Abstract
The increase in antibiotic resistance to Helicobacter pylori (H. pylori) is associated with a decrease in the effectiveness of eradication therapy. Although some success has been achieved by adjusting therapeutic regimens according to local data on resistance to certain antibiotics, a new approach is needed to ensure a better therapeutic response. Tailored therapy, based on sensitivity tests to antibiotics, is increasingly proving to be a superior therapeutic option, even as a first-line therapy. Moreover, the recently published Maastricht VI guidelines emphasize utilizing a susceptibility-guided strategy in respect to antibiotic stewardship as the first choice for eradication therapy. In addition, polymerase chain reaction (PCR) technology is becoming a standard tool in the diagnosis of H. pylori infections through non-invasive testing, which further optimizes the eradication process. We provide a review regarding the current position of the individualized approach in eradication therapy and its future prospects. Based on novel understandings, the personalized approach is an effective strategy to increase the successful eradication of H. pylori infections.
1. Introduction
Helicobacter pylori (H. pylori) infection is the main causal factor in gastritis, gastric and duodenal ulcers, gastric adenocarcinoma and mucosa-associated lymphoid tissue lymphoma (MALT) [1]. Not long after its discovery, H. pylori was recognized as a first-order carcinogen, and therefore by far the most significant aspect of H. pylori infection is its association with the development of gastric cancer [2,3].
Since the discovery of the H. pylori microorganism, eradication regimens have been changed and adapted many times. Triple therapy, which has been known as a standard therapy for years, has not been the therapy of first choice for some time, primarily due to insufficient eradication efficiency [4]. The main cause of this decline in eradication success is increasing antibiotic resistance rates, primarily clarithromycin, worldwide [5,6].
The modern approach to the treatment of H. pylori infections is based on choosing an antibiotic therapy according to the data of local antibiotic resistance. In response to the failure of triple therapy, the 2017 Maastricht guidelines proposed different models of quadruple therapy, primarily concomitant therapy and bismuth-based therapy [7]. Furthermore, different authors proposed additional modifications of the usual antibiotic combination (amoxicillin, clarithromycin, and metronidazole), such as sequential, hybrid and reverse-hybrid therapies [6,8]. The main goal of these alterations was to obtain as high of an eradication rate as possible. However, even with these modifications, the efficacy of eradication in the per protocol analyses of different randomized clinical trials is rarely above 90%, which has been identified as the main goal of antimicrobial therapy [6].
The proposed therapeutic models often affect the quality of life in patients with H. pylori infections [8,9,10,11,12]. Namely, a large number of antibiotics and a complex regimen can have an impact on compliance and the occurrence of potentially adverse events, which can additionally limit the effectiveness of the therapy [10]. However, according to some studies, most primary eradication regimens are expected to fail in as many as 20 to 30% of cases, necessitating the need for a second-line therapy [13,14]. We can assume that this has an even more negative impact on patients’ quality of life, probably due to the complexity of therapeutic regimens and poor compliance. The main factors affecting eradication success are presented in Figure 1.
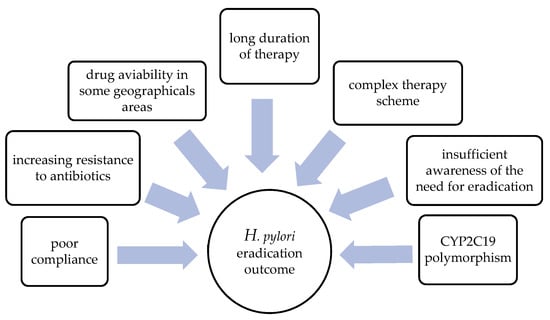
Figure 1.
Factors affecting the efficiency of H. pylori eradication therapy.
Previous studies have shown the importance of CYP2C19 polymorphism in the effectiveness of certain proton pump inhibitors (PPI), which were, until now, an indispensable part of all eradication protocols [15]. One of the main characteristics of CYP2C19 polymorphism is the geographic diversity in its genetic differences: for example, Caucasian populations show a higher prevalence of high metabolizers compared to Asian populations [7].
Nevertheless, in order to achieve a suitable milieu for the action of antibiotics, it seems that there is a potential benefit in implementing the CYP2C19 genotype to guide PPI use and dosing in clinical practice [16].
Finally, the newly published Maastricht VI guidelines offer a different approach than the previous ones when it comes to the first line of eradication therapy [6,7]. In this review, we will present the current findings and the future prospects of the personalized approach in the treatment of H. pylori infections.
2. Current Position and Strategies
There are several proposals to improve the effectiveness of the existing eradication therapy regimens. The following strategies stand out: extending the treatment length of triple therapy to 14 days; the usage of a higher dosage of PPI (proton pump inhibitors) or vonoprazan; the usage of a four-drug regimen; and supplementation with probiotics (Figure 2) [17].
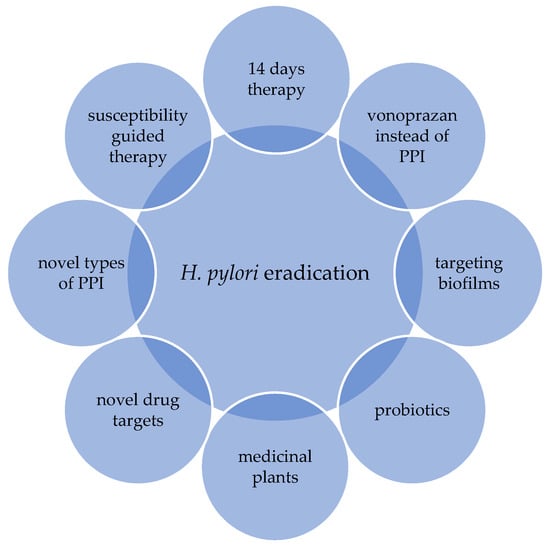
Figure 2.
Current strategies for improving the effectiveness of eradication therapy.
Data from a multicenter prospective non-interventional study that included 21,533 patients from 27 European countries showed that a longer treatment duration, higher acid inhibition and compliance were associated with higher eradication rates [18]. The study revealed that European recommendations are being slowly and heterogeneously incorporated into routine clinical practice. Namely, the results of the same study indicate a reduction in the prescription of triple therapy, a longer duration of treatment and the use of a higher dose of PPI. According to the study, the above resulted in an increase in overall effectiveness (84–90%) [18].
Vonoprazan, a potassium-competitive acid blocker (P-CAB), has a unique pharmacological profile that provides the long-lasting inhibition of gastric acid secretion, even during night-time hours [6]. P-CAB is not dependent on the CYP2C19 genotype or the activation of parietal cells, thus providing an opportunity to improve eradication success rates even in dual therapy regimens [6]. The results of a study combining dual therapy (amoxicillin and P-CAB) showed that the eradication rate in patients with clarithromycin resistance was 95.4% [19]. At first reserved for East Asian countries, today we have studies with P-CAB therapy regimens in Western countries too, although the eradication rate has failed to reach the threshold of 90% [20].
A large number of studies and meta-analyses have studied the role of probiotics in the treatment of H. pylori infections, with conflicting results [6,21,22,23]. Current knowledge only suggests that the use of probiotics could reduce the side effects of eradication therapy, without a direct effect on H. pylori [6]. Such data especially refer to certain probiotics, such as Lactobacillus spp., Bifidobacterium spp. and S. boulardii [21,22,23]. Obviously, more studies are still necessary to assess the direct efficacy of probiotics against H. pylori.
Furthermore, innovative models such as plant extracts, inhibitors of biofilm formation and novel proton pump inhibitors have all been proposed (Figure 2) [17]. However, none of these proposed models have been proven as an optimal treatment option with a satisfying eradication rate. Resistance to different antibiotics remains the main obstacle to effective eradication therapy [6,17].
Emerging Antibiotic Resistance
The resistance of H. pylori to antibiotics has reached alarming levels worldwide [5]. A systematic review and meta-analysis were conducted to assessed the rates of H. pylori resistance to commonly used antibiotics in eradication therapy in World Health Organization (WHO) regions [5]. The analysis included 178 studies, comprising 66,142 isolates from 65 countries. Increasing antibiotic resistance was observed in most WHO regions [5]. Primary and secondary resistance rates to clarithromycin, metronidazole and levofloxacin were ≥15% in all WHO regions, which is the common threshold for choosing alternative empiric regimens [5]. Exceptions were found for primary clarithromycin resistance in the Americas (10%; 95% CI, 4–16%) and the Southeast Asian region (10%; 95% CI, 5–16%), as well as primary levofloxacin resistance in the European region (11%; 95% CI, 9–13%) [5]. As expected, the resistance rates are higher in previously treated individuals than in patients who never received eradication treatment before [5].
