Identification of Novel Inhibitor of Enoyl-Acyl Carrier Protein Reductase (InhA) Enzyme in Mycobacterium tuberculosis from Plant-Derived Metabolites: An In Silico Study
Abstract
:1. Introduction
2. Material and Methods
2.1. Compilation of Bioactive Compounds from Ruta Graveolens and Citrus of Rutaceae Family
2.2. Drugs Target Identification and Ligand Preparation
2.3. Drug-Likeliness Study of the Compounds
2.4. ADME/Tox Profiling of the Filtered Compounds
2.5. Molecular Docking
2.6. Molecular Dynamics Simulation
3. Results
3.1. Compilation of Bioactive Compounds
3.2. Drug-Likeliness Study of Compiled Compounds
3.3. ADMET Study of Screened Compounds Compared with Standard Drug
3.4. Molecular Docking
3.5. Analysis for Molecular Dynamics Simulation
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chakaya, J.; Khan, M.; Ntoumi, F.; Aklillu, E.; Fatima, R.; Mwaba, P.; Kapata, N.; Mfinanga, S.; Hasnain, S.E.; Katoto, P.D.M.C.; et al. Global Tuberculosis Report 2020—Reflections on the Global TB Burden, Treatment and Prevention Efforts. Int. J. Infect. Dis. 2021, 113 (Suppl. S1), S7–S12. [Google Scholar] [CrossRef] [PubMed]
- Global Tuberculosis Report 2020. Available online: https://www.who.int/publications/i/item/9789240013131 (accessed on 7 March 2022).
- Global Tuberculosis Report; WHO: Geneva, Switzerland, 2019.
- Farjallah, A.; Chiarelli, L.R.; Forbak, M.; Degiacomi, G.; Danel, M.; Goncalves, F.; Carayon, C.; Seguin, C.; Fumagalli, M.; Záhorszká, M.; et al. A Coumarin-Based Analogue of Thiacetazone as Dual Covalent Inhibitor and Potential Fluorescent Label of HadA in Mycobacterium tuberculosis. ACS Infect. Dis. 2021, 7, 552–565. [Google Scholar] [CrossRef]
- Espinal, M.A. The Global Situation of MDR-TB. Tuberculosis 2003, 83, 44–51. [Google Scholar] [CrossRef]
- Jayaraman, M.; Loganathan, L.; Muthusamy, K.; Ramadas, K. Virtual Screening Assisted Discovery of Novel Natural Products to Inhibit the Catalytic Mechanism of Mycobacterium Tuberculosis InhA. J. Mol. Liq. 2021, 335, 116204. [Google Scholar] [CrossRef]
- Massengo-Tiassé, R.P.; Cronan, J.E. Diversity in Enoyl-Acyl Carrier Protein Reductases. Cell. Mol. Life Sci. 2009, 66, 1507–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamsri, P.; Hanwarinroj, C.; Phusi, N.; Pornprom, T.; Chayajarus, K.; Punkvang, A.; Suttipanta, N.; Srimanote, P.; Suttisintong, K.; Songsiriritthigul, C.; et al. Discovery of New and Potent InhA Inhibitors as Anti-Tuberculosis Agents: Structure Based Virtual Screening Validated by Biological Assays and X-Ray Crystallography. J. Chem. Inf. Model. 2019, 10, 226–234. [Google Scholar]
- Pakadang, S.R.; Hilaria, M.; Dewi, S.T.R.; Sinala, S.; Jumain. MIC and MKC Analysis of Herbal Medicine in Indonesia Against Mycobacterium tuberculosis. Pharmacogn. J. 2021, 13, 1058–1106. [Google Scholar] [CrossRef]
