Multi-Criteria Decision Analysis for Assessing Social Acceptance of Strategies to Reduce Antimicrobial Use in the French Dairy Industry
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Study Design
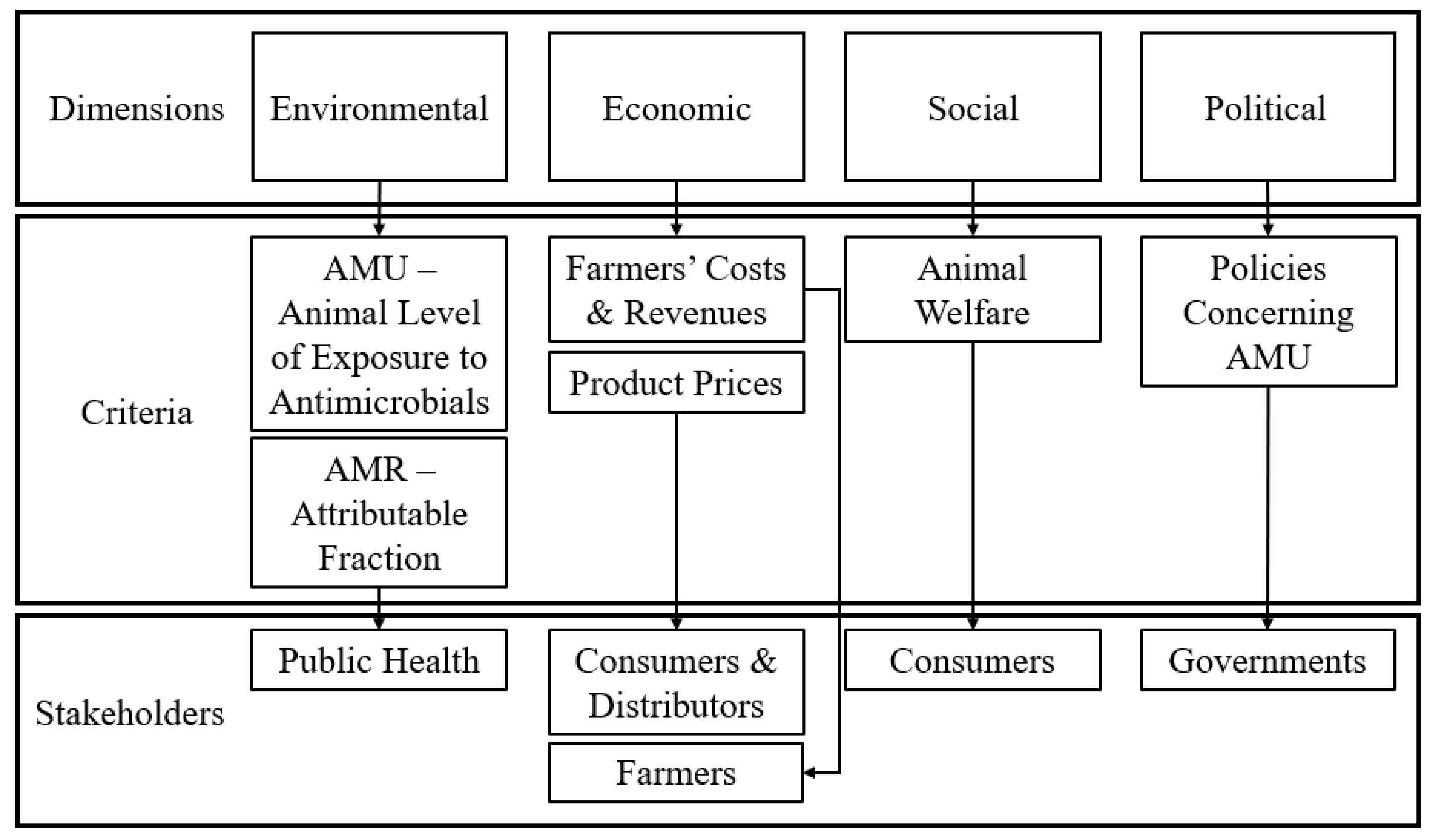
4.2. Development and Assessment of Criteria
4.3. Definitions of Strategies against AMR
4.4. PROMETHEE Implementation
4.4.1. Problem Definition and Identification of Stakeholders
4.4.2. Identification of Key Decision Issues and Definition of Criteria
4.4.3. Weighing Criteria and Criteria Group Ranking
4.4.4. MCDA
4.4.5. Interpretation of Results
4.4.6. Sensitivity Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Current scenario of antibiotic use in the dairy industry (STRA01).
