Abstract
New inhibitors of the bacterial transferase MraY from Aquifex aeolicus (MraYAA), based on the aminoribosyl uridine central core of known natural MraY inhibitors, have been designed to generate interaction of their oxadiazole linker with the key amino acids (H324 or H325) of the enzyme active site, as observed for the highly potent inhibitors carbacaprazamycin, muraymycin D2 and tunicamycin. A panel of ten compounds was synthetized notably thanks to a robust microwave-activated one-step sequence for the synthesis of the oxadiazole ring that involved the O-acylation of an amidoxime and subsequent cyclization. The synthetized compounds, with various hydrophobic substituents on the oxadiazole ring, were tested against the MraYAA transferase activity. Although with poor antibacterial activity, nine out of the ten compounds revealed the inhibition of the MraYAA activity in the range of 0.8 µM to 27.5 µM.
1. Introduction
Infectious diseases are one of the most important causes of human death, and bacterial antimicrobial resistance (AMR) is a major threat for human health [1,2,3]. Based on predictive statistical models, 4.95 million deaths are estimated to be associated with bacterial AMR in 2019, including 1.27 million deaths attributable to bacterial AMR [4]. In addition to its impact on human health [5], antibiotic resistance also has a significant economic cost, notably on the increase in hospital costs due to nosocomial infections [6,7,8,9]. One way to fight bacterial AMR is to focus on biological targets displaying a new mode of action compared to those of the approved antibiotics. Considering their high specificity and their unique occurrence in bacteria, enzymes involved in peptidoglycan biosynthesis [10,11] are promising targets, since each of them is essential for the bacterial growth. In this context, our goal is to focus on the inhibition of the MraY transferase [12], which is an unexploited target. Indeed, even if the discovery of potent MraY inhibitors is the subject of intense research efforts, none of the resulting compounds are being investigated in clinical trials. This represents a major advantage that can delay the emergence of drug resistance. This integral membrane protein, essential to bacterial growth and ubiquitous in the bacterial world [13,14,15], catalyzes the first membrane-associated step of peptidoglycan biosynthesis: the transfer of UDP-MurNAc-pentapeptide on undecaprenylphosphate (C55P) to form the lipid I.
Several families of natural MraY inhibitors are known, notably the widely represented one of peptidonucleosidic antibiotics, such as liposidomycins [16,17,18], muraymycins [19] and caprazamycins [20,21]. All of these compounds share a common aminoribosyl uridine scaffold that has been shown to be important for their biological activity [22,23,24]. The synthesis of the simplified analogs of these complex natural compounds [25,26,27,28] retaining antibacterial activity is a challenge, and significant progress towards this goal has been made [29,30,31,32,33,34,35,36]. The crystallographic structure of MraY from Aquifex aeolicus (MraYAA) in complex with several ligands has been solved (muraymycin D2 (MurD2, PDB: 5CKR) [37] and carbacaprazamycin (PDB: 6OYH) [38]), permitting the S. Y. Lee group to define several hot spots (HS) of the priviledged interaction of the co-crystallized inhibitors with the MraY active site [38]. We also developed the synthesis of several series of inhibitors based on this aminoribosyl uridine core, the chemical diversity being introduced on the scaffold through various linkers, either cyclic such as C- and N-triazoles [39,40], or acyclic, such as urea [41], amide, sulfonamide, squaramide or diamide [42]. The docking of the triazole-containing inhibitors, in either the 5CKR [30] or 6OYH [31] structural models of MraYAA, revealed no significant interactions of the triazole with he aminoacids of the active site (Figure 1, 1A). In the present work, considering this lack of stabilizing interaction, we decided to enlarge the panel of inhibitors with a cyclic linker. We focused on oxadiazole-containing ones because the docking of a representative of the targeted oxadiazole series, with a C10 chain as a hydrophobic substituent, showed the interaction of the oxadiazole ring with the key amino acids (H325) of the enzyme active site (Figure 1, 1B), as observed for the highly potent inhibitors carbacaprazamycin, muraymycin D2 and tunicamycin. We hypothetized that this interaction could contribute to generate more active compounds. Furthermore, a panel of hydrophobic substituents with various chain length was selected to study their influence in promoting antibacterial activity.
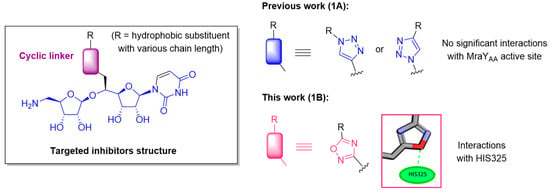
Figure 1.
1A. Structure of the aminoribosyl-uridine-containing MraY inhibitors previously synthetized. 1B. Structure of targeted new MraY inhibitors.
2. Results and Discussion
2.1. Chemistry
The retrosynthetic analysis towards the targeted compounds is outlined in Figure 2. It would rely on the tandem O-acylation-cyclization of the amidoxime A with various acyl chlorides B, either commercially available or synthetized. The amidoxime A could be obtained by a reaction of hydroxylamine with the nitrile C resulting from a diastereselective glycosylation between the nitrile alcohol E and a ribosyl donor D, activated as a fluoride in its anomeric position and bearing an azido group as a masked amine at C-5. Alcohol E should result from the nucleophilic opening of epoxide F resulting from uridine.
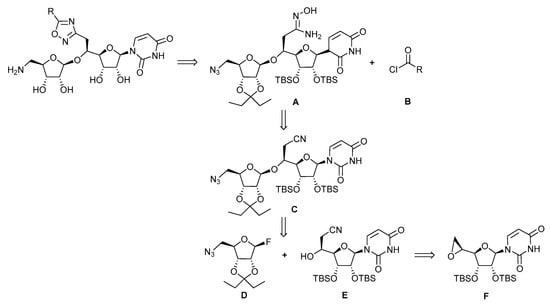
Figure 2.
Retrosynthesis of the targeted oxadiazole inhibitors.
The conveniently protected 5-azidoribosyl fluoride D was readily synthetized from D-ribose [43]. The synthesis of the cyano alcohol E [44] by nucleophilic opening by the potassium cyanide of the epoxide F [44] derived from uridine (Scheme 1) has been optimized (Table 1) by screening the number of equivalents of potassium cyanide, the solvent of the reaction, its duration and the eventual addition of ammonium chloride [45].
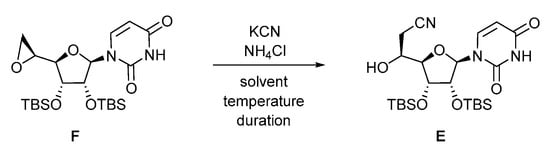
Scheme 1.
Nucleophilic opening of the epoxide F by cyanide ions.

Table 1.
Optimization of the nucleophilic opening of the epoxide F by potassium cyanide.
The previously described conditions [44,45] involving 8 equiv. of KCN and 1.5 equiv. of NH4Cl in DMF at 100 °C for 16 h (entry 1) led to variable percentages of the conversion and isolated yield (up to 68%) of compound E accompanied by the partial deprotection of the silyl ethers. Decreasing the amount of KCN to 6 equiv. and the temperature to 55 °C afforded a moderate 28% of the conversion. Replacing the solvent by refluxing CH3CN with KCN (6 equiv.) in the presence of NH4Cl (1.5 equiv., entry 3) or in its absence (entry 4) only led to poor conversion and degradation. The reaction was also assayed in toluene at 100 °C with KCN (6 equiv.) and NH4Cl (1.5 equiv., entry 5) or without NH4Cl (entry 6) for 16 h. However, only traces of the expected compound E were formed, and the reaction mixture turned out to be brownish, characteristic of silyl ethers deprotection. Finally, the reaction was attempted in a MeOH/H2O 8/1 mixture at 60 °C for 2 h in the presence of KCN (6 equiv.) and NH4Cl (1.5 equiv., entry 7), leading to an improved 84% isolated yield of compound E. Decreasing the amount of KCN (3 equiv.) and increasing the duration of the reaction to 16 h (entry 8) was detrimental to the progress of the reaction, leading to 62% of conversion. The best optimized conditions (entry 9) were revealed to be the use of KCN (5 equiv.) and NH4Cl (2.5 equiv.) in MeOH/H2O 8/1 at 60 °C for 16 h, giving cyanoalcohol E in a reproducible 94% isolated yield.
We next turned to the diastereoselective glycosylation reaction of the phthalimidocyano alcohol E by the fluororibose derivative D as a ribosyl donor (Scheme 2). This reaction was performed according to the conditions we previously described [40]. However, the number of equivalents of the reaction partners has been optimized. The best conditions involved one equivalent of the phthalimido alcohol E and three equivalents of fluorinated azido-ribose D that were dried by the azeotropic removal of water with toluene. Then, an excess of 4 Å molecular sieves was added after activation by vacuum drying. The products were dissolved in freshly distilled DCM before the addition of boron trifluoride etherate (4.0 equiv.) at −78 °C. After ten minutes at −78 °C, followed by sixteen hours at room temperature, the crude product 1 was isolated as a 8/2 mixture of β/α diastereoisomers. After separation by flash chromatography, the pure β isomer of the glycosylated compound 1 was obtained in 67% yield. This derivative 1 was then engaged in the formation of the amidoxime 2 by reaction with 50% aqueous hydroxylamine in refluxing methanol, leading to the expected amidoxime 2 in 87% yield.
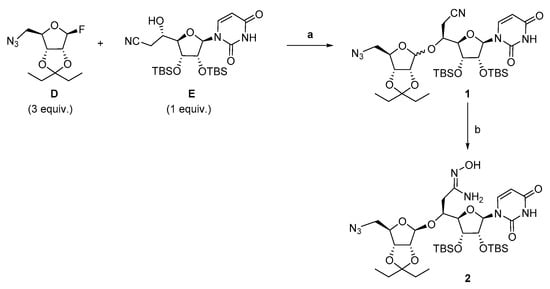
Scheme 2.
Synthesis of the amidoxime 2. Reagents and conditions: (a) 4Å MS, BF3.OEt2 (4.0 equiv.), −78 °C, 10 min then rt, 16 h, 67% of pure β isomer; (b) 50% aqueous NH2OH, MeOH reflux, 6 h, 87%.
Starting from the amidoxime 2 intermediate, the synthesis of a series of MraY inhibitors with an oxadiazole linker was then undertaken by the O-acylation of the amidoxime 2 with a panel of acyl chlorides 3 (Scheme 3), either commercially available, with various alkyl chain lengths, an eventual unsaturation or aromatic moieties (3a–3e), or synthetized according to classical routes (3f–3i).
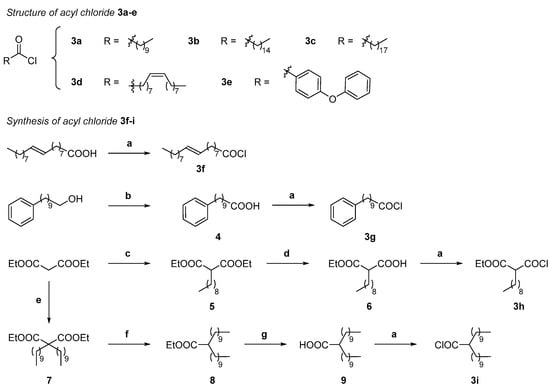
Scheme 3.
Structure and/or synthesis of compounds 3a–i. Reagents and conditions: (a) Oxalyl chloride, dry DMF cat., pentane, r.t., 2 h; (b) CrO3, H2SO4, H2O, acetone, 0 °C to r.t., 3 h, 80%; (c) CH3(CH2)8I (1 equiv.), K2CO3 (5 equiv.), DMF/THF 1/1, overnight, r.t. to 90 °C to r.t., 91%; (d) (1) KOH (1 equiv.), EtOH/H2O 10/1, (2) Aqueous HCl, H2O, 72%; (e) NaH (3 equiv.), 0 °C 30 min, then CH3(CH2)9I (2.5 equiv.), THF, 0 °C to r.t., 48 h, 76%; (f) LiCl (2.5 equiv.), H2O (1.15 equiv.), DMSO, 12 h, r.t., 60%; (g) NaOH, EtOH/H2O 10/1, overnight (90%).
