Ribes nigrum L. Extract-Mediated Green Synthesis and Antibacterial Action Mechanisms of Silver Nanoparticles
Abstract
1. Introduction
2. Results
2.1. The Total Phenolic-Flavonoid Composition of R. nigrum Extracts
2.2. Identification of Major Polyphenols in R. nigrum Extract
2.3. Radical Scavenging Capacity of R. nigrum Extract
2.4. Antioxidant Profiling by HPLC Coupled Post-Column Derivatization
2.5. Metal Chelating Capability of R. nigrum Leaf Extract
2.6. Production of Silver Nanoparticles Using Plant Extract
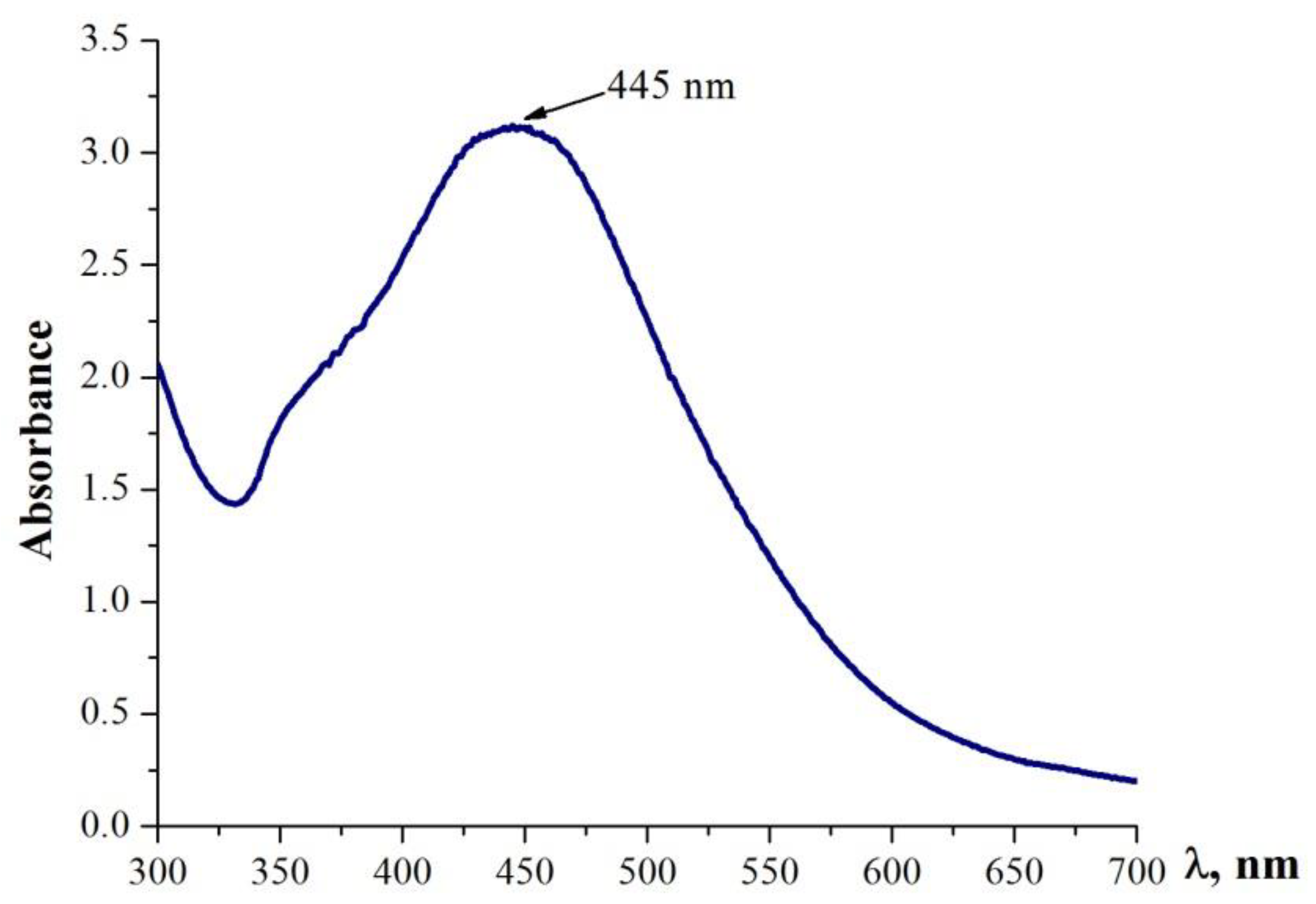
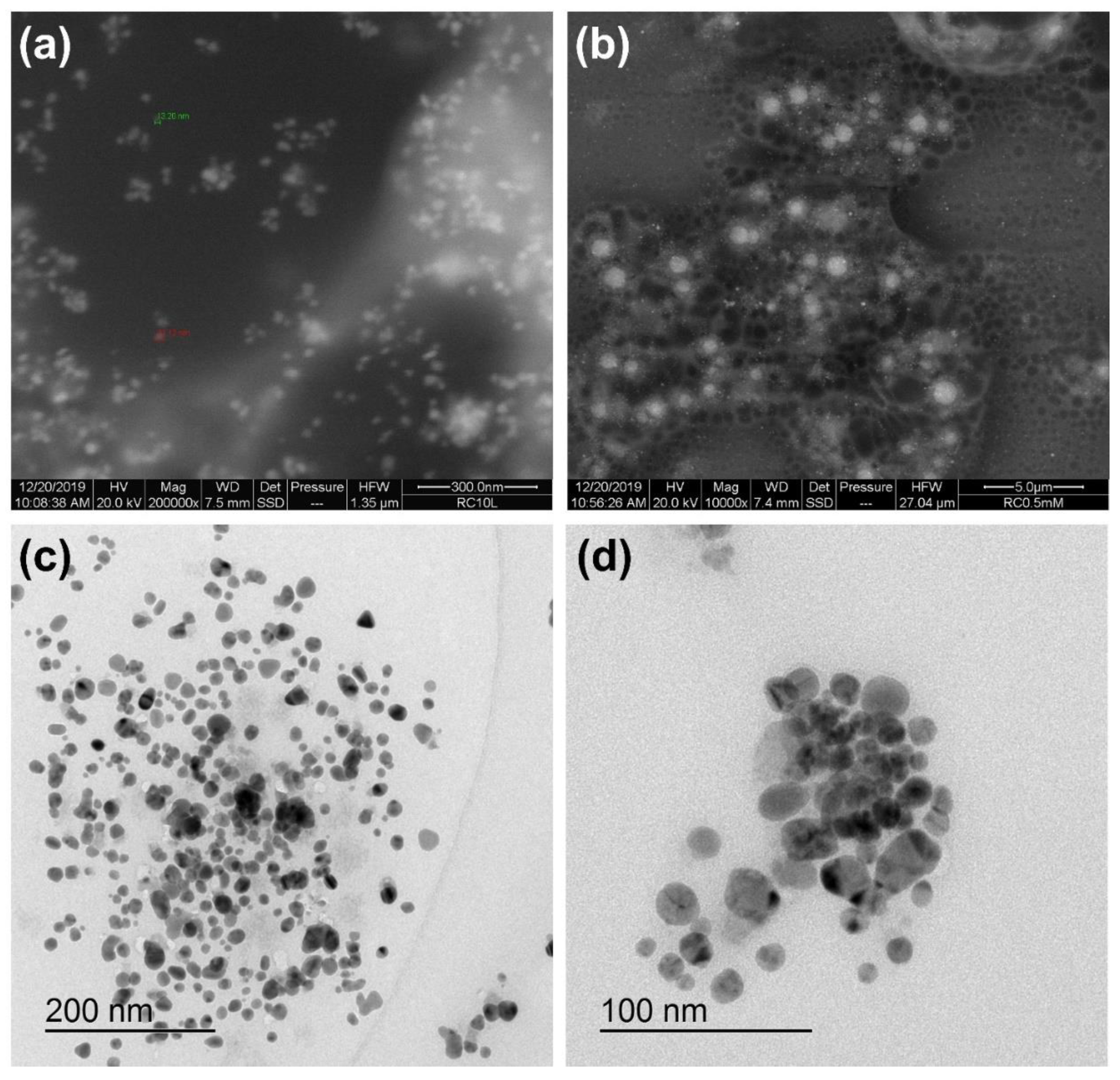
2.7. Characterization of Ag NPs
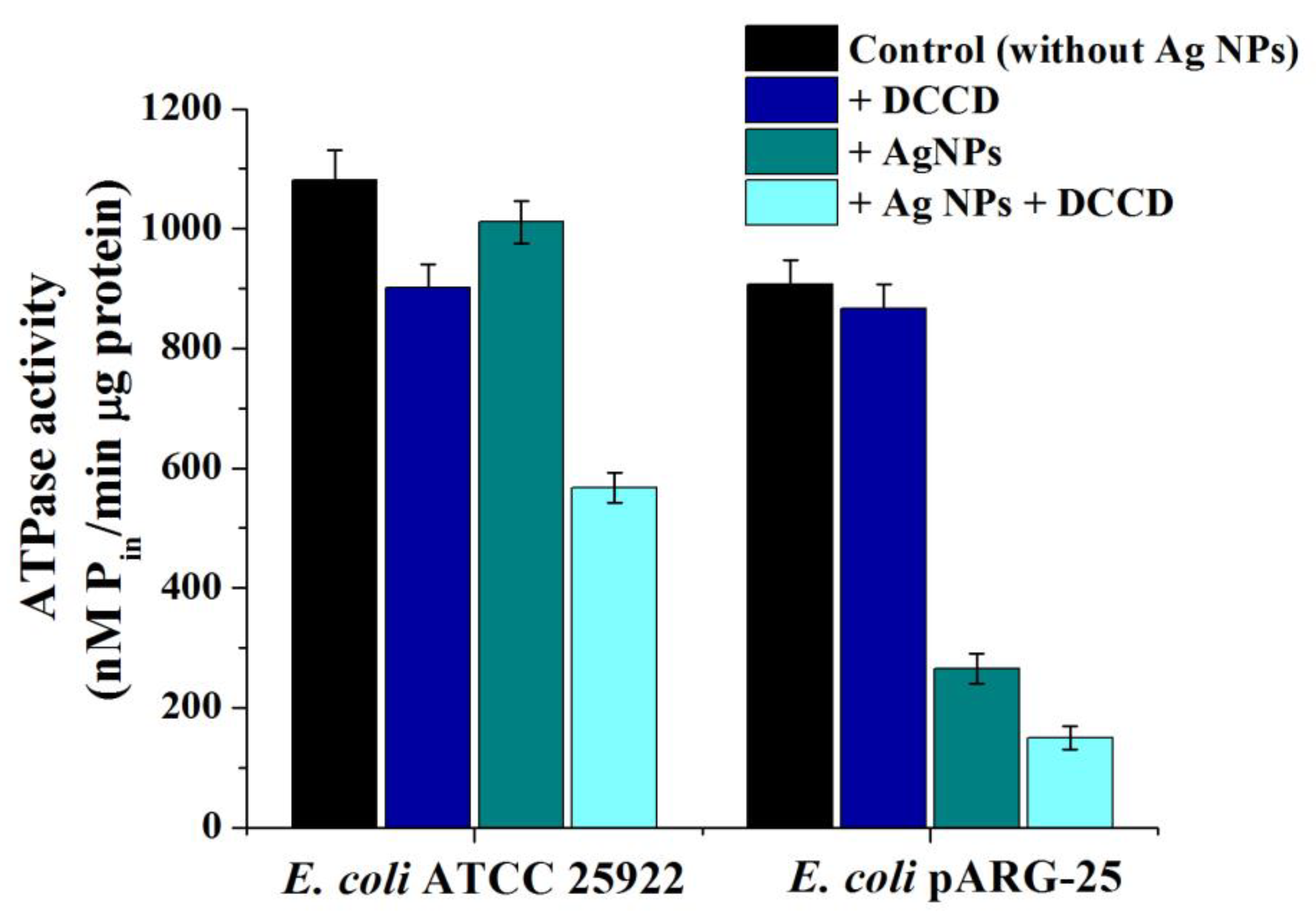
2.8. Effect of Biogenic Ag NPs on Bacterial Growth Rate, FOF1-ATPase Activity, and H+-Fluxes through the Membrane in Escherichia coli ATCC 25922 and Drug-Resistant E. coli pARG-25 Strains
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Plant Material Collection, Identification and Extraction
4.3. Determination of Total Phenolic and Flavonoid Content
4.4. LC-Q-Orbitrap HRMS Analysis
4.5. Post-Column Derivatization with ABTS
4.6. 2,2-Diphenyl-1-picrylhydrazyl Free Radical Scavenging Assay
4.7. Chelating Capability of R. nigrum Leaf Extract
4.8. Synthesis of Ag NPs Using R. nigrum Extracts
4.9. Characterization of Biosynthesized Ag NPs
4.10. Antibacterial Activity of Biosynthesized Ag NPs
4.11. Growth Kinetics of E. coli ATCC 25922 and E. coli pAPG-25 Strains under the Influence of Biosynthesized Ag NPs
4.12. Determination of H+-fluxes
4.13. Determination of FOF1-ATPase Activity in Membrane Vesicles in the Presence of Ag NPs and R. nigrum Leaf Extract
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Munita, J.M.; Cesar, A.A. Mechanisms of Antibiotic Resistance. Annu. Rep. Med. Chem. 2016, 4, 1–24. [Google Scholar] [CrossRef]
- Sahakyan, N.; Petrosyan, M.; Trchounian, A. The Activity of Alkanna Species in Vitro Culture and Intact Plant Extracts Against Antibiotic Resistant Bacteria. Curr. Pharm. Des. 2019, 25, 1861–1865. [Google Scholar] [CrossRef] [PubMed]