- Total interdiction of antimicrobial use (STRA02).
- Interdiction of preventive and metaphylactic antimicrobial use (STRA03).
- Subsidies for farmers committing to reduce the use of antibiotics by 25% (STRA04).
- -
- First, allocate 100 points among the 4 dimensions (Environmental, Economic, Social, and Political) according to the degree of importance of this dimension for the class of stakeholders you represent (in your case: Public Health—construction of public policy). In the table, fill in the total row of each dimension.
- -
- Second, for each dimension, distribute the total number of points allocated to all criteria that make up this dimension. Thus, each criterion will have a score. In the table, fill in the blank cells of the score columns.
- -
- Repeat the process for each strategy.
- -
- Strategy score must per add up to 100 points.
- Cost of production of food of animal origin
- Farmer income
- Cull cow price
- Milk price
- ALEA: indicator of the level of exposure of animals to antibiotics (mass of the treated population divided by the mass of the total population of the animal species). The higher the ALEA, the more animals in a population are treated with antimicrobials.
- Attributable fraction: antibiotic resistance in humans that is attributed to the use of antibiotics in agriculture. The higher this fraction is, the greater is the impact of antimicrobial use in animal farming on public health.
- Mortality rate: number of dairy cows that died in a given period.
- Cull rate: number of cows unfit for calf and/or milk production, due to aging or other criteria, and now fit for fattening and/or slaughter.
- Regulatory framework: public policies related to antibiotic resistance. This frame can take 4 values: weak, moderate, strong, very strong.
- Investments in public policies needed to reduce antibiotic use. Investments can take 4 values: weak, moderate, strong, very strong.
| Dimension | Criteria | STRA01 | Score | STRA02 | Score | STRA03 | Score | STRA04 | Score |
|---|---|---|---|---|---|---|---|---|---|
| Economic | Production costs (€/1000L) | 494 | 9 | 684 | 15 | 667 | 14 | 617.5 | 12 |
| Farmers’ revenues (€/1000L) | 334 | 22 | 473 | 27 | 451 | 26 | 417.5 | 23 | |
| Culled cow price (€/Kg) | 2.4 | 12 | 2.64 | 8 | 2.4 | 8 | 2.4 | 8 | |
| Product price (€/L) | 0.78 | 8 | 1.85 | 19 | 1.05 | 16 | 0.97 | 16 | |
| Total | 51 | 69 | 64 | 59 | |||||
| Environmental | ALEA | 0.273 | 15 | 0 | 1 | 0.177 | 10 | 0.204 | 11 |
| Attributable Fraction (%) | 4 | 15 | 0 | 1 | 2.6 | 10 | 3 | 11 | |
| Total | 30 | 2 | 20 | 22 | |||||
| Social | Mortality rate (%) | 3.8 | 2 | 4.8 | 2 | 4.1 | 2 | 4.04 | 2 |
| Culling rate (%) | 21.3 | 3 | 50.5 | 5 | 31.5 | 5 | 28.6 | 3 | |
| Total | Total | 5 | 7 | 7 | 5 | ||||
| Political | Regulatory framework | Moderate | 12 | Very high | 14 | High | 5 | Moderate | 10 |
| Investment Policies | High | 2 | High | 8 | Moderate | 4 | Very high | 4 | |
| Total | 14 | 22 | 9 | 14 | |||||
| Score Column Total | 100 | 100 | 100 | 100 |
References
- Septimus, E.J. Antimicrobial Resistance: An Antimicrobial/Diagnostic Stewardship and Infection Prevention Approach. Med. Clin. N. Am. 2018, 102, 819–829. [Google Scholar] [CrossRef]
- de Kraker, M.E.A.; Stewardson, A.J.; Harbarth, S. Will 10 million People Die a Year due to Antimicrobial Resistance by 2050? PLoS Med. 2016, 13, e1002184. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial resistance: Prevalence, economic burden, mechanisms of resistance and strategies to overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Antimicrobial Resistance. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 1 January 2022).