The choice of the acyl chloride substituents was directed towards long hydrophobic chains (C10–C18, 3a–3c, 3f) in aiming at filling the hydrophobic groove HS4 of the MraY active site [38] and at obtaining antibacterial activities, since we previously showed that inhibitors with small chains are probably not sufficient to lead to good antibacterial activities [41]. A diphenyl ether (3e) was chosen in agreement with the reported inhibitory activity of MraY [46], and a C9-alkyl chain bearing a terminal phenyl group was also selected (3g). To potentially fill two hot spots of the active site (HS2 and HS4), disubstituted compounds bearing a C9 alkyl chain and an ethyl ester (3h) and two C10 alkyl chains (3i) were also targeted. The synthesis of the acyl chlorides 3f–3i (Scheme 3) was realized by the reaction of the corresponding commercial or synthetized carboxylic acids with oxalyl chloride in the presence of catalytic DMF in pentane [47]. Accordingly, the acyl chloride 3f bearing a long C18 alkyl chain with an E unsaturation was performed from the corresponding commercial acid. The 10-phenyl decanoyl chloride 3g was obtained by the Jones oxidation of the corresponding alcohol into the carboxylic acid 4 that was isolated in 80% yield. The synthesis of the derivative 3h was carried out by reacting the anion of diethyl malonate generated in the presence of an excess of potassium carbonate in a 1/1 DMF/THF mixture with 1-iododecane, leading to the diester, followed by the selective saponification of the one ester group [48] by potassium hydroxide in a 1/10 H2O/EtOH mixture, giving the mono acid mono ester 6 in 72% yield. The dialkylated acid 9 resulted from a three-step sequence involving first the dialkylation by 1-iododecane of diethyl malonate in the presence of sodium hydride in THF [49] that was achieved in 78% yield and led to the dialkylated derivative 7. Then, the decarboethoxylation of this compound 7 in the presence of lithium chloride and H2O in DMSO [50] afforded the monoethyl ester 8, which was followed by the saponification of the ester in the presence of sodium hydroxide in a 10/1 EtOH/H2O mixture, giving the dialkylated acid 9. After treatment of the acids 4, 6 and 9 with oxalyl chloride as previously mentioned, the resulting crude acyl chlorides 3g–3i were sufficiently pure to be engaged in the subsequent O-acylation step of amidoxime 2 without further purification.
With the amidoxime 2 and acyl chlorides 3a–3i in hand, we focused on the synthesis of the oxadiazole-containg inhibitors (Scheme 4). The reaction was first attempted in a two-step sequence involving first the O-acylation of the amidoxime 2 with palmitoyl chloride 3b in the presence of DBU in refluxing CH2Cl2 leading to compound 10b in 45% yield, followed by cyclization in toluene under microwave irradiation at 300 W for 3 h at 135 °C. After purification, the oxadiazole 11b was isolated in 82% yield.
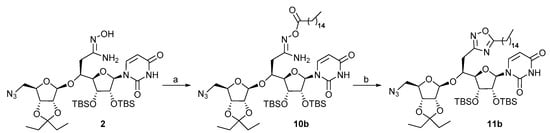
Scheme 4.
Synthesis of the oxadiazole 11b. Reagents and conditions: (a) DBU (2 equiv.), 3b (2 equiv.), CH2Cl2, 0 °C, 15 min, then reflux overnight, 45%; (b) toluene, µW 300 W, 135 °C, 3 h, 82%.
It has to be mentioned that the cyclization reaction performed in thermic conditions involving 4 days of heating in reluxing toluene only led to traces of the expected compound 11b and degradation, while part of the starting O-acylated amidoxime 10b was still present. In order to improve the yield and shorten the duration of this two-step sequence, the reaction was then carried out according to a one-pot procedure under microwave irradiation (Scheme 5, Table 2) at 150 °C in toluene in the presence of variable amounts of DBU for different durations.
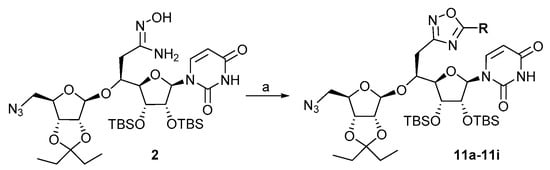
Scheme 5.
Synthesis of the oxadiazoles 11a–11i. Reagents and conditions: (a) 3a–3i (1.1 equiv.), DBU (see Table 2) 30 min, r.t. then, µW 150 °C, toluene.

Table 2.
Conditions for the one-pot synthesis of oxadiazole 11b.
The reaction was first performed in the presence of DBU (2 equiv.) for 15 min leading to the oxadiazole 11b in a modest 28% yield (entry 1). Increasing the amount of DBU (2.5 equiv.) slightly improved the yield of the reaction to 30% (entry 2). Finally, the best conditions involved DBU (2.5 equiv.) for 30 min under microwave irradiation at 150 °C in toluene and led to oxadiazole 11b in 55% yield. Partial degradation was observed with longer reaction time, thus decreasing the yield. These conditions were then applied to the synthesis of the oxadiazoles 11a and 11c–11i that were isolated in yields ranging from 42 to 83% (Table 3).

Table 3.
Isolated yields for the synthesis of compounds 11a–i and 12a–i.
The reduction of the azido group of oxadiazoles 11a–11i was then performed in a 85/15 THF/H2O mixture under Staudinger conditions using polymer-supported triphenylphosphine to efficiently remove the generated supported triphenylphosphine oxide by filtration. The final acidic hydrolysis of the alcohol protective groups of the resulting crude amines was carried out in a cold 4/1 mixture of trifluoroacetic acid/water. The targeted oxadiazoles inhibitors 12a–12i were isolated in 22 to 85% overall yield after flash chromatographic purification on silica gel (Scheme 6, Table 3). The saponification of ester 12h (Scheme 6) was achieved by ammonium hydrogenocarbonate in the presence of triethylamine in methanol leading to the oxadiazole 12j (Scheme 6).
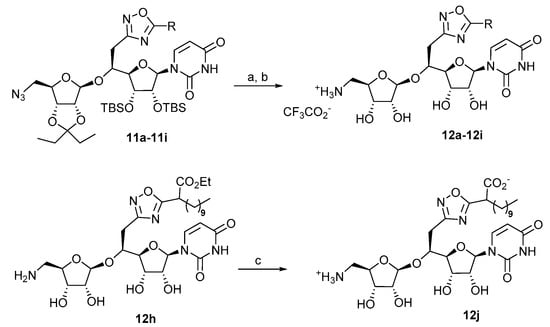
Scheme 6.
Deprotection steps of compounds 11a–11i and 12h. Reagents and conditions: (a) PS-PPh3, THF/H2O 85/15, r.t., 48 h; (b) TFA/H2O 4:1, 0 °C, r.t., 16 h; (c) aqueous NH4HCO3, Et3N, MeOH, 17%.
2.2. Biological Studies
The inhibitory activity of the synthesized compounds 12a–12j was evaluated on MraY transferase purified from Aquifex aeolicus (MraYAA) prepared as previously described by Chung et al. [51]. Their activity (Table 4) was compared to the inhibitory activity of the N-triazole-containing inhibitor 13 (Figure 3) that we previously synthesized [40] (Table 4). Commercially available tunicamycin from Streptomyces sp. was used as a positive control in the test.

Table 4.
Inhibitory activity of compounds 12a–12j against MraYAA.
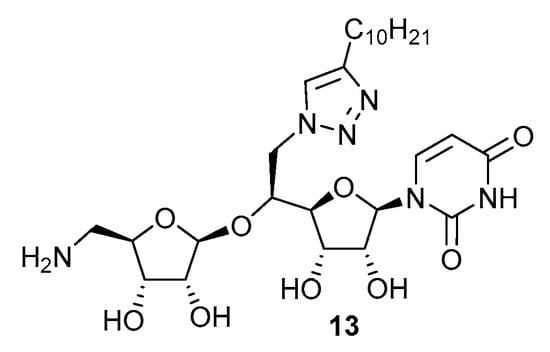
Figure 3.
Structure of the reference N-triazole inhibitor 13.
As shown in Table 4, nine oxadiazole-containing compounds out of the ten tested are relevant inhibitors of the enzymatic activity of the transferase MraYAA with IC50 ranging from 0.8 µM to 27.5 µM, the best MraYAA inhibitory activity being obtained with a saturated C10 alkyl substituent on the oxadiazole moiety (12a). The inhibitory activity of this compound 12a (0.8 µM) is a bit improved compared to that of the reference N-triazole 13 (1.1 µM). Among the compounds with one alkyl chain (12a,12b, 12c), the results show that increasing the alkyl chain length from a C10 chain to C15 and C18 ones is detrimental to inhibitory activity, with IC50 decreasing by a factor of 12 and 20, respectively. The introduction of an unsaturation within the alkyl chain (compounds 12d and 12f) leads to an inhibitory activity in the same range as that of a saturated compound (12c). The configuration of the double bond did not display a significant difference among the obtained IC50 with IC50 equal to 18.9 µM for the Z-unsaturated compound (12d), as compared to 17.3 µM for the E-unsaturated one (12f) and 15.8 µM for the saturated one (12c). The potential filling of the MraYAA hot spots (HS) by aromatic substituents was rather satisfying, with IC50 in the low µM range (3.2 µM and 5.8 µM) for compounds 12e and 12g, respectively. The results concerning the tentative filling of two MraYAA HS by two substituents are contrasted. Indeed, the compound 12i with two C10 alkyl chain revealed no activity, showing that it is probably too hindered for correct positioning within the MraYAA active site, while the oxadiazole, with both a C9 alkyl chain and an ethyl ester, was moderately active with an IC50 equal to 27.5 µM. More interestingly, the corresponding carboxylate 12j is almost tenfold more active (IC50 equal to 2.9 µM) than the parent ester, probably due to its better positioning in the MraYAA active site.
The antibacterial activity of MraY inhibitors 12a–12h was also evaluated against several bacterial strains. Gram-negative (E. coli ATCC 8730, C. freundii ATCC8090 and P. aeruginosa ATCC 27853) and Gram-positive pathogenic bacterial strains (S. aureus ATCC 25923 and E. faecium ATCC 19434), including a methicillin-resistant strain (S. aureus MRSA ATCC 43300), were selected as a representative of pathogen bacterial diversity. Piperacillin and vancomycin were used as positive control in the tests. The results (See Table S1, Supplementary Material) show that, although our previous data on differently substituted urea-containing inhibitors [41], suggested that a linear chain of at least 12 carbon atoms, or a branched substituent, is required to get antibacterial activity, almost none of the oxadiazole inhibitors displayed antibacterial activity, except a modest 50 µg/mL activity for two long-chain-containing compounds, 12c (C18) and 12f (C17 with a E unsaturation), on the three Gram-positive bacterial strains. This disappointing result demonstrates that the further optimization of the lipophilic side chain is still required to increase the antibacterial activity.
2.3. Docking Studies
Molecular modeling studies were performed to rationalize the SAR results for this novel series of MraYAA inhibitors. CDOCKER [52] was used to dock the compounds into the X-ray structures of the MraYAA enzyme in open and close conformations (PDB ID: 5CKR and PDB ID: 6OYH, respectively), following the procedure described previously [41,42]. The top 50 best docking poses, based on the CDOCKER interaction energy function, were retained and then further visually examined.
In the closed conformation of MraYAA (PDB ID: 6OYH), compounds 12a–12d and 12f–12h exhibit a binding mode similar to that of triazole compound 13 (Figure 4). The uracil moiety forms an H-bond with the key residue D196 and π–π stacking interactions with F262 within the small uracil binding pocket. The lipophilic tail anchors deeply in the hydrophobic groove HS4 predicted to be the C55P substrate binding pocket [38] establishing hydrophobic contacts with P322 and L191 in HS2 and HS6 pockets, respectively. In addition, an extended network of H-bond and electrostatic interactions was retrieved between the aminoribosyl part and residues N190 and D193 located in the HS1 domain. No stabilizing H-bond interactions were predicted between the oxadiazole moiety and polar residues H324 and H325 within the HS2 area. Nevertheless, docking in the closed conformation of MraYAA failed to discriminate between active 12a and moderately active compounds (12b–d, 12f–h and 12j). Moreover, rigid or branched tail compounds (12e and 12i, respectively) failed to assume this binding mode due to steric hindrance with the residues of the active site.