- Kakoullis, L.; Papachristodoulou, E.; Chra, P.; Panos, G. Mechanisms of Antibiotic Resistance in Important Gram-Positive and Gram-Negative Pathogens and Novel Antibiotic Solutions. Antibiotics 2021, 10, 415. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.W. History of the Medical Use of Silver. Surg. Infect. 2009, 10, 289–294. [Google Scholar] [CrossRef]
- Sim, W.; Barnard, R.T.; Blaskovich, M.A.T.; Ziora, Z.M. Antimicrobial Silver in Medicinal and Consumer Applications: A Patent Review of the Past Decade (2007–2017). Antibiotics 2018, 7, 93. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Ying, A.N.J.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and Strategies for the Synthesis of Diverse Nanoparticles and Their Applications: A Comprehensive Overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Hambardzumyan, S.; Sahakyan, N.; Petrosyan, M.; Nasim, M.J.; Jacob, C.; Trchounian, A. Origanum vulgare L. Extract-Mediated Synthesis of Silver Nanoparticles, Their Characterization and Antibacterial Activities. AMB Express 2020, 10, 162. [Google Scholar] [CrossRef]
- Kondeti, V.S.S.K.; Gangal, U.; Yatom, S.; Bruggeman, P.J. Ag + Reduction and Silver Nanoparticle Synthesis at the Plasma–Liquid Interface by an RF Driven Atmospheric Pressure Plasma Jet: Mechanisms and the Effect of Surfactant. J. Vac. Sci. Technol. A Vac. Surf. Film. 2017, 35, 061302. [Google Scholar] [CrossRef]
- Timotina, M.; Aghajanyan, A.; Schubert, R.; Trchounian, K.; Gabrielyan, L. Biosynthesis of Silver Nanoparticles Using Extracts of Stevia rebaudiana and Evaluation of Antibacterial Activity. World J. Microbiol. Biotechnol. 2022, 38, 196. [Google Scholar] [CrossRef]
- Xu, D.-P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.-J.; Li, H.-B. Natural Antioxidants in Foods and Medicinal Plants: Extraction, Assessment and Resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Avetisyan, A.; Markosian, A.; Petrosyan, M.; Sahakyan, N.; Babayan, A.; Aloyan, S. Chemical Composition and Some Biological Activities of the Essential Oils from Basil Ocimum Different Cultivars. BMC Complement. Altern. Med. 2017, 17, 60. [Google Scholar] [CrossRef]