Clarithromycin is still considered as a key antibiotic to eradicate H. pylori. However, when resistance is present, not only the probability of the treatment’s success with clarithromycin becomes very low, but it also continues to induce resistance in other bacteria [24]. Moreover, clarithromycin-resistant strains of H. pylori have recently been recognized by the World Health Organization as one of the 12 priority pathogens for which novel antibiotics are urgently needed [25].
In a previously mentioned study in WHO regions by Savoldi et al., resistance to clarithromycin was significantly associated with the failure of clarithromycin-containing regimens (odds ratio, 6.97; 95% CI, 5.23–9.28; p < 0.001) [5]. Moreover, there was a significant increase in resistance to clarithromycin in some regions, crossing the intervention threshold over 10 years: for example, the Southeast Asian region’s resistance increased from 13% in 2006–2008 to 21% in 2012–2016 [5]. Interestingly, in the same study, the pooled prevalence of clarithromycin resistance in the European region had not changed when we compared the same periods: it remained at the level of 28% [5].
On the other hand, Megraud et al. conducted a large prospective study regarding H. pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community [26]. The study was performed in 24 European centers in 18 countries involving 1211 patients with positive H. pylori cultures. The results showed an increase in global primary clarithromycin resistance from 17.5% in 2008 to 21.4% in 2018 (p < 0.05) [26]. However, when we compared these results with the results of previous studies in 1998 and 2008, it is interesting to point out that the global clarithromycin resistance increased between 2008 and 2018 approximately 1% per year, thus it was not as high as we expected [26,27,28].
For clarithromycin resistance, there is still a large heterogeneity from country to country: for example, in Croatia there was an increase in resistance from 22% for the period of 2008–2009 to 34.6% for 2018, according to recent data [26,28]. On the other hand, in France the clarithromycin resistance rate slightly changed, from 21.3% (2008–2009) to 22.5% (2018) [26,28]. We can attribute such changes mainly to different patterns of drug prescription among countries. Some of them have taken important measures for the rational use of antibiotics, following the WHO recommendations, by decreasing the rate of antibiotic consumption, including macrolides, which are now used less for respiratory infections [26]. For example, the overall macrolide consumption decreased by 46% in France in the period from 2000 to 2015, which is obviously not the case in all European countries [26,29].
Worldwide, high metronidazole resistance has been recorded, both primary and secondary [5]. According to recent data, the highest level of metronidazole resistance is recorded in the eastern areas of the world, such as Eastern Mediterranean region (primary resistance 56%, secondary resistance 65%) [5]. However, contrary to other antibiotics, it seems that metronidazole administration in high doses and using 14-day schedules can partially overcome the resistance effect [30].
In the case of levofloxacin, increased primary resistance has also been reported, affecting the efficacy of levofloxacin-based regimens, which are designed mainly for second-line treatment, after the failure of clarithromycin-based first-line therapy [6]. However, in contrast to that of clarithromycin, the H. pylori resistance to levofloxacin has not increased significantly during the last 10 years (14% in 2008–2009 vs. 15.8% in 2018) [26,28]. After the failure of the standard clarithromycin, included in triple therapy, clarithromycin resistance could be expected [6]. According to the Maastricht VI guidelines, second-line eradication therapy should include levofloxacin, which has been proven to be at least as equally effective as the bismuth quadruple regimen [6,31]. It is worth highlighting the safety warning regarding the prescription of levofloxacin and its potential side effects [6]. Based on the above, it is recommended to use levofloxacin judiciously and cautiously, in situations in which the primary lines have already been exhausted.
In contrast to clarithromycin, metronidazole and levofloxacin, resistance to amoxicillin and tetracycline remained <10% in all WHO regions [5].
Increasing H. pylori resistance to key antibiotics, such as clarithromycin and levofloxacin, is leading to treatment failures, forcing us to use quadruple therapies, especially in Europe. Meanwhile, the overall high rate of antimicrobial consumption during the COVID-19 pandemic had a further negative impact on antibiotic resistance worldwide [32]. However, the continuation of policies of decreasing antibiotic consumption could be promising in the stabilization of resistance rates [26]. The establishment and the maintenance of local resistance monitoring systems is essential for determining guidelines and recommendations for the treatment H. pylori infections in specific regions, as some authors have already proposed [33].
3. Tailored Therapy
In order to overcome the problem of empiric therapy and improve the eradication rate, a susceptibility-guided treatment for each patient has been suggested [33,34,35]. This recommendation was finally incorporated in the recently published Maastricht VI guidelines (European Helicobacter and Microbiota Study Group and Consensus panel—Maastricht VI/Florence Consensus Report) [6].
According to many investigators, susceptibility-testing-guided therapy is the best strategy for increasing the eradication rate [36]. By knowing the prevalence of antibiotic resistance in a certain population, we can predict efficacy of different therapy models in susceptible and resistant strains of H. pylori microorganisms [37,38].
The selection of a therapy based on information about the antibiotics that have been used previously represents the origin of the personalized approach in the eradication of H. pylori infections [35]. Romano et al. conducted a prospective study on 401 subjects in a region with high resistance to clarithromycin comparing bismuth quadruple therapy with concomitant therapy; concomitant therapy was prescribed only to those subjects who had not previously used clarithromycin, based on their medical history data [39]. The results showed a high but similar eradication efficiency of concomitant therapy and bismuth-based therapy in patients who had not previously taken clarithromycin: 88.2% vs. 91.5% (p = 0.26) in the ITT analysis and 91.2% vs. 95.8% (p = 0.07) in the PP analysis [39]. The study was an example of how to simply avoid unnecessary proscriptions of macrolides in order to rationalize the use of antibiotics in regions with high resistance to clarithromycin.
Already, therapies based on antibiotic susceptibility testing seem to be an optimal option in reducing and optimizing eradication therapy. The results of a meta-analysis conducted by Chen et al., consisting of 13 controlled clinical trials and including 3512 participants, showed higher eradication rates when tailored therapies were used, compared to empirically chosen regimens [40]. Recently, Mao Q. et al. in a meta-analysis involving 21 studies showed that antibiotic-guided therapy yielded a significantly better efficacy rate compared to that of empirical regimens in first-line treatments (RR, 1.14 (95% CI, 1.08–1.21), I2 = 72.2%) [41]. However, in the second line, they did not find any difference in eradication outcomes (RR, 1.05 [95% CI, 0.84–1.30], I2 = 80.6%), suggesting that empirical therapy could be as efficient as tailored therapy. Still, the results of the study should be interpreted with caution due to the high heterogeneity of the evidence in the research [41].
Gingold-Belfer et al. performed a systematic review and meta-analysis of 16 randomized controlled trials, including 2374 patients who received susceptibility-guided therapy and 2451 patients who received empirical treatments [42]. The results showed that susceptibility-guided treatment may be slightly superior to empirical first-line triple therapy in naive patients. However, susceptibility-guided treatment did not appear to be superior to empirical first-line quadruple therapy or empirical rescue therapy. The superiority of susceptibility-guided therapy to empirical triple therapy with clarithromycin was found only in cases in which the clarithromycin resistance exceeded 20% (RR, 1.18; 95% CI, 1.07–1.30; p = 0.001, I2 = 81%) [42].
Finally, tailored therapy might allow for the prescription of optimized clarithromycin-based triple therapy to patients with clarithromycin-susceptible strains in areas with high overall clarithromycin resistance. It should be emphasized that triple therapy with clarithromycin can be used in areas with high clarithromycin resistance rates only with a previous antibiotic sensitivity test [6]. The global rate of primary clarithromycin resistance is >20%, based on the results of the previously mentioned prospective study conducted in 18 European countries involving 1211 H. pylori culture-positive patients [26]. Accordingly, more than two-thirds of our patients, respecting regional differences, are eligible for clarithromycin-based first-line eradication therapy. By selecting proper patients, we can proscribe standard triple therapy in cases of clarithromycin-sensitive strains, and thus avoid unnecessary quadruple regimens.
