Phytochemical Profiling, In Vitro Biological Activities, and In-Silico Studies of Ficus vasta Forssk.: An Unexplored Plant
Abstract
1. Introduction
2. Results
2.1. Phytochemical Analysis
2.1.1. Preliminary Phytochemical Profiling
2.1.2. Total Bioactive Contents
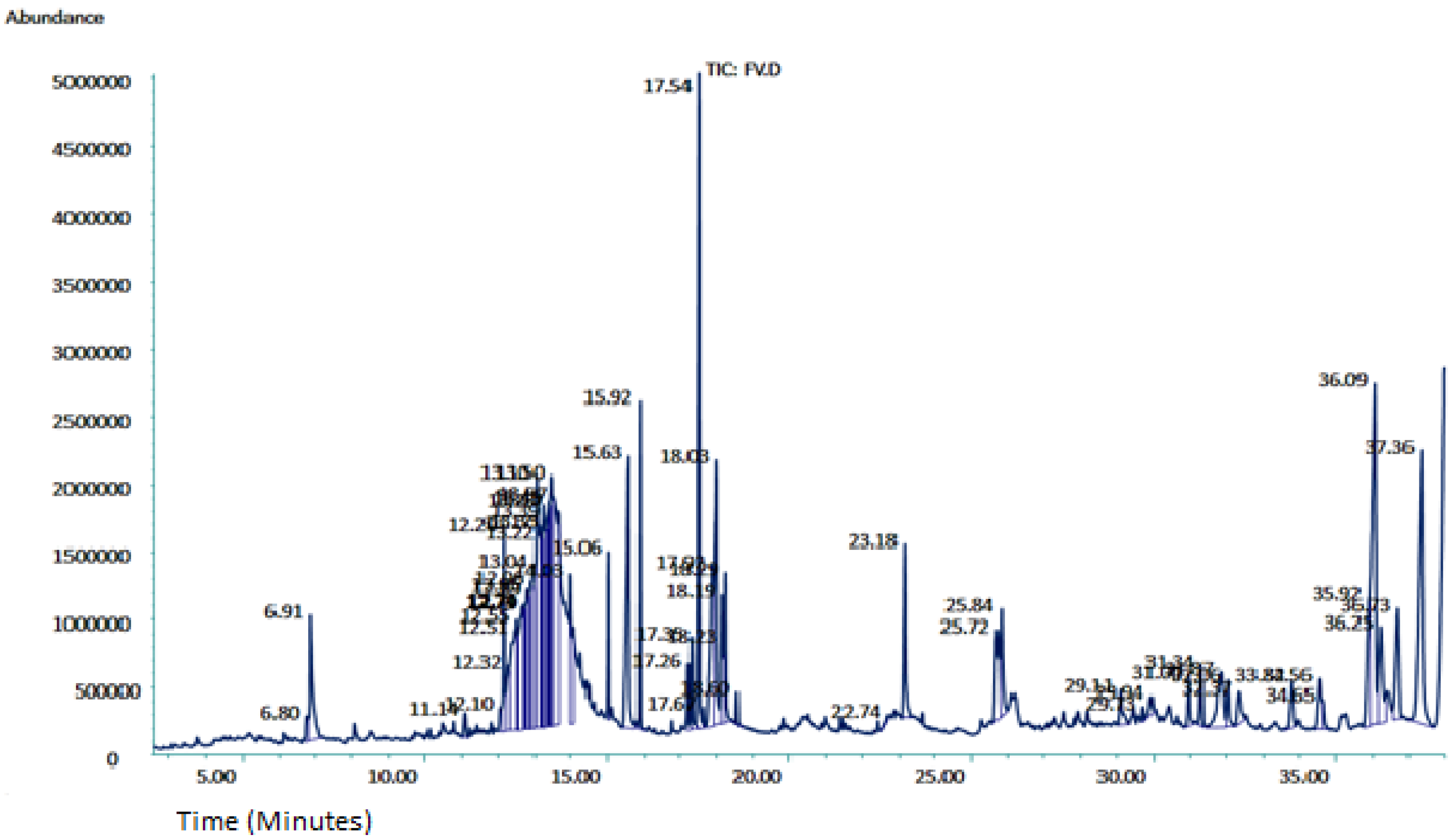
2.1.3. Gas chromatography-Mass Spectroscopy Analysis
2.2. Biological Activities
2.2.1. Antioxidant Activities
2.2.2. Enzyme Inhibition Activities
2.2.3. Antibacterial Activity
2.2.4. Antifungal Activity
2.2.5. Anti-Viral Activity
2.2.6. Thrombolytic Activity
2.2.7. Hemolytic Activity
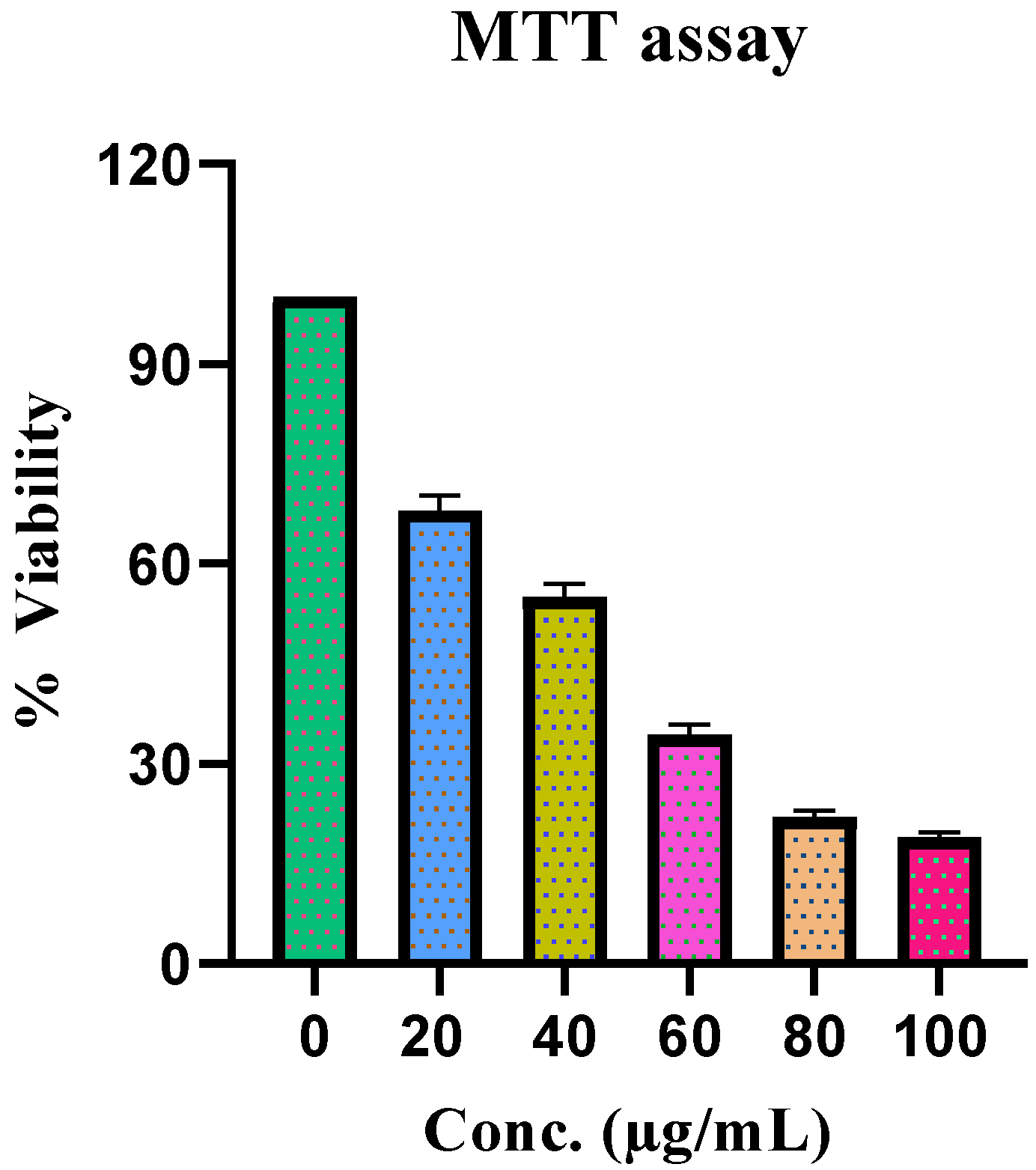
2.2.8. Cytotoxicity
2.3. In Silico Studies
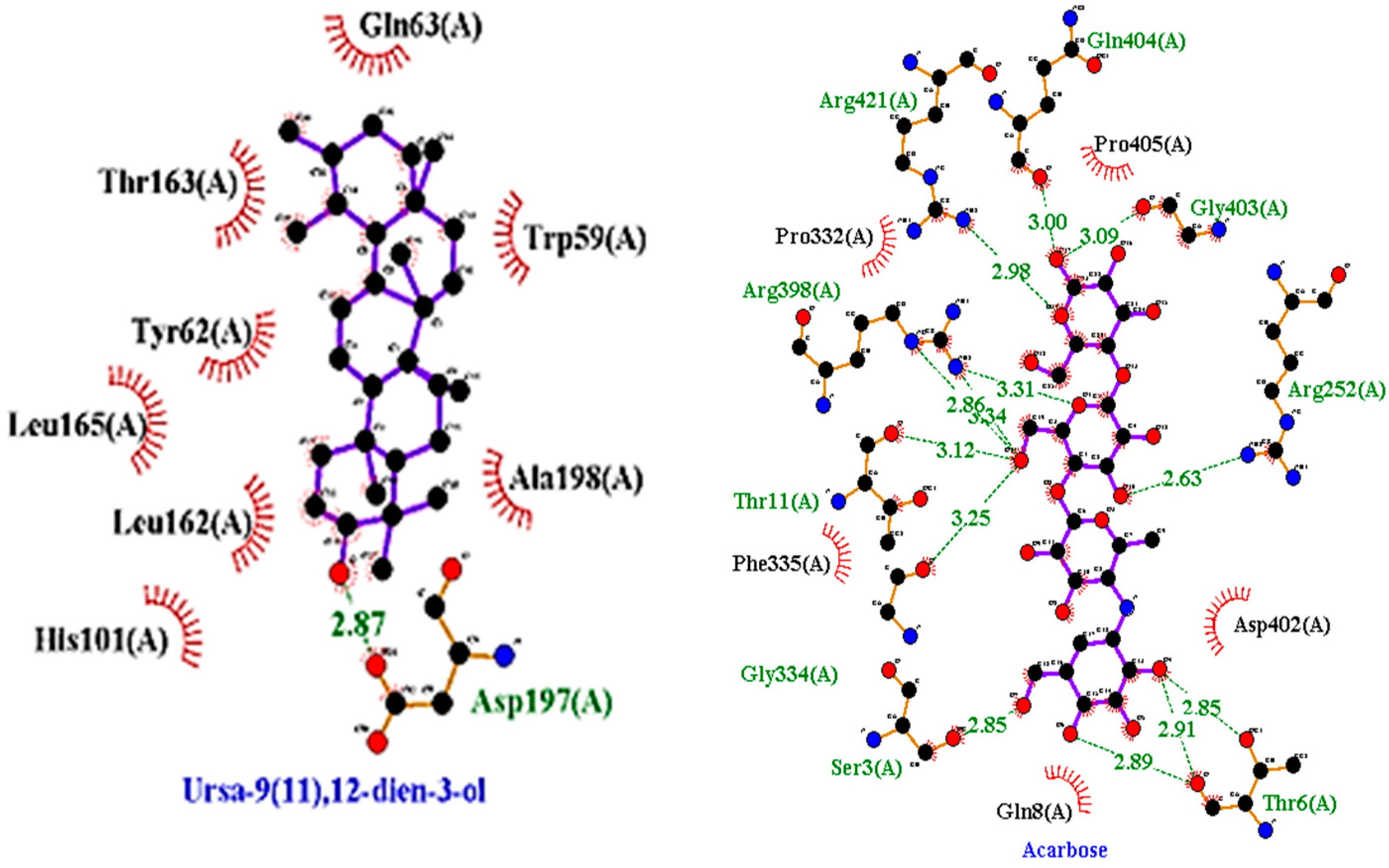
2.3.1. Molecular Docking
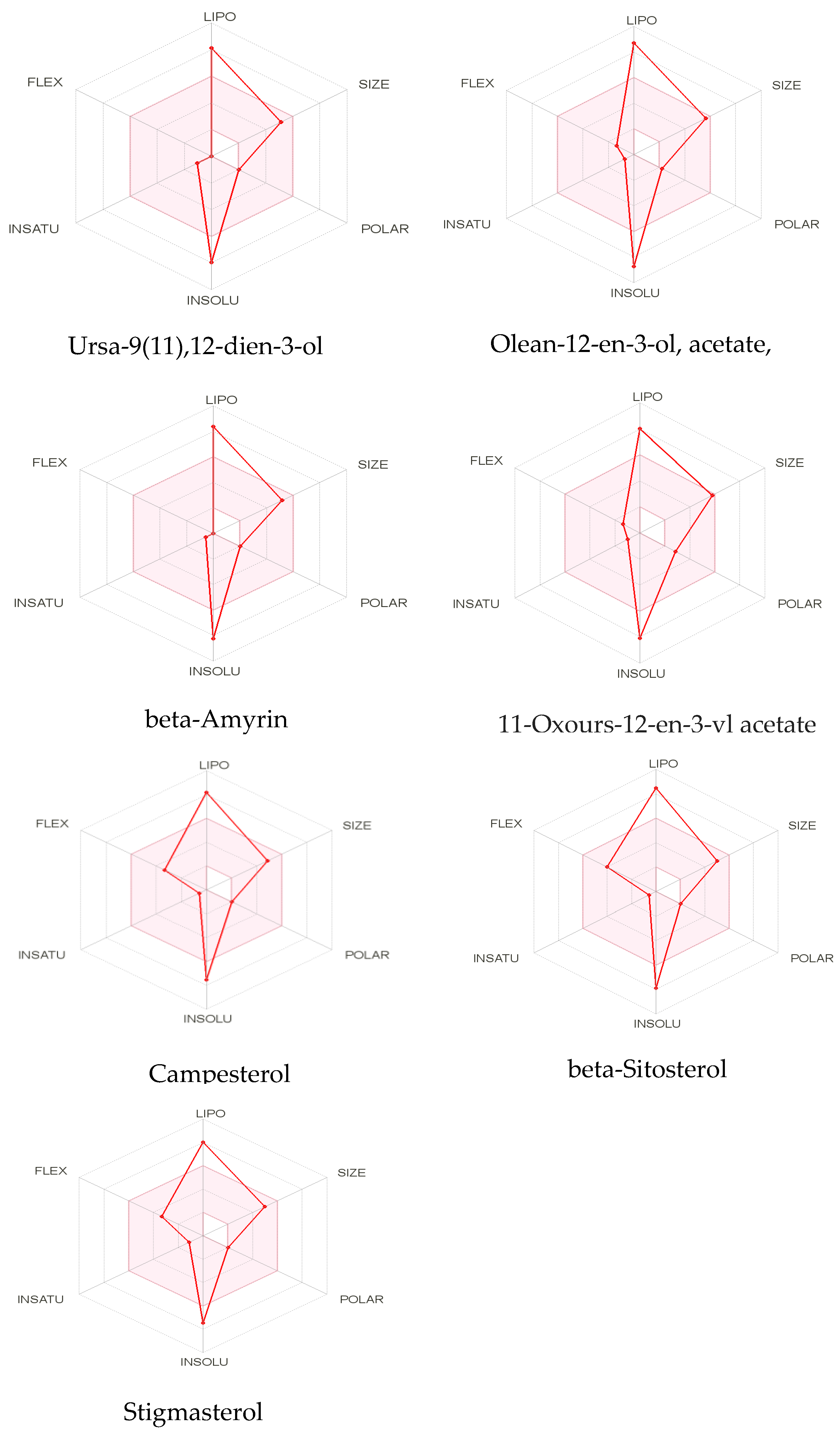
2.3.2. ADMET Analysis
3. Discussion
4. Materials and Methods
4.1. Materials and Reagents
4.2. Collection and Extraction of Plant
4.3. Phytochemical Analysis
4.3.1. Preliminary Phytochemical Profiling
4.3.2. Total bioactive contents (TPC and TFC)
TPC
TFC
4.3.3. Gass Chromatography-Mass Spectroscopy Analysis
4.4. Biological Activities
4.4.1. Antioxidant Activities
DPPH Assay
ABTS Assay
FRAP Assay
CUPRAC Assay
4.4.2. Enzyme Inhibition Activities
α-Glucosidase Inhibition Assay
α-Amylase Inhibition Assay
4.4.3. Antibacterial Activity
Strains of Bacteria
Agar Well Diffusion
4.4.4. Antifungal Activity
Fungal Strains
Agar Tube Dilution
4.4.5. Anti-viral Activity
Viral Strains
Inoculation of Viruses in Chicken Embryonated Eggs
Heamagglutination (HA) Test
4.4.6. Thrombolytic Activity
4.4.7. Hemolytic Activity
4.4.8. Cytotoxicity
Determination of Cell Viability
4.5. In Silico Activities
4.5.1. Molecular Dockings
4.5.2. ADMET Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghalloo, B.A.; Khan, K.-U.-R.; Ahmad, S.; Aati, H.Y.; Al-Qahtani, J.H.; Ali, B.; Mukhtar, I.; Hussain, M.; Shahzad, M.N.; Ahmed, I. Phytochemical Profiling, in Vitro Biological Activities, and In Silico Molecular Docking Studies of Dracaena reflexa. Molecules 2022, 27, 913. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Wirth, S.; Behrendt, U.; Ahmad, P.; Berg, G. Antimicrobial Activity of Medicinal Plants Correlates with the Proportion of Antagonistic Endophytes. Front. Microbiol. 2017, 8, 199. [Google Scholar] [CrossRef]
- Aumeeruddy, M.; Mahomoodally, F. Combating breast cancer using combination therapy with 3 phytochemicals: Piperine, sulforaphane, and thymoquinone: Combination therapy with phytochemicals. Cancer 2019, 125, 1600–1611. [Google Scholar] [CrossRef]
- Ahn, K. The worldwide trend of using botanical drugs and strategies for developing global drugs. BMB Rep. 2017, 50, 111. [Google Scholar] [CrossRef]
- Kumar, S.; Kumar, V.; Prakash, O. Enzymes inhibition and antidiabetic effect of isolated constituents from Dillenia indica. Biomed. Res. Int. 2013, 2013, 382063. [Google Scholar] [CrossRef]
- Salehi, B.; Ata, A.; Anil Kumar, N.V.; Sharopov, F.; Ramírez-Alarcón, K.; Ruiz-Ortega, A.; Abdulmajid Ayatollahi, S.; Tsouh Fokou, P.V.; Kobarfard, F.; Amiruddin Zakaria, Z.; et al. Antidiabetic Potential of Medicinal Plants and Their Active Components. Biomolecules 2019, 9, 551. [Google Scholar] [CrossRef]
- Okechukwu, P.; Sharma, M.; Tan, W.; Hor Kuan, C.; Chirara, K.; Gaurav, A.; Al-Nema, M. In-vitro anti-diabetic activity and in-silico studies of binding energies of palmatine with alpha-amylase, alpha-glucosidase and DPP-IV enzymes. Pharmacia 2020, 67, 363–371. [Google Scholar] [CrossRef]
- Derosa, G.; Maffioli, P. α-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. AMS 2012, 8, 899–906. [Google Scholar] [CrossRef]
- Eruygur, N.; Koçyiğit, U.M.; Taslimi, P.; Ataş, M.; Tekin, M.; Gülçin, İ. Screening the in vitro antioxidant, antimicrobial, anticholinesterase, antidiabetic activities of endemic Achillea cucullata (Asteraceae) ethanol extract. S. Afr. J. Bot. 2019, 120, 141–145. [Google Scholar] [CrossRef]
- Park, J.W.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.J.; Kudo, M.; Johnson, P.; Wagner, S.; Orsini, L.S. Global patterns of hepatocellular carcinoma management from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef]