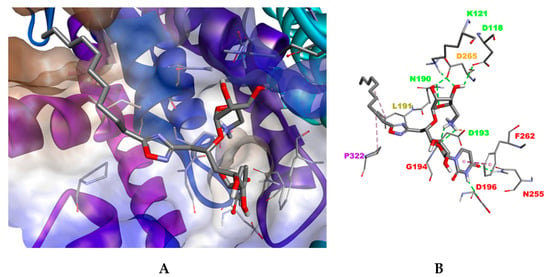
Figure 4.
Docking pose for a compound in the closed conformation of MraYAA (PDB ID: 6OYH). (A). The hydrophobic surface is rendered as brown and the hydrophilic surface as blue. Ligand and residues are shown in stick mode. (B). Interactions between the inhibitor 12a and MraYAA are shown as colored dashed lines: hydrophobic interactions (magenta), electrostatic interactions (orange) and hydrogen bond (green). For clarity, the apolar hydrogen atoms are omitted.
The best docking results were obtained in the open conformation of MraYAA (PDB ID: 5CKR). Although all compounds target the uridine- and uridine-adjacent pockets, as previously seen in closed conformation, significant differences were observed in the interaction network. Two possible binding conformations (I, II) were obtained for the most active compound, 12a (Figure 5A). Both are characterized by two new stabilizing interactions between the uracil moiety and residues K70 and N255 but differ in the spatial orientation of the lipophilic tail, located either in the plane of the membrane (binding mode I, Figure 5B) or exposed in the cytoplasm (binding mode II, Figure 5C). Moreover, a supplementary H-bond with the residue H325 was also predicted for the oxadiazole linker, only for binding mode I. The other compounds bind to the MraYAA active site according to the binding mode II only. For compounds 12e and 12g, the interactions network with the aminoribosyluridine part remained similar to that of compound 12a. Nevertheless, the H-bond interaction of H325 with the oxadiazole moiety was lost, reducing the stability of the compound within the binding site and consequently its activity. Interestingly, the loss of this H-bond was fairly compensated by hydrophobic contacts between the lipophilic tail and residues L191 (HS6) and F309 and between A321 (HS2) and V302 (HS4).
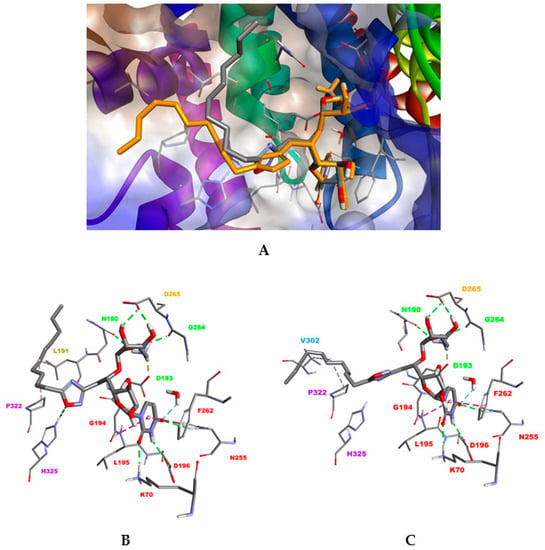
Figure 5.
(A) Superimposition of the two docking conformations I and II, obtained for compound 12a in the open conformation of MraYAA (PDB ID: 5CKR). (B) Binding mode I. (C) Binding mode II. Ligand and residues are shown in stick mode. Interactions between the inhibitor 12a and MraYAA are shown as colored dashed lines: hydrophobic interactions (magenta), electrostatic interactions (orange) and hydrogen bond (green). For clarity, the apolar hydrogen atoms are omitted.
For compound 12b, interaction with H325 seems to be detrimental to the appropriate orientation of the long hydrophobic tail within the HS2 pocket. Less active compounds 12c, 12d and 12f lose at least two significant H-bonds involving N255 in the uridine binding pocket and H325 (HS2). For the branched compound 12i, the HS2 area can accomodate only one branch, leaving the other one exposed towards the solvent surface. Concerning the compounds 2h and 2j with both a C9 alkyl chain and either an ethyl ester or a carboxylate moiety, no clear conclusions could be drawn from docking results. Taken together, these results suggest that tight interactions of compound 12a with residues within the uracil binding pocket, uridine like and HS2 domain in the open conformation of MraYAA could reduce its flexibility providing an explanation for its better activity.
3. Materials and Methods
3.1. Chemical Synthesis
The reactions were carried out under an argon atmosphere, if required, and were monitored by thin-layer chromatography. All microwave-mediated reactions were carried out in G10 or G20 Anton Paar vials, using an Anton Paar Monowave 300 Extra microwave synthesizer. Flash chromatography was performed with silica gel 60 (40–63 μm). Spectroscopic 1H and 13C NMR, MS and/or analytical data were obtained using chromatographically homogeneous samples. 1H NMR (500 MHz) and 13C NMR (125 MHz) spectra were recorded in CDCl3 unless otherwise indicated. Chemical shifts (δ) are reported in ppm, and coupling constants are given in Hz. For each compound, detailed peak assignments have been made according to COSY, HSQC and HMBC experiments. The numbering of molecules is indicated in the Supplementary Material. Optical rotations were measured with a sodium (589 nm) lamp at 20 °C. IR spectra were recorded on an FT–IR spectrophotometer, and the wavelengths are reported in cm−1. High-resolution mass spectra (HRMS) were recorded with a TOF mass analyzer under electrospray ionization (ESI) in positive ionization mode detection [42].
3.1.1. Protected Nitrile 1
To a solution of E (100 mg 0.19 mmol, 1 equiv.) and D (192 mg, 78 mmol, 4 equiv.) in freshly distillated DCM (7 mL) was added 4 Å molecular sieves (1.4 g). After stirring at r.t. for 1 h, boron trifluoride diethyl etherate (96 µL, 0.78 mmol, 4 equiv.) was added at –80 °C. After 15 min at –80 °C, the cooling bath was removed, then the mixture was allowed to warm to r.t. for 16 h. The mixture was filtrated over a pad of celite, then washed with EtOAc. The filtrate was treated with a saturated aqueous solution of sodium bicarbonate. The organic layer was washed with water and brine, then concentrated in vacuo. Flash chromatographic purification (cyclohexane/EtOAc 75:25) afforded the product as a white solid (96 mg, 67%): Rf 0.17 (cyclohexane/EtOAc 7/3); [α]D + 94.1 (c 1.0, CH2Cl2); IR (film) 3675, 2971, 2900, 1696, 1684, 1394, 1229, 1076, 1047, 891, 869; 1H NMR (500 MHz, CDCl3) δ 8.58 (br s, 1H, NH), 7.73 (d, JH6-H5 = 8.2 Hz, 1H, H6), 5.74–5.53 (m, 2H, H5 + H1′), 5.11 (s, 1H, H1″), 4.53 (d, JH2″-H3″ = 6.2 Hz, H2″), 4.43 (d, JH3″-H2″ = 6.2 Hz, 1H, H3″), 4.31 (t, JH4″-H5″b = JH4″-H5″a = 5.4 Hz, 1H, H4″), 4.17–4.03 (m, 2H, H4′ + H2′), 3.98–3.84 (m, 2H, H3′ + H5′), 3.45–3.40 (m, 2H, H5″a, H5″b), 2.93–2.80 (m, 2H, H6′b, H6′a), 1.60 (q, J = 7.4 Hz, 2H, H7″), 1.46 (q, J = 7.4 Hz, 2H, H7″), 0.87 – 0.74 (m, 24H, -C(CH3)3, H8″), 0.16–0.04 (m, 12H, -Si(CH3)2); 13C NMR (125 MHz, CDCl3) δ 162.9 (C4), 149.95 (C2), 140.15 (C6), 118.12 (C6″), 116.63 (C7′), 112.34 (C1″), 101.56 (C5), 90.20 (C1′), 86.00 (C3″), 85.49 (C4″), 84.21 (C4′), 81.56 (C2″), 75.23 (C5′), 75.05 (C2′), 71.05 (C3′), 53.45 (C5″), 29.18 (C7″), 28.85 (C7″), 25.80 (-C(CH3)3), 21.72 (C6′), 18.0, 17.9 (-C(CH3)3), 8.36 (C8″), −4.0, −4.3, −4.9, −4.9 (-Si(CH3)2); HRMS ESI+ Calcd. for C33H57N6O9Si2+ (M + H)+ 737.3720, found 737.3731.
3.1.2. Protected Amidoxime 2
The mixture of nitrile 1 (363 mg, 0.49 mmol, 1 equiv.) and 50% aqueous hydroxylamine (0.23 mL, 3.94 mmol, 8 equiv) in MeOH (8 mL) was heated at reflux for 6 h, then concentrated in vacuo. The oily residue was diluted with EtOAc (30 mL), then washed with water and brine (2 × 10mL). The organic layer was dried (MgSO4), then concentrated in vacuo. Flash chromatographic purification (cyclohexane/EtOAc 75:25) afforded product 2 as a white solid (329 mg, 87%): Rf 0.25 (cyclohexane/EtOAc = 1/1); [α]D +13 (c 1.0, CH2Cl2); IR (film): 1260, 1165, 1091, 911, 834, 735. 1H NMR (500 MHz, CDCl3) δ 7.75 (d, J H6-H5 = 8.1 Hz, 1H, H6), 5.88 (d, JH1′-H2′ = 5.5 Hz, 1H, H1′), 5.73 (d, JH5-H6 = 8.2 Hz, 1H, H5), 5.31 (s, 1H, H1″), 4.62 (dd, JH3″-H2″ = 6.3 Hz, JH3″-H4″ = 1.7 Hz, 1H, H3″), 4.53 (d, JH2″-H3″ = 6.3 Hz, 1H, H2″), 4.34 (td, JH4″-H5″ = 5.6 Hz, JH4″-H3″ = 1.7 Hz, 1H, H4″), 4.25 (t, JH5′-H4′ = 3.0 Hz, 1H, H5′), 4.16-4.09 (m, 2H, H2′, H4′), 4.05 (t, JH3′-H2′ = 3.8 Hz, 1H, H3′), 3.49 (dd, JH6a′-H5′ = 12.8 Hz, JH6a′-H6b′ = 5.6 Hz, 1H, H6a′), 3.45 (dd, JH6b′-H5′ = 12.8 Hz, JH6b′-H6a′ = 5.6 Hz, 1H, H6b′), 2.80 (dd, JH5a′-H6′ = 14.3 Hz, JH5a′-H5b′ = 5.5 Hz, 1H, H5a′), 2.52 (dd, JH5b′-H6′ = 14.3 Hz, JH5b′-H5a′ = 8.8 Hz, 1H, H5b′), 1.69 (q, JH7a″-H8″ = 7.4 Hz, 2H, H7a″), 1.55 (q, JH7b″-H8″ = 7.5 Hz, 2H, H7b″), 0.91-0.83 ( m, 24 H, H8”, SiCH3), 0.10-0.02 (m, 12 H, Si(CH3)2). 13C NMR (125 MHz, CDCl3) δ 171.8 (C7′), 162.8 (C4), 150.1 (C2), 140.5 (C6), 111.8 (C1″), 102.1 (C5), 89.0 (C1′), 86.2 (C2″), 86.1 (C4″), 84.8 (C4′), 81.5 (C5′), 75.3 (C2′), 72.3 (C3′), 53.6 (C5″), 38.7 (C6′ab), 29.4 (C7″), 29.0 (C7″), 25.93, 25.86 (-C(CH3)3), 18.1 (-C(CH3)3), 8.5, 7.7 (C8″), −4.1, −4.6, −4.8, (-SiC); HRMS ESI+ calcd. for C33H60N7O10Si2+ (M + H)+ 770.3935 found 770.3914.
