly Potent and Simpler Antibacterial Agents. J. Am. Chem. Soc. 2017, 139, 15868–15877. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Rigol, S.; Yu, R. Total Synthesis Endeavors and Their Contributions to Science and Society: A Personal Account. CCS Chem. 2019, 1, 3–37. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Rigol, S. A brief history of antibiotics and select advances in their synthesis. J. Antibiot. 2017, 71, 153–184. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Shah, A.A.; Korman, H.; Khan, T.; Shi, L.; Worawalai, W.; Theodorakis, E.A. Total Synthesis and Structural Revision of Antibiotic CJ-16,264. Angew. Chem. Int. Ed. 2015, 54, 9203–9208. [Google Scholar] [CrossRef]
- Lambert, T.H.; Danishefsky, S.J. Synthesis of UCS1025A. Synfacts 2006, 0536. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Shi, L.; Lu, M.; Pattanayak, M.R.; Shah, A.A.; Ioannidou, H.A.; Lamani, M. Total Synthesis of Myceliothermophins C, D, and E. Angew. Chem. Int. Ed. 2014, 126, 11150–11154. [Google Scholar] [CrossRef]
- Martinez, S.T.; Belouezzane, C.; Pinto, A.C.; Glasnov, T. Synthetic strategies towards the azabicyclo 3.3.0-octane core of natural pyrrolizidine alkaloids. an overview. Org. Prep. Proc. Int. 2016, 48, 223–253. [Google Scholar] [CrossRef]
- Uchida, K.; Ogawa, T.; Yasuda, Y.; Mimura, H.; Fujimoto, T.; Fukuyama, T.; Wakimoto, T.; Asakawa, T.; Hamashima, Y.; Kan, T. Stereocontrolled Total Synthesis of (+)-UCS1025A. Chem. Int. Ed. 2012, 51, 12850–12853. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Dvornikovs, V.; Sizova, E. Silylative Dieckmann-Like Cyclizations of Ester-Imides (and Diesters). Org. Lett. 2006, 8, 5191–5194. [Google Scholar] [CrossRef]
- De Figueiredo, R.M.; Oczipka, P.; Fröhlich, R.; Christmann, M. Synthesis of 4-Maleimidobutyric Acid and Related Maleimides. Synthesis 2008, 1316–1318. [Google Scholar] [CrossRef]
- De Figueiredo, R.M.; Voith, M.; Fröhlich, R.; Christmann, M. Synthesis of a Malimide Analogue of the Telomerase Inhibitor UCS1025A Using a Dianionic Aldol Strategy. Synlett 2007, 3, 391–394. [Google Scholar] [CrossRef]
- Ibbotson, L.T.; Christensen, K.E.; Genov, M.; Pretsch, A.; Pretsch, D.; Moloney, M.G. Skeletal Analogues of UCS1025A and B by Cyclization of Maleimides: Synthesis and Biological Activity. Synlett 2022, 33, 396–400. [Google Scholar] [CrossRef]
- Guénin, E.; Monteil, M.; Bouchemal, N.; Prangé, T.; Lecouvey, M. Syntheses of phosphonic esters of alendronate, pamidronate and neridronate. Eur. J. Org. Chem. 2007, 20, 3380–3391. [Google Scholar] [CrossRef]
- Wu, H.; Wu, J.; Zhang, W.; Li, J.; Fang, J.; Lian, X.; Qin, T.; Hao, J.; Zhou, Q.; Wu, S. Discovery and structure-activity relationship study of phthalimide-phenylpyridine conjugate as inhibitor of Wnt pathway. Bioorg. Med. Chem. Lett. 2019, 29, 870–872. [Google Scholar] [CrossRef]
- Dato, F.M.; Sheikh, M.; Uhl, R.Z.; Schüller, A.W.; Steinkrüger, M.; Koch, P.; Neudörfl, J.M.; Gütschow, M.; Goldfuss, B.; Pietsch, M. ω-Phthalimidoalkyl Aryl Ureas as Potent and Selective Inhibitors of Cholesterol Esterase. ChemMedChem 2018, 13, 1833–1847. [Google Scholar] [CrossRef]
- Gabbasov, T.M.; Tsyrlina, E.M.; Spirikhin, L.V.; Yunusov, M.S. Amides of N-Deacetyllappaconitine and Amino Acids. Chem. Nat. Compd. 2018, 54, 951–955. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Henz, A.; Kramer, W.; Lex, J.; Nerowski, F.; Oelgemöller, M.; Peters, K.; Peters, E.M. Synthesis of Medium-and Large-Ring Compounds Initiated by Photochemical Decarboxylation of ω-Phthalimidoalkanoates. Helv. Chim. Acta 1997, 80, 912–933. [Google Scholar] [CrossRef]
- Low temperature single crystal X-ray diffraction data for 6b, 8a, 8b, 8c, 12a, 21, 16b, 23, 27f and 27b were collected using a Rigaku Oxford SuperNova diffractometer and data for 9 were collected at Diamond Light Source, Beamline I19-1. Raw frame data were reduced using CrysAlisPro and the structures were solved using ‘Superflip’ before refinement with CRYSTALS as per the CIF. Full refinement details are given in the Supporting Information (CIF); Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre (CCDC 2160046-56).
