Wide-Scope Target and Suspect Screening of Antibiotics in Effluent Wastewater from Wastewater Treatment Plants in Europe
Abstract
1. Introduction
2. Results and Discussion
2.1. Wide-Scope Target and Suspect Screening and Risk Assessment
2.2. Loads of Antibiotics into Freshwater Ecosystems
3. Materials and Methods
3.1. Investigated Samples
3.2. Sample Preparation and Instrumental Analysis
3.3. Quality Assurance and Quality Control
3.4. Suspect Screening and Semi-Quantification Analysis
3.5. Risk Assessment and Prioritization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meek, R.W.; Vyas, H.; Piddock, L.J. Nonmedical Uses of Antibiotics: Time to Restrict Their Use? PLoS Biol. 2015, 13, e1002266. [Google Scholar] [CrossRef] [PubMed]
- Jendrzejewska, N.; Karwowska, E. The influence of antibiotics on wastewater treatment processes and the development of antibiotic-resistant bacteria. Water Sci. Technol. 2018, 77, 2320–2326. [Google Scholar] [CrossRef] [PubMed]
- Grenni, P.; Ancona, V.; Caracciolo, A.B. Ecological effects of antibiotics on natural ecosystems: A review. Microchem. J. 2018, 136, 25–39. [Google Scholar] [CrossRef]
- Berendonk, T.U.; Manaia, C.M.; Merlin, C.; Fatta-Kassinos, D.; Cytryn, E.; Walsh, F.; Burgmann, H.; Sorum, H.; Norstrom, M.; Pons, M.N.; et al. Tackling antibiotic resistance: The environmental framework. Nat. Rev. Microbiol. 2015, 13, 310–317. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Datta, S.; Pal, N.K.; Nandy, A.K. The antibiotic alarm. Nature 2013, 495, 141. [Google Scholar]
- Rosenblatt-Farrell, N. The landscape of antibiotic resistance. Environ. Health Perspect. 2009, 117, A244–A250. [Google Scholar] [CrossRef]
- Read, A.F.; Woods, R.J. Antibiotic resistance management. Evol. Med. Public Health 2014, 2014, 147. [Google Scholar] [CrossRef]
- Spellberg, B.; Gilbert, D.N. The future of antibiotics and resistance: A tribute to a career of leadership by John Bartlett. Clin. Infect. Dis. 2014, 59 (Suppl. 2), S71–S75. [Google Scholar] [CrossRef]
- Mohanty, D. Rational Use of Antibiotics: Time to Join the War Against Superbugs. Indian J. Surg. 2019, 81, 304–305. [Google Scholar] [CrossRef]
- Domingues, C.P.F.; Rebelo, J.S.; Pothier, J.; Monteiro, F.; Nogueira, T.; Dionisio, F. The Perfect Condition for the Rising of Superbugs: Person-to-Person Contact and Antibiotic Use Are the Key Factors Responsible for the Positive Correlation between Antibiotic Resistance Gene Diversity and Virulence Gene Diversity in Human Metagenomes. Antibiotics 2021, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization: Antibiotic Resistance. 2020. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 15 October 2021).
- Paulus, G.K.; Hornstra, L.M.; Alygizakis, N.; Slobodnik, J.; Thomaidis, N.; Medema, G. The impact of on-site hospital wastewater treatment on the downstream communal wastewater system in terms of antibiotics and antibiotic resistance genes. Int. J. Hyg. Environ. Health 2019, 222, 635–644. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.X.; Aris, A.; Yong, E.L.; Noor, Z.Z. A review of antibiotic removal from domestic wastewater using the activated sludge process: Removal routes, kinetics and operational parameters. Environ. Sci. Pollut. Res. Int. 2022, 29, 4787–4802. [Google Scholar] [CrossRef]
- Elsheikh, A.H.; Saba, A.I.; Panchal, H.; Shanmugan, S.; Alsaleh, N.A.; Ahmadein, M. Artificial Intelligence for Forecasting the Prevalence of COVID-19 Pandemic: An Overview. Healthcare 2021, 9, 1614. [Google Scholar] [CrossRef] [PubMed]
- NORMAN. NORMAN Suspect List Exchange—NORMAN SLE. 2021. Available online: https://www.norman-network.com/nds/SLE/ (accessed on 15 October 2021).
