Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin
Abstract
:1. Introduction
2. Results
2.1. MIC, MBC, and MFC Determination
2.2. Inhibition of Biofilm Formation
2.3. Biofilm Eradication
2.4. Hemolysin Inhibition
2.5. Antiparasitic Action
2.5.1. Cytotoxicity Assay on Host Cells
2.5.2. PPEE, PPHE, and PSEE impaired T. gondii Intracellular Proliferation in BeWo cells
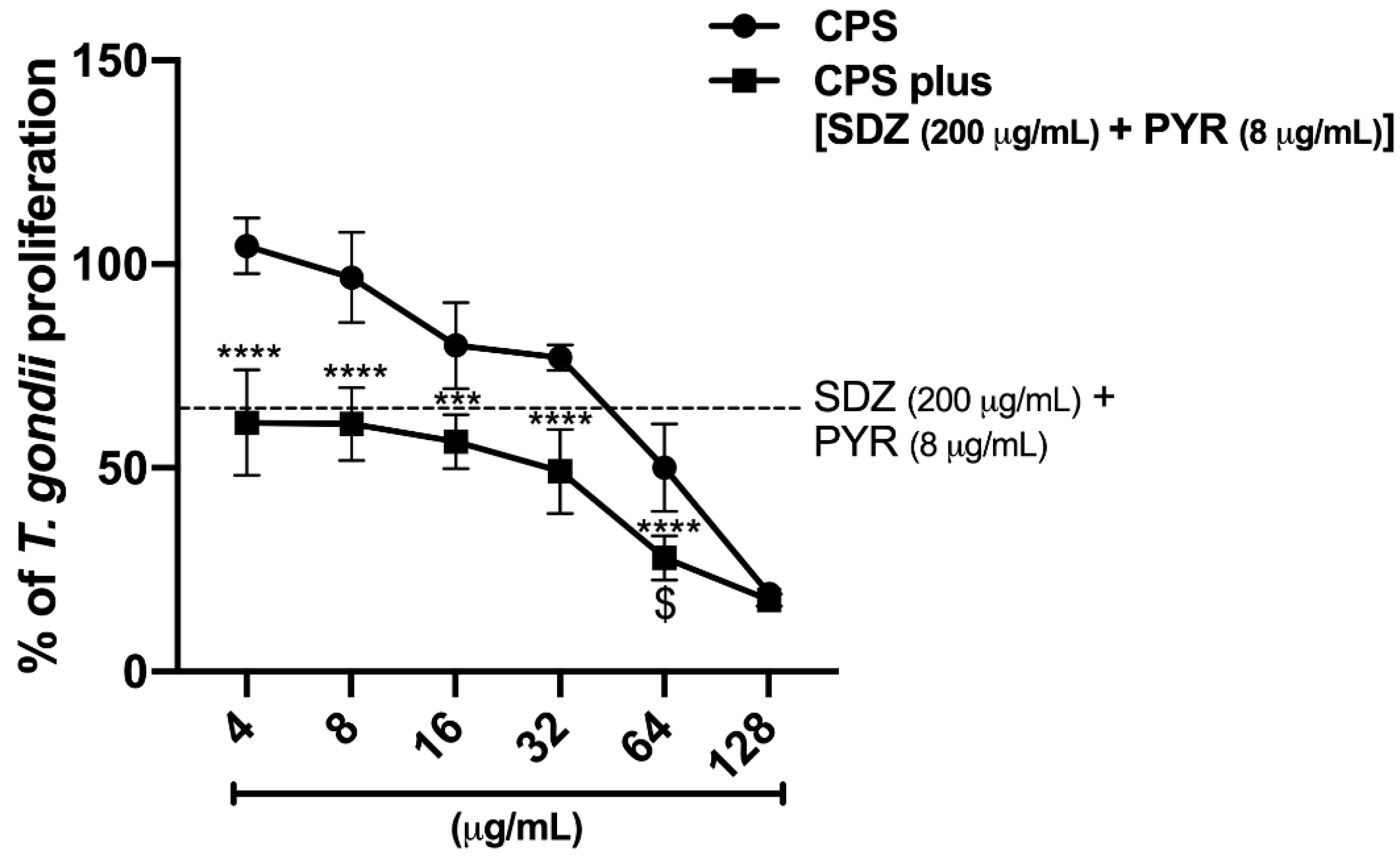
2.5.3. CPS Potentiates the action of SDZ + PYR to Control Parasite Growth
2.6. Analysis of the Chemical Profile of C. chinense Jacq. Extracts by LC-ESI-MS
3. Discussion
4. Materials and Methods
4.1. Obtaining the Extract and Isolated Substances from the Pepper C. chinense Jacq.
4.2. Analysis by High-Performance Liquid Chromatography Coupled to Mass Spectrometry
4.3. Microorganisms
4.4. Determination of Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal/Fungicide Concentration (MBC/MFC)
4.5. Antivirulence Action Evaluation
4.5.1. Candida spp. Antibiofilm Assay
4.5.2. Hemolysin Production Inhibition
4.6. Antiparasitic Effects
4.6.1. Cell Culture and Parasite Maintenance
4.6.2. Host Cell Viability
4.6.3. T. gondii Intracellular Proliferation Assay by β-galactosidase Activity
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jara, M.C.; Frediani, A.V.; Zehetmeyer, F.K.; Bruhn, F.R.P.; Müller, M.R.; Miller, R.G.; Nascente, P.D.S. Multidrug-Resistant Hospital Bacteria: Epidemiological Factors and Susceptibility Profile. Microb. Drug Resist. 2021, 27, 433–440. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive Candidiasis. Nat. Rev. Dis. Primers 2018, 4, 18026. [Google Scholar] [CrossRef]
- Nett, J.E.; Andes, D.R. Contributions of the Biofilm Matrix to Candida Pathogenesis. J. Fungi 2020, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Chakrabarti, A.; Singh, S. Multidrug-resistant Candida auris: An epidemiological review. Expert Rev. Anti Infect. Ther. 2020, 18, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, R.; Milho, C.; Liberal, Â; Silva, J.; Fonseca, C.; Barbosa, A.; Ferreira, I.C.F.R.; Alves, M.J.; Barros, L. Antibiofilm Potential of Medicinal Plants against Candida spp. Oral Biofilms: A Review. Antibiotics 2021, 10, 1142. [Google Scholar] [CrossRef] [PubMed]
- Strang, A.G.; Ferrari, R.G.; Rosário, D.K.D.; Nishi, L.; Evangelista, F.F.; Santana, P.L.; de Souza, A.H.; Mantelo, F.M.; Guilherme, A.L.F. The congenital toxoplasmosis burden in Brazil: Systematic review and meta-analysis. Acta Trop. 2020, 211, 105608. [Google Scholar] [CrossRef]
- Aguirre, A.A.; Longcore, T.; Barbieri, M.; Dabritz, H.; Hill, D.; Klein, P.N.; Lepczyk, C.; Lilly, E.L.; McLeod, R.; Milcarsky, J.; et al. The One Health Approach to Toxoplasmosis: Epidemiology, Control, and Prevention Strategies. EcoHealth 2019, 16, 378–390. [Google Scholar] [CrossRef]
- Doliwa, C.; Escotte-Binet, S.; Aubert, D.; Sauvage, V.; Velard, F.; Schmid, A.; Villena, I. Sulfadiazine resistance inToxoplasma gondii: No involvement of overexpression or polymorphisms in genes of therapeutic targets and ABC transporters. Parasite 2013, 20, 19. [Google Scholar] [CrossRef]
- Silva, L.A.; Fernandes, M.D.; Machado, A.S.; Reis-Cunha, J.L.; Bartholomeu, D.C.; Vitor, R.W.A. Efficacy of sulfadiazine and pyrimetamine for treatment of experimental toxoplasmosis with strains obtained from human cases of congenital disease in Brazil. Exp. Parasitol. 2019, 202, 7–14. [Google Scholar] [CrossRef]
- Teixeira, S.C.; De Souza, G.; Borges, B.C.; De Araújo, T.E.; Rosini, A.M.; Aguila, F.A.; Ambrósio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Silva, M.J.B.; et al. Copaifera spp. oleoresins impair Toxoplasma gondii infection in both human trophoblastic cells and human placental explants. Sci. Rep. 2020, 10, 15158. [Google Scholar] [CrossRef]
- Segura-Campos, M.R.; Ruiz-Ruiz, J.C.; Chel-Guerrero, L.A.; Betancur-Ancona, D.A. Capsicum chinense: Composition and Functional Properties. In Functional Properties of Traditional Foods; Integrating Food Science and Engineering Knowledge into the Food Chain; Kristbergsson, K., Ötles, S., Eds.; Springer: Boston, MA, USA, 2016; Volume 12. [Google Scholar] [CrossRef]
- Rosario, V.N.M.; Chaves, R.P.F.; Pires, I.V.; Santos Filho, A.F.; Toro, M.J.U. Capsicum annuum and Capsicum chinense: Physical, physicalchemical, bioactive characteristics and antioxidante activity. Braz. J. Dev. 2021, 7, 50414–50432. [Google Scholar]
- Batiha, G.E.-S.; Alqahtani, A.; Ojo, O.A.; Shaheen, H.M.; Wasef, L.; Elzeiny, M.; Ismail, M.; Shalaby, M.; Murata, T.; Zaragoza-Bastida, A.; et al. Biological Properties, Bioactive Constituents, and Pharmacokinetics of Some Capsicum spp. and Capsaicinoids. Int. J. Mol. Sci. 2020, 21, 5179. [Google Scholar] [CrossRef]