- Palatinus, L.; Chapuis, G.J. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Parois, P.; Cooper, R.I.; Thompson, A.L. Crystal structures of increasingly large molecules: Meeting the challenges with CRYSTALS software. Chem. Cent. J. 2015, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.I.; Thompson, A.L.; Watkin, D.J. CRYSTALS enhancements: Dealing with hydrogen atoms in refinement. J. Appl. Cryst. 2010, 43, 1100–1107. [Google Scholar] [CrossRef]
- Yoon, U.C.; Lee, C.W.; Oh, S.W.; Mariano, P.S. Exploratory studies probing the intermediacy of azomethine ylides in the photochemistry of N-phthaloyl derivatives of α-amino acids and β-amino alcohols. Tetrahedron 1999, 55, 11997–12008. [Google Scholar] [CrossRef]
- Takahashi, Y.; Miyashi, T.; Yoon, U.C.; Oh, S.W.; Mancheno, M.; Su, Z.; Falvey, D.F.; Mariano, P.S. Mechanistic Studies of the Azomethine Ylide-Forming Photoreactions of N-(Silylmethyl)phthalimides and N-Phthaloylglycine. J. Am. Chem. Soc. 1999, 121, 3926–3932. [Google Scholar] [CrossRef]
- Yoon, U.C.; Kim, D.U.; Lee, Y.J.; Choi, Y.S.; Lee, Y.-J.; Ammon, H.L.; Mariano, P.S. Novel and efficient azomethine ylide forming photoreactions of N-(silylmethyl) phthalimides and related acid and alcohol derivative. J. Am. Chem. Soc. 1995, 117, 2698–2710. [Google Scholar] [CrossRef]
- Muchowski, J.M.; Nelson, P.H. The reaction of carboalkyxyclopropyltriphenylphosphonium salts with imide anions: A three-step synthesis of ±isoretronecanol. Tetrahedron Lett. 1980, 21, 4585–4588. [Google Scholar] [CrossRef]
- Fuchs, P.L. Carboethoxycyclopropyltriphenylphosphonium fluoroborate. Reagent for the facile cycloalkenylation of carbonyl groups J. Am. Chem. Soc. 1974, 96, 1607–1609. [Google Scholar]
- Maury, J.; Mouysset, D.; Feray, L.; Marque, S.R.A.; Siri, D.; Bertrand, M.P. Aminomethylation of Michael Acceptors: Complementary Radical and Polar Approaches Mediated by Dialkylzincs. Chem.—Eur. J. 2012, 18, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Border, S.E.; Pavlović, R.Z.; Lei, Z.; Gunther, M.J.; Wang, H.; Cui, H.; Badjić, J.D. Light Triggered Transformation of Molecular Baskets into Organic Nanoparticles. Chem.—Eur. J. 2019, 25, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Jamel, N.M.; Alheety, K.A.; Ahmed, B.J. Methods of Synthesis Phthalimide Derivatives and Biological Activity—Review. J. Pharm. Sci. Res. 2019, 11, 3348–3354. [Google Scholar]
- Wang, W.; Ding, J.; Xiao, C.; Tang, Z.; Li, D.; Chen, J.; Zhuang, X.; Chen, X. Synthesis of amphiphilic alternating polyesters with oligo (ethylene glycol) side chains and potential use for sustained release drug delivery. Biomacromolecules 2011, 12, 2466–2474. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Goel, O.P.; Magano, J.; Rubin, J.R.; Company, W.-L.; Arbor, A. An efficient stereoselective synthesis of [3S(1S,9S)]-3-[[[9-(benzoylamino)octahydro-6,10-dioxo-6H-pyridazino-(1,2-a)(1,2)-diazepin-1-yl]-carbonyl]amino]-4-oxobutanoic acid, an interleukin converting enzyme (ICE) inhibitor. Bioorg. Med. Chem. Lett. 1999, 9, 1587–1592. [Google Scholar] [CrossRef]