- Palanisamy, P.; Basalingappa, K.M. Phytochemical analysis and antioxidant properties of leaf extracts of Carica papaya. Phytochem. Anal. 2020, 13, 58–62. [Google Scholar]
- Singh, K.; Sharma, A.; Upadhyay, T.K.; Hayat-ul-Islam, M.; Khan, M.K.A.; Dwivedi, U.N.; Sharma, R. Structure-Based In Silico and In Vitro Analysis Reveals Asiatic Acid As Novel Potential Inhibitor of Mycobacterium Tuberculosis Maltosyl Transferase. Curr. Comput. Aided. Drug Des. 2022, 18. (article is ahead of print). [Google Scholar] [CrossRef]
- Samje, M.; Tchoufack, B.; Ngoufo, P.; Dilonga, H.M. Evaluation of Antibacterial Activities and Cytotoxicity of Three Medicinal Plants Used for the Management of Mycobacterium Tuberculosis and Staphylococcus Aureus Infections in the North-West Region of Cameroon. J. Tuberc. Res. 2021, 9, 1–17. [Google Scholar] [CrossRef]
- Coimbra, A.T.; Ferreira, S.; Duarte, A.P. Genus Ruta: A Natural Source of High Value Products with Biological and Pharmacological Properties. J. Ethnopharmacol. 2020, 260, 113076. [Google Scholar] [CrossRef] [PubMed]
- Reyes-Vaquero, L.; Bueno, M.; Ventura-Aguilar, R.I.; Aguilar-Guadarrama, A.B.; Robledo, N.; Sepúlveda-Jiménez, G.; Vanegas-Espinoza, P.E.; Ibáñez, E.; Del Villar-Martínez, A.A. Seasonal Variation of Chemical Profile of Ruta Graveolens Extracts and Biological Activity against Fusarium oxysporum, Fusarium proliferatum and Stemphylium vesicarium. Biochem. Syst. Ecol. 2021, 95, 104223. [Google Scholar] [CrossRef]
- Donadu, M.G.; Peralta-Ruiz, Y.; Usai, D.; Maggio, F.; Molina-Hernandez, J.B.; Rizzo, D.; Bussu, F.; Rubino, S.; Zanetti, S.; Paparella, A.; et al. Colombian Essential Oil of Ruta Graveolens against Nosocomial Antifungal Resistant Candida Strains. J. Fungi 2021, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Lücker, J.; El Tamer, M.K.; Schwab, W.; Verstappen, F.W.; van der Plas, L.H.; Bouwmeester, H.J.; Verhoeven, H.A. Monoterpene Biosynthesis in Lemon (Citrus Limon) cDNA isolation and functional analysis of four monoterpene synthases. Eur. J. Biochem. 2002, 269, 3160–3171. [Google Scholar] [CrossRef] [PubMed]
- Hussain, K.; Nisar, M.F.; Majeed, A.; Nawaz, K.; Bhatti, K. Ethnomedicinal Survey for Important Plants of Jalalpur Jattan, District Gujrat, Punjab, Pakistan. Ethnobot. Leafl. 2010, 2010, 807–825. [Google Scholar]
- Semenya, S.; Maroyi, A. Medicinal Plants Used for the Treatment of Tuberculosis by Bapedi Traditional Healers in Three Districts of the Limpopo Province, South Africa. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 316–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muthuswamy, M.; Asan, P.; Kumar, V. Screening of antitubercular activity of some medicinal plants from Western Ghats, India. Int. J. Pharm. Bio Sci. 2013, 4, 328–334. [Google Scholar]
- Dr. Duke’s Phytochemical and Ethnobotanical Databases at NAL. Available online: https://phytochem.nal.usda.gov/phytochem/search (accessed on 11 March 2022).