- Sahakyan, N.; Bartoszek, A.; Jacob, C.; Petrosyan, M.; Trchounian, A. Bioavailability of Tannins and Other Oligomeric Polyphenols: A Still to Be Studied Phenomenon. Curr. Pharmacol. Rep. 2020, 6, 131–136. [Google Scholar] [CrossRef]
- Ginovyan, M.M.; Sahakyan, N.Z.; Petrosyan, M.T.; Trchounian, A.H. Antioxidant Potential of some Herbs Represented in Armenian Flora and Characterization of Phytochemicals. Proc. YSU B Chem. Biol. Sci. 2021, 55, 25–38. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Liu, Z.-G.; Shen, W.; Gurunathan, S. Silver Nanoparticles: Synthesis, Characterization, Properties, Applications, and Therapeutic Approaches. Int. J. Mol. Sci. 2016, 17, 1534. [Google Scholar] [CrossRef] [PubMed]
- Gabrielyan, L.; Trchounian, A. Antibacterial Activities of Transient Metals Nanoparticles and Membranous Mechanisms of Action. World J. Microbiol. Biotechnol. 2019, 35, 162. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, W.; Xue, J.; Liu, Y.; Liu, Y.; Yan, P.; Liu, J.; Tang, J. Recent Advances in Synthetic Methods and Applications of Silver Nanostructures. Nanoscale Res. Lett. 2018, 13, 54. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Kaur, K. Biological and Physical Applications of Silver Nanoparticles with Emerging Trends of Green Synthesis. Eng. Nanomater. Saf. 2019, 1–25. [Google Scholar] [CrossRef]
- Prabhu, D.; Arulvasu, C.; Babu, G.; Manikandan, R.; Srinivasan, P. Biologically Synthesized Green Silver Nanoparticles from Leaf Extract of Vitex negundo L. Induce Growth-Inhibitory Effect on Human Colon Cancer Cell Line HCT15. Process Biochem. 2013, 48, 317–324. [Google Scholar] [CrossRef]
- Pandian, A.M.K.; Karthikeyan, C.; Rajasimman, M.; Dinesh, M.G. Synthesis of Silver Nanoparticle and Its Application. Ecotoxicol. Environ. Saf. 2015, 121, 211–217. [Google Scholar] [CrossRef]