- King, F.E.; Clark-Lewis, J.W.; Wade, R.; Swindin, W.A. Syntheses from phthalimido-acids. Part VII. Oxazolones and other intermediates in the synthesis of phthalylpeptides, and an investigation on maleic acid. J. Chem. Soc. 1957, 166, 873–880. [Google Scholar] [CrossRef]
- McKay, A.F.; Garmaise, D.L.; Gaudry, R.; Baker, H.A.; Paris, G.Y.; Kay, R.W.; Just, G.E.; Schwartz, R. Bacteriostats. II.1 The Chemical and Bacteriostatic Properties of Isothiocyanates and their Derivatives. J. Am. Chem. Soc. 1959, 81, 4328–4335. [Google Scholar] [CrossRef]
- Schlessinger, R.H.; Poss, M.A.; Richardson, S. Total synthesis of (+)-rosaramicin aglycone and its diacetate. J. Am. Chem. Soc. 1986, 108, 3112–3114. [Google Scholar] [CrossRef]
- Baker, R.; Cummings, W.J.; Hayes, J.F.; Kumar, A. Enantiospecific synthesis of the C-9 to C-18 fragment of macbecins I and II. J. Chem. Soc. Chem. Commun. 1986, 1, 1237–1239. [Google Scholar] [CrossRef]
- Robins, M.J.; Samano, V.; Johnson, M.D. Nucleic acid-related compounds. 58. Periodinane oxidation, selective primary deprotection, and remarkably stereoselective reduction of tert-butyldimethylsilyl-protected ribonucleosides. Synthesis of 9-(.beta.-D-xylofuranosyl)adenine or 3’-deuterioadenosine from adenosine. J. Org. Chem. 1990, 55, 410–412. [Google Scholar] [CrossRef]
- Martin, S.F.; Dodge, J.A.; Burgess, L.E.; Hartmann, M. A formal total synthesis of (+)-macbecin I. J. Org. Chem. 1992, 57, 1070–1072. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Kobayashi, S. Tin(II) Enolates in the Aldol, Michael, and Related Reactions. Org. React. 1994, 46, 1–103. [Google Scholar]
- Banno, K. New Cross Aldol Reactions. Titanium Tetrachloride-promoted Reactions of Silyl Enol Ethers with Carbonyl Compounds Containing A Functional Group. Bull. Chem. Soc. Jpn. 1976, 49, 2284–2291. [Google Scholar] [CrossRef]
- Kalita, H.R.; Borah, A.J.; Phukan, P. Efficient allylation of aldehydes with allyltributylstannane catalyzed by CuI. Tetrahedron Lett. 2007, 48, 5047–5049. [Google Scholar] [CrossRef]
- Downey, C.W.; Johnson, M.W. A tandem enol silane formation-Mukaiyama aldol reaction mediated by TMSOTf. Tetrahedron Lett. 2007, 48, 3559–3562. [Google Scholar] [CrossRef]
- Mahrwald, R. Diastereoselection in Lewis-acid-mediated aldol additions. Chem. Rev. 1999, 99, 1095–1120. [Google Scholar] [CrossRef] [PubMed]
- Phukan, P. Mukaiyama aldol reactions of silyl enolates catalyzed by iodine. Syn. Commun. 2004, 34, 1065–1070. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, S.B.; Mukaiyama, T. A New Catalyst System for the Aldol Type Condensation of Silyl Enol Ethers and Ketene Silyl Acetals. Bull. Korean Chem. Soc. 1994, 15, 529–531. [Google Scholar] [CrossRef]
- Wade Downey, C.; Ingersoll, J.A.; Glist, H.M.; Dombrowski, C.M.; Barnett, A.T. One-Pot Silyl Ketene Acetal-Formation Mukaiyama–Mannich Additions to Imines Mediated by Trimethylsilyl Trifluoromethanesulfonate. Eur. J. Org. Chem. 2015, 2015, 7287–7291. [Google Scholar] [CrossRef]
- Cottrell, I.F.; Davis, P.J.; Moloney, M.G. Stereoselective oxygenation of bicyclic lactams. Tetrahedron Asymmetry 2004, 15, 1239–1242. [Google Scholar] [CrossRef]
