Coumarins as Fungal Metabolites with Potential Medicinal Properties
Abstract
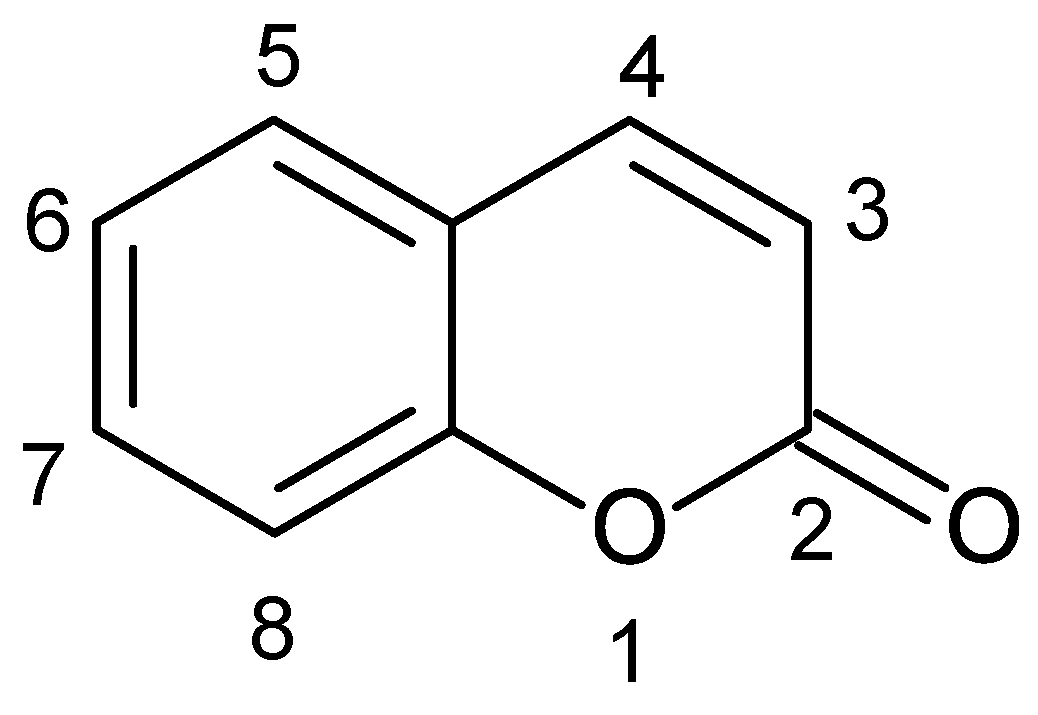
:1. Introduction
1.1. Overview on Fungal Coumarins
1.2. Novelty Statement
2. Coumarins Hold a Special Place among Natural Products
3. Herbal Coumarins Could Be Successfully Bioprospected from Fungi
4. Fungi Is a Promising Source of Functional Coumarins
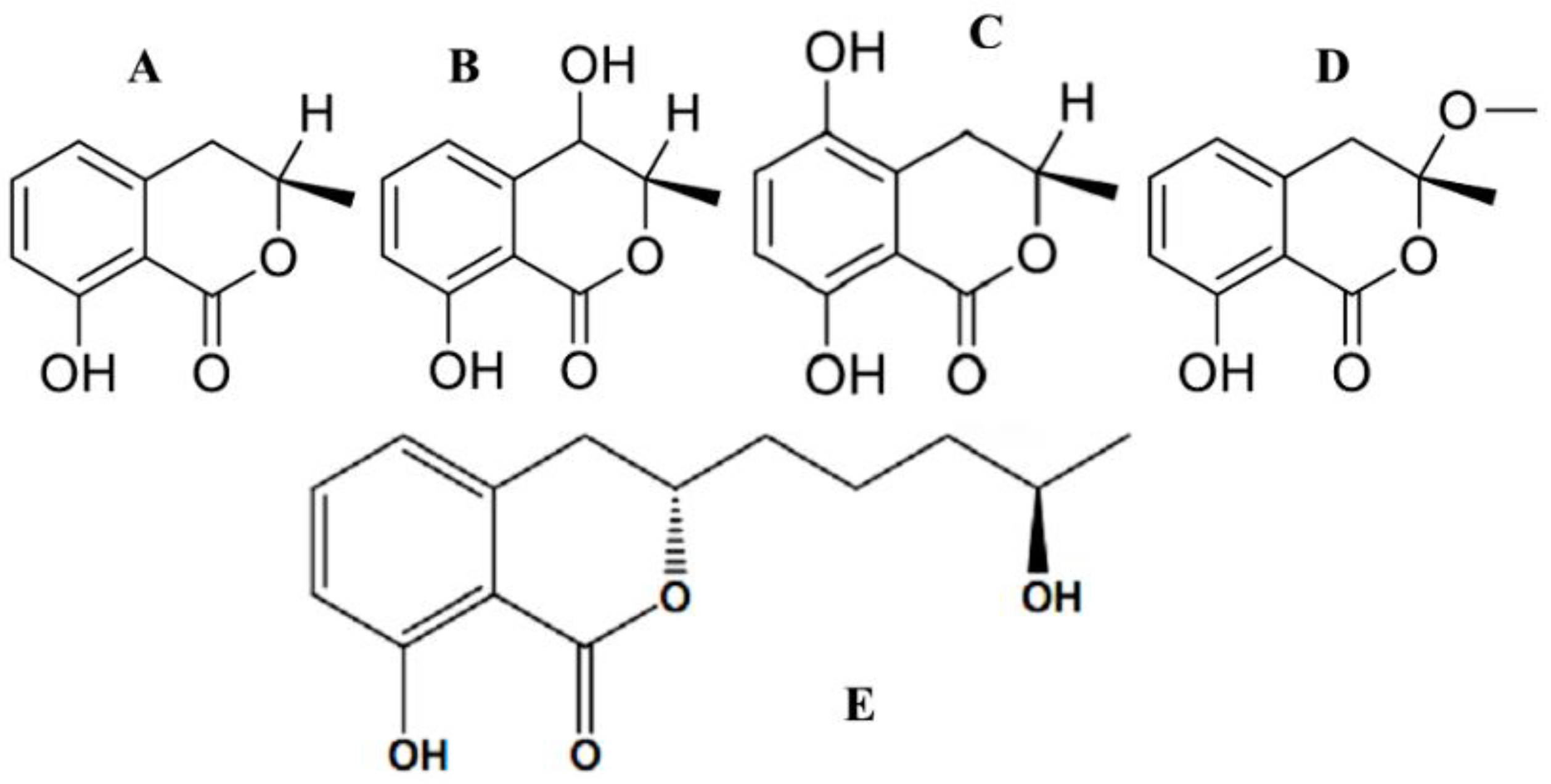
4.1. Fungal Coumarins with Anticancer Action
4.2. Acetylcholinesterase Inhibitory Activity of Coumarins
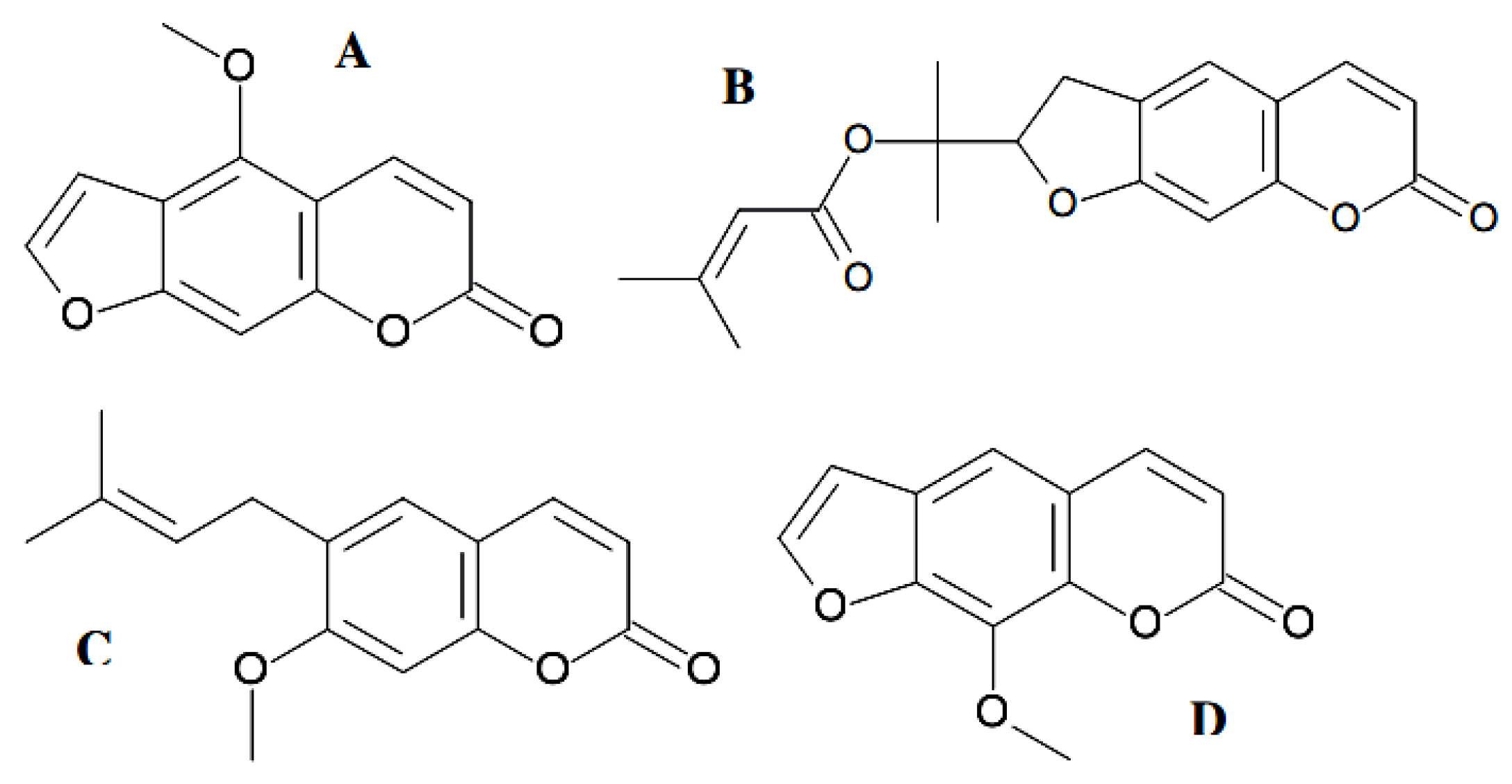
4.3. Antiviral Coumarin-Structured Agents
4.4. Efficacy of Coumarins against Bacterial and Fungal Infections
5. On the Molecular Mechanisms Underlying the Pharmacological Effects of Fungal Coumarins
5.1. Grounds for the Apoptotic/Cytotoxic Activity of Coumarins
5.2. Coumarins Features Aiding in Neurodegenerative Disorders Therapy
5.3. Structural Prerequisites for Coumarins Being Antimicrobials
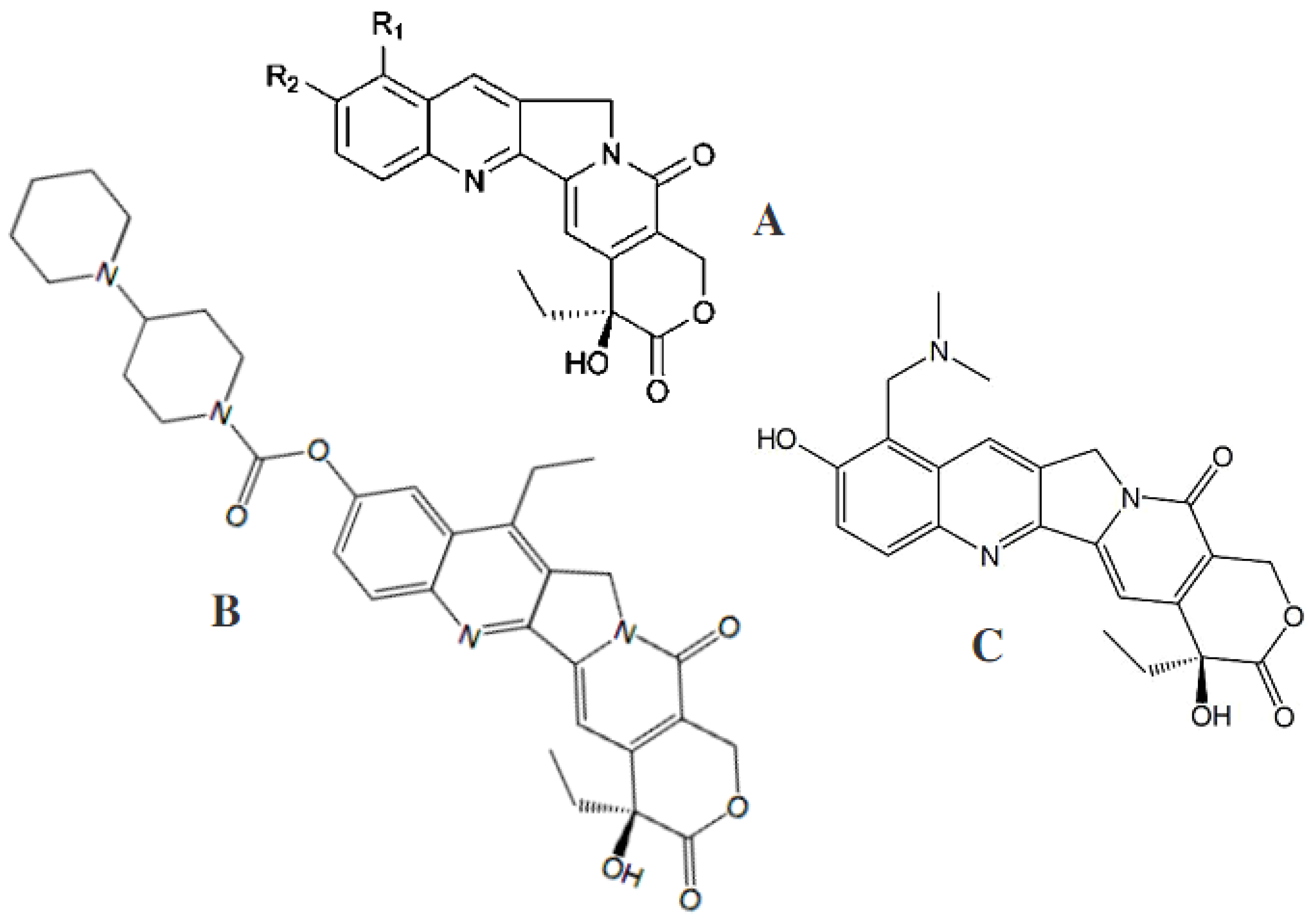
6. Multitarget Ligands of Functionalized Coumarins as Clinically Useful Drugs
6.1. Physicochemical Prerequisites and Toxicity
6.2. The Series of Coumarin Hybrids with Important Class of Natural Products
6.2.1. Anticancer Coumarin-Based Hybrids
6.2.2. Antitubercular Coumarin-Based Hybrids
7. Methodology
7.1. Search Strategy
7.2. Software Tools
8. Concluding Remarks
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Suryanarayanan, T.S.; Thirunavukkarasu, N.; Govindarajulu, M.B.; Sasse, F.; Jansen, R.; Murali, T.S. Fungal endophytes and bioprospecting. Fungal Biol. Rev. 2009, 23, 9–19. [Google Scholar] [CrossRef]
- Kijpornyongpan, T.; Schwartz, A.; Yaguchi, A.; Salvachúa, D. Systems biology-guided understanding of white-rot fungi for biotechnological applications: A review. iScience 2022, 25, 104640. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M. Plant counnarins as allelopathic agents. Int. J. Biol. Chem. 2011, 5, 86–90. [Google Scholar] [CrossRef]
- Reddy, D.S.; Kongot, M.; Kumar, A. Coumarin hybrid derivatives as promising leads to treat tuberculosis: Recent developments and critical aspects of structural design to exhibit anti-tubercular activity. Tuberculosis 2021, 127, 102050. [Google Scholar] [CrossRef]
- Iranshahi, M.; Askari, M.; Sahebkar, A.; Hadjipavlou-Litina, D. Evaluation of antioxidant, anti-inflammatory and lipoxygenase inhibitory activities of the prenylated coumarin umbelliprenin. DARU J. Pharm. Sci. 2009, 17, 99–103. [Google Scholar]
- Ismael, R.N.; Mustafa, Y.F.; Al-Qazaz, H.K. Coumarin-based products: Their biodiversity and pharmacology. Iraqi J. Pharm. 2022, 18, 162–179. [Google Scholar] [CrossRef]
- Braga, R.M.; Padilla, G.; Araújo, W.L. The biotechnological potential of Epicoccum spp.: Diversity of secondary metabolites. Crit. Rev. Microbiol. 2018, 44, 759–778. [Google Scholar] [CrossRef]
- Hussain, M.I.; Syed, Q.A.; Khattak, M.N.K.; Hafez, B.; Reigosa, M.J.; El-Keblawy, A. Natural product coumarins: Biological and pharmacological perspectives. Biologia 2019, 74, 863–888. [Google Scholar] [CrossRef]
- Chandrashekar, S.; Govindappa, M.; Shashank, M.P.; Ramith, R.; Bhargav, S.; Chandan, D.; Shiva, P.K.; Asad, S.; Chandan, S. Plants and endophytes—A partnership for the coumarin production through the microbial systems. Mycology 2022, 13, 2–15. [Google Scholar] [CrossRef]
- Cheke, R.S.; Patel, H.M.; Patil, V.M.; Ansari, I.A.; Ambhore, J.P.; Shinde, S.D.; Kadri, A.; Snoussi, M.; Adnan, M.; Kharkar, P.S.; et al. Molecular insights into coumarin analogues as antimicrobial agents: Recent developments in drug discovery. Antibiotics 2022, 11, 566. [Google Scholar] [CrossRef]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A Natural, Privileged and Versatile Scaffold for Bioactive Compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srikrishna, D.; Godugu, C.; Dubey, P.K. A review on pharmacological properties of coumarins. Mini Rev. Med. Chem. 2018, 18, 113–141. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.J.; Jiang, J.G. Pharmacological and nutritional effects of natural coumarins and their structure–activity relationships. Mol. Nutr. Food Res. 2018, 62, 1701073. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.Q.; Xu, Z.; Zhang, S.; Wu, X.; Ding, J.W.; Lv, Z.S.; Feng, L.S. Recent developments of coumarin-containing derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 136, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.L.; Wu, J.B.; Cheng, X.W.; Zhang, F.Z.; Feng, L.S. Fluoroquinolone derivatives and their anti-tubercular activities. Eur. J. Med. Chem. 2018, 146, 554–563. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Pandey, A.; Manvati, S. Coumarin: An emerging antiviral agent. Heliyon 2020, 6, e03217. [Google Scholar] [CrossRef] [PubMed]
- Umashankar, T.; Govindappa, M.; Ramachandra, Y.L.; Padmalatha Rai, S.; Channabasava, R. Isolation and characterization of coumarin isolated from endophyte, Alternaria species-1 of Crotalaria pallida and its apoptotic action on HeLa cancer cell line. Metabolomics 2015, 5, 1000158. [Google Scholar] [CrossRef]
- Kuriakose, G.C.; Singh, S.; Rajvanshi, P.K.; Surin, W.R.; Jayabaskaran, C. In vitro cytotoxicity and apoptosis induction in human cancer cells by culture extract of an endophytic Fusarium solani strain isolated from Datura metel L. Pharm. Anal. Acta 2014, 5, 1000293. [Google Scholar] [CrossRef]
- Venugopala, K.N.; Rashmi, V.; Odhav, B. Review on natural coumarin lead compounds for their pharmacological activity. BioMed Res. Int. 2013, 2013, 963248. [Google Scholar] [CrossRef]
- Chebrolu, K.K.; Jayaprakasha, G.K.; Jifon, J.; Patil, B.S. Purification of coumarins, including meranzin and pranferin, from grapefruit by solvent partitioning and a hyphenated chromatography. Sep. Purif. Technol. 2013, 116, 137–144. [Google Scholar] [CrossRef]
- Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. Fungal endophytes as efficient sources of plant-derived bioactive compounds and their prospective applications in natural product drug discovery: Insights, avenues, and challenges. Microorganisms 2021, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.; Banerjee, S. Review on synthesis of bio-active coumarin-fused heterocyclic molecules. Curr. Org. Chem. 2020, 24, 2566–2587. [Google Scholar] [CrossRef]
- Nitiema, L.W.; Savadogo, A.; Simpore, J.; Dianou, D.; Traore, A.S. In vitro antimicrobial activity of some phenolic compounds (coumarin and quercetin) against gastroenteritis bacterial strains. Int. J. Microbiol. Res. 2012, 3, 183–187. [Google Scholar] [CrossRef]
- Witaicenis, A.; Seito, L.N.; Di Stasi, L.C. Intestinal anti-inflammatory activity of esculetin and 4-methylesculetin in the trinitrobenzenesulphonic acid model of rat colitis. Chem. Biol. Interact. 2010, 186, 211–218. [Google Scholar] [CrossRef]
- Witaicenis, A.; Seito, L.N.; da Silveira Chagas, A.; de Almeida Junior, L.D.; Luchini, A.C.; Rodrigues-Orsi, P.; Cestari, S.H.; Di Stasi, L.C. Antioxidant and intestinal anti-infammatory effects of plant-derived coumarin derivatives. Phytomedicine 2014, 21, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Kwon, O.S.; Choi, J.S.; Islam, M.N.; Kim, Y.S.; Kim, H.P. Inhibition of 5-lipoxygenase and skin inflammation by the aerial parts of Artemisia capillaris and its constituents. Arch. Pharmacal Res. 2011, 34, 1561–1569. [Google Scholar] [CrossRef]
- Shin, E.; Choi, K.M.; Yoo, H.S.; Lee, C.K.; Hwang, B.Y.; Lee, M.K. Inhibitory effects of coumarins from the stem barks of Fraxinus rhynchophylla on adipocyte differentiation in 3T3-L1 cells. Biol. Pharm. Bull. 2010, 33, 1610–1614. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Aliabadi, A.; Emami, S.; Safavi, M.; Rajabalian, S.; Mohagheghi, M.A.; Khoshzaban, A.; Samzadeh-Kermani, A.; Lamei, N.; Shafiee, A.; et al. Synthesis and in-vitro cytotoxicity of poly-functionalized 4-(2-arylthiazol-4-yl)-4H-chromenes. Arch. Pharm. 2010, 343, 411–416. [Google Scholar] [CrossRef]
- Luo, K.W.; Sun, J.G.; Chan, J.Y.W.; Yang, L.; Wu, S.H.; Fung, K.P.; Liu, F.Y. Anticancer effects of imperatorin isolated from Angelica dahurica: Induction of apoptosis in HepG2 cells through both death-receptor-and mitochondria-mediated pathways. Chemotherapy 2011, 57, 449–459. [Google Scholar] [CrossRef]
- Yun, E.S.; Park, S.S.; Shin, H.C.; Choi, Y.H.; Kim, W.J.; Moon, S.K. p38 MAPK activation is required for esculetin-induced inhibition of vascular smooth muscle cells proliferation. Toxicol. In Vitro 2011, 25, 1335–1342. [Google Scholar] [CrossRef]
- Chiang, C.C.; Cheng, M.J.; Peng, C.F.; Huang, H.Y.; Chen, I.S. A novel dimeric coumarin analog and antimycobacterial constituents from Fatoua pilosa. Chem. Biodivers. 2010, 7, 1728–1736. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.; Spillere, A.R.; das Neves, G.M.; Kagami, L.P.; von Poser, G.L.; Canto, R.F.S.; Eifler-Lima, V.L. Natural and synthetic coumarins as antileishmanial agents: A review. Eur. J. Med. Chem. 2020, 203, 112514. [Google Scholar] [CrossRef] [PubMed]
- Hassan, M.Z.; Osman, H.; Ali, M.A.; Ahsan, M.J. Therapeutic potential of coumarins as antiviral agents. Eur. J. Med. Chem. 2016, 123, 236–255. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhe-Ling, F.E.N.G.; Yi-Tao, W.A.N.G.; Li-Gen, L.I.N. Anticancer carbazole alkaloids and coumarins from Clausena plants: A review. Chin. J. Nat. Med. 2017, 15, 881–888. [Google Scholar] [CrossRef]
- Umashankar, T.; Govindappa, M.; Ramachandra, Y.L.; Chandrappa, C.P.; Padmalatha Rai, S.; Channabasava, R. Isolation, purification and in vitro cytotoxicity activities of coumarin isolated from endophytic fungi, Alternaria species of Crotalaria pallida. Indo Am. J. Pharm. Res. 2015, 5, 926–936. [Google Scholar]
- Schmidt, E.W. Trading molecules and tracking targets in symbiotic interactions. Nat. Chem. Biol. 2008, 4, 466–473. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Kjer, J.; Proksch, P. Fungal endophytes from higher plants: A prolific source of phytochemicals and other bioactive natural products. Fungal Divers. 2010, 41, 1–16. [Google Scholar] [CrossRef]
- Zhou, P.; Takaishi, Y.; Duan, H.; Chen, B.; Honda, G.; Itoh, M.; Takeda, Y.; Kodzhimatov, O.K.; Lee, K.-H. Coumarins and bicoumarin from Ferula sumbul: Anti-HIV activity and inhibition of cytokine release. Phytochemistry 2000, 53, 689–697. [Google Scholar] [CrossRef]
- Devji, T.; Reddy, C.; Woo, C.; Awale, S.; Kadota, S.; Carrico-Moniz, D. Pancreatic anticancer activity of a novel geranylgeranylated coumarin derivative. Bioorgan. Med. Chem. Lett. 2011, 21, 5770–5773. [Google Scholar] [CrossRef]
- Gayathri, V.; Lekshmi, P.; Padmanabhan, R.N. Anti-diabetes activity of ethanol extract of Centella asiatica (L.) Urban (whole plant) in Streptozotocin-induced diabetic rats, isolation of an active fraction and toxicity evaluation of the extract. Int. J. Med. Aromat. Plants 2011, 1, 278–286. [Google Scholar]
- Toghueo, R.M.K.; Eke, P.; Zabalgogeazcoa, Í.; de Aldana, B.R.V.; Nana, L.W.; Boyom, F.F. Biocontrol and growth enhancement potential of two endophytic Trichoderma spp. from Terminalia catappa against the causative agent of Common Bean Root Rot (Fusarium solani). Biol. Control 2016, 96, 8–20. [Google Scholar] [CrossRef]
- Hyde, K.D.; Soytong, K. The fungal endophyte dilemma. Fungal Divers. 2008, 33, 163–173. [Google Scholar]
- Oses, R.; Valenzuela, S.; Freer, J.; Sanfuentes, E.; Rodriguez, J. Fungal endophytes in xylem of healthy Chilean trees and their possible role in early wood decay. Fungal Divers. 2008, 33, 77–86. [Google Scholar]
- Bae, H.; Kim, S.; Sicher-Jr, R.C.; Kim, M.S.; Strem, M.D.; Bailey, B.A. The beneficial endophyte, Trichoderma hamatum, delays the onset of drought stress in Theobroma cacao. Biol. Control 2008, 46, 24–35. [Google Scholar]