- Shaik, M.R.; Khan, M.; Kuniyil, M.; Al-Warthan, A.; Alkhathlan, H.Z.; Siddiqui, M.R.H.; Shaik, J.P.; Ahamed, A.; Mahmood, A.; Khan, M.; et al. Plant-Extract-Assisted Green Synthesis of Silver Nanoparticles Using Origanum vulgare L. Extract and Their Microbicidal Activities. Sustainability 2018, 10, 913. [Google Scholar] [CrossRef]
- Salari, S.; Bahabadi, S.E.; Samzadeh-Kermani, A.; Yosefzaei, F. In-Vitro Evaluation of Antioxidant and Antibacterial Potential of Green Synthesized Silver Nanoparticles Using Prosopis farcta Fruit Extract. Iran. J. Pharm. Res. 2019, 18, 430–445. [Google Scholar] [PubMed]
- Sahakyan, N.Z.; Petrosyan, M.T.; Trchounian, A.H. Increasing of the Superoxide Dismutase Total Activity in Microglial Cells Under the Treatment By Ribes nigrum L. Alcohol Extract. Proc. YSU B Chem. Biol. Sci. 2020, 54, 216–222. [Google Scholar] [CrossRef]
- Luzak, B.; Boncler, M.; Rywaniak, J.; Dudzinska, D.; Rozalski, M.; Krajewska, U.; Balcerczak, E.; Podsedek, A.; Redzynia, M.; Watala, C. Extract from Ribes nigrum Leaves in Vitro Activates Nitric Oxide Synthase (ENOS) and Increases CD39 Expression in Human Endothelial Cells. J. Physiol. Biochem. 2014, 70, 1007–1019. [Google Scholar] [CrossRef] [PubMed]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum l.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef]
- Garbacki, N.; Tits, M.; Angenot, L.; Damas, J. Inhibitory Effects of Proanthocyanidins from Ribes nigrum Leaves on Carrageenin Acute Inflammatory Reactions Induced in Rats. BMC Pharmacol. 2004, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Butnariu, M. Detection of the Polyphenolic Components in Ribes nigrum L. Ann. Agric. Environ. Med. 2014, 21, 11–14. [Google Scholar]
- Paunović, S.M.; Mašković, P.; Nikolić, M.; Miletić, R. Bioactive Compounds and Antimicrobial Activity of Black Currant (Ribes nigrum L.) Berries and Leaves Extract Obtained by Different Soil Management System. Sci. Hortic. (Amst.) 2017, 222, 69–75. [Google Scholar] [CrossRef]
- Jansone, B.; Laekeman, G.; Vlietinck, A. Assessment Report on Ribes nigrum L., Folium; European Medicines Agency: Amsterdam, The Netherlands, 2017; Volume 44.