- Tan, S.W.B.; Chai, C.L.L.; Moloney, M.G. Mimics of pramanicin derived from pyroglutamic acid and their antibacterial activity. Org. Biomol. Chem. 2017, 15, 1889–1912. [Google Scholar] [CrossRef] [PubMed]
- Verho, O.; Maetani, M.; Melillo, B.; Zoller, J.; Schreiber, S.L. Stereospecific palladium-catalyzed C–H arylation of pyroglutamic acid derivatives at the C3 position enabled by 8-aminoquinoline as a directing group. Org. Lett. 2017, 19, 4424–4427. [Google Scholar] [CrossRef]
- Rej, S.; Ano, Y.; Chatani, N. Bidentate directing groups: An efficient tool in C–H bond functionalization chemistry for the expedient construction of C–C bonds. Chem. Rev. 2020, 120, 1788–1887. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Wang, Z.; Zeng, T.; Liu, P.; Engle, K.M. Catalytic intermolecular carboamination of unactivated alkenes via directed aminopalladation. J. Am. Chem. Soc. 2017, 139, 11261–11270. [Google Scholar] [CrossRef] [PubMed]
- Corbet, M.; De Campo, F. 8-Aminoquinoline: A Powerful Directing Group in Metal-Catalyzed Direct Functionalization of C-H Bonds. Angew. Chem.—Int. Ed. 2013, 52, 9896–9898. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.S.; Park, G.; Yu, Y.; Pohl, N.L. Protecting-Group-Based Colorimetric Monitoring of Fluorous-Phase and Solid-Phase Synthesis of Oligoglucosamines. Org. Lett. 2008, 10, 5381–5384. [Google Scholar] [CrossRef] [PubMed]
- Caswell, L.R.; Yang, K.C.C. Nitrophthaloyl and aminophthaloyl derivatives of amino acids. J. Chem. Eng. Data 1968, 13, 291–292. [Google Scholar] [CrossRef]
- Staubli, A.; Ron, E.; Langer, R. Hydrolytically degradable amino acid-containing polymers. J. Am. Chem. Soc. 1990, 112, 4419–4424. [Google Scholar] [CrossRef]
- Richards, J.C.; Spenser, I.D. The stereochemistry of the enzymic decarboxylation of L-arginine and of L-ornithine. Can. J. Chem. 1982, 60, 2810–2820. [Google Scholar] [CrossRef]
- Tomar, R.; Bhattacharya, D.; Babu, S.A. Assembling of medium/long chain-based β-arylated unnatural amino acid derivatives via the Pd(II)-catalyzed sp3 β-C-H arylation and a short route for rolipram-type derivatives. Tetrahedron 2019, 75, 2447–2465. [Google Scholar] [CrossRef]
- Vatulina, G.G.; Tuzhilkova, T.N.; Matveeva, T.V.; Krasnov, V.P.; Burde, N.L.; Alekseeva, L.V. Search for radioprotectors in the series of glutamic acid derivatives. Chem. Pharm. J. 1986, 20, 647–653. [Google Scholar] [CrossRef]
- Robl, J.A. Peptidomimetic synthesis: Utilization of N-acyliminium ion cyclization chemistry in the generation of 7, 6-and 7, 5-fused bicyclic lactams. Tetrahedron Lett. 1994, 35, 393–396. [Google Scholar] [CrossRef]
- Fife, T.H.; Duddy, N.W. Intramolecular aminolysis of esters. Cyclization of esters of (o-aminophenyl) acetic acid. J. Am. Chem. Soc. 1983, 105, 74–79. [Google Scholar] [CrossRef]
- Kim, G.; Keum, G. A new route to quinolone and indole skeletons via ketone-and ester-imide cyclodehydration reactions. Heterocycles 1997, 45, 1979–1988. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).