- Su, Z.; Zeng, Y.; Li, X.; Perumal, A.B.; Zhu, J.; Lu, X.; Dai, M.; Liu, X.; Lin, F. The endophytic fungus Piriformospora Indica-assisted alleviation of cadmium in tobacco. J. Fungi 2021, 7, 675. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Z.Y.; Cui, J.L.; Wang, M.L.; Wang, J.H. Biotransformation ability of endophytic fungi: From species evolution to industrial applications. Appl. Microbiol. Biotechnol. 2021, 105, 7095–7113. [Google Scholar] [CrossRef]
- Zimowska, B.; Bielecka, M.; Abramczyk, B.; Nicoletti, R. Bioactive products from endophytic fungi of sages (Salvia spp.). Agriculture 2020, 10, 543. [Google Scholar] [CrossRef]
- Aly, A.H.; Edrada-Ebel, R.; Indriani, I.D.; Wray, V.; Müller, W.E.G.; Totzke, F.; Schächtele, C.; Kubbutat, M.H.G.; Lin, W.H.; Proksch, P.; et al. Cytotoxic metabolites from the fungal endophyte Alternaria sp. and their subsequent detection in its host plant Polygonum senegalense. J. Nat. Prod. 2008, 71, 972–980. [Google Scholar] [CrossRef]
- Kusari, S.; Lamshöft, M.; Zühlke, S.; Spiteller, M. An endophytic fungus from Hypericum perforatum that produces hypericin. J. Nat. Prod. 2008, 71, 159–162. [Google Scholar] [CrossRef]
- Kumar, A.; Patil, D.; Rajamohanan, P.R.; Ahmad, A. Isolation, purification and characterization of vinblastine and vincristine from endophytic fungus Fusarium oxysporum isolated from Catharanthus roseus. PLoS ONE 2013, 8, e71805. [Google Scholar] [CrossRef]
- Tian, J.F.; Li, P.J.; Li, X.X.; Sun, P.H.; Gao, H.; Liu, X.Z.; Huang, P.; Tang, J.S.; Yao, X.S. New antibacterial isocoumarin glycosides from a wetland soil derived fungal strain Metarhizium anisopliae. Bioorgan. Med. Chem. Lett. 2016, 26, 1391–1396. [Google Scholar] [CrossRef]
- Amna, T.; Puri, S.C.; Verma, V.; Sharma, J.P.; Khajuria, R.K.; Musarrat, J.; Spiteller, M.; Qazi, G.N. Bioreactor studies on the endophytic fungus Entrophospora infrequens for the production of an anticancer alkaloid camptothecin. Can. J. Microbiol. 2006, 52, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.C.; Verma, V.; Amna, T.; Qazi, G.N.; Spiteller, M. An endophytic fungus from Nothapodytes foetida that produces Camptothecin. J. Nat. Prod. 2005, 68, 1717–1719. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Lamshöft, M.; Spiteller, M. Aspergillus fumigatus Fresenius, an endophytic fungus from Juniperus communis L. Horstmann as a novel source of the anticancer pro-drug deoxypodophyllotoxin. J. Appl. Microbiol. 2009, 107, 1019–1030. [Google Scholar] [CrossRef] [PubMed]
- Puri, S.C.; Nazir, A.; Chawla, R.; Arora, R.; Riyaz-ul-Hasan, S.; Amna, T.; Ahmed, B.; Verma, V.; Singh, S.; Sagar, R.; et al. The endophytic fungus Trametes hirsuta as a novel alternative source of podophyllotoxin and related aryl tetralin lignans. J. Biotechnol. 2006, 122, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Eyberger, A.L.; Dondapati, R.; Porter, J.R. Endophyte fungal isolates from Podophyllum peltatum produce podophyllotoxin. J. Nat. Prod. 2006, 69, 1121–1124. [Google Scholar] [CrossRef] [PubMed]
- Kour, A.; Shawl, A.S.; Rehman, S.; Sultan, P.; Qazi, P.H.; Suden, P.; Khajuria, R.K.; Verma, V. Isolation and identification of an endophytic strain of Fusarium oxysporum producing podophyllotoxin from Juniperus recurva. World J. Microbiol. Biotechnol. 2008, 24, 1115–1121. [Google Scholar] [CrossRef]
- Yang, X.; Guo, S.; Zhang, L.; Shao, H. Select of producing podophyllotoxin endophytic fungi from podophyllin plant. Nat. Prod. Res. Dev. 2003, 15, 419–422. [Google Scholar]
- Lu, L.; He, J.; Yu, X.; Li, G.; Zhang, X. Studies on isolation and identification of endophytic fungi strain SC13 from harmaceutical plant Sabina vulgaris Ant. and metabolites. Acta Agric. Borealioccident. Sin. 2006, 15, 85–89. [Google Scholar]
- Cao, L.; Huang, J.; Li, J. Fermentation conditions of Sinopodophyllum hexandrum endophytic fungus on production of podophyllotoxin. Food Ferment. Ind. 2007, 33, 28–32. [Google Scholar]
- Stierle, A.; Strobel, G.; Stierle, D. Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew. Science 1993, 260, 214–216. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.-S.; Jia, M.; Chen, L.; Zhu, B.; Dong, H.-X.; Si, J.-P.; Peng, W.; Han, T. Cytotoxic and antifungal constituents isolated from the metabolites of endophytic fungus DO14 from Dendrobium officinale. Molecules 2016, 21, 14. [Google Scholar] [CrossRef] [PubMed]
- Rai, N.; Keshri, P.K.; Verma, A.; Kamble, S.C.; Mishra, P.; Barik, S.; Singh, S.K.; Gautam, V. Plant associated fungal endophytes as a source of natural bioactive compounds. Mycol. Int. J. Fungal Biol. 2021, 12, 139–159. [Google Scholar] [CrossRef]
- Kharwar, R.N.; Mishra, A.; Gond, S.K.; Stierle, A.; Stierle, D. Anticancer compounds derived from fungal endophytes: Their importance and future challenges. Nat. Prod. Rep. 2011, 28, 1208–1228. [Google Scholar] [CrossRef]
- He, J.; Li, Z.H.; Ai, H.L.; Feng, T.; Liu, J.K. Anti-bacterial chromones from cultures of the endophytic fungus Bipolaris eleusines. Nat. Prod. Res. 2019, 33, 3515–3520. [Google Scholar] [CrossRef] [PubMed]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.P.; Li, X.P.; Zhou, L.L.; Wang, J.W. Endophytes in Artemisia annua L.: New potential regulators for plant growth and artemisinin biosynthesis. Plant Growth Regul. 2021, 95, 293–313. [Google Scholar] [CrossRef]
- Umashankar, T.; Govindappa, M.; Ramachandra, Y.L. In vitro antioxidant and antimicrobial activity of partially purified coumarins from fungal endophytes of Crotalaria pallida. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 58–72. [Google Scholar]
- Rehman, S.; Shawl, A.S.; Kour, A.; Andrabi, R.; Sudan, P.; Sultan, P.; Verma, V.; Qazi, G.N. An endophytic Neurospora sp. from Nothapodytes foetida producing camptothecin. Appl. Biochem. Microbiol. 2008, 44, 203–209. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef]