- Vorobyova, V.; Vasyliev, G.; Skiba, M. Eco-Friendly “Green” Synthesis of Silver Nanoparticles with the Black Currant Pomace Extract and Its Antibacterial, Electrochemical, and Antioxidant Activity. Appl. Nanosci. 2020, 10, 4523–4534. [Google Scholar] [CrossRef]
- Vasyliev, G.; Vorobyova, V.; Skiba, M.; Khrokalo, L. Green Synthesis of Silver Nanoparticles Using Waste Products (Apricot and Black Currant Pomace) Aqueous Extracts and Their Characterization. Adv. Mater. Sci. Eng. 2020, 2020, 4505787. [Google Scholar] [CrossRef]
- Yong, N.L.; Ahmad, A.; Mohammad, A.W. Synthesis and Characterization of Silver Oxide Nanoparticles by a Novel Method. Int. J. Sci. Eng. Res. 2013, 4, 155–158. [Google Scholar]
- Fahmy, H.M.; Mosleh, A.M.; Elghany, A.A.; Shams-Eldin, E.; Abu Serea, E.S.; Ali, S.A.; Shalan, A.E. Coated Silver Nanoparticles: Synthesis, Cytotoxicity, and Optical Properties. RSC Adv. 2019, 9, 20118–20136. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kumar, S.; Bafana, A.; Lin, J.; Dahoumane, S.A.; Je, C. A Mechanistic View of the Light-Induced Synthesis of Silver Nanoparticles Using Extracellular Polymeric Substances of Chlamydomonas reinhardtii. Molecules 2019, 24, 3506. [Google Scholar] [CrossRef] [PubMed]
- Karmakar, S. Particle Size Distribution and Zeta Potential Based on Dynamic Light Scattering: Techniques to Characterise Stability and Surface Distribution of Charged Colloids Particle. Recent Trends Mater. Phys. Chem. 2019, 117–159. [Google Scholar]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef]
- Kumar, A.; Dixit, C.K. 3-Methods for Characterization of Nanoparticles. In Advances in Nanomedicine for the Delivery of Therapeutic Nucleic Acids; Nimesh, S., Chandra, R., Gupta, N., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 43–58. ISBN 978-0-08-100557-6. [Google Scholar]
- Menon, S.; Agarwal, H.; Rajesh Kumar, S.; Venkat Kumar, S. Green Synthesis of Silver Nanoparticles Using Medicinal Plant Acalypha indica Leaf Extracts and Its Application as an Antioxidant and Antimicrobial Agent against Foodborne Pathogens. Int. J. Appl. Pharm. 2017, 9, 42–50. [Google Scholar] [CrossRef]
- Campos, V.; DeAlba-Montero, I.; Ruiz, F.; Butrón-Téllez Girón, C.; García-García, C.E.; Loredo-Tovías, M. Simple and Rapid Method for Silver Nanoparticles Incorporation in Polymethyl Methacrylate (PMMA) Substrates. Superf. Y Vacío 2017, 30, 51–55. [Google Scholar] [CrossRef]
- Genwali, G.R.; Acharya, P.P.; Rajbhandari, M. Isolation of Gallic Acid and Estimation of Total Phenolic Content in Some Medicinal Plants and Their Antioxidant Activity. Nepal J. Sci. Technol. 2013, 14, 95–102. [Google Scholar] [CrossRef]
- Bonarska-Kujawa, D.; Cyboran, S.; Zyłka, R.; Oszmiański, J.; Kleszczyńska, H. Biological Activity of Blackcurrant Extracts (Ribes nigrum L.) in Relation to Erythrocyte Membranes. Biomed Res. Int. 2014, 2014, 783059. [Google Scholar] [CrossRef]
- Moghrovyan, A.; Sahakyan, N.; Babayan, A.; Chichoyan, N.; Petrosyan, M.; Trchounian, A. Essential Oil and Ethanol Extract of Oregano (Origanum vulgare L.) from Armenian Flora as a Natural Source of Terpenes, Flavonoids and Other Phytochemicals with Antiradical, Antioxidant, Metal Chelating, Tyrosinase Inhibitory and Antibacterial Activity. Curr. Pharm. Des. 2019, 25, 1809–1816. [Google Scholar] [CrossRef]