Table A1 shows the randomized clinical trials published in the last 10 years, comparing tailored and empirical therapies in first-line eradication treatment [12,43,44,45,46,47,48,49,50,51]. Although tailored therapy is widely considered as superior to empirical therapy in first-line treatment, the overall results are diverse, in which some studies showed no difference at all between the two therapy groups. However, the heterogeneity of the study’s protocols and reporting should be accounted for [52].
4. Anything New in Diagnostics?
Traditionally, diagnostic tests for H. pylori infections have been divided into noninvasive and invasive ones [6]. However, PCR technology has found its place in both groups, as it can be performed from a gastric biopsy sample and stool, respectively [6,7]. Among noninvasive tests, the 13C-urea breath test (UBT) and stool antigen test (SAT) have been the main tools in clinical practice, both for the diagnosis and confirmation of eradication [6]. IgG serology is not able to differentiate acute infection from past infection, and therefore it is not suitable as a confirmation test [6]. Among invasive tests, the rapid urease test (RUT), histology staining and culture confirmation stand out.
The 13C-UBT is still considered to be a standard tool in practice and studies. More recent data have shown some benefits to using citric acid over other meals in order to improve accuracy and sensitivity [53]. Citric acid helps slow gastric emptying and thus increases the contact time with H. pylori urease [53,54].
The SAT became a suitable diagnostic tool in practice before and after eradication [6]. It is important to highlight that tests based on monoclonal antibodies are superior in comparative studies to polyclonal tests [55]. SATs using enzyme immunoassay (EIA) test kits should be preferred as they have shown better performance than rapid immunochromatography tests [55,56].
When endoscopy is indicated, the RUT represents a fast, cheap and reliable tool for H. pylori diagnosis [6,57]. Although a highly specific test (95–100%), its sensitivity (85–95%) may be affected by small sample sizes, as less than 104 bacterial cells can lead to false-negative results [57,58,59]. The Maastricht VI guidelines suggest the practical reuse of RUT test slides which are meant to be discarded after reading them [6]. Instead of taking additional biopsies for PCR or other tests, those already taken for the RUT can be reused for the purpose of PCR analysis [60,61]. Analyses have shown a high correlation between the reuse of these kinds of test samples [60].
In dyspeptic patients older than 50 years, an upper-GI endoscopy is required, regardless of whether they have alarming symptoms [6,7]. When an endoscopy is indicated, it should involve biopsy sampling, as recommended by the guidelines [6]. A biopsy analysis should result in an etiological diagnosis, gastritis staging and H. pylori status [6]. Gastritis histological staging should be assessed through the OLGA (Operative Link on Gastric Atrophy) and OLGIM (Operative Link on Gastric Intestinal Metaplasia) systems [62,63].
The main limitation of the presented non-invasive diagnostic methods is that they can solely detect H. pylori but cannot provide information on the drug susceptibility of the bacterium. For that purpose, we use antibiotic sensitivity testing.
Antibiotic Sensitivity Testing Issues
Antibiotic sensitivity testing is mainly performed by the following two methods: H. pylori cultures and genotyping by molecular detection [41]. In the first, H. pylori is cultured from a gastric mucosa sample following a biopsy, while the antibiotic sensitivity is detected by agar dilution, disk diffusion or an E-test [41]. Among them, the agar dilution method is considered the gold standard, although it is costly, time-consuming and labor intensive (2–4 weeks) [35,64]. Furthermore, the success rate of cultures and susceptibility testing ranges from 75 to 90% [36,65]. The second method is genotyping by molecular detection (real-time PCR and fluorescence in situ hybridization) from stool and stomach biopsy specimens, whereas point mutations associated with resistance to specific antibiotics are usually detected through commercially available kits [42]. In addition, high-throughput whole-genome sequencing techniques have been used to identify drug-resistant mutations [66,67]. Today, molecular methods (in particular, real time-PCR, whole-genome sequencing and digital PCR) are being improved more and more, thus allowing for the detection of H. pylori mutations associated with resistance to the commonly used antibiotics in eradication regimens with clarithromycin, levofloxacin, tetracycline and rifampicin [6].
The mechanisms of H. pylori bacteria resistance against the antibiotics that are mainly used for eradication are now largely familiar [68]. For example, resistance against clarithromycin is largely because of mutations in the 23S rRNA gene [6,38]. Furthermore, only a few mutations (A2143G, A2142G and A2142C) are responsible for almost all cases of clinical resistance [68]. On the other side, for levofloxacin, data have shown that resistance is mostly due to point mutations in the gyrase gene gyrA [67,69,70]. For tetracycline and rifampicin, fewer data are available; however, according to a known fact, resistance to tetracycline is mostly due to mutations in 16SvrRNA genes and to rifampicin due to mutations in the RNA polymerase gene rpoB [68]. Resistance to amoxicillin, as a basic antibiotic in most of the eradication regimens, is rare, but complex [6]. Moreover, raising resistance to metronidazole is highly complex [6]. Furthermore, metronidazole resistance in vitro may not correspond to what happens in vivo and bismuth-containing therapy may overcome metronidazole resistance [6,24]. Thus, a lot is expected from whole-genome or focused next-generation sequencing, which could predict antibiotic resistance phenotypes with more precision, even in more complicated cases, such as metronidazole or amoxicillin resistance [6].
Despite technological advancements in the PCR diagnostics of H. pylori infections, the successful integration and application of a susceptibility-guided strategy will depend on the rapidity of the spread and acceptability of these methods in clinical practice.
5. Novelties of the Maastricht VI Guidelines
The latest Maastricht VI guidelines from 2022 recommended routinely preforming susceptibility tests (molecular or culture) if available, even before prescribing first-line treatment, in respect to antibiotic stewardship [6]. However, how this strategy will be used in practice remains to be established. For now, it remains a matter of debate whether it is necessary to subject every patient to an upper endoscopy to provide the sample needed to determine antibiotic susceptibility. We need to point out that determination of antibiotic susceptibility is not only benefit for the specific patient, but it also provides the possibility of evaluating the prevalence of antibiotic resistance in naive patients. In this way, we can create and update data on local resistance to antibiotics and contribute to the formation of optimal and effective eradication regimens in a certain region.
Although in theory the recommended approach, based on susceptibility-guided therapy, seems ideal, it is often difficult to implement it in clinical practice. Upper endoscopy is expensive, invasive, often uncomfortable for the patient, and in the end, not even necessary in all patients with dyspepsia [6].
So far, the non-invasive ‘test and treat’ strategy for patients with dyspepsia has proven to be an acceptable method of selection [71]. In practice, non-invasive methods for the confirmation of H. pylori infections are mostly used, such as the stool antigen test and urea breath test [7]. Nevertheless, a positive result, in accordance with the recent Maastricht recommendations, necessitates that the antibiotic resistance is determined [6]. In theory, this would require an endoscopic examination with a sample collection, which ultimately multiplies tests with a questionable cost–benefit effect.
However, recently, non-invasive methods have been developed, such as PCR test from the stool, which makes it possible to detect antibiotic susceptibility and to avoid the invasiveness of an upper endoscopy [72]. A recent meta-analysis showed further progress in DNA extraction methods from stool samples [73]. The study’s results proved there is a high diagnostic accuracy for detecting clarithromycin resistance in H. pylori-positive patients with a sensitivity of 91% and specificity of 97% [73].
The COVID pandemic certainly contributed to the wider use of PCR technology in everyday practice. Thus, real-time PCRs are available and used in most laboratories worldwide [6].
Recently, Japanese authors published the results of a study in which Smart Gene™, a method based on the concept of point-of-care genetic testing, was used [74]. The results showed that the detection performance of Smart Gene™ was comparable with that of real-time PCR and sequencing analyses [74]. Although it was not compared with cultures, mentioned method is considerably shorter than real-time PCR tests, thus making it more appropriate for everyday practice [74].
6. CYP2C19 Polymorphism
CYP2C19 is the main enzyme in the metabolism of PPI [75]. Furthermore, the effects of PPI are determined by the activity of metabolic enzymes, cytochrome P450 enzymes and CYP2C19 with genetic differences (the homozygous EM (HomEM), heterozygous EM (HetEM) and poor metabolizer)) [76]. Thus, the genetic background of a patient and the pharmacological profile of drug can impact the decision whether we should use different PPIs and/or increase doses of PPIs. CPY2C19 genotyping can provide a useful way to further optimize eradication therapy, in that the CYP2C19 genotype is related to the different abilities of PPIs to inhibit gastric acid secretion [6,15].