3.1.3. General Procedure for the Synthesis of Protected Oxadiazoles
In a 10 mL microwave reaction vial, to a solution of amidoxime 2 (1 equiv.) in toluene (3 mL) was added acyl chloride 3a–3i (1.1 equiv.) and DBU (2.5 equiv.). The reaction was stirred at r.t. for 30 min. Then the reaction mixture was irradiated under microwave irradiation at 150 °C for 30 min to 1 h. The reaction mixture was then concentrated in vacuo. Flash chromatographic purifications (cyclohexane/EtOAc 9:1) afforded the products 11a–11i in 42 to 83% yield.
3.1.4. Protected Oxadiazole 11a
Compound 11a was obtained as a white solid (40 mg, 67% yield): Rf 0.25 (cyclohexane/EtOAc = 7/3); [α]D +14 (c 1.0, CH2Cl2); IR (film): 3006, 2873, 1558, 1427, 1368, 1275, 1146, 1016, 988, 935, 887, 820, 764, 750; 1H NMR (500 MHz, CDCl3) δ 7.89 (d, JH6-H5 = 8.2 Hz, 1H, H6), 5.85 (d, JH1′-H2′ = 4.7 Hz, 1H, H1′), 5.73 (dd, JH5-H6 = 8.2 Hz, 1H, H5), 5.24 (s, 1H, H1″), 4.64 (dd, JH3″-H2″ = 6.2 Hz, JH3″-H4″ = 1.5 Hz, 1H, H3″), 4.52 (d, JH2″-H3″ = 6.2 Hz, 1H, H2″), 4.32 (t, JH5′-H4′ = 5.8 Hz, 1H, H5′), 4.24–4.21 (m, 1H, H4″), 4.16 (t, JH2′-H3′ = 4.7 Hz, 1H, H2′), 4.05 (dd, JH4′-H3′ = 4.4 Hz, JH4′-H5′ = 1.6 Hz, 1H, H4′), 3.99 (t, JH3′-H4′ = 4.4 Hz, 1H, H3′), 3.50 (dd, JH6′a-H5′ = 12.7 Hz, JH6′a-H6′b = 5.2 Hz, 1H, H6′a), 3.43 (dd, JH6′b-H5′ = 12.7 Hz, JH6′b-H5′ = 5.2 Hz, 1H, H6′b), 3.38 (dd, JH5″a-H4″ = 15.0 Hz, JH5″a-H5″b = 9.3 Hz, 1H, H5″a), 3.19 (dd, JH5″b-H4″ = 15.0 Hz, JH5″b-H5″a = 9.3 Hz, 1H, H5″b), 2.83 (t, JH2′-H3′ = 7.6 Hz, 1H, H1*), 1.80–1.74 (m, 2H, H2*), 1.69 (q, JH7″-H8″ = 7.5 Hz, 2 H, H7″), 1.56 (q, JH7″-H8″ = 7.5 Hz, 2 H, H7″), 1.30–1.24 (m, 24H, H3*-H14*), 0.92–0.82 (m, 27H, -SiC(CH3)3, H8″, H15*), 0.08–0.05 (12H, Si(CH3)2); 13C NMR (125 MHz, CDCl3) δ 180.2 (C8′), 166.9 (C7′), 163.0 (C4), 150.1 (C2), 140.2 (C6), 118.00 (C6″), 112.2 (C1″), 101.8 (C5), 88.7 (C1′), 86.1 (C2″), 85.0 (C4″), 84.6 (C4′), 81.7 (C3″), 78.2 (C5′), 75.5 (C2′), 72.0 (C3′), 53.4 (C5″), 31.9, 29.6, 29.4, 29.3, 29.2, 29.1, 28.9, 25.8 (C2*-C9*), 26.6, 26.5 (C6′, C1*), 25.76 (SiCCH3), 22.69 (s), 18.0 (SiCCH3), 14.1 (C10*), 8.4, 7.5 (C8″), –4.2, –4.5, –4.8 (SiCH3); HRMS ESI+ calcd. for C44H78N7O10Si2+ (M + H)+ 920.5343 found 920.5326.
3.1.5. Protected Oxadiazole 11b
Compound 11b was obtained as a white soli (53 mg, 55% yield): Rf 0.25 (cyclohexane/EtOAc = 7/3); [α]D +10 (c 1.0, CH2Cl2); IR (film): 3006, 2873, 1558, 1427, 1368, 1275, 1146, 1016, 988, 935, 887, 820, 764, 750; 1H NMR (500 MHz, CDCl3) δ 8.61 (s, 1H, NH), 7.88 (d, JH5-H6 = 8.1Hz, 1H, H6), 5.84 (d, JH1′-H2′ = 4.3Hz, 1H, H1′), 5.72 (d, JH5-H6 = 8.1Hz, 1H, H5), 5.24 (s, 1H, H1″), 4.63 (d, JH2″-H3″ = 6.1Hz, 1H, H3″), 4.52 (d, JH2″-H3″ = 6.0 Hz, 1H, H2″), 4.33–4.29 (m, 1H, H4″), 4.26–4.19 (m, 1H, H5′), 4.18–4.14 (m, 1H, H2′), 4.05 (d, JH3′-H4′ = 3.0 Hz, 1H, H4′), 4.00 (d, JH3′-H4′ = 3.0 Hz, 1H, H3′), 3.50 (dd, JH5″a-H5″b = 12.9 Hz, JH4″-H5″a = 5.1Hz, 1H, H5″a), 3.42 (dd, JH5″a-H5″b = 12.9 Hz, JH4″-H5″b = 5.8 Hz, 1H, H5″b), 3.37 (dd, JH6′a-H6′b = 14.9 Hz, JH6′a-H5′ = 5.0 Hz, 1H, H6′a), 3.19 (dd, JH6′a-H6′b = 14.9 Hz, JH6′b-H5′ = 9.2 Hz, 1H, H6′b), 2.82 (t, JH1*-H2* = 7.6 Hz, 2H, H1*), 1.84–1.62 (m, 4H, H2*, H7″), 1.56 (q, JH7″-H8″ = 7.2 Hz, 2H, H7″), 1.40–1.17 (m, 16H, H2*-H9*), 0.97–0.75 (m, 21H, H10*, H8″, SiC(CH3)3), 0.07, 0.07, 0.06 (s, 12H, SiCH3); 13C NMR (125 MHz, CDCl3) δ 180.3 (C8′), 166.9 (C7′), 162.9 (C4), 150.0 (C2), 140.3 (C6), 118.1 (C6″), 112.3 (C1″), 101.8 (C5), 88.8 (C1′), 86.1 (C2″), 85.0 (C4″), 84.7 (C4′), 81.7 (C3”), 78.3 (C5′), 75.5 (C2′), 72.1 (C3′), 53.4 (C6′ab), 32.0 (C4*), 29.8, 29.7, 29.5(C5”b), 29.3, 29.3, 29.2 (C5”a), 29.1 (C7”), 28.9 (C7”), 26.7 (C1*), 26.6 (C2*), 25.9 (SiC(CH3)3), 25.8 (SiC(CH3)3), 22.8 (C3*), 18.0 (C14*), 14.2 (C15*), 8.5 (C8″), 7.6 (C8″), −4.0 (SiCH3), −4.4 (SiCH3), −4.6 (SiC), −4.7 (SiC). HRMS ESI+ calcd. for C49H88N7O10Si2+ (M + H)+ 990.6125 found 990.6105.
3.1.6. Protected Oxadiazole 11c
Compound 11c was obtained as a colorless oil (50 mg, 75% yield): Rf 0.25 (cyclohexane/EtOAc = 7/3); [α]D +16 (c 1.0, CH2Cl2); IR (film): 2926, 2855, 2105, 1695, 1462, 1378, 1275, 1167, 1100, 925, 867, 839, 764, 750; 1H NMR (500 MHz, CDCl3) δ 7.89 (d, JH6-H5 = 8.0 Hz, 1H, H6), 5.85 (d, JH1′-H2′ = 4.4 Hz, 1H, H1′), 5.73 (dd, JH5-H6 = 8.0 Hz, JH5-H1′ = 2.2 Hz, 1H, H5), 5.24 (s, 1H, H1″), 4.64 (dd, JH3″-H2″ = 6.6 Hz, JH3″-H4″ = 1.4 Hz, 1H, H3″), 4.51 (d, JH2″-H3″ = 6.5 Hz, 1H, H2″), 4.32 (t, JH5′-H4′ = 5.7 Hz, 1H, H5′), 4.24–4.21 (m, 1H, H4″), 4.16 (t, JH2′-H3′ = 4.4 Hz, 1H, H2′), 4.05 (dd, JH4′-H3′ = 4.2 Hz, JH4′-H5′ = 1.5 Hz, 1H, H4′), 3.98 (t, JH3′-H4′ = 4.2 Hz, 1H, H3′), 3.50 (dd, JH6′a-H5′ = 12.8 Hz, JH6′a-H6′b = 5.8 Hz, 1H, H6′a), 3.43 (dd, JH6′b-H5′ = 12.8 Hz, JH6′b-H5′ = 5.8 Hz, 1H, H6′b), 3.38 (dd, JH5″a-H4″ = 15.0 Hz, JH5″a-H5″b = 9.3 Hz, 1H, H5″a), 3.19 (dd, JH5″b-H4″ = 15.0 Hz, JH5″b-H5″a = 9.3 Hz, 1H, H5″b), 2.83 (t, JH2′-H3′ = 7.6 Hz, 1H, H1*), 1.79–1.73 (m, 2H, H2*), 1.69 (q, JH7″-H8″ = 7.4 Hz, 2H, H7″), 1.56 (q, JH7″-H8″ = 7.4 Hz, 2 H, H7″), 1.38–1.24 ( m, 30H, H3*-H17*), 0.92–0.84 (m, 27H, SiC(CH3)3, H8″, H18*), 0.07–0.05 (12 H, Si(CH3)2); 13C NMR (125 MHz, CDCl3) δ 180.1 (C8′), 166.8 (C7′), 162.8 (C4), 149.9 (C2), 140.1 (C6), 117.9 (C6″), 112.1 (C1″), 101.6 (C5), 88.6 (C1′), 85.9 (C2″), 84.9 (C4″), 84.5 (C4′), 81.6 (C3”), 78.1 (C5′), 75.4 (C2′), 71.9 (C3′), 53.2 (C6′ab), 31.9 (C4*), 29.6, 29.3, 29.3(C5”b), 29.2, 29.1, 29.1 (C5”a), 29.0 (C7”), 28.7 (C7”), 26.6 (C1*), 26.4 (C2*), 25.7 (SiC(CH3)3), 25.6 (SiC(CH3)3), 22.6 (C3*), 17.9 (C17*), 14.1 (C18*), 8.3 (C8″), 7.4 (C8″), −4.2 (SiCH3), −4.5 (SiCH3), −4.8 (SiC), −4.8 (SiC); HRMS ESI+ calcd. for C52H94N7O10Si2+ (M + H)+ 1032.6595 found 1032.6576.