- Nascimento, P.L.A.; Nascimento, T.C.; Ramos, N.S.M.; Silva, G.R.; Gomes, J.E.G.; Falcão, R.E.A.; Moreira, K.; Porto, A.L.F.; Silva, T.M.S. Quantification, Antioxidant and Antimicrobial Activity of Phenolics Isolated from Different Extracts of Capsicum frutescens (Pimenta Malagueta). Molecules 2014, 19, 5434–5447. [Google Scholar] [CrossRef] [PubMed]
- Vieira-Araújo, F.M.; Rondon, F.C.M.; Vieira, G.P.; Mendes, F.N.P.; de Freitas, J.C.C.; de Morais, S.M. Sinergism between alkaloids piperine and capsaicin with meglumine antimoniate against Leishmania infantum. Exp. Parasitol. 2018, 188, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Magi, G.; Mingoia, M.; Pugnaloni, A.; Facinelli, B. Antimicrobial and Anti-Virulence Activity of Capsaicin Against Erythromycin-Resistant, Cell-Invasive Group A Streptococci. Front. Microbiol. 2015, 6, 1281. [Google Scholar] [CrossRef]
- Wei, G.; Campagna, A.N.; Bobek, L.A. Effect of MUC7 peptides on the growth of bacteria and on Streptococcus mutans biofilm. J. Antimicrob. Chemother. 2006, 57, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Sebaugh, J.L. Guidelines for accurate EC50/IC50 estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Marcos-Zambrano, L.J.; Escribano, P.; Bouza, E.; Guinea, J. Production of biofilm by Candida and non-Candida spp. isolates causing fungemia: Comparison of biomass production and metabolic activity and development of cut-off points. Int. J. Med. Microbiol. 2014, 304, 1192–1198. [Google Scholar] [CrossRef]
- Metlin. Available online: https://metlin.scripps.edu/landing_page.php?pgcontent=mainPage (accessed on 5 April 2022).
- Mohsen, E.; Younis, I.Y.; Farag, M.A. Metabolites profiling of Egyptian Rosa damascena Mill. flowers as analyzed via ultra-high-performance liquid chromatography-mass spectrometry and solid-phase microextraction gas chromatography-mass spectrometry in relation to its anti-collagenase skin effect. Ind. Crop. Prod. 2020, 155, 112818. [Google Scholar] [CrossRef]
- Gómez-Romero, M.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Metabolite profiling and quantification of phenolic compounds in methanol extracts of tomato fruit. Phytochemistry 2010, 71, 1848–1864. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Ali-Shtayeh, M.S.; Jamous, R.; Arraez-Roman, D.; Carretero, A.S. Comprehensive metabolite profiling of Arum palaestinum (Araceae) leaves by using liquid chromatography–tandem mass spectrometry. Food Res. Int. 2015, 70, 74–86. [Google Scholar] [CrossRef]
- Pubmeda. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/6-Hydroxyhexanoic-acid (accessed on 25 May 2022).
- Kramberger, K.; Barlič-Maganja, D.; Bandelj, D.; Arbeiter, A.B.; Peeters, K.; Višnjevec, A.M.; Pražnikar, Z.J. HPLC-DAD-ESI-QTOF-MS Determination of Bioactive Compounds and Antioxidant Activity Comparison of the Hydroalcoholic and Water Extracts from Two Helichrysum italicum Species. Metabolites 2020, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Pubmedb. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/3-Methoxytyrosine (accessed on 25 May 2022).
- Pubmedc. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/2-Hydroxyhexanoic-acid (accessed on 25 May 2022).
- Pubmedd. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/DL-3-Phenyllactic-acid (accessed on 25 May 2022).
- Beelders, T.; De Beer, D.; Stander, M.A.; Joubert, E. Comprehensive Phenolic Profiling of Cyclopia genistoides (L.) Vent. by LC-DAD-MS and -MS/MS Reveals Novel Xanthone and Benzophenone Constituents. Molecules 2014, 19, 11760–11790. [Google Scholar] [CrossRef]
- Santos, L.S.; Fernandes, C.C.; Santos, L.S.; de Deus, I.P.B.; de Sousa, T.L.; Miranda, M.L.D. Ethanolic extract from Capsicum chinense Jacq. ripe fruits: Phenolic compounds, antioxidant activity and development of biodegradable films. Food Sci. Technol. 2021, 41, 497–504. [Google Scholar] [CrossRef]
- Han, J.; Ye, M.; Qiao, X.; Xu, M.; Wang, B.-R.; Guo, D.-A. Characterization of phenolic compounds in the Chinese herbal drug Artemisia annua by liquid chromatography coupled to electrospray ionization mass spectrometry. J. Pharm. Biomed. Anal. 2008, 47, 516–525. [Google Scholar] [CrossRef] [PubMed]
- Mbakidi-Ngouaby, H.; Pinault, E.; Gloaguen, V.; Costa, G.; Sol, V.; Millot, M.; Mambu, L. Profiling and seasonal variation of chemical constituents from Pseudotsuga menziesii wood. Ind. Crop. Prod. 2018, 117, 34–49. [Google Scholar] [CrossRef]
- Wang, Y.; Vorsa, N.; Harrington, P.D.B.; Chen, P. Nontargeted Metabolomic Study on Variation of Phenolics in Different Cranberry Cultivars Using UPLC-IM—HRMS. J. Agric. Food Chem. 2018, 66, 12206–12216. [Google Scholar] [CrossRef]
- Jackson, S.; Swiner, D.J.; Capone, P.C.; Badu-Tawiah, A.K. Thread spray mass spectrometry for direct analysis of capsaicinoids in pepper products. Anal. Chim. Acta 2018, 1023, 81–88. [Google Scholar] [CrossRef]
- Pubmede. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Oxoepoxyoctadecenoic-acid-I (accessed on 25 May 2022).