- Manjunatha, U.H.; Rao, S.P.S.; Kondreddi, R.R.; Noble, C.G.; Camacho, L.R.; Tan, B.H.; Ng, S.H.; Ng, P.S.; Ma, N.L.; Lakshminarayana, S.B.; et al. Direct Inhibitors of InhA Active against Mycobacterium tuberculosis. Sci. Transl. Med. 2015, 7, 269ra3. [Google Scholar] [CrossRef] [Green Version]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and Computational Approaches to Estimate Solubility and Permeability in Drug Discovery and Development Settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Morris, G.M.; Goodsell, D.S.; Halliday, R.S.; Huey, R.; Hart, W.E.; Belew, R.K.; Olson, A.J. Automated Docking Using a Lamarckian Genetic Algorithm and an Empirical Binding Free Energy Function. J. Comput. Chem. 1998, 19, 1639–1662. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.M.; Goodsell, D.S.; Huey, R.; Olson, A.J. Distributed Automated Docking of Flexible Ligands to Proteins: Parallel Applications of AutoDock 2.4. J. Comput. Aided. Mol. Des. 1996, 10, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Islam, M.H.; Fatima, N.; Upadhyay, T.K.; Khan, M.K.A.; Dwivedi, U.N.; Sharma, R. Elucidation of marine fungi derived anthraquinones as mycobacterial mycolic acid synthesis inhibitors: An in silico approach. Mol. Biol. Rep. 2019, 46, 1715–1725. [Google Scholar] [CrossRef] [PubMed]
- Martyna, G.J.; Klein, M.L.; Tuckerman, M. Nosé–Hoover chains: The canonical ensemble via continuous dynamics. J. Chem. Phys. 1992, 97, 2635–2643. [Google Scholar] [CrossRef]
- Toukmaji, A.Y.; Board, J.A. Ewald summation techniques in perspective: A survey. Comput. Phys. Commun. 1996, 95, 73–92. [Google Scholar] [CrossRef]
- Bowers, K.J.; Chow, D.E.; Xu, H.; Dror, R.O.; Eastwood, M.P.; Gregersen, B.A.; Klepeis, J.L.; Kolossvary, I.; Moraes, M.A.; Sacerdoti, F.D.; et al. Scalable algorithms for molecular dynamics simulations on commodity clusters. In Proceedings of the SC’06: Proceedings of the ACM/IEEE Conference on Supercomputing, Tampa, FL, USA, 11–17 November 2006; p. 43. [Google Scholar]