- Pettit, G.R.; Searcy, J.D.; Tan, R.; Cragg, G.M.; Melody, N.; Knight, J.C.; Chapuis, J.C. Antineoplastic agents. 585. Isolation of Bridelia ferruginea anticancer podophyllotoxins and synthesis of 4-aza-podophyllotoxin structural modifications1. J. Nat. Prod. 2016, 79, 507–518. [Google Scholar] [CrossRef]
- Onocha, P.A.; Okorie, D.A.; Connolly, J.D.; Roycroft, D.S. Monoterpene diol, iridoid glucoside and dibenzo-α-pyrone from Anthocleista djalonensis. Phytochemistry 1995, 40, 1183–1189. [Google Scholar] [CrossRef]
- Aly, A.H.; Edrada-Ebel, R.; Wray, V.; Müller, W.E.G.; Kozytska, S.; Hentschel, U.; Proksch, P.; Ebel, R. Bioactive metabolites from the endophytic fungus Ampelomyces sp. isolated from the medicinal plant Urospermum picroides. Phytochemistry 2008, 69, 1716–1725. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, L.; Wagner, J.; Metzler, M. Estrogenic and clastogenic potential of the mycotoxin alternariol in cultured mammalian cells. Food Chem. Toxicol. 2006, 44, 398–408. [Google Scholar] [CrossRef] [PubMed]
- Palanichamy, P.; Kannan, S.; Murugan, D.; Alagusundaram, P.; Marudhamuthu, M. Purification, crystallization and anticancer activity evaluation of the compound alternariol methyl ether from endophytic fungi Alternaria alternata. J. Appl. Microbiol. 2019, 127, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Davis, R.A.; Longden, J.; Avery, V.M.; Healy, P.C. The isolation, structure determination and cytotoxicity of the new fungal metabolite, trichodermamide C. Bioorgan. Med. Chem. Lett. 2008, 18, 2836–2839. [Google Scholar] [CrossRef]
- Garo, E.; Starks, C.M.; Jensen, P.R.; Fenical, W.; Lobkovsky, E.; Clardy, J. Trichodermamides A and B, cytotoxic modified dipeptides from the marine-derived fungus Trichoderma virens. J. Nat. Prod. 2003, 66, 423–426. [Google Scholar] [CrossRef]
- Capon, R.J.; Ratnayake, R.; Stewart, M.; Lacey, E.; Tennant, S.; Gill, J.H. Aspergillazines A–E: Novel heterocyclic dipeptides from an Australian strain of Aspergillus unilateralis. Org. Biomol. Chem. 2005, 3, 123–129. [Google Scholar] [CrossRef]
- Tawfike, A.F.; Romli, M.; Clements, C.; Abbott, G.; Young, L.; Schumacher, M.; Diederich, M.; Farag, M.; Edrada-Ebel, R. Isolation of anticancer and anti-trypanosome secondary metabolites from the endophytic fungus Aspergillus flocculus via bioactivity guided isolation and MS based metabolomics. J. Chromatogr. B 2019, 1106–1107, 71–83. [Google Scholar] [CrossRef]
- Qi, J.; Shao, C.-L.; Li, Z.-Y.; Gan, L.-S.; Fu, X.-M.; Bian, W.-T.; Zhao, H.-Y.; Wang, C.-Y. Isocoumarin derivatives and benzofurans from a sponge-derived Penicillium sp. fungus. J. Nat. Prod. 2013, 76, 571–579. [Google Scholar] [CrossRef]
- Le Dang, Q.; Shin, T.S.; Park, M.S.; Choi, Y.H.; Choi, G.J.; Jang, K.S.; Kim, I.S.; Kim, J.C. Antimicrobial activities of novel mannosyl lipids isolated from the biocontrol fungus Simplicillium lamellicola BCP against phytopathogenic bacteria. J. Agric. Food Chem. 2014, 62, 3363–3370. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Peng, D.-Y.; Yang, S.-G.; Zhu, X.-L.; Yang, W.-C.; Yang, G.-F. Syntheses of coumarin–tacrine hybrids as dual-site acetylcholinesterase inhibitors and their activity against butylcholinesterase, Aβ aggregation, and β-secretase. Bioorgan. Med. Chem. 2014, 22, 4784–4791. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, J.; Cai, X.; She, Z.; Lin, Y. A new furanocoumarin from the mangrove endophytic fungus Penicillium sp. (ZH16). Nat. Prod. Res. 2012, 26, 1291–1295. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.M.; Hu, D.H.; Zhang, J.J. Umbelliferone exhibits anticancer activity via the induction of apoptosis and cell cycle arrest in HepG2 hepatocellular carcinoma cells. Mol. Med. Rep. 2015, 12, 3869–3873. [Google Scholar] [CrossRef] [PubMed]
- Hamulakova, S.; Janovec, L.; Hrabinova, M.; Spilovska, K.; Korabecny, J.; Kristian, P.; Kuca, K.; Imrich, J. Synthesis and biological evaluation of novel tacrine derivatives and tacrine–coumarin hybrids as cholinesterase inhibitors. J. Med. Chem. 2014, 57, 7073–7084. [Google Scholar] [CrossRef]
- Hornick, A.; Lieb, A.; Vo, N.P.; Rollinger, J.M.; Stuppner, H.; Prast, H. The coumarin scopoletin potentiates acetylcholine release from synaptosomes, amplifies hippocampal long-term potentiation and ameliorates anticholinergic-and age-impaired memory. Neuroscience 2011, 197, 280–292. [Google Scholar] [CrossRef]
- Liu, X.L.; Zhang, L.; Fu, X.L.; Chen, K.; Qian, B.C. Effect of scopoletin on PC3 cell proliferation and apoptosis. Acta Pharmacol. Sin. 2001, 22, 929–933. [Google Scholar]
- Hamulakova, S.; Poprac, P.; Jomova, K.; Brezova, V.; Lauro, P.; Drostinova, L.; Jun, D.; Sepsova, V.; Hrabinova, M.; Soukup, O.; et al. Targeting copper(II)-induced oxidative stress and the acetylcholinesterase system in Alzheimer’s disease using multifunctional tacrine-coumarin hybrid molecules. J. Inorg. Biochem. 2016, 161, 52–62. [Google Scholar] [CrossRef]
- Shikishima, Y.; Takaishi, Y.; Honda, G.; Ito, M.; Takeda, Y.; Kodzhimatov, O.K.; Ashurmetov, O.; Lee, K.H. Chemical constituents of Prangos tschimganica; structure elucidation and absolute configuration of coumarin and furanocoumarin derivatives with anti-HIV activity. Chem. Pharm. Bull. 2001, 49, 877–880. [Google Scholar] [CrossRef]
- Márquez, N.; Sancho, R.; Bedoya, L.M.; Alcamí, J.; López-Pérez, J.L.; San Feliciano, A.; Fiebich, B.L.; Muñoz, E. Mesuol, a natural occurring 4-phenylcoumarin, inhibits HIV-1 replication by targeting the NF-κB pathway. Antivir. Res. 2005, 66, 137–145. [Google Scholar] [CrossRef]