- Pubmedf. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Dihydrocapsaicin (accessed on 25 May 2022).
- Pubmedg. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/12_13_Ep-9-KODE (accessed on 25 May 2022).
- Abu-Reidah, I.M.; Arráez-Román, D.; Al-Nuri, M.; Warad, I.; Segura-Carretero, A. Untargeted metabolite profiling and phytochemical analysis of Micromeria fruticosa L. (Lamiaceae) leaves. Food Chem. 2018, 279, 128–143. [Google Scholar] [CrossRef]
- Buitimea-Cantúa, G.V.; Buitimea-Cantúa, N.E.; Rocha-Pizaña, M.D.R.; Hernández-Morales, A.; Magaña-Barajas, E.; Molina-Torres, J. Inhibitory effect of Capsicum chinense and Piper nigrum fruits, capsaicin and piperine on aflatoxins production in Aspergillus parasiticus by downregulating the expression of aflD, aflM, aflR, and aflS genes of aflatoxins biosynthetic pathway. J. Environ. Sci. Health Part B 2020, 55, 835–843. [Google Scholar] [CrossRef]
- Fieira, C.; Oliveira, F.; Calegari, R.P.; Machado, A.; Coelho, A.R. In vitro and in vivo antifungal activity of nat-ural inhibitors against Penicillium expansum. Food Sci. Technol. 2013, 33, 40–46. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Holetz, F.B.; Pessini, G.L.; Sanches, N.R.; Cortez, D.A.G.; Nakamura, C.V.; Dias Filho, B.P. Screening of some plants used in the Brazilian folk medicine for the treatment of infectious diseases. Mem. Inst. Oswaldo Cruz 2002, 97, 1027–1031. [Google Scholar] [CrossRef] [PubMed]
- Dorantes, L.; Colmenero, R.; Hernandez, H.; Mota, L.; Jaramillo, M.E.; Fernandez, E.; Solano, C. Inhibition of growth of some foodborne pathogenic bacteria by Capsicum annum extracts. Int. J. Food Microbiol. 2000, 57, 125–128. [Google Scholar] [CrossRef]
- Colombo, A.L.; De Almeida Júnior, J.N.; Guinea, J. Emerging multidrug-resistant Candida species. Curr. Opin. Infect. Dis. 2017, 30, 528–538. [Google Scholar] [CrossRef]
- Gonzalez-Lara, M.F.; Ostrosky-Zeichner, L. Invasive Candidiasis. Semin. Respir. Crit. Care Med. 2020, 41, 003–012. [Google Scholar] [CrossRef]
- De Oliveira, D.; Silva, L.; Silva, B.; Borges, T.; Marques, B.; Santos, M.; Oliveira, L.; Bolzani, V.; Rodrigues, A.; Regasini, L.; et al. A new acridone with antifungal properties against Candida spp. and dermatophytes, and antibiofilm activity against C. albicans. J. Appl. Microbiol. 2019, 127, 1362–1372. [Google Scholar] [CrossRef]
- El-Houssaini, H.H.; Elnabawy, O.M.; Nasser, H.A.; Elkhatib, W.F. Influence of subinhibitory antifungal concentrations on extracellular hydrolases and biofilm production by Candida albicans recovered from Egyptian patients. BMC Infect. Dis. 2019, 19, 54. [Google Scholar] [CrossRef]
- Wu, S.; Wang, Y.; Liu, N.; Dong, G.; Sheng, C. Tackling Fungal Resistance by Biofilm Inhibitors. J. Med. Chem. 2017, 60, 2193–2211. [Google Scholar] [CrossRef]
- Palmeira-De-Oliveira, A.; Gaspar, C.; Silva-Dias, A.; Salgueiro, L.; Cavaleiro, C.; Pina-Vaz, C.; Martinez-De-Oliveira, J.; Queiroz, J.; Rodrigues, A. The anti-Candida activity of Thymbra capitata essential oil: Effect upon pre-formed biofilm. J. Ethnopharmacol. 2012, 140, 379–383. [Google Scholar] [CrossRef]