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barcelo, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic residues in final effluents of European wastewater treatment plants and their impact on the aquatic environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Alygizakis, N.A.; Besselink, H.; Paulus, G.K.; Oswald, P.; Hornstra, L.M.; Oswaldova, M.; Medema, G.; Thomaidis, N.S.; Behnisch, P.A.; Slobodnik, J. Characterization of wastewater effluents in the Danube River Basin with chemical screening, in vitro bioassays and antibiotic resistant genes analysis. Environ. Int. 2019, 127, 420–429. [Google Scholar] [CrossRef]
- Wang, K.; Zhuang, T.; Su, Z.; Chi, M.; Wang, H. Antibiotic residues in wastewaters from sewage treatment plants and pharmaceutical industries: Occurrence, removal and environmental impacts. Sci. Total Environ. 2021, 788, 147811. [Google Scholar] [CrossRef]
- Kortesmaki, E.; Ostman, J.R.; Meierjohann, A.; Brozinski, J.M.; Eklund, P.; Kronberg, L. Occurrence of Antibiotics in Influent and Effluent from 3 Major Wastewater-Treatment Plants in Finland. Environ. Toxicol. Chem. 2020, 39, 1774–1789. [Google Scholar] [CrossRef]
- Koch, D.E.; Bhandari, A.; Closb, L.; Hunter, R.P. Azithromycin extraction from municipal wastewater and quantitation using liquid chromatography/mass spectrometry. J. Chromatogr. A 2005, 1074, 17–22. [Google Scholar] [CrossRef]
- Walters, E.; McClellan, K.; Halden, R.U. Occurrence and loss over three years of 72 pharmaceuticals and personal care products from biosolids-soil mixtures in outdoor mesocosms. Water Res. 2010, 44, 6011–6020. [Google Scholar] [CrossRef]
- Sadeghi, M.; Sadeghi, R.; Ghasemi, B.; Mardani, G.; Ahmadi, A. Removal of Azithromycin from Aqueous Solution Using UV- Light Alone and UV Plus Persulfate (UV/Na2S2O8) Processes. Iran. J. Pharm. Res. 2018, 17, 54–64. [Google Scholar] [PubMed]
- Xu, W.; Zhang, G.; Li, X.; Zou, S.; Li, P.; Hu, Z.; Li, J. Occurrence and elimination of antibiotics at four sewage treatment plants in the Pearl River Delta (PRD), South China. Water Res. 2007, 41, 4526–4534. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, P.; Olson, N.D.; Raspanti, G.A.; Rosenberg Goldstein, R.E.; Gibbs, S.G.; Sapkota, A.; Sapkota, A.R. Antibiotic Concentrations Decrease during Wastewater Treatment but Persist at Low Levels in Reclaimed Water. Int. J. Environ. Res. Public Health 2017, 14, 668. [Google Scholar] [CrossRef] [PubMed]
- da Silva Rodrigues, D.A.; da Cunha, C.; do Espirito Santo, D.R.; de Barros, A.L.C.; Pereira, A.R.; de Queiroz Silva, S.; da Fonseca Santiago, A.; de Cassia Franco Afonso, R.J. Removal of cephalexin and erythromycin antibiotics, and their resistance genes, by microalgae-bacteria consortium from wastewater treatment plant secondary effluents. Environ. Sci. Pollut. Res. Int. 2021, 28, 67822–67832. [Google Scholar] [CrossRef]
- Gnida, A.; Felis, E.; Ziembinska-Buczynska, A.; Luczkiewicz, A.; Surmacz-Gorska, J.; Olanczuk-Neyman, K. Evidence of mutations conferring resistance to clarithromycin in wastewater and activated sludge. 3 Biotech 2020, 10, 7. [Google Scholar] [CrossRef]
- Basturk, I.; Varank, G.; Murat-Hocaoglu, S.; Yazici-Guvenc, S.; Can-Güven, E.; Oktem-Olgun, E.E.; Canli, O. Simultaneous degradation of cephalexin, ciprofloxacin, and clarithromycin from medical laboratory wastewater by electro-Fenton process. J. Environ. Chem. Eng. 2021, 9, 104666. [Google Scholar] [CrossRef]
- Kummerer, K.; al-Ahmad, A.; Mersch-Sundermann, V. Biodegradability of some antibiotics, elimination of the genotoxicity and affection of wastewater bacteria in a simple test. Chemosphere 2000, 40, 701–710. [Google Scholar] [CrossRef]