3.1.7. Protected Oxadiazole 11d
Compound 11d was obtained as a colorless oil (47.5 mg, 72% yield): Rf 0.25 (cyclohexane/EtOAc = 7/3); [α]D +33 (c 1.0, CH2Cl2); IR (film): 2928, 2856, 2105, 1695, 1462, 1378, 1275, 1167, 1099, 924, 903, 867, 838, 764, 750; 1H NMR (500 MHz, CDCl3) δ 7.89 (d, JH6-H5 = 8.2 Hz, 1H, H6), 5.85 (d, JH1′-H2′ = 4.7 Hz, 1H, H1′), 5.73 (d, JH5-H6 = 8.2 Hz, 1H, H5), 5.35–5.30 (m, 2 H, H8*, H9*), 5.24 (s, 1H, H1″), 4.64 (dd, JH3″-H2″ = 6.2 Hz, JH3″-H4″ = 1.5 Hz, 1H, H3″), 4.52 (d, JH2″-H3″ = 6.5 Hz, 1H, H2″), 4.31 (t, JH5′-H4′ = 5.8 Hz, 1H, H5′), 4.24–4.21 (m, 1H, H4″), 4.15 (t, JH2′-H3′ = 4.5 Hz, 1H, H2′), 4.05 (dd, JH4′-H3′ = 4.2 Hz, JH4′-H5′ = 1.6 Hz, 1H, H4′), 3.98 (t, JH3′-H4′ = 4.3 Hz, 1H, H3′), 3.50 (dd, JH6′a-H5′ = 13.0 Hz, JH6′a-H6′b = 5.2 Hz, 1H, H6′a), 3.43 (dd, JH6′b-H5′ = 13.0 Hz, JH6′b-H5′ = 5.8 Hz, 1H, H6′b), 3.38 (dd, JH5″a-H4″ = 15.0 Hz, JH5″a-H5″b = 9.2 Hz, 1H, H5″a), 3.19 (dd, JH5″b-H4″ = 15.0 Hz, JH5″b-H5″a = 9.2 Hz, 1H, H5″b), 2.84 (t, JH2′-H3′ = 7.8 Hz, 1H, H1*), 2.02–1.98 (m, 4 H, H7*, H10*), 1.80–1.74 (m, 2 H, H2*), 1.69 (q, JH7″-H8″ = 7.4 Hz, 2 H, H7″), 1.56 (q, JH7″-H8″ = 7.4 Hz, 2 H, H7″), 1.42 –1.24 (m, 20 H, H3*-H6*, H9*-H16*), 0.92–0.84 ( m, 27H, -SiC(CH3)3, H8″, H17*), 0.07–0.05 (12 H, Si(CH3)2); 13C NMR (125 MHz, CDCl3) δ 180.2 (C8′), 166.9 (C7′), 163.0 (C4), 150.0 (C2), 140.2 (C6), 130.2 (C8*), 129.7 (C9*), 118.0 (C6″), 112.3 (C1″), 101.8 (C5), 88.8 (C1′), 86.1 (C2″), 85.0 (C4″), 84.7 (C4′), 81.7 (C3”), 78.3 (C5′), 75.5 (C2′), 72.0 (C3′), 53.4 (C6′ab), 32.0 (C4*), 29.9, 29.8(C5”b), 29.6, 29.4, 29.3 (C5”a), 29.1 (C7”), 28.9 (C7”), 27.3 (C7*), 27.2 (C10*), 26.7 (C1*), 26.6 (C2*), 25.9 (SiC(CH3)3), 25.8 (SiC(CH3)3), 22.8 (C3*), 18.1 (C16* ), 14.2 (C17*), 8.5 (C8″), 7.6 (C8″), −4.0 (SiCH3), −4.4 (SiCH3), −4.6 (SiC), −4.7 (SiC); HRMS ESI+ calcd. for C51H90N7O10Si2+ (M + H)+ 1016.6282 found 1016.6258.
3.1.8. Protected Oxadiazole 11e
Compound 11e was obtained as a colorless oil (23 mg, 42% yield): Rf 0.25 (cyclohexane/EtOAc = 7/3); [α]D +33 (c 1.0, CH2Cl2); IR (film): 2930, 2106, 1697, 1487, 1368, 1275, 1275, 1168, 1100, 924, 870, 839, 764, 750; 1H NMR (500 MHz, CDCl3) δ 8.06 (d, JHa-Hb = 8.8 Hz, 2 H, Ha), 7.91 (dd, JH6-H5 = 8.3 Hz, JH6-H1′ = 3.3 Hz, 1H, H6), 7.41 (t, JHd-He = 7.9 Hz, 2 H, Hd), 7.22 (t, JHe-Hd = 7.4 Hz, 1H, He), 7.09 (d, JHd-Hc = 8.8 Hz, 2 H, Hc), 7.07 (d, JHb-Ha = 8.8 Hz, 2 H, Hb), 5.85 (d, JH1′-H2′ = 4.4 Hz, 1H, H1′), 5.74 (dd, JH5-H6 = 8.3 Hz, JH5-H1′ = 2.0 Hz, 1H, H5), 5.29 (s, 1H, H1″), 4.65 (dd, JH3″-H2″ = 6.2 Hz, JH3″-H4″ = 1.5 Hz, 1H, H3″), 4.54 (d, JH2″-H3″ = 6.2 Hz, 1H, H2″), 4.32–4.21 (m, 2 H, H5′, H4″), 4.18–4.14 (m, 2 H, H2′, H4′), 4.01 (t, JH3′-H4′ = 4.2 Hz, 1H, H3′), 3.52 (dd, JH6′a-H5′ = 12.8 Hz, JH6′a-H6′b = 5.5 Hz, 1H, H6′a), 3.47–3.42 (m, 2 H, H6′b, H5″a), 3.27 (dd, JH5″b-H4″ = 15.0 Hz, JH5″b-H5″a = 9.2 Hz, 1H, H5″b), 1.70 (q, JH7″-H8″ = 7.4 Hz, 2 H, H7″), 1.56 (q, JH7″-H8″ = 7.4 Hz, 2 H, H7″), 0.92–0.82 (m, 24H, -SiC(CH3)3, H8″), 0.07–0.05 (12 H, Si(CH3)2); 13C NMR (125 MHz, CDCl3) δ 175.4 (C8′), 167.6 (C7′), 161.9 (C4), 155.4 (C10′), 150.0 (C2), 130.2 (Ca, Cd), 124.9 (Ce), 120.3 (Cc), 118.3 (Cb), 118.1 (C6″), 112.2 (C1″), 101.8 (C5), 88.8 (C1′), 86.1 (C2″), 85.0 (C4″), 84.8 (C4′), 81.7 (C3”), 78.2 (C5′), 75.5 (C2′), 72.1 (C3′), 53.4 (C6′ab), 32.0 (C4*), 29.9(C5”ab), 29.3 (C7”), 28.9 (C7”), 25.8 (SiC(CH3)3), 25.8 (SiC(CH3)3, 18.1, 18.0 (C9′), 8.5 (C8″), 7.6 (C8″), −4.0 (SiCH3), −4.4 (SiCH3), −4.6 (SiC), −4.7 (SiC). HRMS ESI+ calcd. for C46H66N7O11Si2+ (M + H)+ 948.4353 found 948.4329.
3.1.9. Protected Oxadiazole 11f
Compound 11f was obtained as a colorless oil (55 mg, 83% yield): Rf 0.25 (cyclohexane/EtOAc = 7/3); [α]D +15 (c 1.0, CH2Cl2); IR (film): 2928, 2856, 2105, 1696, 1580, 1462, 1378, 1274, 1167, 1099, 967, 910, 867, 838, 764, 748; 1H NMR (500 MHz, CDCl3) δ 7.89 (d, JH6-H5 = 8.2 Hz, 1H, H6), 5.84 (d, JH1′-H2′ = 4.4 Hz, 1H, H1′), 5.73 (dd, JH5-H6 = 8.2 Hz, JH5-H1′ = 2.0 Hz, 1H, H5), 5.42–5.33 (m, 2 H, H8*, H9*), 5.24 (s, 1H, H1″), 4.63 (dd, JH3″-H2″ = 6.2 Hz, JH3″-H4″ = 1.6 Hz, 1H, H3″), 4.51 (d, JH2″-H3″ = 6.0 Hz, 1H, H2″), 4.31 (t, JH5′-H4′ = 5.7 Hz, 1H, H5′), 4.24–4.20 (m, 1H, H4″), 4.15 (t, JH2′-H3′ = 4.5 Hz, 1H, H2′), 4.05 (dd, JH4′-H3′ = 4.1Hz, JH4′-H5′ = 1.7 Hz, 1H, H4′), 3.98 (t, JH3′-H4′ = 4.4 Hz, 1H, H3′), 3.50 (dd, JH6′a-H5′ = 12.6 Hz, JH6′a-H6′b = 5.1Hz, 1H, H6′a), 3.43 (dd, JH6′b-H5′ = 12.6 Hz, JH6′b-H5′ = 6.1Hz, 1H, H6′b), 3.38 (dd, JH5″a-H4″ = 14.7 Hz, JH5″a-H5″b = 9.0 Hz, 1H, H5″a), 3.19 (dd, JH5″b-H4″ = 15.0 Hz, JH5″b-H5″a = 9.0 Hz, 1H, H5″b), 2.83 (t, JH2′-H3′ = 7.7 Hz, 1H, H1*), 1.97–1.94 (m, 4 H, H7*, H10*), 1.80–1.73 (m, 2 H, H2*), 1.70 (q, JH7″-H8″ = 7.6 Hz, 2 H, H7″), 1.56 (q, JH7″-H8″ = 7.6 Hz, 2 H, H7″), 1.32–1.25 ( m, 20 H, H3*-H6*, H9*-H16*), 0.92–0.84 (m, 27H, -SiC(CH3)3, H8″, H17*), 0.07–0.05 (12 H, Si(CH3)2). 13C NMR (125 MHz, CDCl3) δ 180.2 (C8′), 166.9 (C7′), 162.9 (C4), 150.0 (C2), 140.2 (C6), 130.6 (C8*), 130.2 (C9*), 118.0 (C6″), 112.3 (C1″), 101.8 (C5), 88.8 (C1′), 86.1 (C2″), 85.0 (C4″), 84.7 (C4′), 81.7 (C3”), 78.3 (C5′), 75.5 (C2′), 72.0 (C3′), 53.4 (C6′ab), 32.7 (C7*), 32.6 (C10*), 32.0 (C4*), 29.7, 29.6 (C5”b), 29.5, 29.4, 29.3 (C5”a), 29.1 (C7”), 28.9 (C7”), 26.7 (C1*), 26.6 (C2*), 25.9 (SiC(CH3)3), 25.8 (SiC(CH3)3), 22.8 (C3*), 18.1 (C16*), 14.2 (C17*), 8.5 (C8″), 7.6 (C8″), −4.0 (SiCH3), −4.4 (SiCH3), −4.6 (SiC), −4.7 (SiC); HRMS ESI+ calcd. for C51H90N7O10Si2+ (M + H)+ 1016.6282 found 1016.6274.