- Xu, B.; Wang, L.; González-Molleda, L.; Wang, Y.; Xu, J.; Yuan, Y. Antiviral activity of (+)-rutamarin against Kaposi’s sarcoma-associated herpesvirus by inhibition of the catalytic activity of human topoisomerase II. Antimicrob. Agents Chemother. 2014, 58, 563–573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adianti, M.; Aoki, C.; Komoto, M.; Deng, L.; Shoji, I.; Wahyuni, T.S.; Lusida, M.I.; Soetjipto; Fuchino, H.; Kawahara, N.; et al. Anti-hepatitis C virus compounds obtained from Glycyrrhiza uralensis and other Glycyrrhiza species. Microbiol. Immunol. 2014, 58, 180–187. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.M. Antiviral activity of compounds isolated from plants. Pharm. Biol. 2003, 41, 107–157. [Google Scholar] [CrossRef]
- Singh, I.P.; Bharate, S.B.; Bhutani, K.K. Anti-HIV natural products. Curr. Sci. 2005, 89, 269–290. [Google Scholar]
- Mustafa, Y.F.; Abdulaziz, N.T. Biological potentials of hymecromone-based derivatives: A systematic review. Syst. Rev. Pharm. 2020, 11, 438–452. [Google Scholar]
- Bishnoi, A.; Srivastava, K.; Joshi, M.N. Synthesis, characterization and in vitro anti-JEV activity of N-[p-{3′-(2′-aryl-4′-oxo-1′,3′-thiazolyl)diphenyl]-7-hydroxy-4-methyl-2-oxoquinolines. Indian J. Chem. 2006, 45B, 1961–1964. [Google Scholar] [CrossRef]
- Mazzei, M.; Nieddu, E.; Miele, M.; Balbi, A.; Ferrone, M.; Fermeglia, M.; Mazzei, M.T.; Pricl, S.; Colla, L.; Marongiu, F.; et al. Activity of Mannich bases of 7-hydroxycoumarin against Flaviviridae. Bioorgan. Med. Chem. 2008, 16, 2591–2605. [Google Scholar] [CrossRef]
- Mohammed, A.Y.; Ahamed, L.S. Synthesis and Characterization of New Substituted Coumarin Derivatives and Study Their Biological Activity. Chem. Methodol. 2022, 6, 813–822. [Google Scholar] [CrossRef]
- Abdizadeh, R.; Hadizadeh, F.; Abdizadeh, T. In silico analysis and identification of antiviral coumarin derivatives against 3-chymotrypsin-like main protease of the novel coronavirus SARS-CoV-2. Mol. Divers. 2022, 26, 1053–1076. [Google Scholar] [CrossRef]
- Nishikawa, H. Biochemistry of filamentous fungi III A metabolic product of Aspergillus melleus Yukawa. Part II. Bull. Agric. Chem. Soc. Jpn. 1933, 9, 148–151. [Google Scholar] [CrossRef]
- Findlay, J.A.; Buthelezi, S.; Lavoie, R.; Peña-Rodriguez, L.; Miller, J.D. Bioactive isocoumarins and related metabolites from conifer endophytes. J. Nat. Prod. 1995, 58, 1759–1766. [Google Scholar] [CrossRef]
- Rukachaisirikul, V.; Arunpanichlert, J.; Sukpondma, Y.; Phongpaichit, S.; Sakayaroj, J. Metabolites from the endophytic fungi Botryosphaeria rhodina PSU-M35 and PSU-M114. Tetrahedron 2009, 65, 10590–10595. [Google Scholar] [CrossRef]
- Das, A.J. Moringa oleifera (Lamm.): A plant with immense importance. J. Biol. Act. Prod. Nat. 2012, 2, 307–315. [Google Scholar] [CrossRef]
- Chacón-Morales, P.; Amaro-Luis, J.M.; Bahsas, A. Isolation and characterization of (+)-mellein, the first isocoumarin reported in Stevia genus. Av. Quim. 2013, 8, 145–151. [Google Scholar]
- Zhao, J.H.; Zhang, Y.L.; Wang, L.W.; Wang, J.Y.; Zhang, C.L. Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J. Microbiol. Biotechnol. 2012, 28, 2107–2112. [Google Scholar] [CrossRef]
- Ramos, H.P.; Simão, M.R.; de Souza, J.M.; Magalhães, L.G.; Rodrigues, V.; Ambrósio, S.R.; Said, S. Evaluation of dihydroisocoumarins produced by the endophytic fungus Arthrinium state of Apiospora montagnei against Schistosoma mansoni. Nat. Prod. Res. 2013, 27, 2240–2243. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Krohn, K.; Draeger, S.; Schulz, B. New naphthalene-chroman coupling products from the endophytic fungus, Nodulisporium sp. from Erica arborea. Eur. J. Org. Chem. 2009, 2009, 1564–1569. [Google Scholar] [CrossRef]
- Hussain, H.; Akhtar, N.; Draeger, S.; Schulz, B.; Pescitelli, G.; Salvadori, P.; Antus, S.; Kurtán, T.; Krohn, K. New bioactive 2,3-epoxycyclohexenes and isocoumarins from the endophytic fungus Phomopsis sp. from Laurus azorica. Eur. J. Org. Chem. 2009, 2009, 749–756. [Google Scholar] [CrossRef]
- Kjer, J.; Wray, V.; Edrada-Ebel, R.; Ebel, R.; Pretsch, A.; Lin, W.; Proksch, P. Xanalteric acids I and II and related phenolic compounds from an endophytic Alternaria sp. isolated from the mangrove plant Sonneratia alba. J. Nat. Prod. 2009, 72, 2053–2057. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, J.; She, Z.; Lin, Y. Isoflavones from the mangrove endophytic fungus Fusarium sp. (ZZF41). Nat. Prod. Commun. 2010, 5, 1771–1773. [Google Scholar] [CrossRef]
- Raja, S.B.; Murali, M.R.; Roopa, K.; Devaraj, S.N. Imperatorin a furocoumarin inhibits periplasmic Cu-Zn SOD of Shigella dysenteriae their by modulates its resistance towards phagocytosis during host pathogen interaction. Biomed. Pharmacother. 2011, 65, 560–568. [Google Scholar] [CrossRef] [PubMed]
- Kozioł, E.; Skalicka-Woźniak, K. Imperatorin–pharmacological meaning and analytical clues: Profound investigation. Phytochem. Rev. 2016, 15, 627–649. [Google Scholar] [CrossRef] [PubMed]
- Montagner, C.; de Souza, S.M.; Groposo, C.; Delle Monache, F.; Smânia, E.F.; Smânia Jr, A. Antifungal activity of coumarins. Z. Nat. C 2008, 63, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Tsivileva, O.M.; Koftin, O.V.; Ibragimova, D.N.; Fedotova, O.V.; Nikitina, V.E. Production of biometal complexes based on the systems incorporating 4-hydroxycoumarin fragnment and their effect on microorganisms. Proc. RAS Ufa Sci. Cent. 2019, 2019, 89–98. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Proksch, P. Mangrove derived fungal endophytes—A chemical and biological perception. Fungal Divers. 2013, 61, 1–27. [Google Scholar] [CrossRef]
- Aquila, S.; Santoro, M.; Caputo, A.; Panno, M.L.; Pezzi, V.; De Amicis, F. The tumor suppressor PTEN as molecular switch node regulating cell metabolism and autophagy: Implications in immune system and tumor microenvironment. Cells 2020, 9, 1725. [Google Scholar] [CrossRef] [PubMed]