- Brown, K.D.; Kulis, J.; Thomson, B.; Chapman, T.H.; Mawhinney, D.B. Occurrence of antibiotics in hospital, residential, and dairy effluent, municipal wastewater, and the Rio Grande in New Mexico. Sci. Total Environ. 2006, 366, 772–783. [Google Scholar] [CrossRef]
- Jones-Lepp, T.L.; Stevens, R. Pharmaceuticals and personal care products in biosolids/sewage sludge: The interface between analytical chemistry and regulation. Anal. Bioanal. Chem. 2007, 387, 1173–1183. [Google Scholar] [CrossRef]
- Jelić, A.; Gros, M.; Petrović, M.; Ginebreda, A.; Barceló, D. Occurrence and Elimination of Pharmaceuticals During Conventional Wastewater Treatment. In Emerging and Priority Pollutants in Rivers; Guasch, H., Ginebreda, A., Geiszinger, A., Eds.; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–23. [Google Scholar]
- Yu, R.; Wu, Z. High adsorption for ofloxacin and reusability by the use of ZIF-8 for wastewater treatment. Microporous Mesoporous Mater. 2020, 308, 110494. [Google Scholar] [CrossRef]
- Rodrigues-Silva, C.; Porto, R.; dos Santos, S.; Schneider, J.; Rath, S. Fluoroquinolones in Hospital Wastewater: Analytical Method, Occurrence, Treatment with Ozone and Residual Antimicrobial Activity Evaluation. J. Braz. Chem. Soc. 2019, 30, 1447–1458. [Google Scholar] [CrossRef]
- Minato, Y.; Dawadi, S.; Kordus, S.L.; Sivanandam, A.; Aldrich, C.C.; Baughn, A.D. Mutual potentiation drives synergy between trimethoprim and sulfamethoxazole. Nat. Commun. 2018, 9, 1003. [Google Scholar] [CrossRef] [PubMed]
- Eliopoulos, G.M.; Moellering, R.C., Jr. Antibiotic synergism and antimicrobial combinations in clinical infections. Rev. Infect. Dis. 1982, 4, 282–293. [Google Scholar] [CrossRef]
- Xu, X.; Xu, L.; Yuan, G.; Wang, Y.; Qu, Y.; Zhou, M. Synergistic combination of two antimicrobial agents closing each other’s mutant selection windows to prevent antimicrobial resistance. Sci. Rep. 2018, 8, 7237. [Google Scholar] [CrossRef] [PubMed]
- Hegreness, M.; Shoresh, N.; Damian, D.; Hartl, D.; Kishony, R. Accelerated evolution of resistance in multidrug environments. Proc. Natl. Acad. Sci. USA 2008, 105, 13977–13981. [Google Scholar] [CrossRef]
- Pena-Miller, R.; Lahnemann, D.; Schulenburg, H.; Ackermann, M.; Beardmore, R. The optimal deployment of synergistic antibiotics: A control-theoretic approach. J. R. Soc. Interface 2012, 9, 2488–2502. [Google Scholar] [CrossRef] [PubMed]
- Chait, R.; Craney, A.; Kishony, R. Antibiotic interactions that select against resistance. Nature 2007, 446, 668–671. [Google Scholar] [CrossRef] [PubMed]
- Uluseker, C.; Kaster, K.M.; Thorsen, K.; Basiry, D.; Shobana, S.; Jain, M.; Kumar, G.; Kommedal, R.; Pala-Ozkok, I. A Review on Occurrence and Spread of Antibiotic Resistance in Wastewaters and in Wastewater Treatment Plants: Mechanisms and Perspectives. Front. Microbiol. 2021, 12, 717809. [Google Scholar] [CrossRef]
- Agerstrand, M.; Berg, C.; Bjorlenius, B.; Breitholtz, M.; Brunstrom, B.; Fick, J.; Gunnarsson, L.; Larsson, D.G.; Sumpter, J.P.; Tysklind, M.; et al. Improving environmental risk assessment of human pharmaceuticals. Environ. Sci. Technol. 2015, 49, 5336–5345. [Google Scholar] [CrossRef]
- Bengtsson-Palme, J.; Larsson, D.G. Concentrations of antibiotics predicted to select for resistant bacteria: Proposed limits for environmental regulation. Environ. Int. 2016, 86, 140–149. [Google Scholar] [CrossRef]