- Sahakyan, N.; Andreoletti, P.; Cherkaoui-Malki, M.; Petrosyan, M.; Trchounian, A. Artemisia dracunculus L. Essential Oil Phytochemical Components Trigger the Activity of Cellular Antioxidant Enzymes. J. Food Biochem. 2021, 45, e13691. [Google Scholar] [CrossRef] [PubMed]
- Sahakyan, N.; Andreoletti, P.; Petrosyan, M.; Cherkaoui-Malki, M. Essential Oils of Basil Cultivars Selectively Affect the Activity of Antioxidant Enzymes in Murine Glial Cells. Curr. Nutraceuticals 2022, 3, 9. [Google Scholar] [CrossRef]
- Martinez-Andrade, J.M.; Avalos-Borja, M.; Vilchis-Nestor, A.R.; Sanchez-Vargas, L.O.; Castro-Longoria, E. Dual Function of EDTA with Silver Nanoparticles for Root Canal Treatment–A Novel Modification. PLoS ONE 2018, 13, e0190866. [Google Scholar] [CrossRef] [PubMed]
- La Spina, R.; Mehn, D.; Fumagalli, F.; Rossi, F.; Gilliland, D.; Holland, M.; Reniero, F. Synthesis of Citrate-Stabilized Silver Nanoparticles Modified by Thermal and Ph Preconditioned Tannic Acid. Nanomaterials 2020, 10, 2031. [Google Scholar] [CrossRef]
- Petrosyan, M.; Shcherbakova, Y.; Sahakyan, N.; Vardanyan, Z.; Poladyan, A.; Popov, Y.; Trchounian, A. Alkanna orientalis (L.) Boiss. Plant Isolated Cultures and Antimicrobial Activity of Their Extracts: Phenomenon, Dependence on Different Factors and Effects on Some Membrane-Associated Properties of Bacteria. Plant Cell. Tissue Organ Cult. 2015, 122, 727–738. [Google Scholar] [CrossRef]
- Gabrielyan, L.; Badalyan, H.; Gevorgyan, V.; Trchounian, A. Comparable Antibacterial Effects and Action Mechanisms of Silver and Iron Oxide Nanoparticles on Escherichia coli and Salmonella typhimurium. Sci. Rep. 2020, 10, 13145. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 5th ed.; Council of Europe: Strasbourg, France, 2005. [Google Scholar]
- Ginovyan, M.; Ayvazyan, A.; Nikoyan, A.; Tumanyan, L.; Trchounian, A. Phytochemical Screening and Detection of Antibacterial Components from Crude Extracts of Some Armenian Herbs Using TLC-Bioautographic Technique. Curr. Microbiol. 2020, 77, 1223–1232. [Google Scholar] [CrossRef]
- Ghasemi, K.; Ghasemi, Y.; Ebrahimzadeh, M.A. Antioxidant Activity, Phenol and Flavonoid Contents of 13 Citrus Species Peels and Tissues. Pak. J. Pharm. Sci. 2009, 22, 277–281. [Google Scholar]
- Kusznierewicz, B.; Mróz, M.; Koss-Mikołajczyk, I.; Namieśnik, J. Comparative Evaluation of Different Methods for Determining Phytochemicals and Antioxidant Activity in Products Containing Betalains—Verification of Beetroot Samples. Food Chem. 2021, 362, 130132. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Piasek, A.; Bartoszek, A.; Namiesnik, J. Application of a Commercially Available Derivatization Instrument and Commonly Used Reagents to HPLC On-Line Determination of Antioxidants. J. Food Compos. Anal. 2011, 24, 1073–1080. [Google Scholar] [CrossRef]
- Kusznierewicz, B.; Piasek, A.; Bartoszek, A.; Namiesnik, J. The Optimisation of Analytical Parameters for Routine Profiling of Antioxidants in Complex Mixtures by HPLC Coupled Post-Column Derivatisation. Phytochem. Anal. 2011, 22, 392–402. [Google Scholar] [CrossRef] [PubMed]