- Santoro, M.; Guido, C.; De Amicis, F.; Sisci, D.; Cione, E.; Dolce, V.; Donà, A.; Panno, M.L.; Aquila, S. Bergapten induces metabolic reprogramming in breast cancer cells. Oncol. Rep. 2016, 35, 568–576. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, X.; Hu, S.Y.; Qiu, X.J.; Zhang, Y.J.; Ren, P.; Wang, Y.; Ji, H.; Zhang, C.H.; Xie, W.B.; et al. Meranzin hydrate exhibits anti-depressive and prokinetic-like effects through regulation of the shared alpha 2-adrenoceptor in the brain–gut axis of rats in the forced swimming test. Neuropharmacology 2013, 67, 318–325. [Google Scholar] [CrossRef]
- Zaher, A.M.; Moharram, A.M.; Davis, R.; Panizzi, P.; Makboul, M.A.; Calderón, A.I. Characterisation of the metabolites of an antibacterial endophyte Botryodiplodia theobromae Pat. of Dracaena draco L. by LC–MS/MS. Nat. Prod. Res. 2015, 29, 2275–2281. [Google Scholar] [CrossRef]
- Lekphrom, R.; Kanokmedhakul, S.; Kukongviriyapan, V.; Kanokmedhakul, K. C-7 oxygenated coumarins from the fruits of Micromelum minutum. Arch. Pharmacal Res. 2011, 34, 527–531. [Google Scholar] [CrossRef]
- Li, G.; Lin, P.; Wang, K.; Gu, C.-C.; Kusari, S. Artificial intelligence-guided discovery of anticancer lead compounds from plants and associated microorganisms. Trends Cancer 2022, 8, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Awale, S.; Nakashima, E.M.; Kalauni, S.K.; Tezuka, Y.; Kurashima, Y.; Lu, J.; Esumi, H.; Kadota, S. Angelmarin, a novel anti-cancer agent able to eliminate the tolerance of cancer cells to nutrient starvation. Bioorgan. Med. Chem. Lett. 2006, 16, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Valdivieso, J.; Girotti, A.; Schneider, J.; Arias, F.J. Advanced nanomedicine and cancer: Challenges and opportunities in clinical translation. Int. J. Pharm. 2021, 599, 120438. [Google Scholar] [CrossRef] [PubMed]
- Suksatan, W.; Chupradit, S.; Yumashev, A.V.; Ravali, S.; Shalaby, M.N.; Mustafa, Y.F.; Kurochkin, A.; Siahmansouri, H. Immunotherapy of multisystem inflammatory syndrome in children (MIS-C) following COVID-19 through mesenchymal stem cells. Int. Immunopharmacol. 2021, 101, 108217. [Google Scholar] [CrossRef]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Simunkova, M.; Barbierikova, Z.; Jomova, K.; Hudecova, L.; Lauro, P.; Alwasel, S.H.; Alhazza, I.; Rhodes, C.J.; Valko, M. Antioxidant vs. prooxidant properties of the flavonoid, kaempferol, in the presence of Cu (II) ions: A ROS-scavenging activity, Fenton reaction and DNA damage study. Int. J. Mol. Sci. 2021, 22, 1619. [Google Scholar] [CrossRef]
- Halliwell, B. Are polyphenols antioxidants or pro-oxidants? What do we learn from cell culture and in vivo studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Zheleva, A.M.; Kancheva, V.D.; Nikolova, G.D.; Parmar, V.S.; Gadjeva, V.G. A preliminary study on radical scavenging abilities of two dihydroxy-coumarins by electron paramagnetic resonance (EPR) spectroscopy. Bulg. Chem. Commun. 2018, 50, 315–320. [Google Scholar]
- Dlugosz, A.; Janecka, A. Novobiocin analogs as potential anticancer agents. Mini Rev. Med. Chem. 2017, 17, 728–733. [Google Scholar] [CrossRef]
- Zuehlke, A.D.; Moses, M.A.; Neckers, L. Heat shock protein 90: Its inhibition and function. Philos. Trans. R. Soc. B Biol. Sci. 2018, 373, 20160527. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, M.; Huber, J.; Kramer, J.S.; Heering, J.; Pietsch, L.; Stark, H.; Odadzic, D.; Bischoff, I.; Fürst, R.; Schröder, M.; et al. Demonstrating ligandability of the LC3A and LC3B adapter interface. J. Med. Chem. 2021, 64, 3720–3746. [Google Scholar] [CrossRef] [PubMed]
- McKie, S.J.; Neuman, K.C.; Maxwell, A. DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis. BioEssays 2021, 43, 2000286. [Google Scholar] [CrossRef] [PubMed]
- Dhupal, M.; Chowdhury, D. Phytochemical-based nanomedicine for advanced cancer theranostics: Perspectives on clinical trials to clinical use. Int. J. Nanomed. 2020, 15, 9125. [Google Scholar] [CrossRef]
- Majnooni, M.B.; Fakhri, S.; Shokoohinia, Y.; Mojarrab, M.; Kazemi-Afrakoti, S.; Farzaei, M.H. Isofraxidin: Synthesis, biosynthesis, isolation, pharmacokinetic and pharmacological properties. Molecules 2020, 25, 2040. [Google Scholar] [CrossRef]
- Guévélou, E.; Huvet, A.; Sussarellu, R.; Milan, M.; Guo, X.; Li, L.; Zhang, G.; Quillien, V.; Daniel, J.-Y.; Quéré, C.; et al. Regulation of a truncated isoform of AMP-activated protein kinase α (AMPKα) in response to hypoxia in the muscle of Pacific oyster Crassostrea gigas. J. Comp. Physiol. B 2013, 183, 597–611. [Google Scholar] [CrossRef]
- Zaragozá, C.; Villaescusa, L.; Monserrat, J.; Zaragozá, F.; Álvarez-Mon, M. Potential therapeutic anti-inflammatory and immunomodulatory effects of dihydroflavones, flavones, and flavonols. Molecules 2020, 25, 1017. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Li, X.; Li, Z.; Zhang, L.; Liu, Y.; Ding, H.; Yin, S. Isofraxidin, a coumarin component improves high-fat diet induced hepatic lipid homeostasis disorder and macrophage inflammation in mice. Food Funct. 2017, 8, 2886–2896. [Google Scholar] [CrossRef]
- Yim, S.H.; Tabassum, N.; Kim, W.H.; Cho, H.; Lee, J.H.; Batkhuu, G.J.; Kim, H.J.; Oh, W.K.; Jung, D.W.; Williams, D.R. Isolation and characterization of isofraxidin 7-O-(6′-O-p-coumaroyl)-β-glucopyranoside from Artemisia capillaris Thunberg: A novel, nontoxic hyperpigmentation agent that is effective in vivo. Evid.-Based Complement. Altern. Med. 2017, 2017, 1401279. [Google Scholar] [CrossRef]
- Greenough, M.A.; Camakaris, J.; Bush, A.I. Metal dyshomeostasis and oxidative stress in Alzheimer’s disease. Neurochem. Int. 2013, 62, 540–555. [Google Scholar] [CrossRef]
- Korabecny, J.; Andrs, M.; Nepovimova, E.; Dolezal, R.; Babkova, K.; Horova, A.; Malinak, D.; Mezeiova, E.; Gorecki, L.; Sepsova, V.; et al. 7-Methoxytacrine-p-anisidine hybrids as novel dual binding site acetylcholinesterase inhibitors for Alzheimer’s disease treatment. Molecules 2015, 20, 22084–22101. [Google Scholar] [CrossRef] [PubMed]