- Samaranayake, Y.H.; Cheung, B.P.K.; Yau, J.Y.Y.; Yeung, K.W.; Samaranayake, L.P. Genotypic, phenotypic, and proteomic characterization of Candida glabrata during sequential fluconazole exposure. J. Investig. Clin. Dent. 2011, 2, 117–127. [Google Scholar] [CrossRef] [PubMed]
- Raja, V.; Ahmad, S.I.; Irshad, M.; Wani, W.A.; Siddiqi, W.A.; Shreaz, S. Anticandidal activity of ethanolic root extract of Juglans regia (L.): Effect on growth, cell morphology, and key virulence factors. J. Mycol. MeDicale 2017, 27, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Thiengsusuk, A.; Chaijaroenkul, W.; Na-Bangchang, K. Antimalarial activities of medicinal plants and herbal formulations used in Thai traditional medicine. Parasitol. Res. 2013, 112, 1475–1481. [Google Scholar] [CrossRef] [PubMed]
- Ruangnoo, S.; Itharat, A.; Sakpakdeejaroen, I.; Rattarom, R.; Tappayutpijam, P.; Pawa, K.K. In vitro cytotoxic activity of Benjakul herbal preparation and its active compounds against human lung, cervical and liver cancer cells. J. Med Assoc. Thail. 2012, 95, S127–S134. [Google Scholar]
- Leesombun, A.; Boonmasawai, S.; Shimoda, N.; Nishikawa, Y. Effects of Extracts from Thai Piperaceae Plants against Infection with Toxoplasma gondii. PLoS ONE 2016, 11, e0156116. [Google Scholar] [CrossRef]
- Al-Zanbagi, N.A. IN VIVO EFFECT OF SOME HOME SPICES EXTRACTS ON THE TOXOPLASMA GONDII TACHYZOITES. J. Fam. Community Med. 2009, 16, 59–65. [Google Scholar]
- Zamljen, T.; Medič, A.; Veberič, R.; Hudina, M.; Jakopič, J.; Slatnar, A. Metabolic Variation among Fruits of Different Chili Cultivars (Capsicum spp.) Using HPLC/MS. Plants 2021, 11, 101. [Google Scholar] [CrossRef]
- Jarret, R.L.; Berke, T.; Baldwin, E.A.; Antonious, G.F. Variability for Free Sugars and Organic Acids in Capsicum chinense. Chem. Biodivers. 2009, 6, 138–145. [Google Scholar] [CrossRef]
- Materska, M.; Perucka, I. Antioxidant Activity of the Main Phenolic Compounds Isolated from Hot Pepper Fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756. [Google Scholar] [CrossRef]
- Acunha, T.D.S.; Crizel, R.L.; Tavares, I.B.; Barbieri, R.L.; de Pereira, C.M.P.; Rombaldi, C.V.; Chaves, F.C. Bioactive Compound Variability in a Brazilian Capsicum Pepper Collection. Crop Sci. 2017, 57, 1611–1623. [Google Scholar] [CrossRef]
- Martins, N.; Barros, L.; Henriques, M.; Silva, S.; Ferreira, I.C. Activity of phenolic compounds from plant origin against Candida species. Ind. Crop. Prod. 2015, 74, 648–670. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Aboody, M.S.; Mickymaray, S. Anti-Fungal Efficacy and Mechanisms of Flavonoids. Antibiotics 2020, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Abugri, D.A.; Witola, W.H. Interaction of apigenin-7-O-glucoside with pyrimethamine against Toxoplasma gondii growth. J. Parasit. Dis. 2019, 44, 221–229. [Google Scholar] [CrossRef]
- Veloso, J.; Prego, C.; Varela, M.M.; Carballeira, R.; Bernal, A.; Merino, F.; Díaz, J. Properties of capsaicinoids for the control of fungi and oomycetes pathogenic to pepper. Plant Biol. 2014, 16, 85–177. [Google Scholar] [CrossRef]
- Valera-Vera, E.A.; Reigada, C.; Sayé, M.; Digirolamo, F.A.; Galceran, F.; Miranda, M.R.; Pereira, C.A. Effect of capsaicin on the protozoan parasite Trypanosoma cruzi. FEMS Microbiol. Lett. 2020, 367, fnaa194. [Google Scholar] [CrossRef]