- Machana, K.; Weerapreeyakul, N.; Barusrux, S. Anticancer effect of the extracts from Polyalthia evecta against human hepatoma cell line (HepG2). Asian Pac. J. Trop. Biomed. 2012, 2, 368–374. [Google Scholar] [CrossRef]
- Venkatachalapathy, D.; Shivamallu, C.; Prasad, S.K.; Thangaraj Saradha, G.; Rudrapathy, P.; Amachawadi, R.G.; Patil, S.S.; Syed, A.; Elgorban, A.M.; Bahkali, A.H.; et al. Assessment of Chemopreventive Potential of the Plant Extracts against Liver Cancer Using HepG2 Cell Line. Molecules 2021, 26, 4593. [Google Scholar] [CrossRef]
- Chaudhry, G.-e.-S.; Sohimi, N.; Mohamad, H.; Zafar, M.; Ahmed, A.; Yeong, y.s.; Tengku Muhammad, T.S. Xylocarpus Moluccensis Induces Cytotoxicity in Human Hepatocellular Carcinoma HepG2 Cell Line via Activation of the Extrinsic Pathway. Asian Pac. J. Cancer Prev. 2021, 22, 17–24. [Google Scholar] [CrossRef]
- Joshi, B.; Panda, S.K.; Jouneghani, R.S.; Liu, M.; Parajuli, N.; Leyssen, P.; Neyts, J.; Luyten, W. Antibacterial, Antifungal, Antiviral, and Anthelmintic Activities of Medicinal Plants of Nepal Selected Based on Ethnobotanical Evidence. Evid. Based Complement. Altern. Med. 2020, 2020, 1043471. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Ansari, M.J.; Khan, M.H.U.; Kamal, S.; Rahman, K.; Shoaib, M.; Man, S.; Khan, A.J.; Khan, S.U.; et al. Antimicrobial potentials of medicinal plant’s extract and their derived silver nanoparticles: A focus on honey bee pathogen. Saudi. J. Biol. Sci. 2019, 26, 1815–1834. [Google Scholar] [CrossRef]
- Spengler, G.; Gajdács, M.; Donadu, M.G.; Usai, M.; Marchetti, M.; Ferrari, M.; Mazzarello, V.; Zanetti, S.; Nagy, F.; Kovács, R. Evaluation of the antimicrobial and antivirulent potential of essential oils isolated from Juniperus oxycedrus L. ssp. macrocarpa aerial parts. Microorganisms 2022, 10, 758. [Google Scholar] [CrossRef]
- Takó, M.; Kerekes, E.B.; Zambrano, C.; Kotogán, A.; Papp, T.; Krisch, J.; Vágvölgyi, C. Plant Phenolics and Phenolic-Enriched Extracts as Antimicrobial Agents against Food-Contaminating Microorganisms. Antioxidants 2020, 9, 165. [Google Scholar] [CrossRef]
- Simonetti, G.; Brasili, E.; Pasqua, G. Antifungal Activity of Phenolic and Polyphenolic Compounds from Different Matrices of Vitis vinifera L. against Human Pathogens. Molecules 2020, 25, 3748. [Google Scholar] [CrossRef]
- Montenegro-Landívar, M.F.; Tapia-Quirós, P.; Vecino, X.; Reig, M.; Valderrama, C.; Granados, M.; Cortina, J.L.; Saurina, J. Polyphenols and their potential role to fight viral diseases: An overview. Sci. Total Environ. 2021, 801, 149719. [Google Scholar] [CrossRef]
- Murugesu, S.; Selamat, J.; Perumal, V. Phytochemistry, Pharmacological Properties, and Recent Applications of Ficus benghalensis and Ficus religiosa. Plants 2021, 10, 2749. [Google Scholar] [CrossRef]
- Raju, N.; Yesuf, E.; Bekele, M.; Wabe, N. Investigation of In Vitro Anthelmintic Activity of Ficus vasta leaves. Asian J. Pharm. Biol. Res. 2011, 1, 454–458. [Google Scholar]
- Mosa, O.; Deng, J.; Burhan, S.; Elfatih, M.; Mohamed, S. Evaluation of phytochemical and antimicrobial activities of some Sudanese medicinal plants. J. Pharm. Pharm. Sci. 2014, 3, 1769–1776. [Google Scholar]
- Rashed, K.; Anthonissen, R.; Cappoen, D.; Verschaeve, L. Phytochemical Composition and Potential Genotoxic effects of Important Egyptian Medicinal Plants. Pharmacogn. Commn. 2015, 5, 207–216. [Google Scholar] [CrossRef]
- Rashed, K.; Ono, L. Evaluation of cytotoxicity, anti-herpes simplex virus type 1 (HSV-1) and antibacterial activities of Ficus vasta and phytoconstituents. Int. Curr. Pharm. J. 2013, 3, 211–218. [Google Scholar] [CrossRef]
- Taviano, M.F.; Rashed, K.; Filocamo, A.; Cacciola, F.; Dugo, P.; Mondello, L.; Bisignano, C.; Acquaviva, R.; D’Arrigo, M.; Miceli, N. Phenolic profile and biological properties of the leaves of Ficus vasta Forssk. (Moraceae) growing in Egypt. BMC Complement. Altern. Med. 2018, 18, 161. [Google Scholar] [CrossRef]
- Tejero, I.; González-García, N.; González-Lafont, À.; Lluch, J.M. Tunneling in green tea: Understanding the antioxidant activity of catechol-containing compounds. A variational transition-state theory study. J. Am. Chem. Soc. 2007, 129, 5846–5854. [Google Scholar] [CrossRef]