A difference between Caucasian and Asian subjects, regarding the frequency of genetic deficiencies, has been recorded [77]. Nearly 20% of the Japanese population lacks CYP2C19 activity, but on the other hand only 3% to 4% of Caucasian population are poor metabolizers [77]. Furthermore, the prevalence rate of HomEM is about 70% for Caucasians, but only 30–40% for Asians [78]. Therefore, geographic differences should be considered when selecting a proton pump inhibitor or its dose for eradication treatments.
A homozygous extensive metabolizer (HomEM) is distinguished by two wild-type alleles (or *1/*1) [79,80]. However, a heterozygous EM (HetEM) is characterized by carrying one loss-of-function (LOF) variant allele (frequently *2 or *3), as opposed to a poor metabolizer (PM), in which two LOF variant alleles are found (*2/*2, or *2/*3) [79,80]. A HomEM metabolizes the PPI at high rates by producing excessive amounts of the enzyme [75]. A HetEM, with one wild type and one mutation type, metabolizes the PPI at moderate rates [75]. The differences between the metabolizers have been demonstrated by studies [81]. The results of a meta-analysis performed by Padol et al. showed a significant difference in H. pylori eradication rates between wild-type individuals and carriers of at least one LOF allele (OR = 2.26; 95% CI: 1.58–2.96; p < 0.0001) [81]. A significant difference was also demonstrated between HomEM and HetEM individuals (OR = 2.79; 95% CI: 1.77–4.41; p < 0.0001) [81]. However, another study analyzed individual agents separately, with significant differences only for omeprazole and lansoprazole, whereas no differences between the genotypes were observed for rabeprazole [82].
However, it is reasonable to assume that increasing the dosage might overcome the problem of CYP2C19 polymorphism. A randomized, open-label study demonstrated that increasing the dosage of omeprazole (from 20 to 40 mg daily) would improve the efficacy of eradication, both in homozygous and heterozygous extensive metabolizers [83]. However, other studies have shown contrary results regarding dose-dependent effectiveness using omeprazole, rabeprazole and lansoprazole [84,85]. Therefore, this strategy needs further analysis.
Another possible method might be performing CYP2C19 genotyping before starting eradication therapy. However, such routine examinations are expensive and are not always available. A meta-analysis including six studies with a total of 1703 patients showed that high-dose PPIs (twice the standard dose) increase cure rates by 6–10% in comparison with standard doses in seven-day triple therapy [86]. Possible strategies to avoid CYP2C19 genotype issues could be: (1) selecting PPIs metabolized by the non-enzymatic pathway or; (2) considering increasing the dose of CYP2C19-sensitive PPIs [75].
7. Future Prospects in the Approach to H. pylori Infections and Treatment
Until now, all valid guidelines have defined an H. pylori infection as an infectious disease that should be treated regardless of the presence of symptoms [6,87,88]. Considering the known, long-term consequences of H. pylori infections, it is to be expected that attitudes about the need to treat these infections will remain unchanged in the future.
The increase in antibiotic resistance limits the number of available empiric therapies, which highlights the need for changing the current approach. Despite numerous attempts and proposals to change therapies, none have so far produced the expected increase in the eradication rate. Nevertheless, targeted therapy in the first line of eradication, based on a previous sensitivity test, represents a reliable and directed therapeutic approach. Eradication rates of targeted therapies often exceed 90%, which is considered successful.
With the exception of cultures and the classic determination of sensitivity to certain antibiotics, PCR technology has brought about new dynamics in diagnostics, which enables the detection of bacteria and resistance to certain antibiotics. In fact, today a PCR is possible not only from a stomach mucosa sample, but also from a stool sample; this makes the process of diagnosing and obtaining targeted information about resistance simpler and more accessible.
However, the use of tailored therapy is largely limited by the availability of the necessary tests, as well as the capabilities of local health systems. To that extent, awareness of the rational use of antibiotics is necessary in everyday work, for example avoiding prescribing clarithromycin to anyone who has taken it before. The extension of the empiric regimen to 14 days, the use of PPIs in a higher dose and the promising role of vonoprazan offer a more optimistic picture for future treatment approaches. Additionally, the discovery of new, broader-spectrum antibiotics, as announced by the WHO, remains imperative.
On the other hand, a completely new approach to H. pylori infections has been made through nanotechnology [89]. Drugs used in eradication treatments have many obstacles on their pathway to the site of action, which can seriously undermine their effectiveness [90]. A possible way to overcome these problems might be emerging nanomedicine based on a nanotechnological approach that may enable the efficient and effective targeted delivery of drugs [90]. The use of nanoparticular systems can be beneficial in the prevention of drug degradation in the gastric lumen, as well as in the optimal medication delivery to highly colonized H. pylori areas in the stomach [91]. Compared to antibiotics, nanoparticular systems are proving not only to be less toxic, but also to have a role in decreasing the bacterial resistance against antibiotics [92].
With the recent advancements in the molecular description of H. pylori pathogenesis, more studies are needed to examine the practical application of nanotechnology in eradication treatments [93]. In Table 1, the possible directions of the personalized approach in the treatment of H. pylori infections are shown.

Table 1.
Future directions of personalized approach in treatment of H. pylori infection.
8. Conclusions
The personalized approach in H. pylori eradication treatments reduces unnecessary antibiotic prescription and has a positive effect by limiting the emergence of antibiotic resistance worldwide. Additionally, it allows us to form resistance surveys over time. However, it should be noted that the use of tailored therapy may be limited by availability and also sustainability in some areas. In our opinion, future treatment recommendations for the eradication of H. pylori infections should be based on a personalized approach whenever possible.
Author Contributions
Conceptualization, A.M. and N.P.; methodology, A.M., Z.S. and M.K.; investigation, A.M. and N.P.; writing—original draft preparation, A.M. and N.P.; writing—review and editing, M.K., A.T. and J.B.; visualization, A.M. and Z.S.; supervision, A.T. and J.B.; project administration, A.T. and J.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
Randomized clinical trials comparing tailored and empirical therapy.
Table A1.
Randomized clinical trials comparing tailored and empirical therapy.