- Sora, G.T.D.S.; Souza, A.H.P.; Zielinski, A.A.F.; Haminiuk, C.W.I.; Matsushita, M.; Peralta, R.M. FATTY ACID COMPOSITION OF Capsicum GENUS PEPPERS. Cienc. E Agrotecnologia 2015, 39, 372–380. [Google Scholar] [CrossRef]
- Bonde, C.S.; Bornancin, L.; Lu, Y.; Simonsen, H.T.; Martínez-Valladares, M.; Peña-Espinoza, M.; Mejer, H.; Williams, A.R.; Thamsborg, S.M. Bio-Guided Fractionation and Molecular Networking Reveal Fatty Acids to Be Principal Anti-Parasitic Compounds in Nordic Seaweeds. Front. Pharmacol. 2021, 12, 674520. [Google Scholar] [CrossRef]
- Guimarães, A.; Venâncio, A. The Potential of Fatty Acids and Their Derivatives as Antifungal Agents: A Review. Toxins 2022, 14, 188. [Google Scholar] [CrossRef]
- Silva, T.C.; Justino, A.B.; Prado, D.G.; Koch, G.A.; Martins, M.M.; Santos, P.S.; Morais, S.A.L.; Goulart, L.R.; Cunha, L.C.S.; Sousa, R.M.F.; et al. Chemical composition, antioxidant activity and in-hibitory capacity of α-amylase, α-glucosidase, lipase and non-enzymatic glycation, in vitro, of the leaves of Cassia bakeriana Craib. Ind. Crop. Prod. 2019, 140, 111641. [Google Scholar] [CrossRef]
- CLSI document M07-A9; Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. Approved standard—Ninth Edition. CLSI—Clinical and Laboratory Standards Institute: Wine, PA, USA, 2012.
- CLSI standard M27 Wayne PA; CLSI℄Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts, 4th ed.; Clinical and Laboratory Standards Institute: Wine, PA, USA, 2017. [Google Scholar]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Pierce, C.G.; Uppuluri, P.; Tristan, A.R.; Wormley, F.L., Jr.; Mowat, E.; Ramage, G.; Lopez-Ribot, J.L. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat. Protoc. 2008, 3, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Guan, X.; Zhu, W.; Liu, Z.; Wang, X.; Yu, H.; Wang, H. Capsaicin inhibits Porphyromonas gingivalis growth, biofilm formation, gingivomucosal inflammatory cytokine secretion, and in vitro osteoclastogenesis. Eur. J. Clin. Microbiol. 2013, 33, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Brondani, L.P.; Neto, T.A.d.S.; Freitag, R.A.; Lund, R.G. Evaluation of anti-enzyme properties of Origanum vulgare essential oil against oral Candida albicans. J. Mycol. Med. 2018, 28, 94–100. [Google Scholar] [CrossRef]
- De Melo Riceto, É.B.; de Paula Menezes, R.; Penatti, M.P.; dos Santos Pedroso, R. Enzymatic and hemolytic activity in different Candida species. Rev. Iberoam. Micol. 2014, 32, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, B.F.; Lopes-Maria, J.B.; Gomes, A.O.; Angeloni, M.B.; Castro, A.S.; Franco, P.S.; Fermino, M.L.; Roque-Barreira, M.C.; Ietta, F.; Martins-Filho, O.A.; et al. IL10, TGF Beta1, and IFN Gamma Modulate Intracellular Signaling Pathways and Cytokine Production to Control Toxoplasma gondii Infection in BeWo Trophoblast Cells1. Biol. Reprod. 2015, 92, 82. [Google Scholar] [CrossRef]
- Da Silva, R.J.; Gomes, A.O.; Franco, P.S.; Pereira, A.S.; Milian, I.C.B.; Ribeiro, M.; Fiorenzani, P.; Dos Santos, M.C.; Mineo, J.R.; Da Silva, N.M.; et al. Enrofloxacin and Toltrazuril Are Able to Reduce Toxoplasma gondii Growth in Human BeWo Trophoblastic Cells and Villous Explants from Human Third Trimester Pregnancy. Front. Cell. Infect. Microbiol. 2017, 7, 340. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]