- Kocaçalışkan, I.; Talan, I.; Terzi, I. Antimicrobial activity of catechol and pyrogallol as allelochemicals. Z. Naturforsch. C. 2006, 61, 639–642. [Google Scholar] [CrossRef]
- de Castro Oliveira, L.G.; Brito, L.M.; de Moraes Alves, M.M.; Amorim, L.V.; Sobrinho-Júnior, E.P.C.; de Carvalho, C.E.S.; da Franca Rodrigues, K.A.; Arcanjo, D.D.R.; das Graças Lopes Citó, A.M.; de Amorim Carvalho, F.A. In vitro effects of the neolignan 2, 3-dihydrobenzofuran against Leishmania amazonensis. Basic Clin. Pharmacol. Toxicol. 2017, 120, 52–58. [Google Scholar] [CrossRef]
- Moldovan, Z.; Marincas, O.; Povar, I.; Lupascu, T.; Longree, P.; Rota, J.S.; Singer, H.; Alder, A.C. Environmental exposure of anthropogenic micropollutants in the Prut River at the Romanian-Moldavian border: A snapshot in the lower Danube river basin. Environ. Sci. Pollut. Res. 2018, 25, 31040–31050. [Google Scholar] [CrossRef]
- do Nascimento, M.A.; Vargas, J.P.C.; Rodrigues, J.G.A.; Leão, R.A.C.; de Moura, P.H.B.; Leal, I.C.R.; Bassut, J.; de Souza, R.O.M.A.; Wojcieszak, R.; Itabaiana, I. Lipase-catalyzed acylation of levoglucosan in continuous flow: Antibacterial and biosurfactant studies. RSC Adv. 2022, 12, 3027–3035. [Google Scholar] [CrossRef]
- Sahin, N.; Kula, I.; Erdogan, Y. Investigation of antimicrobial activities of nonanoic acid derivatives. Fresenius Environ. Bull. 2006, 15, 141–143. [Google Scholar]
- Jang, Y.-W.; Jung, J.-Y.; Lee, I.-K.; Kang, S.-Y.; Yun, B.-S. Nonanoic acid, an antifungal compound from Hibiscus syriacus Ggoma. Mycobiology 2012, 40, 145–146. [Google Scholar] [CrossRef]
- Kim, M.-M.; Kim, S.-K. Effect of phloroglucinol on oxidative stress and inflammation. Food Chem. Toxicol. 2010, 48, 2925–2933. [Google Scholar] [CrossRef]
- Dandekar, R.; Fegade, B.; Bhaskar, V. GC-MS analysis of phytoconstituents in alcohol extract of Epiphyllum oxypetalum leaves. J. Pharmacogn. Phytochem. 2015, 4, 149–154. [Google Scholar]
- Wang, G.-F.; Shi, L.-P.; Ren, Y.-D.; Liu, Q.-F.; Liu, H.-F.; Zhang, R.-J.; Li, Z.; Zhu, F.-H.; He, P.-L.; Tang, W.; et al. Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antivir. Res. 2009, 83, 186–190. [Google Scholar] [CrossRef]
- Duke, J.A. Chemicals in Vitis vinifera L. (VItaceae). In Dr. Duke’s Phytochemical and Ethnobotanical Databases; Ag Data Commons: Washington, DC, USA, 2012. [Google Scholar]
- Tyagi, T.; Agarwal, M. Phytochemical screening and GC-MS analysis of bioactive constituents in the ethanolic extract of Pistia stratiotes L. and Eichhornia crassipes (Mart.) solms. J. Pharmacogn. Phytochem. 2017, 6, 195–206. [Google Scholar]
- Ganesh, M.; Mohankumar, M. Extraction and identification of bioactive components in Sida cordata (Burm. f.) using gas chromatography–mass spectrometry. J. Food Sci. Technol. 2017, 54, 3082–3091. [Google Scholar] [CrossRef]
- Islam, M.T.; Ali, E.S.; Uddin, S.J.; Shaw, S.; Islam, M.A.; Ahmed, M.I.; Chandra Shill, M.; Karmakar, U.K.; Yarla, N.S.; Khan, I.N.; et al. Phytol: A review of biomedical activities. Food Chem. Toxicol. 2018, 121, 82–94. [Google Scholar] [CrossRef]
- Santos, C.C.d.M.P.; Salvadori, M.S.; Mota, V.G.; Costa, L.M.; de Almeida, A.A.C.; de Oliveira, G.A.L.; Costa, J.P.; de Sousa, D.P.; de Freitas, R.M.; de Almeida, R.N. Antinociceptive and antioxidant activities of phytol in vivo and in vitro models. Neurosci. J. 2013, 2013, 949452. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Vivek, R.; Boopathy, N.S.; Yaqian, L.; Kathiresan, K.; Chen, J. Anticancer potential of bioactive 16-methylheptadecanoic acid methyl ester derived from marine Trichoderma. J. Appl. Biomed. 2015, 13, 199–212. [Google Scholar] [CrossRef]
- Saikarthik, J.; Ilango, S.; Vijayakumar, J.; Vijayaraghavan, R. Phytochemical analysis of methanolic extract of seeds of Mucuna pruriens by gas chromatography mass spectrometry. Int J. Pharm. Sci Res. 2017, 8, 2916–2921. [Google Scholar]
- Kushwaha, P.; Yadav, S.S.; Singh, V.; Dwivedi, L. Phytochemical screening and GC-MS studies of the methanolic extract of Tridax procumbens. Int. J. Pharm. Sci. 2019, 10, 2492–2496. [Google Scholar]
- Ambrosi, G.D.P. Composition Based on Ethyl Ester of Linoleic acid and Triethyl Ester of Citric Acid for Topical Use in the Treatment of Seborrhea and Acne. U.S. Patent 7,169,811, 30 January 2007. [Google Scholar]