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Haimerl, P.; Arlt, S.; Borchardt, S.; Heuwieser, W. Antibiotic treatment of metritis in dairy cows-A meta-analysis. J. Dairy Sci. 2017, 100, 3783–3795. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial Resistance: A One Health Perspective. Microbiol. Spectr. 2018, 6, 2–6. [Google Scholar] [CrossRef]
- Boireau, C.; Cazeau, G.; Jarrige, N.; Calavas, D.; Madec, J.Y.; Leblond, A.; Haenni, M.; Gay, E. Antimicrobial resistance in bacteria isolated from mastitis in dairy cattle in France, 2006–2016. J. Dairy Sci. 2018, 101, 9451–9462. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Ma, Z.X.; Lee, S.Y.; Jeong, K.C. Mitigating Antibiotic Resistance at the Livestock-Environment Interface: A Review. J. Microbiol. Biotechnol. 2019, 29, 1683–1692. [Google Scholar] [CrossRef]
- Hao, H.H.; Cheng, G.Y.; Iqbal, Z.; Ai, X.H.; Hussain, H.I.; Huang, L.L.; Dai, M.H.; Wang, Y.L.; Liu, Z.L.; Yuan, Z.H. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014, 5, 288. [Google Scholar] [CrossRef] [PubMed]
- Lhermie, G.; Gröhn, Y.T.; Raboisson, D. Addressing Antimicrobial Resistance: An Overview of Priority Actions to Prevent Suboptimal Antimicrobial Use in Food-Animal Production. Front. Microbiol. 2017, 7, 2114. [Google Scholar] [CrossRef] [PubMed]
- Zwald, A.G.; Ruegg, P.L.; Kaneene, J.B.; Warnick, L.D.; Wells, S.J.; Fossler, C.; Halbert, L.W. Management practices and reported antimicrobial usage on conventional and organic dairy farms. J. Dairy Sci. 2004, 87, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Farrell, S.; McKernan, C.; Benson, T.; Elliott, C.; Dean, M. Understanding farmers’ and veterinarians’ behavior in relation to antimicrobial use and resistance in dairy cattle: A systematic review. J. Dairy Sci. 2021, 104, 4584–4603. [Google Scholar] [CrossRef]
- Hernando-Amado, S.; Coque, T.; Baquero, F.; Martínez, J. Defining and combating antibiotic resistance from One Health and Global Health perspectives. Nat. Microbiol. 2021, 4, 1432–1442. [Google Scholar] [CrossRef] [PubMed]
- Ministère de L’agriculture et de L’alimentation (ECOANTIBIO). Plan National de Réduction des Risques D’antibiorésistance en Médecine Vétérinaire (2017–2021). 2019. Available online: https://agriculture.gouv.fr/le-plan-ecoantibio-2-2017-2021 (accessed on 12 February 2020).
- L’agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail (ANSES). Antimicrobial Resistance: A Major Issue for Animals and Humans. 2022. Available online: https://www.anses.fr/en/content/antimicrobial-resistance (accessed on 5 December 2022).
- Legifrance. Public Health Code. 2020. Available online: https://www.legifrance.gouv.fr/codes/id/LEGIARTI000032256759/2016-04-01 (accessed on 5 December 2022).
- Poizat, A.; Bonnet-Beaugrand, F.; Rault, A.; Fourichon, C.; Bareille, N. Antibiotic Use by Farmers to Control Mastitis as Influenced by Health Advice and Dairy Farming Systems. Prev. Vet. Med. 2017, 146, 61–72. [Google Scholar] [CrossRef]
- Lhermie, G.; Wernli, D.; Jørgensen, P.S.; Kenkel, D.; Lawell, C.Y.C.L.; Tauer, L.W.; Gröhn, Y.T. Tradeoffs between Resistance to Antimicrobials in Public Health and Their Use in Agriculture: Moving towards Sustainability Assessment. Ecol. Econ. 2019, 166, 106427. [Google Scholar] [CrossRef]
- Aenishaenslin, C.; Belanger, D.; Fertel, C.; Hongoh, V.; Mareschal, B.; Waaub, J. Practical Guide to Establishing a Multi-Criteria and Multi-Actor Decision-Making Process: Steps and Tools; Les Cahiers du GERAD G-2019-03; GERAD HEC: Montreal, QC, Canada, 2019. [Google Scholar]
- Figueira, J.; Greco, S.; Ehrgott, M. Multiple Criteria Decision Analysis: State of The Art Surveys. Introduction. Int. Ser. Oper. Res. Man 2005, 78, Xxi–Xxxvi. [Google Scholar] [CrossRef]