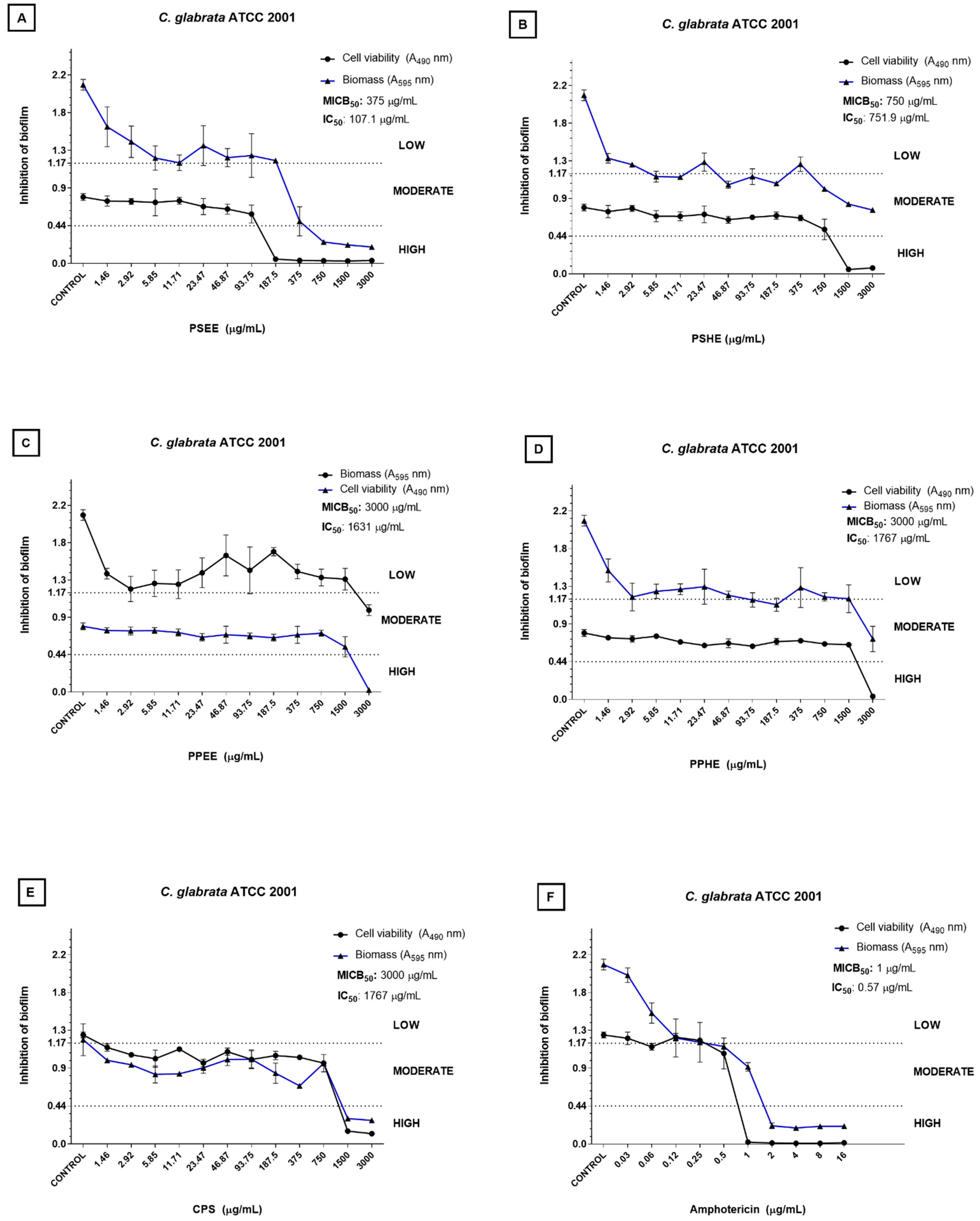
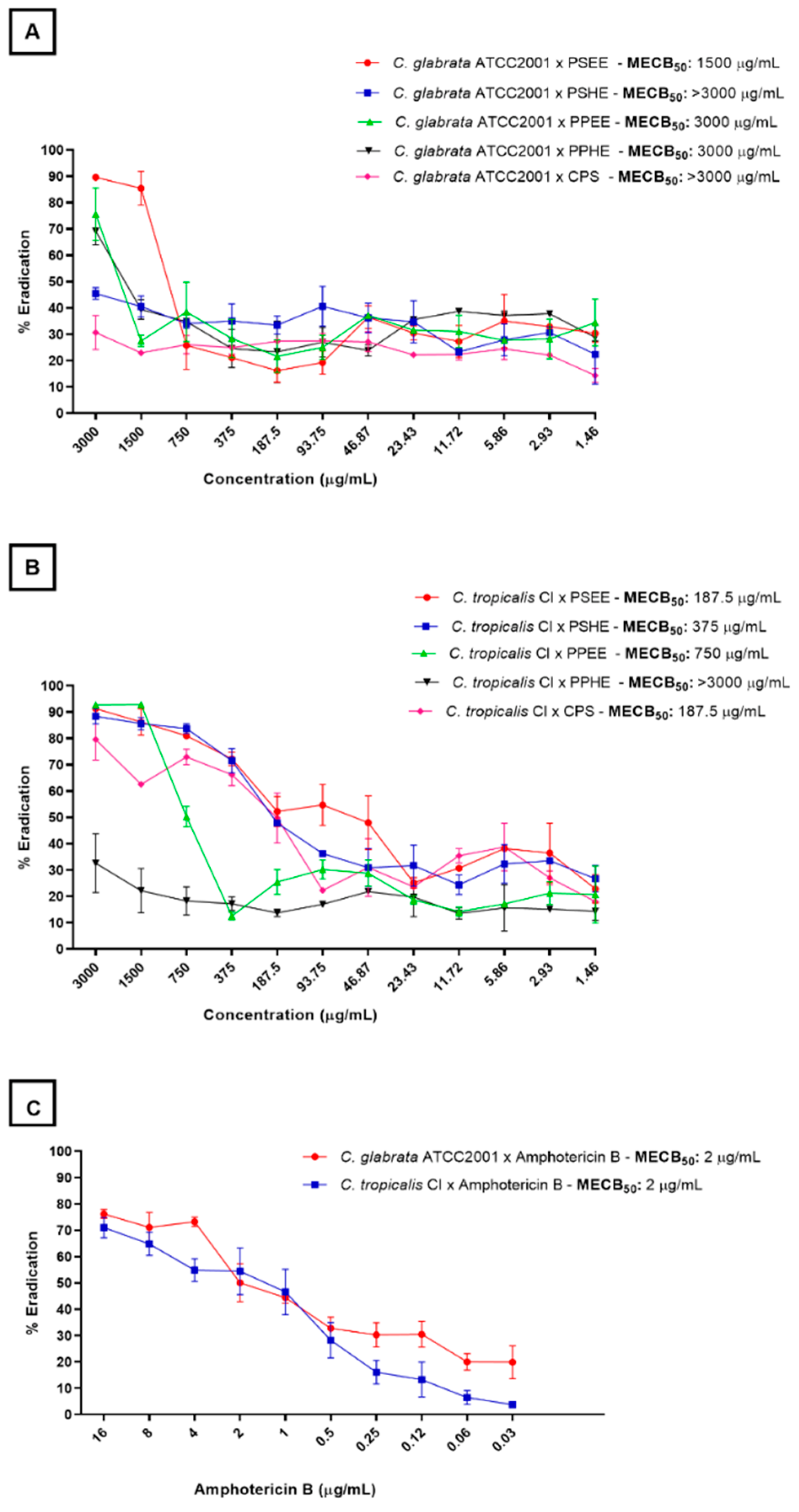
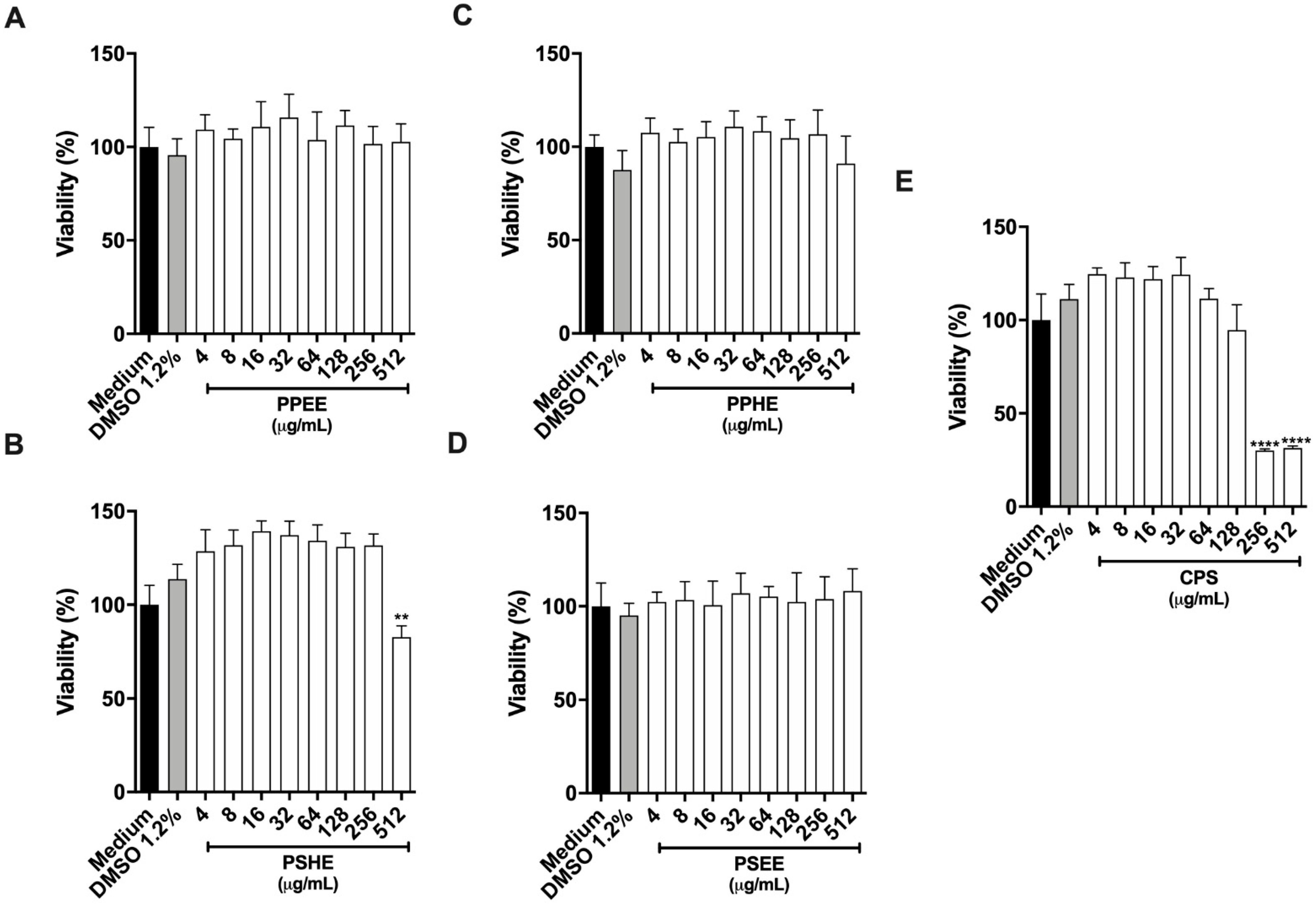
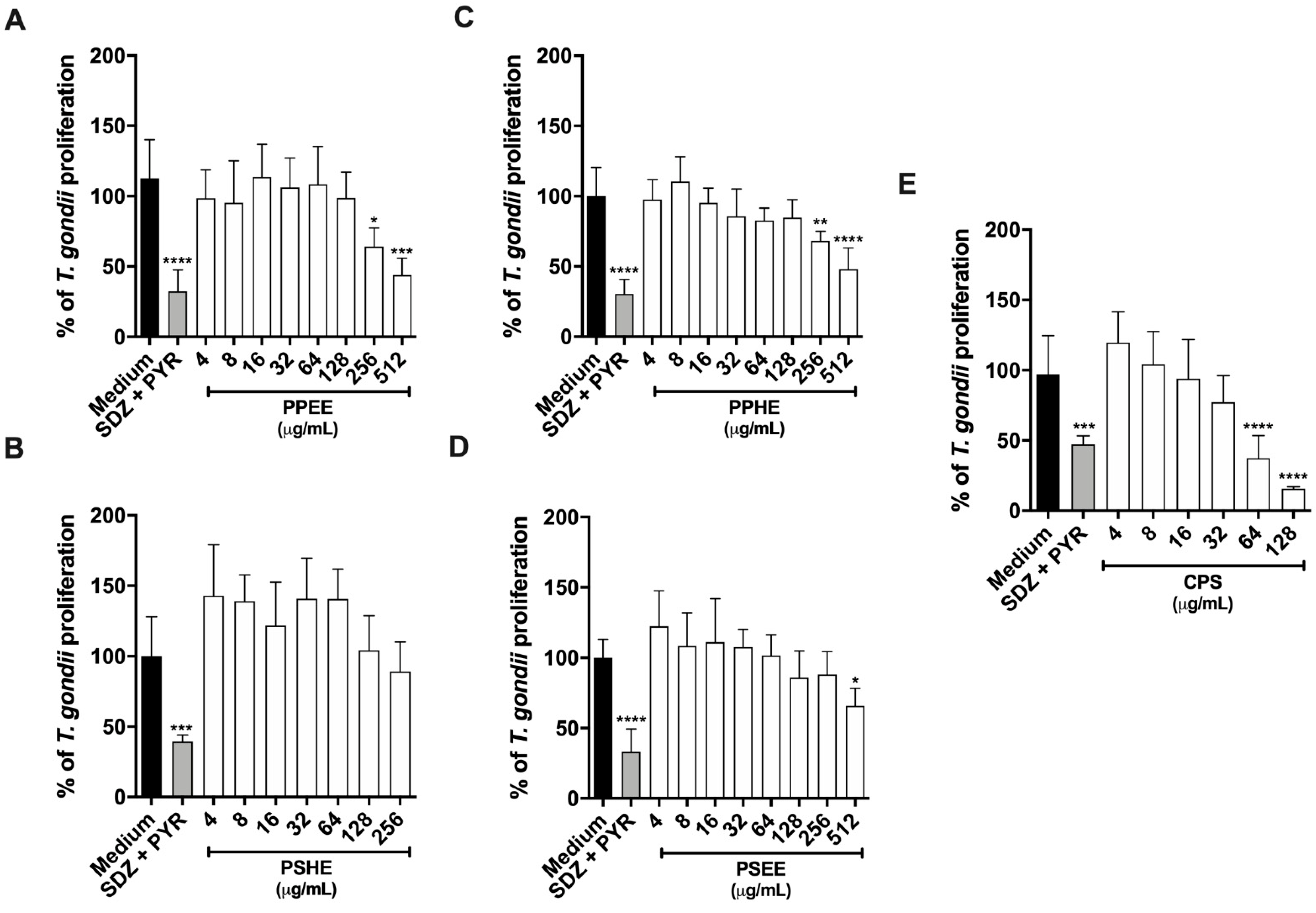
| Isolates | Minimum Inhibitory Concentration/Minimum Fungicide Concentration (μg/mL) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPS 1 | PPHE 2 | PPEE 3 | PSHE 4 | PSEE 5 | Amphotericin B | |||||||
| MIC 6 | MFC 7 | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| C. albicans—ATCC 90028 | 1500 | 1500 | 1500 | 1500 | 3000 | 3000 | 3000 | 3000 | 1500 | 1500 | 0.5 | 0.5 |
| C. albicans—CI 8 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 3000 | 0.25 | 0.25 |
| C. parapsilosis—ATCC 22019 | - | - | 3000 | - | - | - | - | - | 1500 | 1500 | 0.5 | 0.5 |
| C. parapsilosis—CI | - | - | 3000 | - | - | - | - | - | 3000 | 3000 | 0.125 | 0.125 |
| C. tropicalis—ATCC 13803 | - | - | 3000 | 3000 | 3000 | - | 3000 | - | 3000 | - | 0.5 | 0.5 |
| C. tropicalis—CI | 187.5 | 187.5 | 187.5 | 750 | 750 | 750 | 187.5 | 375 | 93.75 | 93.75 | 0.125 | 0.125 |
| C. glabrata—ATCC2001 | 187.5 | 187.5 | 1500 | 1500 | 3000 | 3000 | 375 | 375 | 187.5 | 187.5 | 0.25 | 0.25 |
| C. glabrata—CI | 1500 | 1500 | 1500 | 1500 | 3000 | 3000 | 1500 | 1500 | 750 | 1500 | 0.25 | 0.25 |
| C. krusei—ATCC 6258 | 1500 | 1500 | 1500 | 750 | 3000 | 3000 | 750 | 1500 | 3000 | 3000 | 1.0 | 1.0 |
| C. krusei—CI | 1500 | 1500 | 750 | 750 | 750 | 750 | 187.5 | 1500 | 1500 | 1500 | 0.06 | 0.06 |
| C. glabrata (ATCC 2001) | |||||||
|---|---|---|---|---|---|---|---|
| Control | CPS 1 | PPHE 2 | PPEE 3 | PSHE 4 | PSEE 5 | Amphotericin B | |
| Average Hi | 0.35 | 0.47 | 0.5 | 0.47 | 0.48 | 0.52 | 0.42 |
| % inhibition | - | 34.3% | 42.8% | 34.3% | 37.1% | 48.6% | 20% |
| p valor | 0.13 | 0.002 * | 0.71 | 0.03 * | 0.006 * | >0.99 | |
| C. tropicalis (CI 6) | |||||||
| Control | CPS | PPHE | PPEE | PSHE | PSEE | Amphotericin B | |
| Average Hi | 0.53 | 0.74 | 0.70 | 0.61 | 0.67 | 0.69 | 0.70 |
| % inhibition | - | 39.6% | 32.1% | 15.1% | 26.4% | 30.2% | 32.1% |
| p valor | <0.0001 * | 0.0002 * | 0.99 | 0.0067 * | 0.0012 * | 0.0004 * | |
| N. | Rt (Min) | [M-H]– | Exact Mass | Error (ppm) | Fragmentos MS2 | Molecular Formula | Tentative Identity | References |
|---|---|---|---|---|---|---|---|---|
| 1 | 0.75 | 181.0740 | 181.0745 | −2.7 | 10 eV: 181, 163, 119, 101, 89, 71, 59 | C6H14O6 | Sorbitol 1 | [20] |
| 2 | 0.81 | 191.0563 | 191.0561 | 1.04 | 10 eV: 173, 158, 127, 109, 93, 85 | C7H12O6 | Quinic acid 1,2 | [20,21] |
| 3 | 0.96 | 128.0354 | 128.0353 | 0.78 | 10 eV: 128, 112, 99, 88 | C5H7NO3 | Pyroglutamic acid 1,2 | [20] |
| 4 | 1.10 | 117.0194 | 117.0193 | 0.85 | 10 eV: 99, 73 | C4H6O4 | Succinic acid 1,2 | [20,21] |
| 5 | 1.18 | 292.1429 | 292.1435 | −2.0 | 10 eV: 202, 130 | C12H22NO7 | Fructosyl-leucine/isoleucine 1 | [22] |
| 6 | 1.37 | 169.0143 | 169.0142 | 0.59 | 10 eV: 125 | C7H6O5 | Gallic acid 1,2 | [20,21] |
| 7 | 1.59 | 164.0724 | 164.0723 | 0.60 | 10 eV: 147, 103, 72 | C9H11NO2 | Phenylalanine 1,2 | [20] |
| 8 | 2.27 | 153.0194 | 153.0193 | 0.65 | 10 eV: 109 | C7H6O4 | 3,4-dihydroxybenzoic acid (Protocatechuic acid) 1,2 | [20,23] |
| 9 | 2.52 | 117.0561 | 117.0557 | 3.41 | 10 eV: 99, 87, 71 | C5H10O3 | Hydroxyisovaleric acid 1,2 | [20] |