- Jasim, H.; Hussein, A.O.; Hameed, I.H.; Kareem, M.A. Characterization of alkaloid constitution and evaluation of antimicrobial activity of Solanum nigrum using gas chromatography mass spectrometry (GC-MS). J. Pharmacogn. Phytotherapy 2015, 7, 56–72. [Google Scholar]
- Novitasari, M.R.; Febrina, L.; Agustina, R.; Rahmadani, A.; Rusli, R. Analisis GC-MS senyawa aktif antioksidan fraksi etil asetat daun libo (Ficus variegata Blume.). J. Sains. Kes. 2016, 1, 221–225. [Google Scholar] [CrossRef]
- Sivakumaran, G.; Prabhu, K.; Rao, M.; Jones, S.; Sundaram, R.L.; Ulhas, V.R.; Vijayalakshmi, N. Gas chromatography-mass spectrometry analysis of one Ayurvedic oil, Ksheerabala Thailam. Drug Invent. Today. 2019, 11, 2661–2665. [Google Scholar]
- Akash, S.S.; Singh, S.K. Phytoconstituents estimation of Lepidium sativum L. seed extract using GC-MS spectroscopy. World J. Pharm Res. 2017, 7, 1360–1367. [Google Scholar]
- Kaur, N.; Chaudhary, J.; Jain, A.; Kishore, L. Stigmasterol: A comprehensive review. Int. J. Pharm. Sci. 2011, 2, 2259. [Google Scholar]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar] [CrossRef]
- Panda, S.; Jafri, M.; Kar, A.; Meheta, B. Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma. Fitoterapia 2009, 80, 123–126. [Google Scholar] [CrossRef]
- Sushma, V.; Pal, S.M.; Viney, C. GC-MS Analysis of Phytocomponents in the Various Extracts of Shorea robusta Gaertn F. Int. J. Pharmacogn. Phytochem. Res. 2017, 9, 783–788. [Google Scholar] [CrossRef][Green Version]
- Saeidnia, S.; Manayi, A.; Gohari, A.R.; Abdollahi, M. The story of beta-sitosterol-a review. Eur. J. Med. Plants 2014, 4, 590. [Google Scholar] [CrossRef]
- Manorenjitha, M.; Norita, A.; Norhisham, S.; Asmawi, M. GC-MS analysis of bioactive components of Ficus religiosa (Linn.) stem. Int J. Pharm. Bio. Sci. 2013, 4, 99–103. [Google Scholar]
- Duraiswamy, A.; Shanmugasundaram, D.; Sasikumar, C.S. Evaluation of the phytochemical constituents in ADJ6, an anti-diabetic polyherbal formulation by GC-MS. J. Pharmacogn. Phytochem. 2016, 5, 173. [Google Scholar]
- Musaddiq, S.; Imran Shahzad, M.; Firdous, F.; Iqbal, A.; Tanveer, M.; Ashraf, A.; Aslam, S.; Khakwani, S. Thiazolidines: Potential anti-viral agents against avian influenza and infectious bronchitis viruses. Vet. Res. Forum 2020, 11, 415–421. [Google Scholar]
- Greco, I.; Molchanova, N.; Holmedal, E.; Jenssen, H.; Hummel, B.D.; Watts, J.L.; Håkansson, J.; Hansen, P.R.; Svenson, J. Correlation between hemolytic activity, cytotoxicity and systemic in vivo toxicity of synthetic antimicrobial peptides. Sci. Rep. 2020, 10, 13206. [Google Scholar]
- Demir, T.; Akpınar, Ö. Biological activities of phytochemicals in plants. Turk. J. Agric.-Food Sci. Technol. 2020, 8, 1734–1746. [Google Scholar]
- Cahlíková, L.; Breiterová, K.; Opletal, L. Chemistry and Biological Activity of Alkaloids from the Genus Lycoris (Amaryllidaceae). Molecules 2020, 25, 4797. [Google Scholar] [CrossRef]
- Muniyandi, K.; George, E.; Sathyanarayanan, S.; George, B.P.; Abrahamse, H.; Thamburaj, S.; Thangaraj, P. Phenolics, tannins, flavonoids and anthocyanins contents influenced antioxidant and anticancer activities of Rubus fruits from Western Ghats, India. Food Sci. Hum. Wellness. 2019, 8, 73–81. [Google Scholar] [CrossRef]
- Sparg, S.G.; Light, M.E.; van Staden, J. Biological activities and distribution of plant saponins. J. Ethnopharmacol. 2004, 94, 219–243. [Google Scholar] [CrossRef]
- Nawaz, H.; Waheed, R.; Nawaz, M. Phytochemical Composition, Antioxidant Potential, and Medicinal Significance of Ficus. Mod. Fruit Ind. 2020, 1, 20. [Google Scholar]
- Chavan, J.J.; Gaikwad, N.B.; Kshirsagar, P.R.; Dixit, G.B. Total phenolics, flavonoids and antioxidant properties of three Ceropegia species from Western Ghats of India. S. Afr. J. Bot. 2013, 88, 273–277. [Google Scholar] [CrossRef]
- Hara, Y.; Honda, M. The inhibition of α-amylase by tea polyphenols. Agric. Biol. Chem. 1990, 54, 1939–1945. [Google Scholar] [CrossRef]
- Matsui, T.; Tanaka, T.; Tamura, S.; Toshima, A.; Tamaya, K.; Miyata, Y.; Tanaka, K.; Matsumoto, K. α-Glucosidase inhibitory profile of catechins and theaflavins. J. Agric. Food Chem. 2007, 55, 99–105. [Google Scholar] [CrossRef]