- Schrödinger Release 2018-4: Desmond Molecular Dynamics System; D. E. Shaw Research: New York, NY, USA; Maestro-Desmond Interoperability Tools, Schrödinger: New York, NY, USA, 2018.
- Singh, S.; Gupta, A.K.; Verma, A. Molecular Properties and Bioactivity Score of the Aloe Vera Antioxidant Compounds—In Order to Lead Finding. Res. J. Pharm. Biol. Chem. Sci. 2013, 4, 876–881. [Google Scholar]
- Bonate, P.L.; Howard, D.R. Pharmacokinetics in Drug Development: Regulatory and Development Paradigms; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2005; Volume 2, ISBN 0971176736. [Google Scholar]
- Heath, R.J.; White, S.W.; Rock, C.O. Lipid Biosynthesis as a Target for Antibacterial Agents. Prog. Lipid Res. 2001, 40, 467–497. [Google Scholar] [CrossRef]
- Heath, R.J.; Rubin, J.R.; Holland, D.R.; Zhang, E.; Snow, M.E.; Rock, C.O. Mechanism of Triclosan Inhibition of Bacterial Fatty Acid Synthesis *. J. Biol. Chem. 1999, 274, 11110–11114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vosátka, R.; Krátký, M.; Vinšová, J. Triclosan and Its Derivatives as Antimycobacterial Active Agents. Eur. J. Pharm. Sci. 2018, 114, 318–331. [Google Scholar] [CrossRef]
- Nedyalkova, M.; Vasighi, M.; Sappati, S.; Kumar, A.; Madurga, S.; Simeonov, V. Inhibition Ability of Natural Compounds on Receptor-Binding Domain of SARS-CoV2: An In Silico Approach. Pharmaceuticals 2021, 14, 1328. [Google Scholar] [CrossRef]
- Pauli, I.; dos Santos, R.N.; Rostirolla, D.C.; Martinelli, L.K.; Ducati, R.G.; Timmers, L.F.S.M.; Basso, L.A.; Santos, D.S.; Guido, R.V.C.; Andricopulo, A.D.; et al. Discovery of New Inhibitors of Mycobacterium Tuberculosis InhA Enzyme Using Virtual Screening and a 3D-Pharmacophore-Based Approach. J. Chem. Inf. Model. 2013, 53, 2390–2401. [Google Scholar] [CrossRef]