- Link, M.; von der Ohe, P.C.; Voss, K.; Schafer, R.B. Comparison of dilution factors for German wastewater treatment plant effluents in receiving streams to the fixed dilution factor from chemical risk assessment. Sci. Total Environ. 2017, 598, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Freeling, F.; Alygizakis, N.A.; von der Ohe, P.C.; Slobodnik, J.; Oswald, P.; Aalizadeh, R.; Cirka, L.; Thomaidis, N.S.; Scheurer, M. Occurrence and potential environmental risk of surfactants and their transformation products discharged by wastewater treatment plants. Sci. Total Environ. 2019, 681, 475–487. [Google Scholar] [CrossRef] [PubMed]
- Thomaidis, N.S.; Gago-Ferrero, P.; Ort, C.; Maragou, N.C.; Alygizakis, N.A.; Borova, V.L.; Dasenaki, M.E. Reflection of Socioeconomic Changes in Wastewater: Licit and Illicit Drug Use Patterns. Environ. Sci. Technol. 2016, 50, 10065–10072. [Google Scholar] [CrossRef] [PubMed]
- Schymanski, E.L.; Jeon, J.; Gulde, R.; Fenner, K.; Ruff, M.; Singer, H.P.; Hollender, J. Identifying small molecules via high resolution mass spectrometry: Communicating confidence. Environ. Sci. Technol. 2014, 48, 2097–2098. [Google Scholar] [CrossRef] [PubMed]
- Mohammed Taha, H.; Aalizadeh, R.; Alygizakis, N.; Antignac, J.P.; Arp, H.P.H.; Bade, R.; Baker, N.; Belova, L.; Bijlsma, L.; Bolton, E.E.; et al. The NORMAN Suspect List Exchange (NORMAN-SLE): Facilitating European and worldwide collaboration on suspect screening in high resolution mass spectrometry. Environ. Sci. Eur. 2022, 34, 104. [Google Scholar] [CrossRef] [PubMed]
- Alygizakis, N.A.; Oswald, P.; Thomaidis, N.S.; Schymanski, E.L.; Aalizadeh, R.; Schulze, T.; Oswaldova, M.; Slobodnik, J. NORMAN digital sample freezing platform: A European virtual platform to exchange liquid chromatography high resolution-mass spectrometry data and screen suspects in “digitally frozen” environmental samples. TrAC Trends Anal. Chem. 2019, 115, 129–137. [Google Scholar] [CrossRef]
- Aalizadeh, R.; Alygizakis, N.A.; Schymanski, E.L.; Krauss, M.; Schulze, T.; Ibanez, M.; McEachran, A.D.; Chao, A.; Williams, A.J.; Gago-Ferrero, P.; et al. Development and Application of Liquid Chromatographic Retention Time Indices in HRMS-Based Suspect and Nontarget Screening. Anal. Chem. 2021, 93, 11601–11611. [Google Scholar] [CrossRef]
- Djoumbou-Feunang, Y.; Pon, A.; Karu, N.; Zheng, J.; Li, C.; Arndt, D.; Gautam, M.; Allen, F.; Wishart, D.S. CFM-ID 3.0: Significantly Improved ESI-MS/MS Prediction and Compound Identification. Metabolites 2019, 9, 72. [Google Scholar] [CrossRef]
- Alygizakis, N.; Galani, A.; Rousis, N.I.; Aalizadeh, R.; Dimopoulos, M.A.; Thomaidis, N.S. Change in the chemical content of untreated wastewater of Athens, Greece under COVID-19 pandemic. Sci. Total Environ. 2021, 799, 149230. [Google Scholar] [CrossRef]
- Chen, X.; Reynolds, C.H. Performance of Similarity Measures in 2D Fragment-Based Similarity Searching: Comparison of Structural Descriptors and Similarity Coefficients. J. Chem. Inf. Comput. Sci. 2002, 42, 1407–1414. [Google Scholar] [CrossRef]
- von der Ohe, P.C.; Dulio, V.; Slobodnik, J.; De Deckere, E.; Kuhne, R.; Ebert, R.U.; Ginebreda, A.; De Cooman, W.; Schuurmann, G.; Brack, W. A new risk assessment approach for the prioritization of 500 classical and emerging organic microcontaminants as potential river basin specific pollutants under the European Water Framework Directive. Sci. Total Environ. 2011, 409, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Dulio, V.; von der Ohe, P.C. NORMAN Prioritisation Framework for Emerging Substances. 2013. Available online: http://www.norman-network.net/sites/default/files/norman_prioritisation_manual_15%20April2013_final_for_website.pdf (accessed on 21 October 2021).