- Gong, L.; Feng, D.; Wang, T.; Ren, Y.; Liu, Y.; Wang, J. Inhibitors of α-amylase and α-glucosidase: Potential linkage for whole cereal foods on prevention of hyperglycemia. Food Sci. Nutr. 2020, 8, 6320–6337. [Google Scholar] [CrossRef] [PubMed]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed]
- Ткаченкo, Г.М.; Буюн, Л.И.; Осадoвский, З.; Гoнчаренкo, В.И.; Прoкoпив, А.И. Antimicrobial activity of ethanolic extract obtained from Ficus vasta Forssk. (Moraceae). Территoрия науки 2017, 5, 12–17. [Google Scholar]
- Kim, J.H.; Lee, J.; Kang, S.; Moon, H.; Chung, K.H.; Kim, K.R. Antiplatelet and Antithrombotic Effects of the Extract of Lindera obtusiloba Leaves. Biomol. Ther. 2016, 24, 659–664. [Google Scholar] [CrossRef]
- Tabassum, S.; Ahmad, S.; Rehman Khan, K.U.; Tabassum, F.; Khursheed, A.; Zaman, Q.U.; Bukhari, N.A.; Alfagham, A.; Hatamleh, A.A.; Chen, Y. Phytochemical Profiling, Antioxidant, Anti-Inflammatory, Thrombolytic, Hemolytic Activity In Vitro and In Silico Potential of Portulacaria afra. Molecules 2022, 27, 2377. [Google Scholar] [CrossRef]
- Sowemimo-Coker, S.O. Red blood cell hemolysis during processing. Transfus Med. Rev. 2002, 16, 46–60. [Google Scholar] [CrossRef]
- Zohra, M.; Fawzia, A. Hemolytic activity of different herbal extracts used in Algeria. Int. J. Pharm. Sci. 2014, 5, 495–500. [Google Scholar]
- Khursheed, A.; Ahmad, S.; Khan, K.-u.-R.; Tousif, M.I.; Aati, H.Y.; Ovatlarnporn, C.; Rao, H.; Khurshid, U.; Ghalloo, B.A.; Tabassum, S. Efficacy of Phytochemicals Derived from Roots of Rondeletia odorata as Antioxidant, Antiulcer, Diuretic, Skin Brightening and Hemolytic Agents—A Comprehensive Biochemical and In Silico Study. Molecules 2022, 27, 4204. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.H.; Ahmad, K.; Rabbani, G.; Danishuddin, M.; Choi, I. Computer Aided Drug Design and its Application to the Development of Potential Drugs for Neurodegenerative Disorders. Curr. Neuropharmacol. 2018, 16, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Eid, E.E.; Azam, F.; Hassan, M.; Taban, I.M.; Halim, M.A. Zerumbone binding to estrogen receptors: An in-silico investigation. J. Recept. Signal. Transduct. 2018, 38, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Patil, R.; Das, S.; Stanley, A.; Yadav, L.; Sudhakar, A.; Varma, A. Optimized Hydrophobic Interactions and Hydrogen Bonding at the Target-Ligand Interface Leads the Pathways of Drug-Designing. PLoS ONE 2010, 5, e12029. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Lipinski, C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today Technol. 2004, 1, 337–341. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A Simple, Robust, and Efficient Description of n-Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef]
- Duffy, F.J.; Devocelle, M.; Shields, D.C. Computational approaches to developing short cyclic peptide modulators of protein-protein interactions. Methods Mol. Biol. 2015, 1268, 241–271. [Google Scholar]
- Al Azzam, K.; Negim, E.-S.; Aboul-Enein, H. ADME studies of TUG-770 (a GPR-40 inhibitor agonist) for the treatment of type 2 diabetes using SwissADME predictor: In silico study. J. Appl. Pharm. Sci. 2022, 12, 159–169. [Google Scholar]
- Banerjee, P.; Eckert, A.O.; Schrey, A.K.; Preissner, R. ProTox-II: A webserver for the prediction of toxicity of chemicals. Nucleic Acids Res. 2018, 46, W257–W263. [Google Scholar] [CrossRef]
- Sivanandham, V. Phytochemical techniques—A review. World J. Sci. Res. 2015, 1, 80–91. [Google Scholar]
- Sembiring, E.; Elya, B.; Sauriasari, R. Phytochemical Screening, Total Flavonoid and Total Phenolic Content and Antioxidant Activity of Different Parts of Caesalpinia bonduc (L.) Roxb. Pharmacogn. J. 2017, 10, 123–127. [Google Scholar] [CrossRef]
- Dilshad, R.; Ahmad, S.; Aati, H.; Al-qahtani, J.H.; Sherif, A.E.; Hussain, M.; Ghalloo, B.A.; Tahir, H.; Basit, A.; Ahmed, M. Phytochemical Profiling, In Vitro Biological Activities, and In-Silico Molecular Docking Studies of Typha domingensis. Arab. J. Chem. 2022, 15, 104133. [Google Scholar] [CrossRef]
- Benarfa, A.; Gourine, N.; Mahfoudi, R.; Harrat, M.; Yousfi, M. Effect of Seasonal and Regional Variations on Phenolic Compounds of Deverra scoparia (Flowers/Seeds) Methanolic Extract and the Evaluation of Its in Vitro Antioxidant Activity. Chem. Biodivers. 2019, 16, e1900420. [Google Scholar] [CrossRef] [PubMed]