- Minarini, A.; Milelli, A.; Simoni, E.; Rosini, M.; Laura Bolognesi, M.; Marchetti, C.; Tumiatti, V. Multifunctional tacrine derivatives in Alzheimer’s disease. Curr. Med. Chem. 2013, 13, 1771–1786. [Google Scholar] [CrossRef] [PubMed]
- Golfakhrabadi, F.; Shams Ardakani, M.R.; Saeidnia, S.; Akbarzadeh, T.; Yousefbeyk, F.; Jamalifar, H.; Khanavi, M. In vitro antimicrobial and acetylcholinesterase inhibitory activities of coumarins from Ferulago carduchorum. Med. Chem. Res. 2016, 25, 1623–1629. [Google Scholar] [CrossRef]
- Karakaya, S.; Şimşek, D.; Özbek, H.; Güvenalp, Z.; Altanlar, N.; Kazaz, C.; Kiliç, C.S. Antimicrobial activities of extracts and isolated coumarins from the roots of four Ferulago species growing in Turkey. Iran. J. Pharm. Res. IJPR 2019, 18, 1516–1529. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, A.; Lawson, D.M. The ATP-Binding Site of Type II Topoisomerases as a Target for Antibacterial Drugs. Curr. Top. Med. Chem. 2003, 3, 283–303. [Google Scholar] [CrossRef]
- Phillips, J.W.; Goetz, M.A.; Smith, S.K.; Zink, D.L.; Polishook, J.; Onishi, R.; Salowe, S.; Wiltsie, J.; Allocco, J.; Sigmund, J.; et al. Discovery of kibdelomycin, a potent new class of bacterial type II topoisomerase inhibitor by chemical-genetic profiling in Staphylococcus aureus. Chem. Biol. 2011, 18, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Pojer, F.; Wemakor, E.; Kammerer, B.; Chen, H.; Walsh, C.T.; Li, S.M.; Heide, L. CloQ, a prenyltransferase involved in clorobiocin biosynthesis. Proc. Natl. Acad. Sci. USA 2003, 100, 2316–2321. [Google Scholar] [CrossRef]
- Basile, A.; Sorbo, S.; Spadaro, V.; Bruno, M.; Maggio, A.; Faraone, N.; Rosselli, S. Antimicrobial and antioxidant activities of coumarins from the roots of Ferulago campestris (Apiaceae). Molecules 2009, 14, 939–952. [Google Scholar] [CrossRef]
- Soine, T.O. Naturally Occurring Coumarins and Related Physiological Activities. J. Pharm. Sci. 1964, 53, 231–264. [Google Scholar] [CrossRef]
- Steffensky, M.; Mühlenweg, A.; Wang, Z.X.; Li, S.M.; Heide, L. Identification of the novobiocin biosynthetic gene cluster of Streptomyces spheroides NCIB 11891. Antimicrob. Agents Chemother. 2000, 44, 1214–1222. [Google Scholar] [CrossRef]
- Pacholec, M.; Hillson, N.J.; Walsh, C.T. NovJ/NovK catalyze benzylic oxidation of a β-hydroxyl tyrosyl-S-pantetheinyl enzyme during aminocoumarin ring formation in novobiocin biosynthesis. Biochemistry 2005, 44, 12819–12826. [Google Scholar] [CrossRef] [PubMed]
- Metwally, N.H.; Abdallah, S.O.; Mohsen, M.M.A. Design, green one-pot synthesis and molecular docking study of novel N,N-bis(cyanoacetyl)hydrazines and bis-coumarins as effective inhibitors of DNA gyrase and topoisomerase IV. Bioorgan. Chem. 2020, 97, 103672. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Walsh, C.T. Coumarin formation in novobiocin biosynthesis: β-hydroxylation of the aminoacyl enzyme tyrosyl-S-NovH by a cytochrome P450 NovI. Chem. Biol. 2001, 8, 301–312. [Google Scholar] [CrossRef]
- Mustafa, Y.F.; Abdulaziz, N.T. Hymecromone and its products as cytotoxic candidates for brain cancer: A brief review. NeuroQuantology 2021, 19, 175–186. [Google Scholar] [CrossRef]
- Hansen, T.M.; Gu, Y.-G.; Rehm, T.M.; Dandliker, P.J.; Chovan, L.E.; Bui, M.H.; Nilius, A.M.; Beutel, B.A. Synthesis and antibacterial activity of 5-methoxy- and 5-hydroxy-6-fluoro-1,8-naphthyridone-3-carboxylic acid derivatives. Bioorgan. Med. Chem. Lett. 2005, 15, 2716–2719. [Google Scholar] [CrossRef]
- Philip, B.M.; John, J.S.; Shyni, V.; Kuruvilla, T.K.; Paulose, T.A.P.; Sajan, D. Vibrational spectra and molecular docking studies of bergapten isolated from Melicopedenhamii leaves as anti-breast cancer agents. J. Mol. Struct. 2022, 1258, 132656. [Google Scholar] [CrossRef]
- Sashidhara, K.V.; Kumar, A.; Kumar, M.; Sarkar, J.; Sinha, S. Synthesis and in vitro evaluation of novel coumarin–chalcone hybrids as potential anticancer agents. Bioorgan. Med. Chem. Lett. 2010, 20, 7205–7211. [Google Scholar] [CrossRef]
- Lacy, A.; O’Kennedy, R. Studies on coumarines and coumarin related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef]
- Abraham, K.; Woehrlin, F.; Lindtner, O.; Heinemeyer, G.; Lampen, A. Toxicology and risk assessment of coumarin: Focus on human data. Mol. Nutr. Food Res. 2010, 54, 228–239. [Google Scholar] [CrossRef]
- Weigta, S.; Hueblera, N.; Streckerb, R.; Braunbeckb, T.; Broscharda, T.H. Developmental effects of coumarin and the anticoagulant coumarin derivative warfarin on zebrafish (Danio rerio) embryos. Reprod. Toxicol. 2012, 33, 133–141. [Google Scholar] [CrossRef]
- Belluti, F.; Fontana, G.; Dal Bo, L.; Carenini, N.; Giommarelli, C.; Zunino, F. Design, synthesis and anticancer activities of stilbene-coumarin hybrid compounds: Identification of novel proapoptotic agents. Bioorgan. Med. Chem. 2010, 18, 3543–3550. [Google Scholar] [CrossRef] [PubMed]
- Gobbi, S.; Belluti, F.; Rampa, A.; Bisi, A. Flavonoid-inspired vascular disrupting agents: Exploring flavone-8-acetic acid and derivatives in the new century. Molecules 2021, 26, 4228. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wang, B.; Yang, J.; Liu, T.; Sun, J.; Wang, X. Synthesis and biological evaluation of 3-arylcoumarin derivatives as potential anti-diabetic agents. J. Enzym. Inhib. Med. Chem. 2019, 34, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Elkanzi, N.A.; Hrichi, H.; Alolayan, R.A.; Derafa, W.; Zahou, F.M.; Bakr, R.B. Synthesis of Chalcones Derivatives and Their Biological Activities: A Review. ACS Omega 2022, 7, 27769–27786. [Google Scholar] [CrossRef]
- Vermeulen, K.; Van Bockstaele, D.R.; Berneman, Z.N. The cell cycle: A review of regulation, deregulation and therapeutic targets in cancer. Cell Prolif. 2003, 36, 131–149. [Google Scholar] [CrossRef]