- Vilchèze, C.; Morbidoni, H.R.; Weisbrod, T.R.; Iwamoto, H.; Kuo, M.; Sacchettini, J.C.; Jacobs, W.R. Inactivation of the InhA-Encoded Fatty Acid Synthase II (FASII) Enoyl-Acyl Carrier Protein Reductase Induces Accumulation of the FASI End Products and Cell Lysis of Mycobacterium Smegmatis. J. Bacteriol. 2000, 182, 4059–4067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agüero, F.; Al-Lazikani, B.; Aslett, M.; Berriman, M.; Buckner, F.S.; Campbell, R.K.; Carmona, S.; Carruthers, I.M.; Chan, A.W.E.; Chen, F.; et al. Genomic-Scale Prioritization of Drug Targets: The TDR Targets Database. Nat. Rev. Drug Discov. 2008, 7, 900–907. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Siddiqi, M.I. CoMFA Based de Novo Design of Pyrrolidine Carboxamides as Inhibitors of Enoyl Acyl Carrier Protein Reductase from Mycobacterium tuberculosis. J. Mol. Model. 2008, 14, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Rozwarski, D.A.; Grant, G.A.; Barton, D.H.R.; Jacobs, W.R.; Sacchettini, J.C. Modification of the NADH of the Isoniazid Target (InhA) from Mycobacterium tuberculosis. Science 1998, 279, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Yu, H.; Yu, S.; Wang, F.; Sacchettini, J.C.; Magliozzo, R.S. Hydrogen Peroxide-Mediated Isoniazid Activation Catalyzed by Mycobacterium Tuberculosis Catalase-Peroxidase (KatG) and Its S315T Mutant. Biochemistry 2006, 45, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Timmins, G.S.; Deretic, V. Mechanisms of Action of Isoniazid. Mol. Microbiol. 2006, 62, 1220–1227. [Google Scholar] [CrossRef]
- Ramaswamy, S.V.; Reich, R.; Dou, S.J.; Jasperse, L.; Pan, X.; Wanger, A.; Quitugua, T.; Graviss, E.A. Single Nucleotide Polymorphisms in Genes Associated with Isoniazid Resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2003, 47, 1241–1250. [Google Scholar] [CrossRef] [Green Version]
- Peacock, C.S.; Seeger, K.; Harris, D.; Murphy, L.; Ruiz, J.C.; Quail, M.A.; Peters, N.; Adlem, E.; Tivey, A.; Aslett, M.; et al. Comparative Genomic Analysis of three Leishmania Species that Cause Diverse Human Disease. Nat. Genet. 2007, 39, 839–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahua, K.M.; Ioset, J.R.; Ransijn, A.; Mauël, J.; Mavi, S.; Hostettmann, K. Antileishmanial and Antifungal Acridone Derivatives from the Roots of ThamnosmaRhodesica. Phytochemistry 2004, 65, 963–968. [Google Scholar] [CrossRef]