3.1.10. Protected Oxadiazole 11g
Compound 11g was obtained as a colorless oil (38 mg, 58% yield): Rf 0.25 (cyclohexane/EtOAc = 7/3); [α]D +6 (c 1.0, CH2Cl2); IR (film): 2929, 2856, 2105, 1695, 1580, 1462, 1377, 1274, 1167, 1100, 924, 865, 839, 764, 750; 1H NMR (500 MHz, CDCl3) δ 7.89 (d, JH6-H5 = 8.1Hz, 1H, H6), 7.28–7.16 (m, 5 H, Habc), 5.85 (d, JH1′-H2′ = 4.7 Hz, 1H, H1′), 5.73 (dd, JH5-H6 = 8.1Hz, JH5-H1′ = 2.0 Hz, 1H, H5), 5.24 (s, 1H, H1″), 4.64 (dd, JH3″-H2″ = 6.1Hz, JH3″-H4″ = 1.4 Hz, 1H, H3″), 4.52 (d, JH2″-H3″ = 6.1Hz, 1H, H2″), 4.32 (t, JH5′-H4′ = 5.7 Hz, 1H, H5′), 4.24–4.21 (m, 1H, H4″), 4.16 (t, JH2′-H3′ = 4.4 Hz, 1H, H2′), 4.05 (dd, JH4′-H3′ = 4.3 Hz, JH4′-H5′ = 1.7 Hz, 1H, H4′), 3.98 (t, JH3′-H4′ = 4.5 Hz, 1H, H3′), 3.50 (dd, JH6′a-H5′ = 12.8 Hz, JH6′a-H6′b = 5.8 Hz, 1H, H6′a), 3.43 (dd, JH6′b-H5′ = 12.8 Hz, JH6′b-H5′ = 5.8 Hz, 1H, H6′b), 3.38 (dd, JH5″a-H4″ = 15.0 Hz, JH5″a-H5″b = 9.3 Hz, 1H, H5″a), 3.19 (dd, JH5″b-H4″ = 15.0 Hz, JH5″b-H5″a = 9.3 Hz, 1H, H5″b), 2.82 (t, JH2′-H3′ = 7.6 Hz, 1H, H1*), 2.59 (t, JH9′-H10′ = 7.6 Hz, 1H, H9*), 1.79–1.73 (m, 2H, H2*), 1.70 (q, JH7″-H8″ = 7.6 Hz, 2 H, H7″), 1.63–1.58 (m, 2H, H8*), 1.56 (q, JH7″-H8″ = 7.6 Hz, 2H, H7″), 1.36–1.26 ( m, 10 H, H3*-H7*), 0.92–0.84 ( m, 24H, -SiC(CH3)3, H8″, H18*), 0.07–0.05 (12 H, Si(CH3)2); 13C NMR (125 MHz, CDCl3) δ 180.2 (C8′), 166.9 (C7′), 162.9 (C4), 150.0 (C2), 142.9 (C10*), 140.2 (C6), 128.5 (Ca), 128.3 (Cb), 125.7 (Cc), 118.0 (C6″), 112.3 (C1″), 101.8 (C5), 88.8 (C1′), 86.1 (C2″), 85.0 (C4″), 84.7 (C4′), 81.7 (C3”), 78.3 (C5′), 75.5 (C2′), 72.0 (C3′), 53.4 (C6′ab), 36.1 (C9*), 31.6 (C4*), 29.5, 29.4, 29.4 (C5”b), 29.3, 29.3, 29.2 (C5”a), 29.1 (C7”), 28.9 (C7”), 26.7 (C1*), 26.6 (C2*), 25.9 (SiC(CH3)3), 25.8 (SiC(CH3)3), 17.9 (C8*), 8.5 (C8″), 7.6 (C8″), −4.0 (SiCH3), −4.4 (SiCH3), −4.6 (SiC), −4.7 (SiC). HRMS ESI− calcd. for C49H78N7O10Si2− (M + H)− 980.5354 found 980.5297.
3.1.11. Protected Oxadiazole 11h
Compound 11h was obtained as a colorless oil and as a 1/1 mixture of stereoisomers (38 mg, 58% yield): Rf 0.25 (cyclohexane/EtOAc = 7/3); [α]D +6 (c 1.0, CH2Cl2); IR (film): 2929, 2856, 2105, 1695, 1580, 1462, 1377, 1274, 1167, 1100, 924, 865, 839, 764, 750; 1H NMR (500 MHz, CDCl3) δ 7.90 (d, JH5-H6 = 8.1 Hz, 1H, H6), 5.85 (d, JH1′-H2′ = 3.1 Hz, 0.5H, H1′), 5.84 (d, JH1′-H2′ = 3.4 Hz, 0.5H, H1′), 5.74 (d, JH5-H6 = 8.1 Hz, 1H, H5), 5.22 (s, 0.5H, H1″), 5.22 (s, 0.5 H, H1″), 4.63 (d, JH2″-H3″ = 6.1 Hz, 1H, H3″), 4.51 (d, JH2″-H3″ = 6.1 Hz, 1H, H2″), 4.35–4.23 (m, 2H, H4″, H5′), 4.23–4.14 (m, 3 H, H3**, H2′), 4.08 (dd, JH4′-H5′ = 8.5 Hz, JH3′-H4′ = 4.4 Hz, 0.5H, H4′), 4.07 (dd, JH4′-H5′ = 8.5 Hz, JH3′-H4′ = 4.4 Hz, 0.5 H, H4′), 4.00 (t, JH3′-H4′ = JH2′-H3′ = 4.4 Hz, 1H, H3′), 3.95 (t, J = 7.6 Hz, 1H, H1*), 3.50 (dd, JH5″a-H5″b = 12.7, JH4″-H5″a = 4.9 Hz, 0.5H, H5″a), 3.49 (dd, JH5″a-H5″b = 12.7, JH4″-H5″a = 4.9 Hz, 0.5H, H5″a), 3.46–3.34 (m, 2 H, H6′a, H5″b), 3.26–3.16 (m, 1H, H6′b), 2.18–1.96 (m, 2 H, H2*), 1.75–1.65 (m, 2H, H7″), 1.59–1.51 (m, 2H, H7″), 1.40–1.5 (m, 17H, H3*-H9*, H4**), 0.94–0.78 (m, 21 H, H10*, H8″, SiC(CH3)3), 0.07, 0.07, 0.06 (s, 12H, SiCH3); 13C NMR (125 MHz, CDCl3) δ 176.74, 176.67 (C2**), 168.66, 168.63 (C8′), 167.23, 167.20 (C7′), 162.9, 162.8 (C4), 150.00, 149.95 (C2), 140.2 (C6), 117.96, 117.93 (C6″), 112.34, 112.29 (C1″), 101.76 (C5), 88.81 (C1′), 86.03 (C2″), 84.98, 84.86 (C4′, C4″), 81.7 (C3″), 78.2, 78.1 (C5′), 75.5 (C2′), 71.9 (C3′), 62.02 (C3**), 53.27, 53.25 (C5″), 44.60, 44.55 (C1*), 31.89 (C2*), 30.2, 29.7, 29.5, 29.4, 29.3, 29.2, 29.1, 28.8, 27.20, 22.7 (C3*-C9*, C7″), 25.81, 25.76 (SiCCH3), 18.0 (SiCCH3), 14.15, 14.12 (C4**), 8.4, 7.5 (C8″), −4.2, −4.5, −4.8 (SiCH3); HRMS ESI− calcd. for C47H80N7O12Si2− (M + H)− 990,5409 found 990.5455.
3.1.12. Protected Oxadiazole 11i
Compound 11i was obtained as a colorless oil (57 mg, 74% yield): Rf 0.25 (cyclohexane/EtOAc = 7/3); [α]D +10 (c 1.0, CH2Cl2); IR (film): 2928, 2856, 1697, 1463, 1260, 1168, 1102, 840, 779, 740; 1H NMR (500 MHz, CDCl3) δ 7.93 (d, JH6-H5 = 8.5 Hz, 1H, H6), 5.83 (d, JH1′-H2′ = 4.3 Hz, 1H, H1′), 5.73 (dd, JH5-H6 = 8.3 Hz, JH5-H1′ = 2.2 Hz, 1H, H5), 5.23 (s, 1H, H1″), 4.63 (dd, JH3″-H2″ = 6.3 Hz, JH3″-H4″ = 1.2 Hz, 1H, H3″), 4.51 (d, JH2″-H3″ = 6.4 Hz, 1H, H2″), 4.31-4.24 (m, 2 H, H5′,H4″), 4.16 (d, JH2′-H3′ = 3.9 Hz, 1H, H2′), 4.17 (q, JH3***-H4*** = 7.1Hz, 1H, H3***), 4.09 (dd, JH4′-H3′ = 4.9 Hz, JH4′-H5′ = 1.4 Hz, 1H, H4′), 4.01 (t, JH3′-H4′ = 4.6 Hz, 1H, H3′), 3.52 (dd, JH5″a-H5″b = 13 Hz, JH5″a-H6′ = 4.9 Hz, 1H, H5″a), 3.43 (dd, JH5″b-H5″a = 13 Hz, JH5″b-H4″ = 5.8 Hz, 1H, H5″b), 3.40 (dd, JH6″a-H6″b = 15.4 Hz, JH6″a-H5′ = 5.5 Hz, 1H, H6″a), 3.21 (dd, JH6″b-H6″a = 15.4 Hz, JH6″b-H5″ = 8.4 Hz, 1H, H6″b), 2.12-2.00 (m, 4 H, H2*,H2**), 1.68 (q, JH7″a-H8″ = 7.4 Hz, 2 H, H7″a), 1.55 (q, JH7″b-H8″ = 7.4 Hz, 2 H, H7″b), 1.28–1.19 ( m, 33 H, H3**-H10**, H4*-H10*, H4***), 1.14–1.03 ( m, 3H, H3*), 0.91–0.83 ( m, 30H, -SiC(CH3)3, H8″, H11*, H11**), 0.09–0.07 (12 H, Si(CH3)2). 13C NMR (125 MHz, CDCl3) δ 183.4 (C8′), 166.8 (C7′), 163.0 (C4), 150.0 (C2), 140.3 (C6), 118.0 (C6″), 112.3 (C1″), 101.7 (C5), 89.0 (C1′), 86.1 (C2″), 85.1 (C4″), 84.7 (C4′), 81.7 (C3”), 78.3 (C5′), 75.6 (C2′), 71.8 (C3′), 53.4 (C5”), 38.5 (C1*), 33.5 (C2*), 33.4 (C2**), 32.0, 29.7, 29.7, 29.5, 29.4, 29.3, 28.9 (C7”), 27.3 (C7”), 26.0 (SiC(CH3)3), 25.9 (SiC(CH3)3), 22.8 (C3*), 18.0 (C3** ), 14.2 (C4***,C11**,C11*), 8.5 (C8″), 7.6 (C8″), −4.0 (SiCH3), −4.3(SiCH3), −4.6(SiC), −4.7(SiC). HRMS ESI+ calcd. for C55H100N7O10Si2+ (M + H)+ 1074.7064 found 1074.7060.
3.1.13. General Procedure for Compounds Deprotection
The deprotection of compounds 11a–11i was performed according to reference [42] and afforded the fully deprotected compounds 12a–12i in 22 to 85% yield over two steps.
3.1.14. Oxadiazole 12a
Compound 12a was obtained from the protected oxadiazole 11a (30 mg, 32 μmol, 1 equiv.) as a white solid (16.7 mg, 85% yield): Rf 0.15 (DCM/MeOH/NH4OH 14% 80/18/2); [α]D + 15 (c 1.0, CH2Cl2); IR (film): 2912, 2888, 2240, 1750, 1700, 1650, 1400, 1275, 1260, 1000, 764, 750.; 1H NMR (500 MHz, MeOD) δ 7.80 (d, JH6-H5 = 8.1Hz, 1H, H6), 5.77 (d, JH1′-H2′ = 3.1Hz, 1H, H1′), 5.73 (d, JH5-H6 = 8.1Hz, 1H, H5), 5.14 (s, 1H, H1″), 4.33–4.27 (m, 1H, H2′), 4.18–4.12 (m, 2 H, H3′, H4′), 4.09 (t, JH4″-H5″ = 7.1Hz, 1H, H4″), 4.04 (m, 2 H, H2″, H3″), 3.98 (m, 1H, H5′), 3.37–3.32 (m, 1H, H6′a) 3.29–3.26 (m,1H, H5″a), 3.20 (dd, JH6′b-H6′a = 15.0, JH6′b-H5′ = 5.7 Hz, 1H, H6′b), 3.11 (dd, JH5″b-H5″a = 13.0, JH5″b-H4″ = 7.1Hz,1H, H5″b), 2.91 (t, JH2*-H3* = 7.5 Hz, 2 H, H2*), 1.79 (dt, JH3*-H2* = JH3*-H4* = 7.4 Hz, 2 H, H3*), 1.44–1.20 (m,14 H), 0.89 (t, JH11*-H10* = 6.8 Hz, 3 H, H11*).; 13C NMR (125 MHz, MeOD) δ 181.9 (C1*), 168.7 (C7′), 166.1(C4), 152.1 (C2), 142.2 (C6), 111.0 (C1″), 102.5 (C5), 91.6 (C1′), 85.7 (C3′), 80.0 (C4″), 78.1 (C5′), 76.3 (C3″), 75.5 (C2′), 73.8 (C2″), 71.3 (C4′), 44.3 (C5″), 33.0, 30.6 (C6′), 30.39, 30.20, 30.02, 27.6 (C3*), 27.1 (C2*),23.7, 14.4; HRMS ESI+ calcd. for C27H43N5O10 (M + H)+ 598.3083, found 598.3082.