- Baudoin, F.; Hogeveen, H.; Wauters, E. Reducing Antimicrobial Use and Dependence in Livestock Production Systems: A Social and Economic Sciences Perspective on an Interdisciplinary Approach. Front. Vet. Sci. 2021, 8, 584593. [Google Scholar] [CrossRef]
- Raboisson, D.; Ferchiou, A.; Pinior, B.; Gautier, T.; Sans, P.; Lhermie, G. The Use of Meta-Analysis for the Measurement of Animal Disease Burden: Losses Due to Clinical Mastitis as an Example. Front. Vet. Sci. 2020, 7, 149. [Google Scholar] [CrossRef]
- Fourichon, C.; Seegers, H.; Beaudeau, F.; Verfaille, L.; Bareille, N. Health-Control Costs in Dairy Farming Systems in Western France. Livest. Prod. Sci. 2001, 68, 141–156. [Google Scholar] [CrossRef]
- Gay, E.; Cazeau, G.; Jarrige, N.; Calavas, D. Utilisation des antibiotiques chez les ruminants domestiques en France: Résultats d’enquêtes de pratiques auprès d’éleveurs et de vétérinaires. Bulletin épidémiologique, santé animale et alimentation n.53. Spécial Antibiotiques et Antibiorésistances. ANSES 2012, 53, 8–11. [Google Scholar]
- Pinedo, P.J.; Velez, J. Invited Review: Unique reproductive challenges for certified organic dairy herds. Appl. Anim. Sci. 2019, 35, 416–425. [Google Scholar] [CrossRef]
- Casewell, M.; Friis, C.; Marco, E.; McMullin, P.; Phillips, I. The European ban on growth-promoting antibiotics and emerging consequences for human and animal health. J. Antimicrob. Chemother. 2003, 52, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Parsonage, B.; Hagglund, P.K.; Keogh, L.; Wheelhouse, N.; Brown, R.E.; Dancer, S.J. Control of Antimicrobial Resistance Requires an Ethical Approach. Front. Microbiol. 2017, 8, 2124. [Google Scholar] [CrossRef] [PubMed]
- Bowman, M.; Marshall, K.K.; Kuchler, F.; Lynch, L. Raised without Antibiotics: Lessons from Voluntary Labeling of Antibiotic Use Practices in the Broiler Industry. Am. J. Agric. Econ. 2016, 98, 622–642. [Google Scholar] [CrossRef]
- Andersen, V.; Hald, T. Interventions Aimed at Reducing Antimicrobial Usage and Resistance in Production Animals in Denmark. NAM Perspect. 2017. Available online: https://nam.edu/interventions-aimed-at-reducing-antimicrobial-usage-and-resistance-in-production-animals-in-denmark/ (accessed on 14 February 2022).
- Dennis, E.J.; Schroeder, T.C.; Renter, D.G.; Pendell, D.L. Value of arrival metaphylaxis in U.S. cattle industry. J. Agric. Resour. Econ. 2018, 43, 233–250. [Google Scholar] [CrossRef]
- Lhermie, G.; Tauer, L.W.; Gröhn, Y.T. The farm cost of decreasing antimicrobial use in dairy production. PLoS ONE 2018, 13, e0194832. [Google Scholar] [CrossRef]
- Vose, D.; Acar, J.; Anthony, F.; Franklin, A.; Gupta, R.; Nicholls, T.; Tamura, Y.; Thompson, S.; Threlfall, E.J.; Van Vuuren, M.; et al. Antimicrobial resistance: Risk analysis methodology for the potential impact on public health of antimicrobial resistant bacteria of animal origin. Rev. Sci. Tech. 2001, 20, 811–827. [Google Scholar] [CrossRef]
- Cinelli, M.; Coles, S.R.; Kirwan, K. Analysis of the Potentials of Multi-Criteria Decision Analysis Methods to Conduct Sustainability Assessment. Ecol. Indic. 2014, 46, 138–148. [Google Scholar] [CrossRef]
- Brans, J.P.; Mareschal, B. PROMETHEE methods. In Multiple Criteria Decision Analysis: State of the Art Surveys; Figueira, J., Greco, S., Ehrgott, M., Eds.; Springer: New York, NY, USA, 2005; pp. 163–195. [Google Scholar]
- Macharis, C.; Springael, J.; Brucker, K.D.; Verbeke, A. PROMETHEE and AHP: The Design of Operational Synergies in Multicriteria Analysis. Eur. J. Oper. Res. 2004, 153, 307–317. [Google Scholar] [CrossRef]
- Bradford, H.; McKernan, C.; Elliott, C.; Dean, M. Consumers’ perceptions and willingness to purchase pork labelled ‘raised without antibiotics’. Appetite 2022, 171, 105900. [Google Scholar] [CrossRef]
- WHO. Global Antimicrobial Resistance Surveillance System (GLASS). 2015. Available online: https://www.who.int/initiatives/glass (accessed on 12 February 2022).