- Aalizadeh, R.; von der Ohe, P.C.; Thomaidis, N.S. Prediction of acute toxicity of emerging contaminants on the water flea Daphnia magna by Ant Colony Optimization-Support Vector Machine QSTR models. Environ. Sci. Process Impacts 2017, 19, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Slobodnik, J.; Mrafkova, L.; Carere, M.; Ferrara, F.; Pennelli, B.; Schüürmann, G.; von der Ohe, P.C. Identification of river basin specific pollutants and derivation of environmental quality standards: A case study in the Slovak Republic. TrAC Trends Anal. Chem. 2012, 41, 133–145. [Google Scholar] [CrossRef]
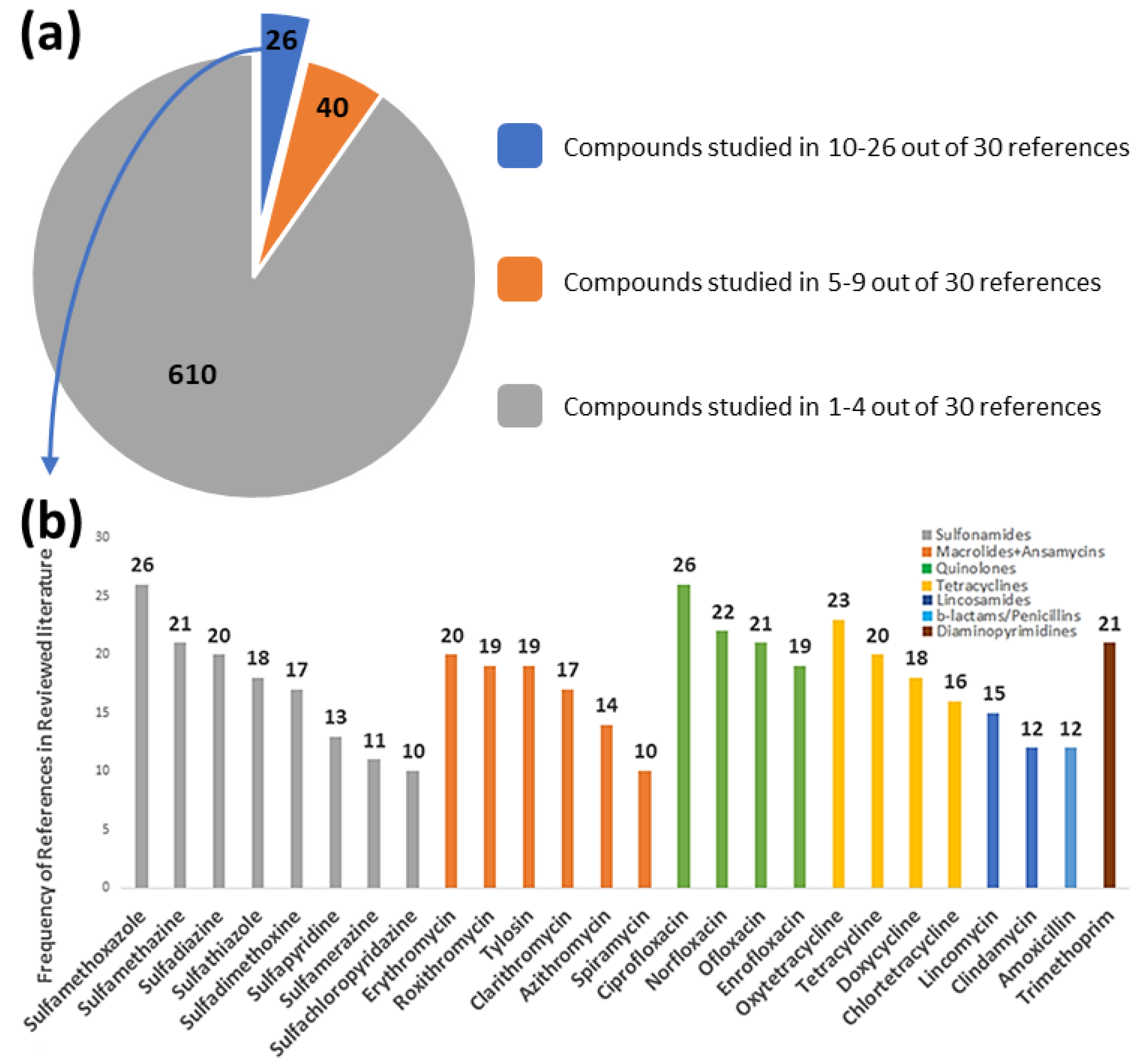
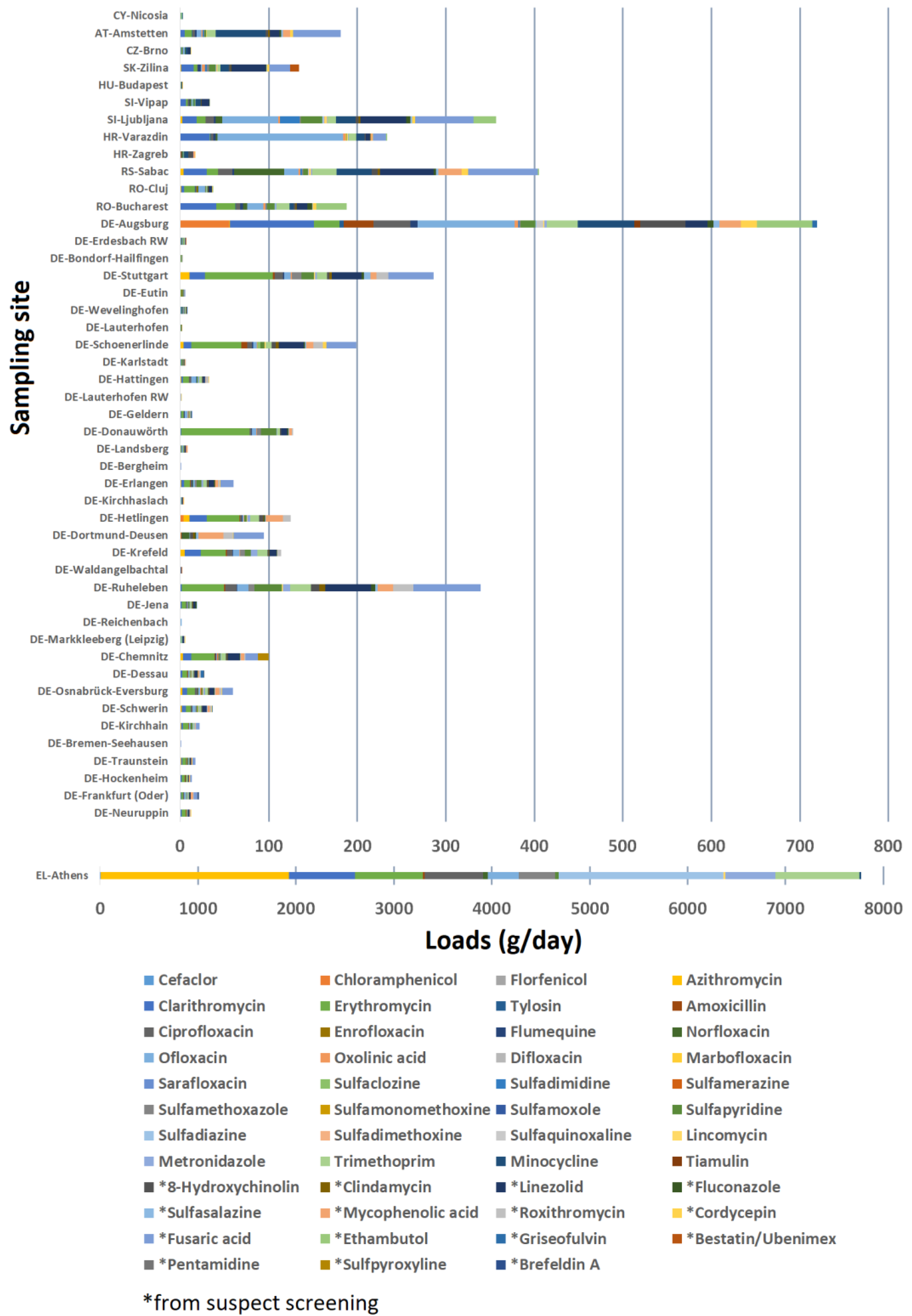
| Antibiotic | InChIKey | Antibiotics Class | FoA | FoE | EoE | Risk Score |
|---|---|---|---|---|---|---|
| Ofloxacin | GSDSWSVVBLHKDQ-UHFFFAOYSA-N | Quinolones | 1.00 | 0.85 | 1.00 | 2.85 |
| Azithromycin | MQTOSJVFKKJCRP-BICOPXKESA-N | Macrolides | 0.83 | 0.63 | 0.43 | 1.89 |
| Erythromycin | ULGZDMOVFRHVEP-RWJQBGPGSA-N | Macrolides | 0.96 | 0.54 | 0.16 | 1.66 |
| Clarithromycin | AGOYDEPGAOXOCK-KCBOHYOISA-N | Macrolides | 0.98 | 0.35 | 0.10 | 1.43 |
| Ciprofloxacin | MYSWGUAQZAJSOK-UHFFFAOYSA-N | Quinolones | 0.96 | 0.10 | 0.03 | 1.09 |
| Sulfapyridine | GECHUMIMRBOMGK-UHFFFAOYSA-N | Sulfonamides | 0.98 | 0.00 | 0.00 | 0.98 |
| Trimethoprim | IEDVJHCEMCRBQM-UHFFFAOYSA-N | Dihydrofolate reductase inhibitor | 0.98 | 0.00 | 0.00 | 0.98 |
| * 8-Hydroxyquinoline | MCJGNVYPOGVAJF-UHFFFAOYSA-N | Other | 0.91 | 0.00 | 0.00 | 0.91 |