3.1.15. Oxadiazole 12b
Compound 12b was obtained from the protected oxadiazole 11b (27 mg, 27 μmol, 1 equiv.) as a white solid (10.7 mg, 59% yield): Rf 0.15 (DCM/MeOH/NH4OH 14% 80/18/2); [α]D + 1 (c 1.0, CH3OH); IR (film): 2924, 2853, 1673, 1466, 1272, 1203, 1136, 800, 724; 1H NMR (500 MHz, MeOD) δ 7.81 (d, JH6-H5 = 8.1Hz, 1H, H6), 5.77 (d, JH1′-H2′ = 3.2 Hz, 1H, H1′), 5.72 (d, JH5-H6 = 8.1Hz, 1H, H5), 5.14 (s, 1H, H1″), 4.30 (td, JH5′-H6′ = 6.5 Hz, JH5′-H4′ = 2.5 Hz, 1H, H5′), 4.19-4.01 (m, 5 H, H2′, H3′, H4′, H3″, H2″), 3.98 (d, JH4″-H5″ = 4.3 Hz, 1H, H4″), 3.30–3.27 (m, 2 H, H6′ab), 3.21 (dd, JH5″a-H4″ = 14.9 Hz, JH5″a-H5″b = 5.7 Hz, 1H, H5″a), 3.12 (dd, JH5″b-H4″ = 13.0 Hz, JH5″b-H5″a = 9.0 Hz, 1H, H5″b), 2.91 (t, JH1*-H2* = 7.5 Hz, 2H, H1*), 1.82–1.76 (m, 2H, H2*), 1.38–1.29 ( m, 24 H, H3*-H14*), 0.90 (t, JH15*-H14* = 7.0 Hz, 3H, H15*); 13C NMR (125 MHz, MeOD) δ 181.1 (C8′), 168.7 (C7′), 166.1 (C4), 152.0 (C2), 142.2 (C6), 110.9 (C1″), 102.4 (C5), 91.5 (C1′), 85.6 (C2″), 79.9 (C4′), 78.0 (C5′), 76.2 (C4”), 75.5 (C2′), 73.8 (C3″), 71.2 (C3′), 44.3 (C5”), 33.0 (C6′), 30.8, 30.7, 30.7, 30.5, 30.4, 30.3, 30.2, 30.0 (C4*-C14*), 27.6 (C2*), 27.1 (C1*), 23.7 (C3*), 14.4 (C15*). HRMS ESI+ calcd. for C32H54N5O10+ (M + H)+ 668.3865 found 668.3860.
3.1.16. Oxadiazole 12c
Compound 12c was obtained from the protected oxadiazole 11c (28 mg, 27 μmol, 1 equiv.) as a colorless oil (9.2 mg, 40% yield): Rf 0.15 (DCM/MeOH/NH4OH 14% 80/18/2); [α]D + 0.8 (c 1.0, CH3OH); IR (film): 2921, 2851, 1672, 1432, 1275, 1204, 1138, 961, 800, 764.; 1H NMR (500 MHz, MeOD) δ 7.81 (d, JH6-H5 = 8.0 Hz, 1H, H6), 5.76 (d, JH1′-H2′ = 3.2 Hz, 1H, H1′), 5.72 (d, JH5-H6 = 8.0 Hz, 1H, H5), 5.14 (s, 1H, H1″), 4.30 (td, JH5′-H6′ = 6.5 Hz, JH5′-H4′ = 2.5 Hz, 1H, H5′), 4.17–4.01 (m, 5 H, H2′, H3′, H4′, H3″, H2″), 3.98 (d, JH4″-H5″ = 4.1Hz, 1H, H4″), 3.30–3.25 (m, 2 H, H6′ab), 3.21 (dd, JH5″a-H4″ = 15.0 Hz, JH5″a-H5″b = 5.6 Hz, 1H, H5″a), 3.12 (dd, JH5″b-H4″ = 13.0 Hz, JH5″b-H5″a = 9.0 Hz, 1H, H5″b), 2.91 (t, JH1*-H2* = 7.5 Hz, 2 H, H1*), 1.80–1.76 (m, 2 H, H2*), 1.36–1.29 ( m, 30 H, H3*-H17*), 0.90 (t, JH15*-H14* = 6.9 Hz, 3H, H18*); 13C NMR (125 MHz, MeOD) δ 181.8 (C8′), 168.7 (C7′), 166.1 (C4), 152.0 (C2), 142.2 (C6), 110.9 (C1″), 102.4 (C5), 91.6 (C1′), 85.6 (C2″), 79.9 (C4′), 78.1 (C5′), 76.2 (C4”), 75.5 (C2′), 73.8 (C3″), 71.2 (C3′), 44.3 (C5”), 33.0 (C6′), 30.8, 30.5, 30.4, 30.3, 30.2, 30.0 (C4*-C17*), 27.6 (C2*), 27.1 (C1*), 23.7 (C3*), 14.4 (C18*); HRMS ESI+ calcd. for C35H60N5O10+ (M + H)+ 710.4335 found 710.4325.
3.1.17. Oxadiazole 12d
Compound 12d was obtained from the protected oxadiazole 11d (22 mg, 21 μmol, 1 equiv.) as a colorless oil (12 mg, 66% yield): Rf 0.15 (DCM/MeOH/NH4OH 14% 80/18/2); [α]D + 0.9 (c 1.0, CH3OH); IR (film): 3007, 2920, 2850, 1670, 1435, 1275, 1204, 1138, 960, 801, 764, 750; 1H NMR (500 MHz, MeOD) δ 7.81 (d, JH6-H5 = 8.1Hz, 1H, H6), 5.76 (d, JH1′-H2′ = 3.3 Hz, 1H, H1′), 5.73 (d, JH5-H6 = 8.1Hz, 1H, H5), 5.34 (t, JH8*-H9* = 4.6 Hz, 2 H, H8*, H9*), 5.14 (s, 1H, H1″), 4.30 (td, JH5′-H6′ = 6.5 Hz, JH5′-H4′ = 2.5 Hz, 1H, H5′), 4.15–4.01 (m, 5 H, H2′, H3′, H4′, H3″, H2″), 3.98 (d, JH4″-H5″ = 4.3 Hz, 1H, H4″), 3.29–3.25 (m, 2 H, H6′ab), 3.21 (dd, JH5″a-H4″ = 14.6 Hz, JH5″a-H5″b = 5.8 Hz, 1H, H5″a), 3.12 (dd, JH5″b-H4″ = 12.3 Hz, JH5″b-H5″a = 9.2 Hz, 1H, H5″b), 2.91 (t, JH1*-H2* = 7.3 Hz, 2 H, H1*), 2.06–2.01 (m, 4 H, H7*, H10*), 1.81–1.76 (m, 2 H, H2*), 1.35–1.29 ( m, 20 H, H3*-H6*, H11*-H16*), 0.91 (t, JH15*-H14* = 6.7 Hz, 3H, H17*); 13C NMR (125 MHz, MeOD) δ 181.8 (C8′), 168.7 (C7′), 166.1 (C4), 152.0 (C2), 142.2 (C6), 130.9 (C8*), 130.7 (C9*), 110.9 (C1″), 102.3 (C5), 91.6 (C1′), 85.5 (C2″), 80.0 (C4′), 78.1 (C5′), 76.2 (C4”), 75.5 (C2′), 73.8 (C3″), 71.2 (C3′), 44.3 (C5”), 33.0 (C6′), 30.8, 30.7, 30.6, 30.4, 30.3, 30.3, 30.1, 30.0 (C4*-C6*, C11*-C16*), 28.1 (C7*), 28.0 (C10*), 27.6 (C2*), 27.1 (C1*), 23.7 (C3*), 14.4 (C17*). HRMS ESI− calcd. for C34H54N5O10− (M + H)− 692.3876 found 692.3890.
3.1.18. Oxadiazole 12e
Compound 12e was obtained from the protected oxadiazole 11e (20 mg, 21 μmol, 1 equiv.) as a colorless oil (8 mg, 60% yield): Rf 0.15 (DCM/MeOH/NH4OH 14% 80/18/2); [α]D + 1 (c 1.0, CH3OH); IR (film): 3058, 2912, 2840, 1658, 1435, 1275, 1244, 1198, 993, 799, 751; 1H NMR (500 MHz, MeOD) δ 8.10 (d, JHa-Hb = 8.8 Hz, 2 H, Ha), 7.81 (d, JH6-H5 = 8.4 Hz, 1H, H6), 7.45 (t, JHd-He = 8.0 Hz, 2H, Hd), 7.25 (t, JHe-Hd = 7.5 Hz, 1H, He), 7.13–7.10 (m, 4 H, Hc, Hb), 5.78 (d, JH1′-H2′ = 3.0 Hz, 1H, H1′), 5.70 (d, JH5-H6 = 8.4 Hz, 1H, H5), 5.18 (s, 1H, H1″), 4.38 (td, JH5′-H6′ = 5.7 Hz, JH5′-H4′ = 3.1Hz, 1H, H5′), 4.17–4.14 (m, 2 H, H2′, H3′), 4.09–4.05 (m, 3 H, H4′, H3″, H2″), 3.99 (d, JH4″-H5″ = 3.5 Hz, 1H, H4″), 3.38–3.32 (m, 2H, H6′ab), 3.29–3.27 (m, JH5″a-H5″b = 5.8 Hz, 1H, H5″a), 3.17–3.11 (m, 1H, H5″b); 13C NMR (125 MHz, MeOD) δ 176.6 (C8′), 169.5 (C7′), 166.0 (C4), 163.6 (C9*), 156.6 (C10*), 152.1 (C2), 142.2 (C6), 131.3 (Cd), 131.2 (Ca), 126.0 (Ce), 121.3 (Cc), 119.0 (Cb), 110.8 (C1″), 102.4 (C5), 91.5 (C1′), 85.7 (C2″), 79.9 (C4′), 78.0 (C5′), 76.2 (C4”), 75.4 (C2′), 73.9 (C3″), 71.3 (C3′), 44.4 (C5”), 30.4 (C11*); HRMS ESI+ calcd. for C29H32N5O11+ (M + H)+ 626.2092 found 626.2078.
3.1.19. Oxadiazole 12f
Compound 12f was obtained from the protected oxadiazole 11f (44 mg, 41 μmol, 1 equiv.) as a colorless oil (22.8 mg, 63% yield): Rf 0.15 (DCM/MeOH/NH4OH 14% 80/18/2); [α]D + 8 (c 1.0, CH3OH); IR (film): 3052, 2926, 2854, 1698, 1410, 1275, 1203, 1132, 965, 799, 764, 750; 1H NMR (500 MHz, MeOD) δ 7.81 (d, JH6-H5 = 8.1Hz, 1H, H6), 5.77 (d, JH1′-H2′ = 3.2 Hz, 1H, H1′), 5.73 (d, JH5-H6 = 8.1Hz, 1H, H5), 5.41–5.36 (m, 2 H, H8*, H9*), 5.14 (s, 1H, H1″), 4.30 (td, JH5′-H6′ = 6.5 Hz, JH5′-H4′ = 2.5 Hz, 1H, H5′), 4.16–4.02 (m, 5 H, H2′, H3′, H4′, H3″, H2″), 3.98 (d, JH4″-H5″ = 4.8 Hz, 1H, H4″), 3.30–3.25 (m, 2 H, H6′ab), 3.21 (dd, JH5″a-H4″ = 14.9 Hz, JH5″a-H5″b = 5.7 Hz, 1H, H5″a), 3.12 (dd, JH5″b-H4″ = 13.1Hz, JH5″b-H5″a = 9.0 Hz, 1H, H5″b), 2.91 (t, JH1*-H2* = 7.6 Hz, 2H, H1*), 2.00–1.95 (m, 4H, H7*, H10*), 1.81–1.76 (m, 2H, H2*), 1.37–1.29 ( m, 20H, H3*-H6*, H11*-H16*), 0.90 (t, JH15*-H14* = 6.9 Hz, 3H, H17*); 13C NMR (125 MHz, MeOD) δ 181.8 (C8′), 168.7 (C7′), 166.1 (C4), 152.1 (C2), 142.2 (C6), 131.6 (C8*), 131.4 (C9*), 110.9 (C1″), 102.4 (C5), 91.5 (C1′), 85.6 (C2″), 80.0 (C4′), 78.0 (C5′), 76.2 (C4”), 75.5 (C2′), 73.8 (C3″), 71.3 (C3′), 44.3 (C5”), 33.6 (C7*), 33.5 (C10*), 33.0 (C6′), 30.7, 30.6, 30.5, 30.4, 30.3, 30.2, 30.0, 29.9 (C4*-C6*, C11*-C16*), 27.6 (C2*), 27.1 (C1*), 23.7 (C3*), 14.4 (C17*); HRMS ESI+ calcd. for C34H56N5O10+ (M + H)+ 694.4022 found 694.4012.