- Rehman, T.; Ahmad, S.; Ghauri, A.O.; Abbasi, W.M.; Arshad, M.A. Evaluation of α-glucosidase Inhibitory Potential of Some Homeopathic Mother Tinctures. J. Pharm. Pharm. Sci. 2018, 6, 190–193. [Google Scholar]
- Anyanwu, G.; Onyeneke, E.; Okoli, J.; Johannes, M.; Rehman, S.U.; Zaib, S.; Rauf, K. Pharmacological activities of a novel phthalic acid ester and iridoid glycoside isolated from the root bark of Anthocleista vogelii Planch. Trop. Biomed. 2019, 36, 35–43. [Google Scholar]
- Khan, N.; Hwa, C.; Perveen, N.; Paliwal, N. Phytochemical screening, antimicrobial and antioxidant activity determination of Trigonella foenum-graecum seeds. Pharm. Pharmacol. Int. 2019, 7, 175–186. [Google Scholar]
- Mahmood, A.; Mahmood, A.; Qureshi, R.A. Antimicrobial activities of three species of family mimosaceae. Pak. J. Pharm. Sci. 2012, 25, 203–206. [Google Scholar]
- Shahzad, M.i.; Anwar, S.; Aslam, Z.; Saba, N.; Ashraf, H.; Kamran, Z. Antiviral activities of Cholistani plants against common poultry viruses. Trop. Biomed. 2020, 37, 1129–1140. [Google Scholar]
- Ahmed, M.; Khan, K.-u.-R.; Ahmad, S.; Aati, H.Y.; Ovatlarnporn, C.; Rehman, M.S.-u.; Javed, T.; Khursheed, A.; Ghalloo, B.A.; Dilshad, R. Comprehensive Phytochemical Profiling, Biological Activities, and Molecular Docking Studies of Pleurospermum candollei: An Insight into Potential for Natural Products Development. Molecules 2022, 27, 4113. [Google Scholar] [CrossRef]
- Hemthanon, T.; Ungcharoenwiwat, P. Antibacterial activity, stability, and hemolytic activity of heartwood extract from Caesalpinia sappan for application on nonwoven fabric. Electron. J. Biotechnol. 2022, 55, 9–17. [Google Scholar] [CrossRef]
- Khan, M.; Li, T.; Ahmad Khan, M.K.; Rasul, A.; Nawaz, F.; Sun, M.; Zheng, Y.; Ma, T. Alantolactone Induces Apoptosis in HepG2 Cells through GSH Depletion, Inhibition of STAT3 Activation, and Mitochondrial Dysfunction. Biomed. Res. Int. 2013, 2013, 719858. [Google Scholar] [CrossRef] [PubMed]
- Idris, M.O.; Adeniji, S.E.; Habib, K.; Adeiza, A.A. Molecular Docking of Some Novel Quinoline Derivatives as Potent Inhibitors of Human Breast Cancer Cell Line. Lab-in-Silico 2021, 2, 30–37. [Google Scholar]
- Laskowski, R.; Swindells, M. LigPlot+: Multiple Ligand–Protein Interaction Diagrams for Drug Discovery. J. Chem. Inf. Model. 2011, 51, 2778–2786. [Google Scholar] [CrossRef] [PubMed]



| Sr. No. | Metabolites | Tests | Results |
|---|---|---|---|
| 1 | Carbohydrates | MolischTest | + |
| Iodine Test | + | ||
| 2 | Proteins | Biurette Test | − |
| 3 | Lipids | Saponification Test | + |
| 4 | Flavonoids | Reaction with NaOH | + |
| 5 | Tannins | Lead Acetate Test | + |
| 6 | Saponins | Frothing | + |
| 7 | Amino acids | Ninhydrin Test | − |
| 8 | Steroids/Terpenes | SalkowaskiTest | + |
| 9 | Glycosides | Erdmann Test | + |
| 10 | Alkaloids | Hager Test | + |
| Wagner Test | + | ||
| Mayer Test | + | ||
| 11 | Phenols | Ferric chloride Test | + |
| 12 | Resins | Acetic Anhydride Test | − |
| Sample | TPC mg GAE/g | TFC mg QE/g | Antioxidant Activities (mg/mL) IC50 | |||
|---|---|---|---|---|---|---|
| DPPH | FRAP | ABTS | CUPRAC | |||
| F. vasta | 89.47 ± 3.21 | 129.2 ± 4.14 | 1.75 ± 0.08 | 1.91 ± 0.11 | 1.63 ± 0.06 | 1.51 ± 0.04 |
| Sr No. | RT (minutes) | Compounds Identified | M. Formula | Mol. Wt (g/mol) | Chemical Class | Area (%) | Reported Activities of Compounds |
|---|---|---|---|---|---|---|---|
| 1 | 6.80 | Catechol | C6H6O2 | 110.11 | Benzenediol | 0.25 | Antioxidant [26], antibacterial, and antifungal [27] |
| 2 | 6.91 | 2,3-Dihydrobenzofuran | C8H8O | 120.15 | 1-Benzofurans | 2.17 | Antileishmania [28] |
| 3 | 12.20 | 4,4,5,8-Tetramethylchroman-2-ol | C13H18O2 | 206.28 | Vitamin E analog | 1.39 | Anti-inflammatory [29] |
| 4 | 12.74 | Levoglucosan | C6H10O5 | 162.14 | Carbohydrate | 0.55 | Antibacterial [30] |
| 5 | 12.76 | Nonanoic acid | C9H18O2 | 158.24 | Ester | 0.58 | Antimicrobial [31], antifungal [32] |
| 6 | 12.79 | Phloroglucinol | C6H6O3 | 126.11 | Benzene triol | 0.40 | Oxidative stress [33] |
| 7 | 13.10 | 4-((1E)-3-Hydroxy-1-propenyl)-2-methoxyphenol | C10H12O3 | 180.2005 | Organic compound | 3.71 | Anti-inflammatory, antimicrobial, and antioxidant [34] |