| 10 | 2.73 | 329.0877 | 329.0878 | −0.30 | 10 eV: 269, 209, 167 | C14H18O9 | Dihydroxybenzoic acid methyl ether-O-hexoside 1 | [21] |
| 11 | 2.92 | 181.0509 | 181.0506 | 1.65 | 10 eV: 163, 135, 119 | C9H10O4 | Hydroxyphenyllactic acid 1 | [20,24] |
| 12 | 3.33 | 137.0246 | 137.0244 | 1.45 | 10 eV: 123, 93, 65 | C7H6O3 | Hydroxybenzoic acid 1,2 | [20,25] |
| 13 | 3.66 | 131.0713 | 131.0714 | −0.76 | 10 eV: 131, 99, 59 | C6H12O3 | Hydroxycaproic acid I 1,2 | [24] |
| 14 | 4.03 | 210.0774 | 210.0772 | 0.95 | 20 eV: 163, 124, 94 | C10H13NO4 | Methoxytyrosine 1 | [26] |
| 15 | 4.34 | 131.0713 | 131.0714 | −0.76 | 10 eV: 131, 99, 85, 69 | C6H12O3 | Hydroxycaproic acid II 1,2 | [27] |
| 16 | 4.80 | 165.0559 | 165.0557 | 1.21 | 10 eV: 165, 147, 119, 103, 91, 73 | C9H10O3 | 3-phenyllactic acid 1,2 | [28,29] |
| 17 | 4.91 | 163.0402 | 163.0401 | 0.61 | 10 eV: 147, 119, 103 | C9H8O3 | 2-hydroxycinnamic acid (p-coumaric acid) 1 | [23] |
| 18 | 5.03 | 563.1409 | 563.1406 | 0.53 | 20 eV: 563, 503, 473, 443, 425, 383, 353 | C26H28O14 | Apigenin-6-glucoside-8-arabinoside (Isoshaftoside) 1,2 | [23] |
| 19 | 5.27 | 447.0931 | 447.0933 | −0.44 | 20 eV: 357, 327, 285 | C21H20O11 | Luteolin-6-C-glucoside or Luteolin-8-C-glucoside (Iso)orientin 1 | [23] |
| 20 | 5.24 | 193.0505 | 193.0506 | −0.51 | 10 ev: 178, 149, 134 | C10H10O4 | Ferulic acid 1 | [30] |
| 21 | 5.46 | 431.0983 | 431.0984 | −0.23 | 10 eV: 431, 342, 311, 183 | C21H20O10 | Apigenin-8-C-glucoside (Vitexin) 1 | [23,31] |
| 22 | 6.32 | 187.0974 | 187.0976 | −1.06 | 5 eV: 187, 125, 97 | C9H16O4 | Azelaic acid 1,2,3,4 | [32] |
| 23 | 6.37 | 447.0933 | 447.0933 | 0.0 | 20 eV: 343, 300, 301, 271, 227, 179, 151, 109 | C21H20O11 | Quercetin 3-O-rhamnoside 1 | [20,21] |
| 24 | 7.01 | 461.1089 | 461.1089 | 0.0 | 20 eV: 357, 315, 314, 299, 295, 271, 199, 151 | C22H22O11 | Isorhamnetin 3-O-rhamnoside 1 | [33] |
| 25 | 8.34 | 327.2179 | 327.2177 | 0.61 | 20 eV: 291, 211, 171, 137, 85 | C18H32O5 | Trihydroxyoctadecdienoic acid I 1,2 | [22] |
| 26 | 8.49 | 327.2175 | 327.2177 | −0.61 | 10 eV: 273, 201, 171, 137, 85 | C18H32O5 | Trihydroxyoctadecdienoic acid II 1,2 | [22] |
| 27 | 8.75 | 329.2333 | 329.2333 | 0.0 | 20 eV: 329, 311, 293, 275, 229, 211 201, 183, 171, 139, 127 | C18H34O5 | Hydroxyoctadecanedioic acid I or Trihydroxyoctadecenoic acid I 1,2 | [21,22,23] |
| 28 | 8.85 | 329.2332 | 329.2333 | −0.30 | 20 eV: 329, 311, 293, 275, 229, 211, 201, 183, 171, 139 | C18H34O5 | Hydroxy-octadecanedioic acid II or Trihydroxy-octadecenoic acid II 1,2 | [21,22,23] |
| 29 | 8.97 | 329.2334 | 329.2333 | 0.30 | 20 eV: 329, 311, 293, 275, 229, 211, 201, 183, 171, 139 | C18H34O5 | Hydroxyoctadecanedioic acid III or Trihydroxyoctadecenoic acid III 1,2 | [21,22,23] |
| 30 | 9.05 | 287.2231 | 287.2228 | 1.00 | 20 eV: 287, 269, 241, 211 | C16H32O4 | Dihydroxy-hexadecanoic acid 1 | [22] |
| 31 | 9.12 | 304.1916 | 304.1918 | −0.65 | 10 eV: 289, 168, 116 | C18H27NO3 | Capsaicin 1,2,3,4 | [34] |
| 32 | 9.19 | 329.2335 | 329.2333 | 0.60 | 20 eV: 329, 311, 293, 275, 229, 201, 171, 139 | C18H34O5 | Hydroxyoctadecanedioic acid IV or Trihydroxyoctadecenoic acid IV 1,2 | [22,23] |