| Lincomycin | OJMMVQQUTAEWLP-KIDUDLJLSA-N | Lincosamides | 0.88 | 0.00 | 0.00 | 0.88 |
| Flumequine | DPSPPJIUMHPXMA-UHFFFAOYSA-N | Quinolones | 0.85 | 0.00 | 0.00 | 0.85 |
| * Clindamycin | KDLRVYVGXIQJDK-NOWPCOIGSA-N | Other | 0.83 | 0.04 | 0.01 | 0.84 |
| * Linezolid | TYZROVQLWOKYKF-ZDUSSCGKSA-N | Other | 0.83 | 0.00 | 0.00 | 0.83 |
| * Fluconazole | RFHAOTPXVQNOHP-UHFFFAOYSA-N | Other | 0.79 | 0.00 | 0.00 | 0.83 |
| Metronidazole | VAOCPAMSLUNLGC-UHFFFAOYSA-N | Other | 0.81 | 0.00 | 0.00 | 0.81 |
| Sulfamethoxazole | JLKIGFTWXXRPMT-UHFFFAOYSA-N | Sulfonamides | 0.79 | 0.00 | 0.00 | 0.79 |
| * Sulfasalazine | NCEXYHBECQHGNR-UHFFFAOYSA-N | Sulfonamides | 0.79 | 0.00 | 0.00 | 0.79 |
| Oxolinic acid | KYGZCKSPAKDVKC-UHFFFAOYSA-N | Quinolones | 0.77 | 0.00 | 0.00 | 0.77 |
| * Mycophenolic acid | HPNSFSBZBAHARI-RUDMXATFSA-N | Other | 0.70 | 0.06 | 0.01 | 0.73 |
| * Roxithromycin | RXZBMPWDPOLZGW-XMRMVWPWSA-N | Macrolides | 0.66 | 0.00 | 0.00 | 0.70 |
| Amoxicillin | LSQZJLSUYDQPKJ-NJBDSQKTSA-N | Penicillins | 0.67 | 0.00 | 0.00 | 0.67 |
| * Cordycepin | OFEZSBMBBKLLBJ-BAJZRUMYSA-N | Other | 0.57 | 0.00 | 0.00 | 0.57 |
| * Fusaric acid | DGMPVYSXXIOGJY-UHFFFAOYSA-N | Other | 0.43 | 0.00 | 0.00 | 0.43 |
| Sulfadimidine (Sulfamethazine) | ASWVTGNCAZCNNR-UHFFFAOYSA-N | Sulfonamides | 0.42 | 0.00 | 0.00 | 0.42 |
| Minocycline | DYKFCLLONBREIL-KVUCHLLUSA-N | Tetracycline | 0.29 | 0.04 | 0.01 | 0.34 |
| Florfenicol | AYIRNRDRBQJXIF-NXEZZACHSA-N | Amphenicols | 0.29 | 0.00 | 0.00 | 0.29 |