| 8 | 13.50 | Quinic acid | C7H12O6 | 192.17 | Cyclitol/Cyclohexane carboxylic acid | 4.07 | Anti-carcinogenic and antioxidant [35] |
| 9 | 15.63 | n-Hexadecanoic acid | C16H32O2 | 256.42 | Fatty acid | 4.27 | Antioxidant, and nematicide [36] |
| 10 | 15.92 | Hexadecanoicacid, ethyl ester | C18H36O2 | 284.5 | Fatty acid ester | 1.82 | Antioxidant, and nematicide [37] |
| 11 | 17.35 | 9,12,15-Octadecatrienoic acid, methyl ester, (Z,Z,Z)- | C19H32O2 | 292.5 | Fatty acid ester | 0.72 | Anti-inflammatory, anti-cancer, hepatoprotective, [38] |
| 12 | 17.54 | Phytol | C20H40O | 296.5 | Diterpenoid | 5.13 | Cytotoxic, antioxidant, and antimicrobial [39,40] |
| 13 | 17.67 | 16-Methylheptadecanoic acid methyl ester | C19H38O2 | 298.5 | Ester of isostearic acid | 0.13 | Anti-cancer [41] |
| 14 | 17.92 | 9 12-Octadecadienoic acid (z z)- methyl ester | C19H34O2 | 294.4 | Fatty acid | 2.80 | Hepatoprotective [42] |
| 15 | 18.03 | 11 14 17-eicosatrienoic acid methyl ester | C21H36O2 | 320.5 | Fatty acid methyl ester | 3.76 | Anti-inflammatory, anti-arthritic [43] |
| 16 | 18.19 | Linoleic acid ethyl ester | C20H36O2 | 308.5 | Linoleic acid | 0.99 | Anti-acne [44] |
| 17 | 18.23 | Octadecanoic acid | C18H36O2 | 284.5 | Fatty acid (stearic acid) | 0.43 | Antioxidant, antimicrobial [45] |
| 18 | 22.74 | 12-Oleanene-3-yl acetate (3.α.)- | C32H52O2 | 468.8 | Triterpenoid | 0.01 | Antioxidant and cytotoxic [46] |
| 19 | 23.18 | Hexadecanoicacid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C19H38O4 | 330.5 | Fatty acid | 1.77 | Antioxidant, and anti-inflammatory [47] |
| 20 | 25.84 | Methyl (Z)-5,11,14,17-eicosatetraenoate | C21H34O2 | 318.5 | Fatty acid methyl ester | 1.31 | Antibacterial [48] |
| 21 | 32.06 | Vitamin E | C29H50O2 | 430.7 | Vitamins | 0.65 | Anti-cancer, hepatoprotective, and antispasmodic [36] |
| 22 | 33.82 | Campesterol | C28H48O | 400.7 | Phytosterol | 0.83 | Anti-inflammatory, antidiabetic, and anti-cancer [36] |
| 23 | 34.56 | Stigmasterol | C29H48O | 412.7 | Sterol | 0.90 | Anti-tumor, hypoglycemic, and anti-inflammatory [49,50,51] |
| 24 | 34.65 | Ursa-9(11),12-dien-3-ol | C30H48O | 424.7 | Triterpene | 0.36 | Anti-inflammatory, and antioxidant [52] |
| 25 | 35.92 | beta-Sitosterol | C29H50O | 414.7 | Phytosterol | 1.54 | Anti-cancer, and hypocholestremia [53] |
| 26 | 36.09 | Stigmasterol, 22,23-dihydro | C29H50O | 414.7 | Steroid | 8.04 | Anti-cancer, antioxidant, hypoglycemic, and anti-viral [54] |
| 27 | 37.36 | 11-Oxours-12-en-3-yl acetate | C32H50O3 | 482.7 | Ester of acetic acid | 6.46 | Antidiabetic [55] |
| 28 | 36.73 | beta-Amyrin | C30H50O | 426.7 | Triterpenoid | 2.26 | Antioxidant, antimalarial, and antiulcer [52] |
| Zone of Inhibition (mm) | |||||
|---|---|---|---|---|---|
| Bacterial Strains | Conc. 25 mg/mL | Conc. 50 mg/mL | Conc. 75 mg/mL | Conc. 100 mg/mL | Standard Ceftriaxone 1 mg/mL |
| Staphylococcus aureus | 18 | 20 | 21 | 22 | 26 |
| Staphylococcus epidermidis | 16 | 17 | 20 | 21 | 26 |
| Escherichia coli | 20 | 21 | 22 | 24 | 25 |
| Bordetella bronchiseptica | 12 | 13 | 15 | 18 | 20 |
| Bacillus subtilis | 12 | 16 | 17 | 19 | 22 |
| Bacillus pumilus | 13 | 14 | 16 | 17 | 21 |
| Micrococcus luteus | 15 | 17 | 18 | 20 | 21 |
| Sample | Fungal Strains | Linear Growth in Test Tubes (mm) | Linear Growth in Control (mm) | % Age Inhibition |
|---|---|---|---|---|
| F. vasta | Aspergillus niger | 42 | 98 | 42% |
| Fusarium avenaceum | 50 | 91 | 54.94% | |
| Fusarium brachygibbosum | 49 | 101 | 48.51% |
| Strains | Titer Count in Control | Titer Count in Acyclovir | Titer Count in F. vasta |
|---|---|---|---|
| IBV | 1024 | 00 | 00 |
| NDV | 2048 | 00 | 00 |
| H9 | 2048 | 00 | 16 |
| Extract/Standard | Hemolytic Activity % |
|---|---|
| F. vasta | 6.39 ± 0.45 |
| Triton X-100 | 92.27 ± 4.71 |
| Sr. No. | Ligand | Structures | α-Amylase | α-Glucosidase | ||||
|---|---|---|---|---|---|---|---|---|
| Binding Energy | Amino Acids H-Bond Interactions | Amino Acids Hydrophobic Interactions | Binding Energy | Amino Acids H-Bond Interactions | Amino Acids Hydrophobic Interactions | |||