| Authors | Year | Country | N | Susceptibility Testing | Tailored Therapy | Empirical Therapy | Results Tailored vs. Empirical | Comments | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Regime (PPI Included) | Duration (Days) | Regime (PPI Included) | Duration (Days) | |||||||
| Delchier et al. [43] | 2019 | France | 526 | GenoT ype HelicoDR® PCR | AM (1000 mg bd) + CL (500 mg bd) AM (1000 mg bd) + LE (250 mg bd) AM (1000 mg bd) + MT (500 mg bd) | 7–14 | AM (1000 mg bd) + CL (500 mg bd) | 7 | ITT: 85.5% vs. 73.1%, p = 0.003 PP: 74.4% vs. 86.5%, P = 0.003 | tailored therapy superior |
| Dong et al. [44] | 2015 | China | 90 | Culture with E test and PCR | AM + CL + BI AM + BI + LE AM + BI + FU BI + CL + MT (doses n/a) | 7 | BI (600 mg bd) + AM (1000 mg bd) + CL (500 mg bd) | 7 | ITT: 91.1 % vs. 73.3%, p = 0.027 PP: 96.3% vs. 78.6%, p = 0.021 | tailored therapy superior |
| Zhou et al. [40] | 2016 | China | 1050 | Culture with E test | AM (1000 mg bd) + CL (500 mg bd) AM (1000 mg tid) + TI (500 mg tid) | 10 | AM (1000 mg bd) + CL (500 mg bd) + BI (220 mg bd) AM (1000 mg bd) + CL (500 mg bd) + TI (500 mg bd) | 10 | ITT: 88.7% vs. 77.4% vs. 78.3 %, p < 0.001 PP: 93.3% vs. 87.0% vs. 87.4%, p = 0.021 | tailored therapy superior than triple plus bismuth and concomitant groups |
| Chen et al. [46] | 2019 | China | 382 | Culture with agar dilution | AM (1000 mg bd) + CL (500 mg bd) AM (1000 mg bd) + MT (400 mg bd) AM (1000 mg bd) + LE (500 mg bd) AM (1000 mg bd) + BI (600 mg bd) + MT (400 mg qid) | 14 | AM (1000 mg tid) + MT (400 mg tid) + BI (600 mg bd) | 14 | ITT: 91.6% vs. 85.4%, p = 0.12 PP: 97.7% vs. 97.6%, p = 1.00 | no significant difference |
| Ong et al. [47] | 2019 | South Korea | 397 | Culture with agar dilution and PCR | AM (1000 mg bd) + CL (500 mg bd) AM (1000 mg bd) + MT (500 mg bd) | 14 | AM (1000 mg bd) + CL (500 mg bd) + MT (500 mg bd) | 14 | ITT: 86.2% vs. 81.6%, p = 0.132 PP: 90.2% vs. 86.5%, p = 0.179 | no significant difference |
| Pan et al. [48] | 2020 | China | 467 | Culture with agar dilution | AM (1000 mg bd) + CL (500 mg bd) AM (1000 mg bd) + LE (200 mg bd) AM (1000 mg bd) + FU (100 mg bd) BI (200–220 mg bd) + AM (100 mg bd)/CL (500 mg bd)/LE (200 mg bd)/FU (100 mg bd) * | 14 | AM (1000 mg bd) + CL (500 mg bd) + BI (200–220 mg bd) | 14 | ITT: 85.99% vs. 67.32% vs. 63.69%, p < 0.001 PP: 91.22% vs. 74.64% vs. 68.49%, p < 0.001 | no significant differences between tailored triple therapy and empirical bismuth therapy tailored bismuth therapy was the most efficacious regimen |
| Perkovic et al. [12] | 2021 | Croatia | 80 | Culture with E test | AM/CL/MT/ TT/LE * (doses n/a) | 14 | AM (1000 mg bd) + CL (500 mg bd) + MT (500 mg bd) | 14 | ITT= 92.5% vs. 70%, p = 0.010 PP = 100% vs. 87.5%, p = 0.030 | tailored therapy superior |
| Cho et al. [49] | 2021 | South Korea | 282 | dual priming oligonucleotide-based PCR | PPI + AM + CL PPI + TT + MT + BI (doses n/a) | 7 | AM (1000 mg bd) + MT (750 mg bd) + BI (600 mg bd) | 14 | ITT: 80.9% vs. 85.8%, p = 0.262 PP: 89.0% vs. 93.5%, p = 0.198 | empirical bismuth therapy exhibits similar efficacy and improved cost-effectiveness compared to tailored |
| Choi et al. [51] | 2021 | South Korea | 217 | dual-priming oligonucleotide-based PCR | MT (500 mg bd) + BI (300 mg qid) + TT (500 mg qid) AM (1000 mg bd) + CL (500 mg bd) | 10 - 14 | AM (1000 mg bd) + CL (500 mg bd) + MT (500 mg bd) | 10 | ITT: 82.7% vs. 82.2%, p = 095 PP: 90.1% vs. 91.6%, p = 0.72 | no significant difference in eradication rates; adverse events were significantly lower in tailored group |
| Kim et al. [50] | 2022 | South Korea | 290 | dual-priming oligonucleotide-based PCR | AM (1000 mg bd) + CL (500 mg bd) MT (500 mg tid) + BI (120 mg qid) + TT (500 mg qid) | 14 | AM (1000 mg bd) + CL (500 mg bd) + MT (500 mg bd) | 14 | ITT: 85.5% vs. 82.8%, p = 0.520 PP: 94.6% vs. 88.6%, p = 0.084 | empirical and tailored therapy showed similar overall eradication rates |
* Two of the listed antibiotics were given, based on the susceptibility testing results. Abbreviations: PPI, proton pump inhibitor; AM, amoxicillin; CL, clarithromycin; MT, metronidazole; LE, levofloxacin; BI, bismuth; FU, furazolidone; TI, tinidazole; TT, tetracycline; PCR, polymerase chain reaction; ITT, intention-to-treat; PP, per-protocol; bd, twice a day; tid, three times a day; qid; four times a day; n/a, not available.
References
- Fischbach, W.; Malfertheiner, P. Helicobacter pylori infection. Dtsch. Arztebl. Int. 2018, 115, 429–436. [Google Scholar] [CrossRef] [PubMed]
- IARC. Working Group on the Evaluation of Carcinogenic Risks to Humans. Helicobacter pylori. In Schistosomes, Liver Flukes and Helicobacter Pylori Views and Expert Opinions of an IARC Working Group on the Evaluation of Carcinogenic Risks to Humans; IARC: Lyon, France, 1994; pp. 177–240. [Google Scholar]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Yamaguchi, S.; Mashiba, H.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 34, 784–789. [Google Scholar] [CrossRef]
- Yeo, Y.H.; Shiu, S.I.; Ho, H.J.; Zou, B.; Lin, J.T.; Wu, M.S.; Liou, J.M.; Wu, C.Y. First-line Helicobacter pylori eradication therapies in countries with high and low clarithromycin resistance: A systematic review and network meta-analysis. Gut 2018, 67, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Savoldi, A.; Carrara, E.; Graham, D.Y.; Conti, M.; Tacconelli, E. Prevalence of Antibiotic Resistance in Helicobacter pylori: A Systematic Review and Meta-analysis in World Health Organization Regions. Gastroenterology 2018, 155, 1372–1382. [Google Scholar] [CrossRef]
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.M.; Schulz, C.; Gasbarrini, A.; Hunt R., H.; Leja, M.; O'Morain, C.; et al. European Helicobacter and Microbiota Study group. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipres, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—The Maastricht V/Florence Consensus Report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed]
- Mestrovic, A.; Perkovic, N.; Bozic, J.; Pavicic Ivelja, M.; Vukovic, J.; Kardum, G.; Puljiz, Z.; Tonkic, A. Randomised clinical trial comparing concomitant and hybrid therapy for eradication of Helicobacter pylori infection. PLoS ONE 2020, 15, e0244500. [Google Scholar] [CrossRef]
- Hirata, K.; Suzuki, H.; Matsuzaki, J.; Masaoka, T.; Saito, Y.; Nishizawa, T.; Iwasaki, E.; Fukuhara, S.; Okada, S.; Hibi, T. Improvement of reflux symptom related quality of life after Helicobacter pylori eradication therapy. J. Clin. Biochem. Nutr. 2013, 52, 172–178. [Google Scholar] [CrossRef]
- Taguchi, H.; Kanmura, S.; Maeda, T.; Iwaya, H.; Arima, S.; Sasaki, F.; Nasu, Y.; Tanoue, S.; Hashimoto, S.; Ido, A. Helicobacter pylori eradication improves the quality of life regardless of the treatment outcome: A multicenter prospective cohort study. Medicine 2017, 96, e9507. [Google Scholar] [CrossRef] [PubMed]