- Arun, A.; Ansari, M.I.; Popli, P.; Jaiswal, S.; Mishra, A.K.; Dwivedi, A.; Hajela, K.; Konwar, R. New piperidine derivative DTPEP acts as dual-acting anti-breast cancer agent by targeting ERα and downregulating PI3K/Akt-PKCα leading to caspase-dependent apoptosis. Cell Prolif. 2018, 51, e12501. [Google Scholar] [CrossRef]
- Kamath, P.R.; Sunil, D.; Joseph, M.M.; Salam, A.A.A.; Sreelekha, T.T. Indole-coumarin-thiadiazole hybrids: An appraisal of their MCF-7 cell growth inhibition, apoptotic, antimetastatic and computational Bcl-2 binding potential. Eur. J. Med. Chem. 2017, 136, 442–451. [Google Scholar] [CrossRef]
- Giacomini, E.; Rupiani, S.; Guidotti, L.; Recanatini, M.; Roberti, M. The use of stilbene scaffold in medicinal chemistry and multi-target drug design. Curr. Med. Chem. 2016, 23, 2439–2489. [Google Scholar] [CrossRef]
- Xiao, C.F.; Tao, L.Y.; Sun, H.Y.; Wei, W.; Chen, Y.; Fu, L.W.; Zou, Y. Design, synthesis and antitumor activity of a series of novel coumarin–stilbenes hybrids, the 3-arylcoumarins. Chin. Chem. Lett. 2010, 21, 1295–1298. [Google Scholar] [CrossRef]
- Krstic, A.; Pavic, A.; Avdovic, E.; Markovic, Z.; Stevanovic, M.; Petrovic, I. Coumarin-Palladium (II) Complex Acts as a Potent and Non-Toxic Anticancer Agent against Pancreatic Carcinoma Cells. Molecules 2022, 27, 2115. [Google Scholar] [CrossRef]
- Luo, G.; Li, X.; Zhang, G.; Wu, C.; Tang, Z.; Liu, L.; You, Q.; Xiang, H. Novel SERMs based on 3-aryl-4-aryloxy-2H-chromen-2-one skeleton—A possible way to dual ERα/VEGFR-2 ligands for treatment of breast cancer. Eur. J. Med. Chem. 2017, 140, 252–273. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Kulkarni, S.; Singh, Y.; Kumar, P.; Thareja, S. Selective Estrogen Receptor Modulators (SERMs) for the Treatment of ER+ Breast Cancer: An Overview. J. Mol. Struct. 2022, 1270, 133853. [Google Scholar] [CrossRef]
- Batran, R.Z.; Dawood, D.H.; El-Seginy, S.A.; Ali, M.M.; Maher, T.J.; Gugnani, K.S.; Rondon-Ortiz, A.N. New coumarin derivatives as anti-breast and anti-cervical cancer agents targeting VEGFR-2 and p38α MAPK. Arch. Pharm. 2017, 350, 1700064. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, E.Y.; Latif, N.A.A.; El-Mansy, M.F.; Elserwy, W.S.; Abdelhafez, O.M. VEGFR-2 inhibiting effect and molecular modeling of newly synthesized coumarin derivatives as anti-breast cancer agents. Bioorgan. Med. Chem. 2020, 28, 115328. [Google Scholar] [CrossRef]
- Mohamed, T.K.; Batran, R.Z.; Elseginy, S.A.; Ali, M.M.; Mahmoud, A.E. Synthesis, anticancer effect and molecular modeling of new thiazolylpyrazolyl coumarin derivatives targeting VEGFR-2 kinase and inducing cell cycle arrest and apoptosis. Bioorgan. Chem. 2019, 85, 253–273. [Google Scholar] [CrossRef]
- Rusu, A.; Lungu, I.A.; Moldovan, O.L.; Tanase, C.; Hancu, G. Structural characterization of the millennial antibacterial (fluoro) quinolones—Shaping the fifth generation. Pharmaceutics 2021, 13, 1289. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Zhang, S.; Xu, Z.; Lv, Z.-S.; Liu, M.-L.; Feng, L.-S. 4-Quinolone hybrids and their antibacterial activities. Eur. J. Med. Chem. 2017, 141, 335–345. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, M.L.; Feng, L.S.; Lv, K.; Guan, Y.; Guo, H.Y.; Xiao, C.L. Synthesis and In-Vitro Antimycobacterial Activity of Fluoroquinolone Derivatives Containing a Coumarin Moiety. Arch. Pharm. 2011, 344, 802–809. [Google Scholar] [CrossRef]
- McKay, C.D.; O’Bryan, E.; Gubhaju, L.; McNamara, B.; Gibberd, A.J.; Azzopardi, P.; Eades, S. Potential Determinants of Cardio-Metabolic Risk among Aboriginal and Torres Strait Islander Children and Adolescents: A Systematic Review. Int. J. Environ. Res. Public Health 2022, 19, 9180. [Google Scholar] [CrossRef]
- Pace, E.; Cerveri, I.; Lacedonia, D.; Paone, G.; Sanduzzi Zamparelli, A.; Sorbo, R.; Allegretti, M.; Lanata, L.; Scaglione, F. Clinical Efficacy of Carbocysteine in COPD: Beyond the Mucolytic Action. Pharmaceutics 2022, 14, 1261. [Google Scholar] [CrossRef]
- Fonseca, G.P.; Reniers, B.; Landry, G.; White, S.; Bellezzo, M.; Antunes, P.C.G.; de Sales, C.P.; Welteman, E.; Yoriyaz, H.; Verhaegen, F. A medical image-based graphical platform—Features, applications and relevance for brachytherapy. Brachytherapy 2014, 13, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Himmler, H.J.; Kos, A. Free chemical structure search in MDL SDFiles using the open source Personal Chemistry Client (PCC). Chem. Cent. J. 2008, 2, 43. [Google Scholar] [CrossRef]
- Eweas, A.F.; Maghrabi, I.A.; Namarneh, A.I. Advances in molecular modeling and docking as a tool for modern drug discovery. Pharm. Chem. 2014, 6, 211–228. [Google Scholar]
- Tallapragada, K.; Chewning, J.; Kombo, D.; Ludwick, B. Making SharePoint® Chemically Aware™. J. Cheminform. 2012, 4, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, S. Articles, Books, and Internet Documents with Structural Formulas Drawn by XΥMTEX—Writing, Submission, Publication, and Internet Communication in Chemistry. Asian J. TEX 2009, 3, 89–108. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsivileva, O.M.; Koftin, O.V.; Evseeva, N.V. Coumarins as Fungal Metabolites with Potential Medicinal Properties. Antibiotics 2022, 11, 1156. https://doi.org/10.3390/antibiotics11091156
Tsivileva OM, Koftin OV, Evseeva NV. Coumarins as Fungal Metabolites with Potential Medicinal Properties. Antibiotics. 2022; 11(9):1156. https://doi.org/10.3390/antibiotics11091156
Chicago/Turabian StyleTsivileva, Olga M., Oleg V. Koftin, and Nina V. Evseeva. 2022. "Coumarins as Fungal Metabolites with Potential Medicinal Properties" Antibiotics 11, no. 9: 1156. https://doi.org/10.3390/antibiotics11091156
APA StyleTsivileva, O. M., Koftin, O. V., & Evseeva, N. V. (2022). Coumarins as Fungal Metabolites with Potential Medicinal Properties. Antibiotics, 11(9), 1156. https://doi.org/10.3390/antibiotics11091156