- Schelz, Z.; Ocsovszki, I.; Bózsity, N.; Hohmann, J.; Zupkó, I. Antiproliferative Effects of Various Furanoacridones Isolated from Ruta Graveolens on Human Breast Cancer Cell Lines. Anticancer Res. 2016, 36, 2751–2758. [Google Scholar]
- Réthy, B.; Hohmann, J.; Minorics, R.; Varga, A.; Ocsovszki, I.; Molnár, J.; Juhász, K.; Falkay, G.; Zupkó, I. Antitumour Properties of Acridone Alkaloids on a Murine Lymphoma Cell Line. Anticancer Res. 2008, 28, 2737–2743. [Google Scholar] [PubMed]
- Shivekar, S.S.; Kaliaperumal, V.; Brammacharry, U.; Sakkaravarthy, A.; Raj, C.K.; Alagappan, C.; Muthaiah, M. Prevalence and factors associated with multidrug-resistant tuberculosis in South India. Sci. Rep. 2020, 10, 17552. [Google Scholar] [CrossRef] [PubMed]


| S. No. | Name of Compound | PubChem ID | Molecular Weight (g/mol) (≤500) | Xlog P3 (≤5) | H-Bond Donor (≤5) | H-Bond Acceptor (≤10) | Rotational Bond (≤10) | n VIOLATION |
|---|---|---|---|---|---|---|---|---|
| 1. | α-Limonene diepoxide | 232703 | 68.23 | 1.1 | 0 | 2 | 1 | 0 |
| 2. | Nobiletin | 72344 | 402.4 | 3 | 0 | 8 | 7 | 1 |
| 3. | Sinensetin | 145659 | 372.4 | 3 | 0 | 7 | 6 | 1 |
| 4. | Tangeretin | 68077 | 372.4 | 3 | 0 | 7 | 6 | 1 |
| 5. | Diosmetin | 5281612 | 300.26 | 1.7 | 3 | 6 | 2 | 0 |
| 6. | Graveoline | 353825 | 279.29 | 3.1 | 0 | 4 | 1 | 0 |
| 7. | Rutacridone | 5281849 | 307.3 | 4.6 | 1 | 4 | 1 | 0 |
| 8. | Daphnoretin-methyl-ether | 5318544 | 366.3 | 3.6 | 0 | 7 | 4 | 0 |
| 9. | Rutaretin | 44146779 | 262.26 | 1.6 | 2 | 5 | 1 | 0 |
| 10. | Gravacridonediol | 5317836 | 341.4 | 2.3 | 3 | 6 | 2 | 0 |
| S.No. | Compound Name | Toxicity | Absorption | Distributions | Metabolism Cyp 2d6 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mutagenicity (Ames Test) | Carcinogenicity | HIA% | Pcaco-2 (nm/s) | Pmdck (nm/s) | Pskin (nm/s) | PPB% | BBB% | |||
| 1. | α-Limonene diepoxide | Mutagenic | Non-carcinogenic | 100 | 57.6 | 28.79 | −2.5 | 54.15 | 0.23 | Non-inhibitor |