- Mestrovic, A.; Bozic, J.; Vukojevic, K.; Tonkic, A. Impact of Different Helicobacter pylori Eradication Therapies on Gastrointestinal Symptoms. Medicina 2021, 57, 803. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, N.; Mestrovic, A.; Bozic, J.; Ivelja, M.P.; Vukovic, J.; Kardum, G.; Sundov, Z.; Tonkic, M.; Puljiz, Z.; Vukojevic, K.; et al. Randomized Clinical Trial Comparing Concomitant and Tailored Therapy for Eradication of Helicobacter pylori Infection. J. Pers. Med. 2021, 11, 534. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Fischbach, L. Helicobacter pylori infection. N. Engl. J. Med. 2010, 363, 595–596. [Google Scholar] [PubMed]
- Gisbert, J.P.; Calvet, X. Review article: The effectiveness of standard triple therapy for Helicobacter pylori has not changed over the last decade, but it is not good enough. Aliment. Pharmacol. Ther. 2011, 34, 1255–1268. [Google Scholar] [CrossRef] [PubMed]
- Furuta, T.; Shirai, N.; Sugimoto, M.; Nakamura, A.; Hishida, A.; Ishizaki, T. Influence of CYP2C19 pharmacogenetic polymorphism on proton pump inhibitor-based therapies. Drug Metab. Pharmacokinetics 2005, 20, 153–167. [Google Scholar] [CrossRef]
- Lima, J.J.; Thomas, C.D.; Barbarino, J.; Desta, Z.; Van Driest, S.L.; El Rouby, N.; Johnson, J.A.; Cavallari, L.H.; Shakhnovich, V.; Thacker, D.L.; et al. Clinical Pharmacogenetics Implementation Consortium (CPIC) Guideline for CYP2C19 and Proton Pump Inhibitor Dosing. Clin. Pharmacol. Ther. 2021, 109, 1417–1423. [Google Scholar] [CrossRef]
- Roszczenko-Jasińska, P.; Wojtyś, M.I.; Jagusztyn-Krynicka, E.K. Helicobacter pylori treatment in the post-antibiotics era-searching for new drug targets. Appl. Microbiol. Biotechnol. 2020, 104, 9891–9905. [Google Scholar] [CrossRef]
- Nyssen, O.P.; Bordin, D.; Tepes, B.; Pérez-Aisa, Á.; Vaira, D.; Caldas, M.; Bujanda, L.; Castro-Fernandez, M.; Lerang, F.; Leja, M.; et al. European Registry on Helicobacter pylori management (Hp-EuReg): Patterns and trends in first-line empirical eradication prescription and outcomes of 5 years and 21,533 patients. Gut 2021, 70, 40–54. [Google Scholar] [CrossRef]
- Graham, D.Y.; Lu, H.; Shiotani, A. Vonoprazan-containing Helicobacter pylori triple therapies contribution to global antimicrobial resistance. J. Gastroenterol. Hepatol. 2021, 36, 1159–1163. [Google Scholar] [CrossRef]
- Chey, W.D.; Mégraud, F.; Laine, L.; López, L.J.; Hunt, B.J.; Howden, C.W. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology 2022, 163, 608–619. [Google Scholar] [CrossRef]
- Zhou, B.G.; Chen, L.X.; Li, B.; Wan, L.Y.; Ai, Y.W. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: A systematic review and meta-analysis with trial sequential analysis. Helicobacter 2019, 24, e12651. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, C.; Zhao, J.; Zhang, H.; Zhai, Q.; Chen, W. Meta-analysis of the efficacy of probiotic-supplemented therapy on the eradication of H. pylori and incidence of therapy-associated side effects. Microb. Pathog. 2020, 147, 104403. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Wang, B.; Zhou, X.; Wang, F.; Xie, Y.; Zheng, H.; Lv, N. Efficacy and safety of probiotics as adjuvant agents for Helicobacter pylori infection: A meta-analysis. Exp. Ther. Med. 2015, 9, 707–716. [Google Scholar] [CrossRef]
- Mégraud, F. H pylori antibiotic resistance: Prevalence, importance, and advances in testing. Gut 2004, 53, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Megraud, F.; Bruyndonckx, R.; Coenen, S.; Wittkop, L.; Huang, T.D.; Hoebeke, M.; Bénéjat, L.; Lehours, P.; Goossens, H.; Glupczynski, Y.; et al. Helicobacter pylori resistance to antibiotics in Europe in 2018 and its relationship to antibiotic consumption in the community. Gut 2021, 70, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Glupczynski, Y.; Mégraud, F.; Lopez-Brea, M.; Andersen, L.P. European multicentre survey of in vitro antimicrobial resistance in Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 820–823. [Google Scholar] [CrossRef]
- Megraud, F.; Coenen, S.; Versporten, A.; Kist, M.; Lopez-Brea, M.; Hirschl, A.M.; Andersen, L.P.; Goossens, H.; Glupczynski, Y.; Study Group participants. Helicobacter pylori resistance to antibiotics in Europe and its relationship to antibiotic consumption. Gut 2013, 62, 34–42. [Google Scholar] [CrossRef]
- Jukic, I.; Vukovic, J.; Rusic, D.; Bozic, J.; Bukic, J.; Leskur, D.; Seselja Perisin, A.; Modun, D. Adherence to Maastricht V/Florence consensus report for the management of Helicobacter pylori infection among primary care phy-sicians and medical students in Croatia: A cross-sectional study. Helicobacter 2021, 26, e12775. [Google Scholar] [CrossRef]
- Gisbert, J.P.; McNicholl, A.G. Optimization strategies aimed to increase the efficacy of H. pylori eradication therapies. Helicobacter 2017, 22, 12392. [Google Scholar] [CrossRef]
- Gisbert, J.P. Optimization strategies aimed to increase the efficacy of Helicobacter pylori eradication therapies with quinolones. Molecules 2020, 25, 5084. [Google Scholar] [CrossRef]
- Khan, S.; Hasan, S.S.; Bond, S.E.; Conway, B.R.; Aldeyab, M.A. Antimicrobial consumption in patients with COVID-19: A systematic review and meta-analysis. Expert Rev. Anti-Infect. Ther. 2022, 20, 749–772. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Shiotani, A.; Graham, D.Y. Current and Future Treatment of Helicobacter pylori Infections. Adv. Exp. Med. Biol. 2019, 1149, 211–225. [Google Scholar] [PubMed]
- Fallone, C.A.; Chiba, N.; Van Zanten, S.V.; Fischbach, L.; Gisbert, J.P.; Hunt, R.H.; Jones, N.L.; Render, C.; Leontiadis, G.I.; Moayyedi, P.; et al. The Toronto consensus for the treatment of Helicobacter pylori infection in adults. Gastroenterology 2016, 151, 51–69. [Google Scholar] [CrossRef] [PubMed]
- Liou, J.M.; Chen, P.Y.; Kuo, Y.T.; Wu, M.S. Toward population specific and personalized treatment of Helicobacter pylori infection. J. Biomed. Sci. 2018, 25, 70. [Google Scholar] [CrossRef]
- Megraud, F.; Lehours, P. Helicobacter pylori detection and antimicrobial susceptibility testing. Clin. Microbiol. Rev. 2007, 20, 280–322. [Google Scholar] [CrossRef]
- Liou, J.M.; Wu, M.S.; Lin, J.T. Treatment of Helicobacter pylori infection: Where are we now? J. Gastroenterol. Hepatol. 2016, 31, 1918–1926. [Google Scholar] [CrossRef]
- Graham, D.Y. Hp-normogram (normo-graham) for assessing the outcome of H. pylori therapy: Effect of resistance, duration, and CYP2C19 genotype. Helicobacter 2016, 21, 85–90. [Google Scholar] [CrossRef]
- Romano, M.; Gravina, A.G.; Nardone, G.; Federico, A.; Dallio, M.; Martorano, M.; Mucherino, C.; Romiti, A.; Avallone, L.; Granata, L. Non-bismuth and bismuth quadruple therapies based on previous clarithromycin exposure are as effective and safe in an area of high clarithromycin resistance: A real-life study. Helicobacter 2020, 25, e12694. [Google Scholar] [CrossRef]
- Chen, H.; Dang, Y.; Zhou, X.; Liu, B.; Liu, S.; Zhang, G. Tailored Therapy Versus Empiric Chosen Treatment for Helicobacter pylori Eradication: A Meta-Analysis. Medicine 2016, 95, e2750. [Google Scholar] [CrossRef]
- Ma, Q.; Li, H.; Liao, J.; Cai, Z.; Zhang, B. Tailored therapy for Helicobacter pylori eradication: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 908202. [Google Scholar] [CrossRef]