- Rautela, A.; Rani, J.; Debnath (Das), M. Green Synthesis of Silver Nanoparticles from Tectona grandis Seeds Extract: Characterization and Mechanism of Antimicrobial Action on Different Microorganisms. J. Anal. Sci. Technol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Soghomonyan, D.; Mnatsakanyan, N.; Trchounian, A. To the Mechanism of Antibacterial Action of Colloidal Silver: Changes in Membrane-Associated Proton ATPase Activity in Escherichia Coli and Enterococcus Faecalis. Biol. J. Armen. 2019, 71, 73–78. [Google Scholar]
- Szermer-Olearnik, B.; Sochocka, M.; Zwolinska, K.; Ciekot, J.; Czarny, A.; Szydzik, J.; Kowalski, K.; Boratynski, J. Comparison of Microbiological and Physicochemical Methods for Enumeration of Microorganisms. Postepy Hig. Med. Dosw. 2014, 68, 1392–1396. [Google Scholar] [CrossRef] [PubMed]
- Gabrielyan, L.; Hakobyan, L.; Hovhannisyan, A.; Trchounian, A. Effects of Iron Oxide (Fe3O4) Nanoparticles on Escherichia Coli Antibiotic-Resistant Strains. J. Appl. Microbiol. 2019, 126, 1108–1116. [Google Scholar] [CrossRef] [PubMed]
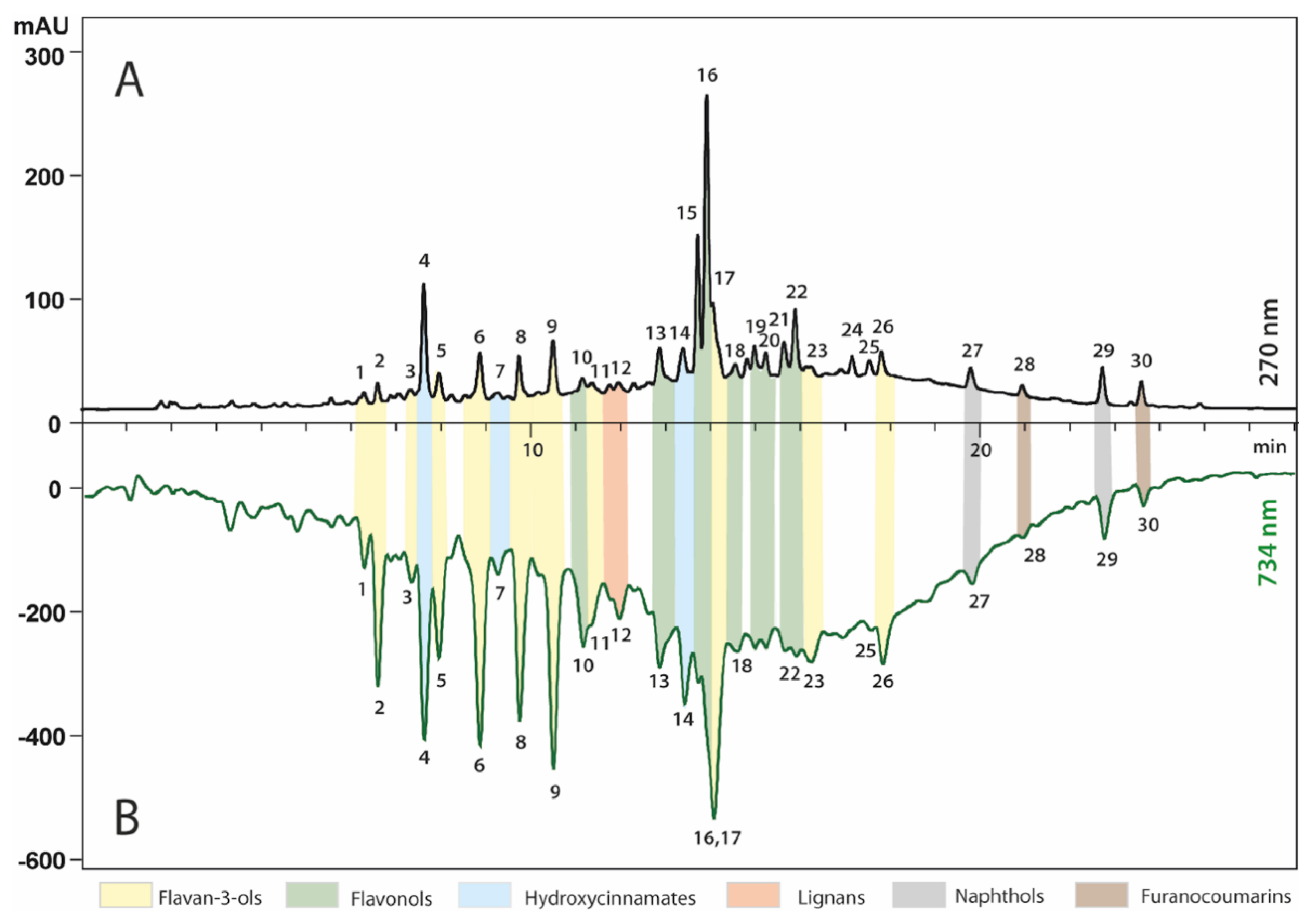




| No. | RT (min) | Tentative Identification | Molecular Formula | Molecular Weight | Λmax (nm) | Theoretical (m/z) | Observed (m/z) | Mass Error (ppm) | Fragments (m/z) |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 5.96 | B-typeprocyanidin dimer | C30H26O12 | 578.14243 | 279 | 577.13460 | 577.13547 | −1.49 | 125.02; 289.07; 407.08 |
| 2. | 6.23 | (epi)Gallocatechin | C15H14O7 | 306.07396 | 272 | 305.06613 | 305.06659 | −1.49 | 125.02; 137.02; 167.03 |
| 3. | 6.92 | B-typeprocyanidin dimer | C30H26O12 | 578.14243 | 281 | 577.13460 | 577.13533 | −1.25 | 125.02; 289.07; 407.08 |
| 4. | 7.28 | Caffeoylquinic acid | C16H18O9 | 354.09509 | 325 | 353.08726 | 353.08772 | −1.29 | 191.05 |
| 5. | 7.54 | (+)-Catechin | C15H14O6 | 290.07904 | 279 | 289.07122 | 289.07171 | −1.69 | 109.03; 123.04; 125.02; 151.04 |
| 6. | 8.40 | B-typeprocyanidin dimer | C30H26O12 | 578.14243 | 279 | 577.13461 | 577.13531 | −1.21 | 125.02; 289.07; 407.08 |
| 7. | 9.30 | (-)-Epicatechin | C15H14O6 | 290.07904 | 279 | 289.07122 | 289.07163 | −1.41 | 109.03; 123.04; 125.02; 151.04 |
| 8. | 9.93 | A-typeprocyanidin trimer | C45H36O18 | 864.19017 | 279 | 863.18235 | 863.18375 | −1.62 | 289.07; 451.10; 573.10; 711.13 |
| 9. | 10.42 | Rhamnetin glucoside | C22H22O12 | 478.11113 | 366 | 477.10331 | 477.10395 | −1.34 | 315.05 |
| 10. | 10.55 | A-typeprocyanidin tetramer | C60H50O24 | 1154.26921 | 279 | 1153.26139 | 1153.26251 | −0.97 | 575.12; 865.20; 1001.21 |
| 11. | 11.53 | Lariciresinol glucoside | C26H34O11 | 522.21012 | 280 | 521.20229 | 521.20294 | −1.24 | 359.15 |
| 12. | 12.48 | Quercetin rutinoside | C27H30O16 | 610.15339 | 354 | 609.14557 | 609.14633 | −1.25 | 301.03 |
| 13. | 12.95 | Coumaric acid derivative | C25H28O13 | 536.15299 | 311 | 535.14517 | 535.14578 | −1.13 | 147.04; 163.04 |
| 14. | 13.25 | Quercetin hexoside | C21H20O12 | 464.09548 | 354 | 463.08766 | 463.08829 | −1.38 | 300.03; 301.03 |
| 15. | 13.40 | Quercetin glucuronide | C21H18O13 | 478.07474 | 354 | 477.06692 | 477.06729 | −0.76 | 151.00; 178.99; 301.03 |