| 2. | Nobiletin | Mutagenic | Non-carcinogenic | 99.07 | 54.02 | 0.06 | −3.6 | 84.85 | 0.02 | Non-inhibitor |
| 3. | Sinensetin | Mutagenic | Non-carcinogenic | 98.8 | 51.22 | 0.06 | −3.5 | 86.24 | 0.02 | Non-inhibitor |
| 4. | Tangeretin | Mutagenic | Non-carcinogenic | 98.8 | 53.6 | 0.62 | −3.4 | 87.17 | 0.02 | Non-inhibitor |
| 5. | Diosmetin | Mutagenic | Non-carcinogenic | 88.18 | 7.02 | 23.85 | −4.1 | 90.16 | 0.2 | Non-inhibitor |
| 6. | Graveolinine | Mutagenic | Non-carcinogenic | 97.83 | 56.13 | 37.77 | −3.47 | 89.96 | 0.04 | Non-inhibitor |
| 7. | Guaiacol | Mutagenic | Non-carcinogenic | 96.47 | 29.44 | 362.86 | −1.9 | 99.18 | 0.9 | Non-inhibitor |
| 8. | Rutacridone | Mutagenic | Non-carcinogenic | 95.74 | 35.63 | 8.75 | −3.28 | 89.6 | 0.87 | Non-inhibitor |
| 9. | Daphnoretine-methyl-ether | Mutagenic | Non-carcinogenic | 99.11 | 25.71 | 0.42 | −3.65 | 87.02 | 0.11 | Non-inhibitor |
| 10. | Rutaretin | Non-Mutagenic | Non-carcinogenic | 90.96 | 5.84 | 253.44 | −3.18 | 72.17 | 0.59 | Non-inhibitor |
| 11. | Gravacridonediol | Non- Mutagenic | Non-carcinogenic | 100 | 19.17 | 45.96 | −4.02 | 79.22 | 0.26 | Non-inhibitor |
| S.No. | Plants | Compound | Binding Energy | Ki (Inhibition Constant) | H-Bond Interacting Amino Acids within a Distance of 3Å | Distance (Å) |
|---|---|---|---|---|---|---|
| 1. | Citrus | α-Limonene diepoxide | −4.95 | 235.01µm | Ile194 | 2.8574 |
| 2. | Citrus | Nobiletin | −6.87 | 9.19 µm | Ile194 | 3.13498 |
| 3. | Citrus | Sinensetin | −7.98 | 1.42 µm | ________ | |
| 4. | Citrus | Tangeretin | −7.04 | 6.96 µm | Ile 194, Tyr 158 | 2.74349 2.53957 |
| 5. | Citrus | Diosmetin | −7.92 | 1.98 µm | Ile194, Pro156 | 2.01273 1.89617 |
| 6. | Ruta graveolens | Daphnoretin-methyl-ether | −7.62 | 2.61µm | Ile21, Ala22 | 2.64217 2.99582 |
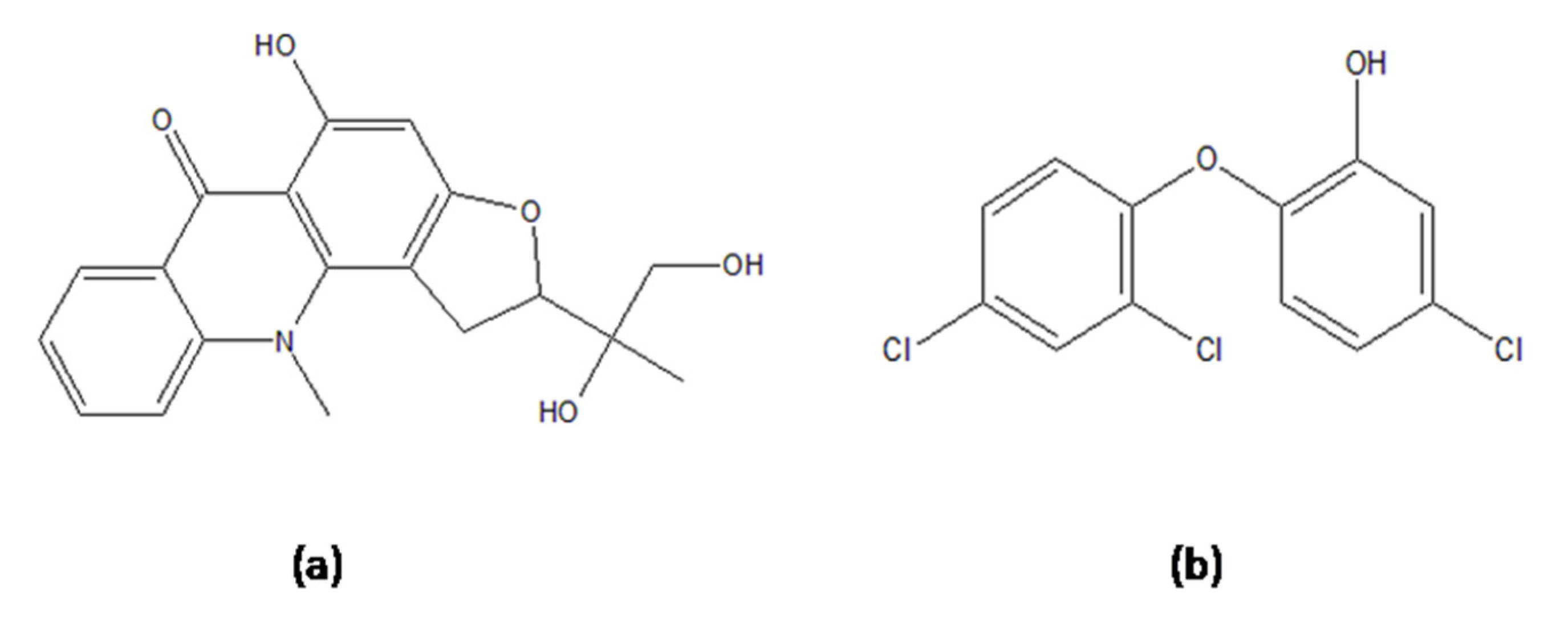
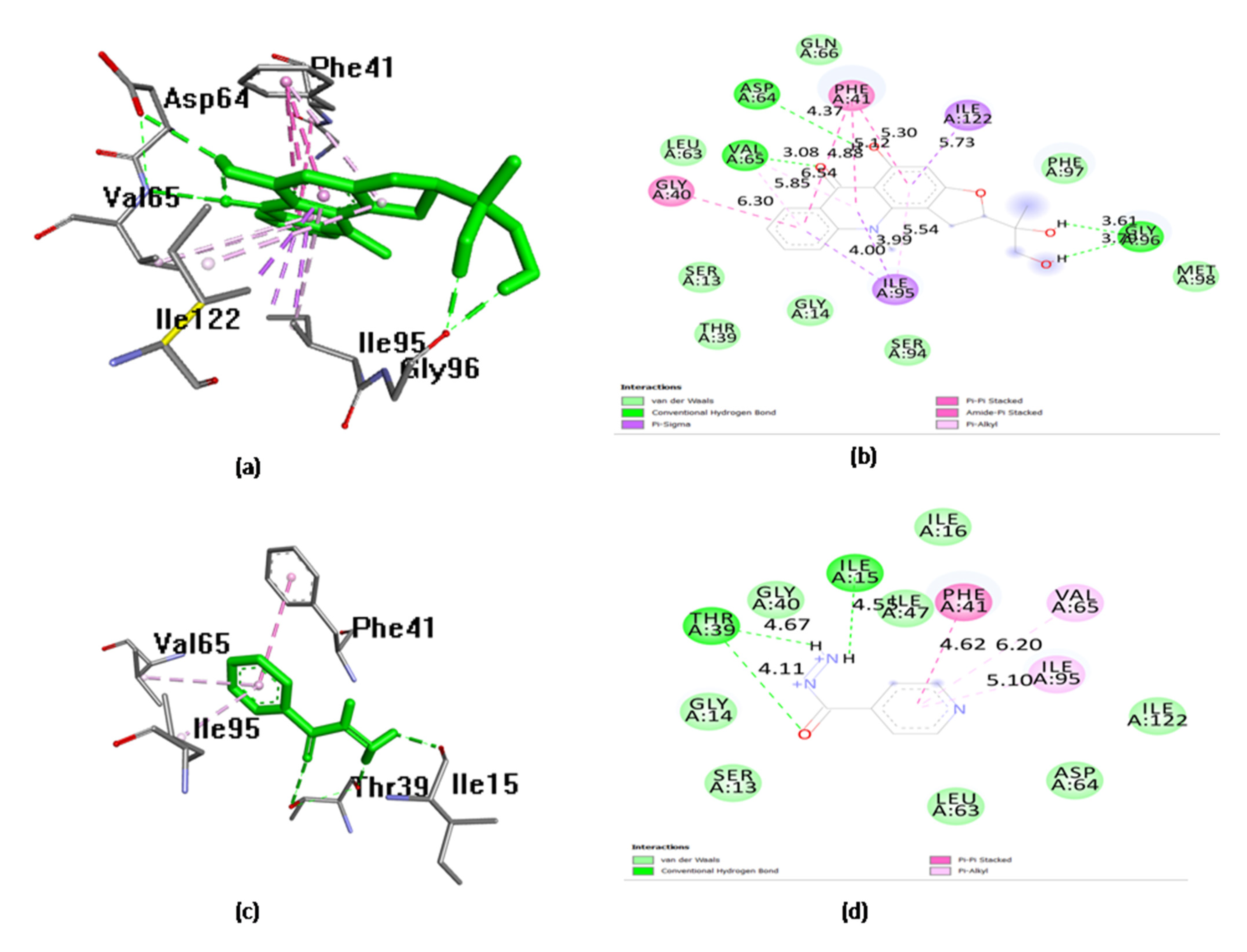
| 7. | Ruta graveolens | Gravacridonediol | −10.49 | 600.24 nm | Val 65, Gly 96 | 2.92375 1.98401 |
| 8. | Ruta graveolens | Graveolinine | −7.69 | 2.33 µm | Ile 194 | 2.7123 |