| 1 | Ursa- 9(11),12-dien-3-ol |  | −10.5 | ASP 197 | TRP 59, HIS 101, LEU 162, TYR 62, ALA 198, TYR 62, THR 163, GLN 63, LEU 165 | −8 | - | PRO 223, PHE 225, LEU 219, GLU 141, GLN 392, TYR 388, LYS 290, ASP 289, TRP 288 |
| 2 | Olean-12-en-3-ol, acetate, (3beta)- |  | −10.4 | - | TRP 59, GLU 233, ASP 356, HIS 305, TRP 58, ASP 300, THR 163, ASP 197, HIS 299, TYR 62, LEU 165, HIS 101 | −8.1 | LEU 287, ASN 258 | PHE 225, GLU 226, VAL 222, PRO 214, PRO 223, GLY 286 |
| 3 | beta-Amyrin | 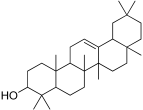 | −10.1 | GLU 233, ASP 197 | TRP 59, THR 163, ASP 300, LEU 162, TYR 62, HIS 299, TRP 58 | −8.4 | GLU 113 | TYR 122, PRO 109, TRP 110, GLU 183, GLU 180, TYR 68, LYS 69 |
| 4 | 11-Oxours-12-en-3-yl acetate |  | −9.6 | - | TRP 58, GLU 233, ASP 197, TRP 59, LEU 162, TYR 62, THR 163 | −8.1 | - | LYS 395, TYR 221, LEU 219, GLU 141, GLN 392, PRO 223, PHE 225, ASP 289, TRP 288, LYS 290, ILE 391 |
| 5 | Campesterol |  | −9.3 | GLU 240 | TYR 151, HIS 299, ASP 197, LEU 162, ILE 235, ALA 198, HIS 201, TYR 62, GLU 233, TRP 59, TRP 58, LYS 200, ASP 300 | −6.9 | LYS 205 | LYS 206, GLU 173, ASN 171, PHE 210, ILE 127, TRP 128, ASP 124, HIS 129, LYS 118, GLY 209, ALA 208 |
| 6 | beta-Sitosterol | 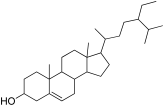 | −9.3 | GLU 240 | ILE 235, HIS 201, TYR 62, TYR 151, HIS 299, TRP 58, ALA 198, ARG 195, ASP 197, LEU 162, TRP 59, LYS 200, GLU 233 | −6.9 | ASN 258 | LYS 290, PRO 223, PHE 225, MET 229, LEU 287, ASP 289, TRP 288, SER 145, GLU 141, ILE 143 |
| 7 | Stigmasterol | 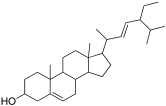 | −9.1 | GLU 233 | ALA 198, TRP 59, LEU 162, ASP 197, LEU 165, GLN 63, GLY 104, THR 163 | −7.5 | GLN 392, LYS 395 | MET 229, PHE 225, GLU 141, PRO 223, ILE 391, SER 145, TYR 388, LYS 290, TRP 288, LEU 287 |
| 8 | Acarbose (standard) |  | −7.7 | ARG 252, GLY 403, ARG 421, THR 11, ARG 398, THR 6, GLN 404, GLY 334, SER 3 | PRO 332, PRO 405, PHE 335, ASP 402, GLN 8 | −6.7 | ASN 58, ARG 17, ASP 59, ASN 61, ASP 381, ASP 379, LYS 436, PRO 433 | VAL 383, TRP 434, MET 435, ASP 378, |
| Sr no. | Best-Docked Compounds | Lipinski’s Rule | Solubility | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HBD | HBA | MWT | Lipophilicity | M.R | LR | ESOL Class | Ali Class | Silicos-IT Class | ||
| 1 | Ursa-9(11),12-dien-3-ol | 1 | 1 | 424.7 | 4.81 | 134.67 | 1 | PS | PS | PS |
| 2 | Olean-12-en-3-ol, acetate, (3beta)- | 0 | 2 | 468.75 | 5.19 | 144.62 | 1 | PS | IS | PS |
| 3 | beta-Amyrin | 1 | 1 | 426.72 | 4.74 | 134.88 | 1 | PS | PS | PS |
| 4 | 11-Oxours-12-en-3-yl acetate | 0 | 3 | 482.74 | 4.79 | 145.08 | 1 | PS | PS | PS |
| 5 | Campesterol | 1 | 1 | 400.68 | 4.92 | 131.23 | 1 | PS | PS | MS |
| 6 | beta-Sitosterol | 1 | 1 | 414.71 | 4.79 | 133.23 | 1 | PS | PS | PS |
| 7 | Stigmasterol | 1 | 1 | 412.69 | 5.01 | 132.75 | 1 | PS | PS | MS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aati, H.Y.; Anwar, M.; Al-Qahtani, J.; Al-Taweel, A.; Khan, K.-u.-R.; Aati, S.; Usman, F.; Ghalloo, B.A.; Asif, H.M.; Shirazi, J.H.; et al. Phytochemical Profiling, In Vitro Biological Activities, and In-Silico Studies of Ficus vasta Forssk.: An Unexplored Plant. Antibiotics 2022, 11, 1155. https://doi.org/10.3390/antibiotics11091155
Aati HY, Anwar M, Al-Qahtani J, Al-Taweel A, Khan K-u-R, Aati S, Usman F, Ghalloo BA, Asif HM, Shirazi JH, et al. Phytochemical Profiling, In Vitro Biological Activities, and In-Silico Studies of Ficus vasta Forssk.: An Unexplored Plant. Antibiotics. 2022; 11(9):1155. https://doi.org/10.3390/antibiotics11091155
Chicago/Turabian StyleAati, Hanan Y., Mariyam Anwar, Jawaher Al-Qahtani, Areej Al-Taweel, Kashif-ur-Rehman Khan, Sultan Aati, Faisal Usman, Bilal Ahmad Ghalloo, Hafiz Muhammad Asif, Jafir Hussain Shirazi, and et al. 2022. "Phytochemical Profiling, In Vitro Biological Activities, and In-Silico Studies of Ficus vasta Forssk.: An Unexplored Plant" Antibiotics 11, no. 9: 1155. https://doi.org/10.3390/antibiotics11091155
APA StyleAati, H. Y., Anwar, M., Al-Qahtani, J., Al-Taweel, A., Khan, K.-u.-R., Aati, S., Usman, F., Ghalloo, B. A., Asif, H. M., Shirazi, J. H., & Abbasi, A. (2022). Phytochemical Profiling, In Vitro Biological Activities, and In-Silico Studies of Ficus vasta Forssk.: An Unexplored Plant. Antibiotics, 11(9), 1155. https://doi.org/10.3390/antibiotics11091155