- Gingold-Belfer, R.; Niv, Y.; Schmilovitz-Weiss, H.; Levi, Z.; Boltin, D. Susceptibility-guided versus empirical treatment for Helicobacter pylori infection: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 2021, 36, 2649–2658. [Google Scholar] [CrossRef]
- Delchier, J.C.; Bastuji-Garin, S.; Raymond, J.; Megraud, F.; Amiot, A.; Cambau, E.; Burucoa, C.; HELICOSTIC Study Group. Efficacy of a tailored PCR-guided triple therapy in the treatment of Helicobacter pylori infection. Med. Mal. Infect. 2020, 50, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Ji, D.; Huang, R.; Zhang, F.; Huang, Y.; Xiang, P.; Kong, M.; Nan, L.; Zeng, X.; Wu, Y. Multiple Genetic Analysis System-Based Antibiotic Susceptibility Testing in Helicobacter pylori and High Eradication Rate with Phenotypic Resistance-Guided Quadruple Therapy. Medicine 2015, 94, e2056. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhang, J.; Song, Z.; He, L.; Li, Y.; Qian, J.; Bai, P.; Xue, Y.; Wang, Y.; Lin, S. Tailored versus Triple plus Bismuth or Concomitant Therapy as Initial Helicobacter pylori Treatment: A Randomized Trial. Helicobacter 2016, 21, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Long, X.; Ji, Y.; Liang, X.; Li, D.; Gao, H.; Xu, B.; Liu, M.; Chen, Y.; Sun, Y.; et al. Randomised controlled trial: Susceptibility-guided therapy versus empiric bismuth quadruple therapy for first-line Helicobacter pylori treatment. Aliment. Pharmacol. Ther. 2019, 49, 1385–1394. [Google Scholar] [CrossRef]
- Ong, S.; Kim, S.E.; Kim, J.H.; Yi, N.H.; Kim, T.Y.; Jung, K.; Park, M.I.; Jung, H.Y. Helicobacter pylori eradication rates with concomitant and tailored therapy based on 23S rRNA point mutation: A multicenter randomized controlled trial. Helicobacter 2019, 24, e12654. [Google Scholar] [CrossRef]
- Pan, J.; Shi, Z.; Lin, D.; Yang, N.; Meng, F.; Lin, L.; Jin, Z.; Zhou, Q.; Wu, J.; Zhang, J.; et al. Is tailored therapy based on antibiotic susceptibility effective? A multicenter, open-label, randomized trial. Front. Med. 2020, 14, 43–50. [Google Scholar] [CrossRef]
- Cho, J.H.; Jin, S.Y.; Park, S. Comparison of tailored Helicobacter pylori eradication versus modified bismuth quadruple therapy in Korea: A randomized controlled trial. Expert Rev. Anti-Infect. Ther. 2022, 20, 923–929. [Google Scholar] [CrossRef]
- Kim, S.J.; Jee, S.R.; Park, M.I.; Jung, K.; Kim, G.H.; Lee, M.W.; Lee, J.; Jang, J.S.; Koh, M.; Busan and Gyeongnam Society of Helicobacter and Upper Gastrointestinal Research. Busan and Gyeongnam Society of Helicobacter and Upper Gastrointestinal Research. A randomized controlled trial to compare Helicobacter pylori eradication rates between the empirical concomitant therapy and tailored therapy based on 23S rRNA point mutations. Medicine 2022, 101, e30069. [Google Scholar] [CrossRef]
- Choi, Y.I.; Chung, J.W.; Kim, K.O.; Kwon, K.A.; Kim, Y.J.; Kim, J.H.; Seo, J.Y.; Park, D.K. Tailored eradication strategy vs concomitant therapy for Helicobacter pylori eradication treatment in Korean patients. World J. Gastroenterol. 2021, 27, 5247–5258. [Google Scholar] [CrossRef]
- Vrebalov Cindro, P.; Bukic, J.; Pranic, S.; Leskur, D.; Rusic, D.; Seselja Perisin, A.; Božić, J.; Vuković, J.; Modun, D. Did an introduction of CONSORT for abstracts guidelines improve reporting quality of randomised controlled trials’ abstracts on Helicobacter pylori infection? Observational study. BMJ Open 2022, 12, e054978. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y.; Runke, D.; Anderson, S.Y.; Malaty, H.M.; Klein, P.D. Citric acid as the test meal for the 13C-urea breath test. Am. J. Gastroenterol. 1999, 94, 1214–1217. [Google Scholar] [CrossRef]
- Leodolter, A.; Domínguez-Muñoz, J.E.; Von Arnim, U.; Malfertheiner, P. Citric acid or orange juice for the 13C-urea breath test: The impact of pH and gastric emptying. Aliment. Pharmacol. Ther. 1999, 13, 1057–1062. [Google Scholar] [CrossRef] [PubMed]
- Gisbert, J.P.; de la Morena, F.; Abraira, V. Accuracy of monoclonal stool antigen test for the diagnosis of H. pylori infection: A systematic review and meta-analysis. Am. J. Gastroenterol. 2006, 101, 1921–1930. [Google Scholar] [CrossRef]
- Lario, S.; Ramírez-Lázaro, M.J.; Montserrat, A.; Quílez, M.E.; Junquera, F.; Martínez-Bauer, E.; Sanfeliu, I.; Brullet, E.; Campo, R.; Segura, F.; et al. Diagnostic accuracy of three monoclonal stool tests in a large series of untreated Helicobacter pylori infected patients. Clin. Biochem. 2016, 49, 682–687. [Google Scholar] [CrossRef]
- Pohl, D.; Keller, P.M.; Bordier, V.; Wagner, K. Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J. Gastroenterol. 2019, 25, 4629–4660. [Google Scholar] [CrossRef]
- Monteiro, L.; de Mascarel, A.; Sarrasqueta, A.M.; Bergey, B.; Barberis, C.; Talby, P.; Roux, D.; Shouler, L.; Goldfain, D.; Lamouliatte, H.; et al. Diagnosis of Helicobacter pylori infection: Noninvasive methods compared to invasive methods and evaluation of two new tests. Am. J. Gastroenterol. 2001, 9, 353–358. [Google Scholar] [CrossRef]
- Tseng, C.A.; Wang, W.M.; Wu, D.C. Comparison of the clinical feasibility of three rapid urease tests in the diagnosis of Helicobacter pylori infection. Dig. Dis. Sci. 2005, 50, 449–452. [Google Scholar] [CrossRef]
- Li, Y.; Rimbara, E.; Thirumurthi, S.; Trespalacios, A.; Reddy, R.; Sabounchi, S.; Attumi, T.A.; Graham, D.Y. Detection of clarithromycin resistance in Helicobacter pylori following noncryogenic storage of rapid urease tests for 30 days. J. Dig. Dis. 2012, 13, 54–59. [Google Scholar] [CrossRef]
- Chung, W.C.; Jung, S.H.; Oh, J.H.; Kim, T.H.; Cheung, D.Y.; Kim, B.W.; Kim, S.S.; Kim, J.I.; Sin, E.Y. Dual-priming oligonucleotide-based multiplex PCR using tissue samples in rapid urease test in the detection of Helicobacter pylori infection. World J. Gastroenterol. 2014, 20, 6547–6553. [Google Scholar] [CrossRef]
- Pimentel-Nunes, P.; Libânio, D.; Lage, J.; Abrantes, D.; Coimbra, M.; Esposito, G.; Hormozdi, D.; Pepper, M.; Drasovean, S.; White, J.R.; et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy 2016, 48, 723–730. [Google Scholar] [CrossRef]
- Marcos, P.; Brito-Gonçalves, G.; Libânio, D.; Pita, I.; Castro, R.; Sá, I.; Dinis-Ribeiro, M.; Pimentel-Nunes, P. Endoscopic grading of gastric intestinal metaplasia on risk assessment for early gastric neoplasia: Can we replace histology assessment also in the West? Gut 2020, 69, 1762–1768. [Google Scholar] [CrossRef]
- Ogata, S.K.; Gales, A.C.; Kawakami, E. Antimicrobial susceptibility testing for Helicobacter pylori isolates from Brazilian children and adolescents: Comparing agar dilution, E-test, and disk diffusion. Braz. J. Microbiol. 2014, 45, 1439–1448. [Google Scholar] [CrossRef]
- Liou, J.M.; Chang, C.Y.; Sheng, W.H.; Wang, Y.C.; Chen, M.J.; Lee, Y.C.; Hung, H.W.; Chian, H.; Chang, S.C.; Wu, M.S.; et al. Genotypic resistance in Helicobacter pylori strains correlates with susceptibility test and treatment outcomes after levofloxacin- and clarithromycin-based therapies. Antimicrob. Agents Chemother. 2011, 55, 1123–1129. [Google Scholar] [CrossRef]