- Hemonic, A.; Chauvin, C.; Delzescaux, D.; Verliat, F.; Correge, I.; The French Working Group ‘Antimicrobials in the Swine Industry. Reliable estimation of antimicrobial use and its evolution between 2010 and 2013 in French swine farms. Porc. Health Manag. 2018, 4, 8. [Google Scholar] [CrossRef]
- ANSES; L’agence Nationale de Sécurité Sanitaire de L’alimentation, de L’environnement et du Travail. Rapport Annuel Médicaments Vétérinaires Contenant des Antibiotiques en France. 2018. Available online: https://www.anses.fr/fr/content/rapport-de-lanses-suivi-des-ventes-de-m%C3%A9dicaments-v%C3%A9t%C3%A9rinaires-contenant-des-antibiotiques-8 (accessed on 7 February 2022).
- CDC. Antibiotic Resistance Threats in the United States. 2019. Available online: www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 23 March 2020).
- Bywater, R.J.; Casewell, M.W. An assessment of the impact of antibiotic resistance in different bacterial species and of the contribution of animal sources. J. Antimicrob. Chemother. 2000, 46, 643–644. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prescott, J.F. The Resistance Tsunami, Antimicrobial Stewardship, and the Golden Age of Microbiology. Vet. Microbiol. 2014, 171, 273–278. [Google Scholar] [CrossRef] [PubMed]
- IDELE. Production Cost of 1000 Liters of Milk in 2018. 2018. Available online: https://idele.fr/detail-article/prix-de-revient-du-lait-de-vache-rica#:~:text=Tant%20en%20plaine%20avec%201.41,salari%C3%A9e%20contre%201%2C17).idele.fr/services/outils/prix-de-revient-du-lait-de-vache-rica.html (accessed on 18 February 2020).
- IDELE. Average Selling Price of a Liter of Cow’s Milk on the Market in 2020. 2020. Available online: https://idele.fr/inosys-reseaux-elevage/?eID=cmis_download&oID=workspace%3A%2F%2FSpacesStore%2Ff7c5b70f-6210-4e1f-a13f-bfa182379f73&cHash=443a418b5465dc8dc23ca324bc64b973idele.fr/filieres/bovin-lait/publication/idelesolr/recommends/resultats-economiques-des-fermes-laitieres-de-louest.html (accessed on 10 February 2020).
- Web-Agri. Average Price of the Culled Cow on 02/18/2020. 2020. Available online: http://www.web-agri.fr/gros-bovins-boucherie/334 (accessed on 18 February 2020).
- Raboisson, D.; Cahuzac, E.; Sans, P.; Allaire, G. Herd-Level and Contextual Factors Influencing Dairy Cow Mortality in France in 2005 and 2006. J. Dairy Sci. 2011, 94, 1790–1803. [Google Scholar] [CrossRef] [PubMed]
- Reducing the Use of Veterinary Antibiotics (ECOANTIBIO). 2017. Available online: https://agriculture.gouv.fr/plan-ecoantibio-2012-2017-lutte-contre-lantibioresistancefile:///C:/Users/dmanr/Downloads/plaq-ecoantibio-gb-2017.pdf (accessed on 8 February 2022).