| 33 | 9.45 | 309.2065 | 309.2071 | −1.9 | 10 eV: 309, 291, 265, 209, 185, 171, 149, 113 | C18H30O4 | Oxoepoxyoctadecenoic acid I 1,2 | [35] |
| 34 | 9.65 | 306.2072 | 306.2075 | −0.97 | 20 eV: 247, 170 | C18H29NO3 | Dihydrocapsaicin 1,2,3,4 | [20,36] |
| 35 | 9.76 | 309.2072 | 309.2071 | 0.32 | 20 eV: 309, 291, 273, 249, 201, 185, 171, 155, 137 | C18H30O4 | Oxoepoxyoctadecenoic acid II 1,2 | [37] |
| 36 | 9.91 | 311.2230 | 311.2228 | 0.64 | 20 eV: 293, 275, 249, 201, 185, 171, 155, 139 | C18H32O4 | Dihydroxy-octadecadienoic acid or Linoleic acid hydroperoxide 1,2,3,4 | [38] |
| 37 | 10.18 | 311.2229 | 311.2228 | 0.32 | 20 eV: 293, 275, 249, 201, 185, 171, 155, 139 | C18H32O4 | Dihydroxyoctadecadienoic acid or Linoleic acid hydroperoxide 1,2,3,4 | [38] |
| 38 | 10.56 | 293.2124 | 293.2122 | 0.68 | 20 eV: 275, 235, 171, 121 | C18H30O3 | Hydroxyoctadecatrienoic acid I or Oxooctadeca-dienoic acid I 1,2 | [22,38] |
| 39 | 291.1968 | 291.1966 | 0.68 | 20 eV: 291, 273, 185, 121 | C18H28O3 | Oxooctadecatrienoic acid 3 | [20] | |
| 40 | 10.85 | 293.2119 | 293.2122 | −1.0 | 20 eV: 275, 235, 171, 121 | C18H30O3 | Hydroxyoctadecatrienoic acid II or Oxooctadeca-dienoic acid II 1,2,3,4 | [22,38] |
| 41 | 295.2278 | 295.2279 | −0.33 | 20 eV: 277, 259, 233, 195, 171, 151, 123 | C18H32O3 | Hydroxyoctadecadienoic acid I 1,2 | [22] | |
| 42 | 10.90 | 293.2119 | 293.2122 | −1.0 | 20 eV: 293, 275, 249, 221, 197, 185, 149, 125 | C18H30O3 | Oxooctadecadienoic acid III 1,2,3,4 | [22,38] |
| 43 | 295.2277 | 295.2279 | −0.67 | 20 eV: 277, 235, 183, 171 | C18H32O3 | Hydroxyoctadecadienoic acid II 1,2,4 | [22] | |
| 44 | 11.86 | 277.2170 | 277.2173 | −1.0 | 20 eV: 277, 233 | C18H30O2 | Octadecatrienoic acid 3,4 | [21] |
| 45 | 12.23 | 279.2331 | 279.2330 | 0.35 | 20 eV: 279, 254, 218, 185, 151, 171, 211 | C18H32O2 | Octadecadienoic acid 2,3,4 (Linoleic acid) | [21] |
| 46 | 12.58 | 255.2334 | 255.2330 | −1.56 | 25 eV: 255, 237, 201 | C16H32O2 | Hexadecanoic acid 3,4 (Palmitic acid) | [21] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menezes, R.d.P.; Bessa, M.A.d.S.; Siqueira, C.d.P.; Teixeira, S.C.; Ferro, E.A.V.; Martins, M.M.; Cunha, L.C.S.; Martins, C.H.G. Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin. Antibiotics 2022, 11, 1154. https://doi.org/10.3390/antibiotics11091154
Menezes RdP, Bessa MAdS, Siqueira CdP, Teixeira SC, Ferro EAV, Martins MM, Cunha LCS, Martins CHG. Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin. Antibiotics. 2022; 11(9):1154. https://doi.org/10.3390/antibiotics11091154
Chicago/Turabian StyleMenezes, Ralciane de Paula, Meliza Arantes de Souza Bessa, Camila de Paula Siqueira, Samuel Cota Teixeira, Eloisa Amália Vieira Ferro, Mário Machado Martins, Luis Carlos Scalon Cunha, and Carlos Henrique Gomes Martins. 2022. "Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin" Antibiotics 11, no. 9: 1154. https://doi.org/10.3390/antibiotics11091154
APA StyleMenezes, R. d. P., Bessa, M. A. d. S., Siqueira, C. d. P., Teixeira, S. C., Ferro, E. A. V., Martins, M. M., Cunha, L. C. S., & Martins, C. H. G. (2022). Antimicrobial, Antivirulence, and Antiparasitic Potential of Capsicum chinense Jacq. Extracts and Their Isolated Compound Capsaicin. Antibiotics, 11(9), 1154. https://doi.org/10.3390/antibiotics11091154