| Norfloxacin | OGJPXUAPXNRGGI-UHFFFAOYSA-N | Quinolones | 0.27 | 0.00 | 0.00 | 0.27 |
| Sulfadiazine | SEEPANYCNGTZFQ-UHFFFAOYSA-N | Sulfonamides | 0.21 | 0.02 | 0.00 | 0.23 |
| Chloramphenicol | WIIZWVCIJKGZOK-RKDXNWHRSA-N | Amphenicols | 0.21 | 0.00 | 0.00 | 0.21 |
| Cefaclor | QYIYFLOTGYLRGG-GPCCPHFNSA-N | Cefalosporines | 0.21 | 0.00 | 0.00 | 0.21 |
| Sulfamerazine | QPPBRPIAZZHUNT-UHFFFAOYSA-N | Sulfonamides | 0.21 | 0.00 | 0.00 | 0.21 |
| Tylosin | WBPYTXDJUQJLPQ-VMXQISHHSA-N | Macrolides | 0.17 | 0.00 | 0.00 | 0.17 |
| Sulfaclozine | QKLPUVXBJHRFQZ-UHFFFAOYSA-N | Sulfonamides | 0.17 | 0.00 | 0.00 | 0.17 |
| Enrofloxacin | SPFYMRJSYKOXGV-UHFFFAOYSA-N | Quinolones | 0.15 | 0.00 | 0.00 | 0.15 |
| * Ethambutol | AEUTYOVWOVBAKS-UWVGGRQHSA-N | Other | 0.13 | 0.00 | 0.00 | 0.13 |
| Marbofloxacin | BPFYOAJNDMUVBL-UHFFFAOYSA-N | Quinolones | 0.06 | 0.00 | 0.00 | 0.06 |
| Sulfadimethoxine | ZZORFUFYDOWNEF-UHFFFAOYSA-N | Sulfonamides | 0.06 | 0.00 | 0.00 | 0.06 |
| * Griseofulvin | DDUHZTYCFQRHIY-RBHXEPJQSA-N | Other | 0.06 | 0.00 | 0.00 | 0.06 |
| * Bestatin/Ubenimex | VGGGPCQERPFHOB-RDBSUJKOSA-N | Other | 0.06 | 0.00 | 0.00 | 0.06 |
| Sarafloxacin | XBHBWNFJWIASRO-UHFFFAOYSA-N | Quinolones | 0.04 | 0.00 | 0.00 | 0.04 |
| Sulfamonomethoxine | WMPXPUYPYQKQCX-UHFFFAOYSA-N | Sulfonamides | 0.04 | 0.00 | 0.00 | 0.04 |
| Sulfamoxole | CYFLXLSBHQBMFT-UHFFFAOYSA-N | Sulfonamides | 0.04 | 0.00 | 0.00 | 0.04 |
| Sulfaquinoxaline | NHZLNPMOSADWGC-UHFFFAOYSA-N | Sulfonamides | 0.04 | 0.00 | 0.00 | 0.04 |