- Skjolstrup, N.K.; Nielsen, L.R.; Jensen, C.S.; Lastein, D.B. Veterinary Herd Health Consultancy and Antimicrobial Use in Dairy Herds. Front. Vet. Sci. 2020, 7, 547975. [Google Scholar] [CrossRef]
- Behzadian, M.; Kazemadeh, R.B.; Albadvi, A.; Aghdasi, M. PROMETHEE: A comprehensive literature review on methodologies and applications. Eur. J. Oper. Res. 2010, 200, 198–215. [Google Scholar] [CrossRef]
- Aenishaenslin, C.; Hongoh, V.; Cisse, H.D.; Hoen, A.G.; Samoura, K.; Michel, P.; Waaub, J.P.; Belanger, D. Multi-criteria decision analysis as an innovative approach to managing zoonoses: Results from a study on Lyme disease in Canada. BMC Public Health 2013, 13, 897. [Google Scholar] [CrossRef]
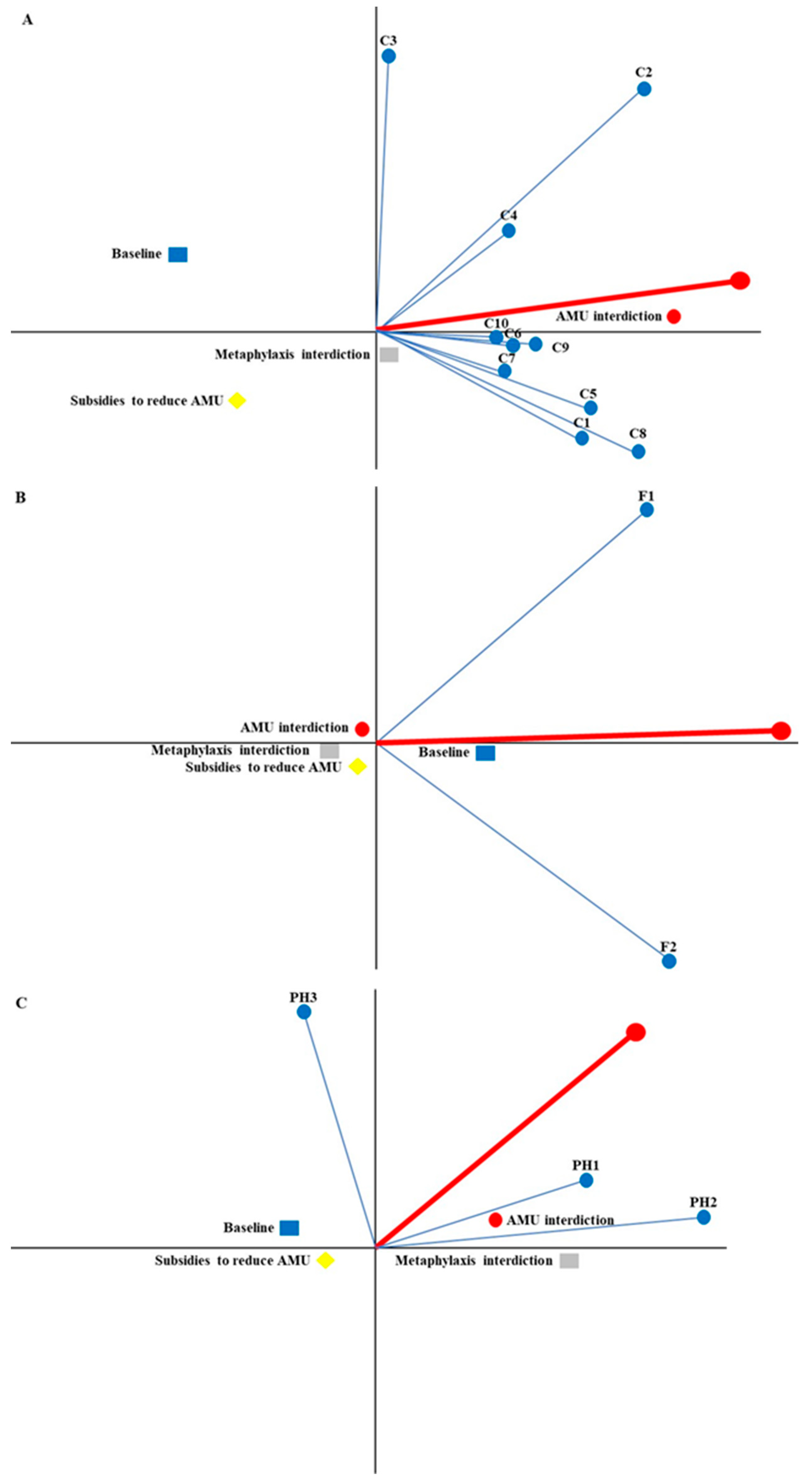


| Stakeholders | Weighted Ranking | Strategy | Phi | Phi+ | Phi− |
|---|---|---|---|---|---|
| Consumers | 1 | AMU interdiction | 0.23 | 0.58 | 0.22 |
| 2 | Preventive AMU interdiction | 0.007 | 0.24 | 0.25 | |
| 3 | Subsides to reduce AMU | −0.10 | 0.27 | 0.37 | |
| 4 | Baseline strategy | −0.19 | 0.19 | 0.32 | |
| Farmers | 1 | Baseline strategy | 0.1 | 0.36 | 0.26 |
| 2 | AMU interdiction | −0.02 | 0.34 | 0.36 | |
| 3 | Subsides to reduce AMU | −0.03 | 0.29 | 0.32 | |
| 4 | Preventive AMU interdiction | −0.05 | 0.28 | 0.33 | |
| Public health representatives | 1 | AMU interdiction | 0.12 | 0.45 | 0.33 |
| 2 | Preventive AMU interdiction | −0.004 | 0.25 | 0.26 | |
| 3 | Baseline strategy | −0.03 | 0.34 | 0.35 | |
| 4 | Subsides to reduce AMU | −0.09 | 0.21 | 0.30 |
| Criteria | Weight Stability Interval | ||
|---|---|---|---|
| Minimum | Maximum | Difference | |
| Regulatory framework | 9.28 | 11.12 | 1.84 |
| Farmer’s revenues | 15.84 | 18.1 | 2.26 |
| Production cost | 12.92 | 15.22 | 2.3 |
| Culling rate | 5.75 | 8.24 | 2.49 |
| Attributable fraction 2 | 2.99 | 8.84 | 5.85 |
| Product price | 1.31 | 12.86 | 11.55 |
| ALEA 1 | 0 | 11.99 | 11.99 |
| Mortality rate | 5.8 | 17.94 | 12.14 |