| 16. | 13.50 | Epicatechin gallate | C22H18O10 | 442.09000 | 279 | 441.08218 | 441.08274 | −1.27 | 169.01; 289.07 |
| 17. | 14.36 | Quercetin pentoside | C20H18O11 | 434.08492 | 354 | 433.07709 | 433.07765 | −1.28 | 300.03; 301.03 |
| 18. | 14.49 | Quercetin malonyl glucoside | C24H22O15 | 550.09588 | 354 | 549.08805 | 549.08885 | −1.45 | 301.03 |
| 19. | 14.77 | Quercetin pentoside | C20H18O11 | 434.08492 | 351 | 433.07709 | 433.07752 | −0.98 | 300.03; 301.03 |
| 20. | 15.09 | Quercetin pentoside | C20H18O11 | 434.08492 | 347 | 433.07709 | 433.07755 | −1.05 | 300.03; 301.03 |
| 21. | 15.47 | Quercitrin | C21H20O11 | 448.10057 | 349 | 447.09274 | 447.09332 | −1.29 | 300.03; 301.03 |
| 22. | 15.59 | B-type galloylated procyanidin dimer | C37H30O16 | 730.15339 | 279 | 729.14557 | 729.14679 | −1.69 | 289.07; 407.08 |
| 23. | 16.60 | Coumaric acid derivative | C20H28O9 | 412.17334 | 334 | 411.16551 | 411.16622 | −1.71 | 145.03; 163.04 |
| 24. | 17.15 | Coumaric acid derivative | C20H28O9 | 412.17334 | 310 | 411.16551 | 411.16616 | −1.57 | 119.05; 145.03; 163.04 |
| 25. | 17.33 | B-type digalloylated procyanidin dimer | C44H34O20 | 882.16435 | 278 | 881.15653 | 881.15809 | −1.78 | 287.06; 407.08;729.15 |
| 26. | 19.54 | Musizin glucoside | C19H22O8 | 378.13147 | 334 | 377.12365 | 377.12408 | −1.17 | 215.07 |
| 27. | 20.73 | Marmesin glucoside | C20H24O9 | 408.14204 | 336 | 407.13421 | 407.13468 | −1.17 | 230.06; 245.08 |
| 29. | 22.42 | Musizin acetyl glucoside | C21H24O9 | 420.14204 | 334 | 419.13421 | 419.13471 | −1.187 | 215.07 |
| 30. | 23.30 | Marmesin acetyl glucoside | C22H26O10 | 450.1526 | 337 | 449.14478 | 449.14526 | −1.097 | 245.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hovhannisyan, Z.; Timotina, M.; Manoyan, J.; Gabrielyan, L.; Petrosyan, M.; Kusznierewicz, B.; Bartoszek, A.; Jacob, C.; Ginovyan, M.; Trchounian, K.; et al. Ribes nigrum L. Extract-Mediated Green Synthesis and Antibacterial Action Mechanisms of Silver Nanoparticles. Antibiotics 2022, 11, 1415. https://doi.org/10.3390/antibiotics11101415
Hovhannisyan Z, Timotina M, Manoyan J, Gabrielyan L, Petrosyan M, Kusznierewicz B, Bartoszek A, Jacob C, Ginovyan M, Trchounian K, et al. Ribes nigrum L. Extract-Mediated Green Synthesis and Antibacterial Action Mechanisms of Silver Nanoparticles. Antibiotics. 2022; 11(10):1415. https://doi.org/10.3390/antibiotics11101415
Chicago/Turabian StyleHovhannisyan, Zaruhi, Marina Timotina, Jemma Manoyan, Lilit Gabrielyan, Margarit Petrosyan, Barbara Kusznierewicz, Agnieszka Bartoszek, Claus Jacob, Mikayel Ginovyan, Karen Trchounian, and et al. 2022. "Ribes nigrum L. Extract-Mediated Green Synthesis and Antibacterial Action Mechanisms of Silver Nanoparticles" Antibiotics 11, no. 10: 1415. https://doi.org/10.3390/antibiotics11101415
APA StyleHovhannisyan, Z., Timotina, M., Manoyan, J., Gabrielyan, L., Petrosyan, M., Kusznierewicz, B., Bartoszek, A., Jacob, C., Ginovyan, M., Trchounian, K., Sahakyan, N., & Nasim, M. J. (2022). Ribes nigrum L. Extract-Mediated Green Synthesis and Antibacterial Action Mechanisms of Silver Nanoparticles. Antibiotics, 11(10), 1415. https://doi.org/10.3390/antibiotics11101415