| Tiamulin | UURAUHCOJAIIRQ-QGLSALSOSA-N | Other | 0.04 | 0.00 | 0.00 | 0.04 |
| * Pentamidine | XDRYMKDFEDOLFX-UHFFFAOYSA-N | Other | 0.04 | 0.00 | 0.00 | 0.04 |
| Difloxacin | NOCJXYPHIIZEHN-UHFFFAOYSA-N | Quinolones | 0.02 | 0.00 | 0.00 | 0.02 |
| * Sulfpyroxyline | FBFBRAFXKGRRHI-UHFFFAOYSA-N | Sulfonamides | 0.02 | 0.00 | 0.00 | 0.02 |
| * Brefeldin A | KQNZDYYTLMIZCT-KQPMLPITSA-N | Other | 0.02 | 0.00 | 0.00 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, K.; Alygizakis, N.A.; Thomaidis, N.S.; Slobodnik, J. Wide-Scope Target and Suspect Screening of Antibiotics in Effluent Wastewater from Wastewater Treatment Plants in Europe. Antibiotics 2023, 12, 100. https://doi.org/10.3390/antibiotics12010100
Ng K, Alygizakis NA, Thomaidis NS, Slobodnik J. Wide-Scope Target and Suspect Screening of Antibiotics in Effluent Wastewater from Wastewater Treatment Plants in Europe. Antibiotics. 2023; 12(1):100. https://doi.org/10.3390/antibiotics12010100
Chicago/Turabian StyleNg, Kelsey, Nikiforos A. Alygizakis, Nikolaos S. Thomaidis, and Jaroslav Slobodnik. 2023. "Wide-Scope Target and Suspect Screening of Antibiotics in Effluent Wastewater from Wastewater Treatment Plants in Europe" Antibiotics 12, no. 1: 100. https://doi.org/10.3390/antibiotics12010100
APA StyleNg, K., Alygizakis, N. A., Thomaidis, N. S., & Slobodnik, J. (2023). Wide-Scope Target and Suspect Screening of Antibiotics in Effluent Wastewater from Wastewater Treatment Plants in Europe. Antibiotics, 12(1), 100. https://doi.org/10.3390/antibiotics12010100