| 9. | Ruta graveolens | Gvaiacol | −4.09 | 996.61 µm | Tyr158, Pro 156 | 2.99138 1.9058 |
| 10. | Ruta graveolens | Rutacridone | −7.43 | 3.59 µm | ______ | |
| 11. | Ruta graveolens | Rutaretin | −7.23 | 5.01 µm | Pro156 | 2.33402 |
| 12. | Drugs | Isoniazid | −5.49 | 549.74 µm | Thr39, Ile15, Gly14 | 2.9347 2.09599 2.9347 |
| 13. | Natural inhibitor | Triclosan | −6.69 | 12.43 µm | Gly 14, Thr 39, Ile 15 | 2.89647 1.92469 2.22936 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, K.; Pandey, N.; Ahmad, F.; Upadhyay, T.K.; Islam, M.H.; Alshammari, N.; Saeed, M.; Al-Keridis, L.A.; Sharma, R. Identification of Novel Inhibitor of Enoyl-Acyl Carrier Protein Reductase (InhA) Enzyme in Mycobacterium tuberculosis from Plant-Derived Metabolites: An In Silico Study. Antibiotics 2022, 11, 1038. https://doi.org/10.3390/antibiotics11081038
Singh K, Pandey N, Ahmad F, Upadhyay TK, Islam MH, Alshammari N, Saeed M, Al-Keridis LA, Sharma R. Identification of Novel Inhibitor of Enoyl-Acyl Carrier Protein Reductase (InhA) Enzyme in Mycobacterium tuberculosis from Plant-Derived Metabolites: An In Silico Study. Antibiotics. 2022; 11(8):1038. https://doi.org/10.3390/antibiotics11081038
Chicago/Turabian StyleSingh, Kratika, Niharika Pandey, Firoz Ahmad, Tarun Kumar Upadhyay, Mohammad Hayatul Islam, Nawaf Alshammari, Mohd Saeed, Lamya Ahmed Al-Keridis, and Rolee Sharma. 2022. "Identification of Novel Inhibitor of Enoyl-Acyl Carrier Protein Reductase (InhA) Enzyme in Mycobacterium tuberculosis from Plant-Derived Metabolites: An In Silico Study" Antibiotics 11, no. 8: 1038. https://doi.org/10.3390/antibiotics11081038
APA StyleSingh, K., Pandey, N., Ahmad, F., Upadhyay, T. K., Islam, M. H., Alshammari, N., Saeed, M., Al-Keridis, L. A., & Sharma, R. (2022). Identification of Novel Inhibitor of Enoyl-Acyl Carrier Protein Reductase (InhA) Enzyme in Mycobacterium tuberculosis from Plant-Derived Metabolites: An In Silico Study. Antibiotics, 11(8), 1038. https://doi.org/10.3390/antibiotics11081038