| Price culled cow | 0 | 12.54 | 12.54 |
| Policies investments | 7.53 | 100 | 92.47 |
| Criteria | STRA01 | STRA02 | STRA03 | STRA04 | |
|---|---|---|---|---|---|
| Environmental | ALEA 1 | 0.27 | 0 | 0.17 | 0.20 |
| Attributable fraction 2 (%) | 0.04 | 0 | 0.026 | 0.03 | |
| Economic | Production costs (€/1000 L) | 494 | 684 | 667 | 617.5 |
| Farmers’ revenues (€/1000 L) | 334 | 473 | 451 | 417.5 | |
| Culled cow price (€/Kg) | 2.4 | 2.64 | 2.4 | 2.4 | |
| Product price (€/L) | 0.78 | 1.85 | 1.05 | 0.96 | |
| Social | Mortality rate (%) | 3.8 | 4.8 | 4.1 | 4.04 |
| Culling rate (%) | 21.3 | 50.5 | 31.5 | 28.6 | |
| Political | Regulatory framework | Moderate | Very high | High | Moderate |
| Investment Policies | High | High | Moderate | Very high |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manriquez, D.; Costa, M.; Ferchiou, A.; Raboisson, D.; Lhermie, G. Multi-Criteria Decision Analysis for Assessing Social Acceptance of Strategies to Reduce Antimicrobial Use in the French Dairy Industry. Antibiotics 2023, 12, 8. https://doi.org/10.3390/antibiotics12010008
Manriquez D, Costa M, Ferchiou A, Raboisson D, Lhermie G. Multi-Criteria Decision Analysis for Assessing Social Acceptance of Strategies to Reduce Antimicrobial Use in the French Dairy Industry. Antibiotics. 2023; 12(1):8. https://doi.org/10.3390/antibiotics12010008
Chicago/Turabian StyleManriquez, Diego, Maiara Costa, Ahmed Ferchiou, Didier Raboisson, and Guillaume Lhermie. 2023. "Multi-Criteria Decision Analysis for Assessing Social Acceptance of Strategies to Reduce Antimicrobial Use in the French Dairy Industry" Antibiotics 12, no. 1: 8. https://doi.org/10.3390/antibiotics12010008
APA StyleManriquez, D., Costa, M., Ferchiou, A., Raboisson, D., & Lhermie, G. (2023). Multi-Criteria Decision Analysis for Assessing Social Acceptance of Strategies to Reduce Antimicrobial Use in the French Dairy Industry. Antibiotics, 12(1), 8. https://doi.org/10.3390/antibiotics12010008