- Chen, J.; Ye, L.; Jin, L.; Xu, X.; Xu, P.; Wang, X.; Li, H. Application of next generation sequencing to characterize novel mutations in clarithromycin susceptible Helicobacter pylori strains with A2143G of 23S rRNA gene. Ann. Clin. Microbiol. Antimicrob. 2018, 17, 10. [Google Scholar] [CrossRef]
- Nezami, B.G.; Jani, M.; Alouani, D.; Rhoads, D.D.; Sadri, N. Helicobacter pylori mutations detected by next-generation sequencing in formalinfixed, paraffin-embedded gastric biopsy specimens are associated with treatment failure. J. Clin. Microbiol. 2018, 57, e01834-18. [Google Scholar]
- Hu, Y.; Zhang, M.; Lu, B.; Dai:, J. Helicobacter pylori and Antibiotic Resistance, A Continuing and Intractable Problem. Helicobacter 2016, 21, 349–363. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Li, Z.; Wang, L.; Zhu-Ge, L.Y.; Zhao, R.L.; Wu, S.; Wang, Y.; An, Y.; Xie, Y. A systematic review and meta-analysis of genotypic methods for detecting antibiotic resistance in Helicobacter pylori. Helicobacter 2018, 23, e12467. [Google Scholar] [CrossRef]
- Egli, K.; Wagner, K.; Keller, P.M.; Risch, L.; Risch, M.; Bodmer, T. Comparison of the Diagnostic Performance of qPCR, Sanger Sequencing, and Whole-Genome Sequencing in Determining Clarithromycin and Levofloxacin Resistance in Helicobacter pylori. Front. Cell. Infect. Microbiol. 2020, 10, 596371. [Google Scholar] [CrossRef]
- Beresniak, A.; Malfertheiner, P.; Franceschi, F.; Liebaert, F.; Salhi, H.; Gisbert, J.P. Helicobacter pylori “Test-and-Treat” strategy with urea breath test: A cost-effective strategy for the management of dyspepsia and the prevention of ulcer and gastric cancer in Spain-Results of the Hp-Breath initiative. Helicobacter 2020, 25, e12693. [Google Scholar] [CrossRef]
- Graham, D.Y.; Moss, S.F. Antimicrobial susceptibility testing for Helicobacter pylori is now widely available: When, how, why. Am. J. Gastroenterol. 2022, 117, 524–528. [Google Scholar] [CrossRef]
- Gong, R.J.; Xu, C.X.; Li, H.; Liu, X.M. Polymerase chain reaction-based tests for detecting Helicobacter pylori clarithromycin resistance in stool samples: A meta-analysis. World J. Clin. Cases 2021, 9, 133–147. [Google Scholar] [CrossRef]
- Kakiuchi, T.; Okuda, M.; Matsuo, M.; Fujimoto, K. Smart Gene™ as an effective non-invasive point-of-care test to detect Helicobacter pylori clarithromycin-resistant mutation. J. Gastroenterol. Hepatol. 2022, 37, 1719–1725. [Google Scholar] [CrossRef]
- Kuo, C.H.; Lu, C.Y.; Shih, H.Y.; Liu, C.J.; Wu, M.C.; Hu, H.M.; Hsu, W.H.; Yu, F.J.; Wu, D.C.; Kuo, F.C. CYP2C19 polymorphism influences Helicobacter pylori eradication. World J. Gastroenterol. 2014, 20, 16029–16036. [Google Scholar] [CrossRef]
- Ma Win, T.M.; Htun, M.; Phyu Myint, W.P.; Aung, M.M.; Ni, N. High-dose dual therapy and CYP2C19 polymorphism in Helicobacter pylori eradication. GastroHep 2021, 3, 379–383. [Google Scholar] [CrossRef]
- Kuo, C.H.; Wang, S.S.; Hsu, W.H.; Kuo, F.C.; Weng, B.C.; Li, C.J.; Hsu, P.I.; Chen, A.; Hung, W.C.; Yang, Y.C.; et al. Rabeprazole can overcome the impact of CYP2C19 polymorphism on quadruple therapy. Helicobacter 2010, 15, 265–272. [Google Scholar] [CrossRef]
- Ishizaki, T.; Sohn, D.R.; Kobayashi, K.; Chiba, K.; Lee, K.H.; Shin, S.G.; Andersson, T.; Regårdh, C.G.; Lou, Y.C.; Zhang, Y.; et al. Interethnic differences in omeprazole metabolism in the two S-mephenytoin hydroxylation phenotypes studied in Caucasians and Orientals. Ther. Drug Monit. 1994, 16, 214–215. [Google Scholar] [CrossRef]
- Tomalik-Scharte, D.; Lazar, A.; Fuhr, U.; Kirchheiner, J. The clinical role of genetic polymorphisms in drug-metabolizing enzymes. Pharm. J. 2008, 8, 4–15. [Google Scholar] [CrossRef]
- Hagymási, K.; Müllner, K.; Herszényi, L.; Tulassay, Z. Update on the pharmacogenomics of proton pump inhibitors. Pharmacogenomics 2011, 12, 873–888. [Google Scholar] [CrossRef]
- Padol, S.; Yuan, Y.; Thabane, M.; Padol, I.T.; Hunt, R.H. The effect of CYP2C19 polymorphisms on H. pylori eradication rate in dual and triple first-line PPI therapies: A meta-analysis. Am. J. Gastroenterol. 2006, 101, 1467–1475. [Google Scholar] [CrossRef]
- Shi, S.; Klotz, U. Proton pump inhibitors: An update of their clinical use and pharmacokinetics. Eur. J. Clin. Pharmacol. 2008, 64, 935–951. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Wang, H.L.; Chern, H.D.; Shun, C.T.; Lin, B.R.; Lin, C.J.; Wang, T.H. Role of omeprazole dosage and cytochrome P450 2C19 genotype in patients receiving omeprazole-amoxicillin dual therapy for Helicobacter pylori eradication. Pharmacotherapy 2011, 31, 227–238. [Google Scholar] [CrossRef]
- Pan, X.; Li, Y.; Qiu, Y.; Tang, Q.; Qian, B.; Yao, L.; Shi, R.; Zhang, G. Efficacy and tolerability of first-line triple therapy with levofloxacin and amoxicillin plus esomeprazole or rabeprazole for the eradication of Helicobacter pylori infection and the effect of CYP2C19 genotype: A 1-week, randomized, open-label study in Chinese adults. Clin. Ther. 2010, 32, 2003–2011. [Google Scholar]
- Lee, J.H.; Jung, H.Y.; Choi, K.D.; Song, H.J.; Lee, G.H.; Kim, J.H. The Influence of CYP2C19 Polymorphism on Eradication of Helicobacter pylori: A Prospective Randomized Study of Lansoprazole and Rabeprazole. Gut Liver 2010, 4, 201–206. [Google Scholar] [CrossRef]
- Villoria, A.; Garcia, P.; Calvet, X.; Gisbert, J.P.; Vergara, M. Meta-analysis: High-dose proton pump inhibitors vs. standard dose in triple therapy for Helicobacter pylori eradication. Aliment. Pharmacol. Ther. 2008, 28, 868–877. [Google Scholar] [CrossRef]
- Chey, W.D.; Leontiadis, G.I.; Howden, C.W.; Moss, S.F. ACG Clinical Guideline: Treatment of Helicobacter pylori Infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef]
- Sugano, K.; Tack, J.; Kuipers, E.J.; Graham, D.Y.; El-Omar, E.M.; Miura, S.; Haruma, K.; Asaka, M.; Uemura, N.; Malfertheiner, P.; et al. Kyoto global consensus report on Helicobacter pylori gastritis. Gut 2015, 64, 1353–1367. [Google Scholar] [CrossRef]
- Safarov, T.; Kiran, B.; Bagirova, M.; Allahverdiyev, A.M.; Abamor, E.S. An overview of nanotechnology-based treatment approaches against Helicobacter Pylori. Expert Rev. Anti-Infect. Ther. 2019, 17, 829–840. [Google Scholar] [CrossRef]
- Qin, Y.; Lao, Y.H.; Wang, H.; Zhang, J.; Yi, K.; Chen, Z.; Han, J.; Song, W.; Tao, Y.; Li, M. Combatting Helicobacter pylori with oral nanomedicines. J. Mater. Chem. B 2021, 9, 9826–9838. [Google Scholar] [CrossRef]
- Lopes, D.; Nunes, C.; Martins, M.C.; Sarmento, B.; Reis, S. Eradication of Helicobacter pylori: Past, present and future. J. Control. Release 2014, 189, 169–186. [Google Scholar] [CrossRef]
- Kroll, A.V.; Fang, R.H.; Zhang, L. Biointerfacing and Applications of Cell Membrane-Coated Nanoparticles. Bioconj. Chem. 2017, 28, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Debraekeleer, A.; Remaut, H. Future perspective for potential Helicobacter pylori eradication therapies. Future Microbiol. 2018, 13, 671–687. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).