3.1.20. Oxadiazole 12g
Compound 12g was obtained from the protected oxadiazole 11g (20 mg, 20 μmol, 1 equiv.) as a colorless oil (12 mg, 63% yield): Rf 0.15 (DCM/MeOH/NH4OH 14% 80/18/2); [α]D + 1 (c 1.0, CH3OH); IR (film): 3028, 2913, 2841, 1660, 1435, 1275, 1133, 993, 800, 764, 750; 1H NMR (500 MHz, MeOD) δ 7.81 (d, JH6-H5 = 8.3 Hz, 1H, H6), 7.23 (d, JHa-Hb = 7.1Hz, 2 H, Ha), 7.16–7.11 (m, 3 H, Hb, Hc), 5.76 (d, JH1′-H2′ = 3.1Hz, 1H, H1′), 5.72 (d, JH5-H6 = 8.2 Hz, 1H, H5), 5.14 (s, 1H, H1″), 4.29 (td, JH5′-H6′ = 6.5 Hz, JH5′-H4′ = 2.7 Hz, 1H, H5′), 4.15–4.01 (m, 5 H, H2′, H3′, H4′, H3″, H2″), 3.98 (d, JH4″-H5″ = 3.9 Hz, 1H, H4″), 3.30–3.26 (m, 2H, H6′ab), 3.20 (dd, JH5″a-H4″ = 15.3 Hz, JH5″a-H5″b = 5.3 Hz, 1H, H5″a), 3.11 (dd, JH5″b-H4″ = 13.1Hz, JH5″b-H5″a = 9.0 Hz, 1H, H5″b), 2.90 (t, JH1*-H2* = 7.4 Hz, 2 H, H1*), 2.59 (t, JH9*-H10* = 7.7 Hz, 2 H, H9*), 1.80–1.74 (m, 2 H, H2*), 1.63–1.57 (m, 2 H, H8*), 1.37–1.28 ( m, 10 H, H3*-H7*); 13C NMR (125 MHz, MeOD) δ 181.8 (C8′), 168.7 (C7′), 166.1 (C4), 152.1 (C2), 143.9 (C10*), 142.2 (C6), 129.4 (Ca), 129.2 (Cb), 126.6 (Cc), 110.9 (C1″), 102.4 (C5), 91.5 (C1′), 85.6 (C2″), 79.9 (C4′), 78.1 (C5′), 76.2 (C4”), 75.5 (C2′), 73.8 (C3″), 71.2 (C3′), 44.3 (C5”), 36.9 (C9*), 32.7 (C6′), 30.5, 30.4, 30.3, 30.2, 30.1, 29.9 (C3*-C8*), 27.6 (C2*), 27.1 (C1*); HRMS ESI+ calcd. for C32H46N5O10+ (M + H)+ 660.3239 found 660.3226.
3.1.21. Oxadiazole 12h
Compound 12h was obtained from the protected oxadiazole 11h (42 mg, 42 μmol, 1 equiv.) as a white solid (19 mg, 68% yield) and as a 1/1 mixture of stereoisomers: Rf 0.15 (DCM/MeOH/NH4OH 14% 80/18/2); [α]D + 1.4 (c 1.0, CH3OH); IR (film): 2925, 2855, 1688, 1579, 1464, 1274, 1126, 748; 1H NMR (500 MHz, MeOD) δ 7.80 (d, JH5-H6 = 8.1 Hz, 0.5H, H6), 7.79 (d, JH5-H6 = 8.1 Hz, 0.5H, H6), 5.75 (d, JH1′-H2′ = 3.1 Hz, 1H, H1′), 5.72 (d, JH5-H6 = 8.1 Hz, 1H, H5), 5.14 (s, 0.5H, H1″), 5.12 (s, 0.5H, H1″), 4.31 (td, JH5′-H6′a = JH5′-H6′b = 6.0, JH4′-H5′ = 3.0 Hz, 1H, H5′), 4.26–4.16 (m, 2H, H3**), 4.16–4.09 (m, 2H, H1*, H2′), 4.09–4.01 (m, 3H, H4″, H4′, H3″), 3.97 (d, JH2″-H3″ = 3.5 Hz, 1H, H2″), 3.38–3.32 (m, 1H, H6′a), 3.30–3.26 (m, 1H, H5″a), 3.23 (dd, JH6′a-H6′b = 15.0, JH5′-H6′b = 6.0 Hz, 1H, H6′b), 3.14 (dd, JH5″a-H5″b = 13.0, JH4″-H5″b = 8.2 Hz, 1H, H5″b), 2.17–1.95 (m, 2H, H2*), 1.43–1.27 (m, 14H, H3*-H9*), 1.25 (t, JH3**-H4** = 7.1 Hz, 3H, H4**), 0.90 (t, JH9*-H10* = 6.9 Hz, 3H, H10*); 13C NMR (125 MHz, MeOD) δ 178.28, 178.22 (C2**), 170.5, 170.4 (C8′), 169.22, 169.18 (C7′), 166.16 (C4), 152.1 (C2), 142.2 (C6), 111.1, 111.0 (C1″), 102.4 (C5), 91.60, 91.57 (C1′), 85.9 (C4′), 80.0 (C4″), 78.3, 78.1 (C5′), 76.3 (C2″), 75.5 (C1*), 73.9 (C3′), 71.34, 71.30 (C2′), 63.2 (C3**), 44.3 (C5″), 33.1, 31.2, 31.0, 30.6, 30.5, 30.2, 28.1, 23.7 (C2*-C9*), 30.4 (C6′), 14.5, 14.4 (C4**, C10*); HRMS ESI+ calcd. for C30H48N5O12+ (M + H)+ 670.3294 found 670.3320.
3.1.22. Oxadiazole 12i
Compound 12i was obtained from the protected oxadiazole 11i (34 mg, 32 μmol, 1 equiv.) as a colorless oil (5.3 mg, 22% yield): Rf 0.15 (DCM/MeOH/NH4OH 14% 80/18/2); [α]D + 1 (c 1.0, CH3OH); IR (film): 2923, 1690, 1275, 1260, 764, 750; 1H NMR (500 MHz, MeOD) δ 7.89 (d, JH6-H5 = 8.1Hz, 1H, H6), 5.81 (d, JH1′-H2′ = 2.7 Hz, 1H, H1′), 5.74 (d, JH5-H6 = 8.1Hz, 1H, H5), 5.03 (s, 1H, H1″), 4.30 (td, JH5′-H6′ = 6.3 Hz, JH5′-H4′ = 2.2 Hz, 1H, H5′), 4.14–4.11 (m, 2 H, H2′, H3′), 4.07–4.04 (m, 1H, H4′), 3.98 (dd, JH3″-H2″ = 6.6 Hz, JH3″-H4″ = 4.8 Hz, 1H, H3″), 3.93 (dd, JH2″-H3″ = 4.6 Hz, JH2″-H1″ = 1.2 Hz, 1H, H2″), 3.88 (td, JH4″-H5″ = 7.1 Hz, JH4″-H3″ = 3.5 Hz, 1H, H4″), 3.24 (dd, JH6′a-H5′ = 8.7 Hz, JH6′a-H6′b = 6.3 Hz, 1H, H6′a), 3.19 (dd, JH6′b-H5′ = 8.7 Hz, JH6′b-H5′ = 6.3 Hz, 1H, H6′b), 3.06–3.00 (m, 1H, H1*), 2.91 (dd, JH5″a-H4″ = 13.3 Hz, JH5″a-H5″b = 3.7 Hz, 1H, H5″a), 2.79 (dd, JH5″b-H4″ = 13.3 Hz, JH5″b-H5″a = 7.5 Hz, 1H, H5″b), 1.79–1.69 (m, 4 H, H2*,H2**), 1.35–1.17 ( m, 34 H, H3**-H10**, H3*-H10*), 0.91–0.88 ( 6H, H11*, H11**); 13C NMR (125 MHz, MeOD) δ 184.5 (C8′), 168.6 (C7′), 166.2 (C4), 152.2 (C2), 142.0 (C6), 111.1 (C1″), 102.4 (C5), 91.0 (C1′), 86.3 (C4′), 84.5 (C4″), 78.5 (C5′), 76.8 (C2”), 75.8 (C2′), 73.3 (C3″), 71.3 (C3′), 45.3 (C5”), 39.6 (C1*), 34.6 (C2*), 34.5 (C2**), 33.0 (C9*, C9**), 30.8, 30.7, 30.4, 30.4 (C4*-C8*, C4**-C8**), 28.2 (C3*, C3**), 23.7 (C10*, C10**), 14.4 (C11*, C11**); HRMS ESI+ calcd. for C38H66N5O10+ (M + H)+ 752,4804 found 752.4804.
3.1.23. Oxadiazole 12j
To a solution of oxadiazole 12h (2.5 mg, 3.7 µmol, 1 equiv.) in methanol (0.4 mL) at 0 °C was added a solution of NH4HCO3 in water (0.1 M, 0.5 mL) and trimethylamine (14 µL, 99 µmol, 27 equiv.). After 48 h at 0 °C, the reaction mixture was diluted in 5 mL water, and the solution was freeze-dried. The residue was purified on a reverse phase column (Sep-Pak Cartridges C18) using a mixture of an aqueous solution NH4HCO3 (0.1 M) and acetonitrile (1/0 to 1/1, v/v). The fractions containing the product were freeze-dried to afford product 12j as a white powder (0.4 mg) and as a 1/1 mixture of stereoisomers in 17% yield: Rf 0.07 (DCM/MeOH/NH4OH 14% 80/18/2); 1H NMR (500 MHz, MeOD) δ 7.87 (d, JH5-H6 = 8.1 Hz, 0.5H, H6), 7.85 (d, JH5-H6 = 8.1 Hz, 0.5H, H6), 5.80 (d, JH1′-H2′ = 3.4 Hz, 0.5H, H1′), 5.79 (d, JH1′-H2′ = 3.4 Hz, 0.5H, H1′), 5.74 (d, JH5-H6 = 8.1 Hz, 0.5H, H5), 5.74 (d, JH5-H6 = 8.1 Hz, 0.5H, H5), 5.12 (s, 0.5H, H1″), 5.09 (s, 0.5H, H1″), 4.37–4.31 (m, 1H, H5′), 4.18–3.85 (m, 6H, H1*, H2′, H4″, H4′, H3″, H2″), 3.14–2.72 (m, 4H, H6′a, H5″a, H6′b, H5″b), 2.18–1.98 (m, 2H, H2*), 1.40–1.22 (m, 14H, H3*-H9*), 0.90 (t, JH9*-H10* = 6.9 Hz, 3H, H10*); 13C NMR (125 MHz, MeOD) δ 181.4 (C2**), 170.3 (C8′), 169.0 (C7′), 166.3 (C4), 152.3, 152.2(C2), 142.4, 142.2 (C6), 110.4, 110.0 (C1″), 102.57, 102.51 (C5), 91.7 (C1′), 86.4 (C4′), 80.0 (C4″), 78.1 (C5′), 76.5 (C2″), 75.55, 75.49 (C1*), 73.7, 73.6 (C3′), 71.4, 71.3 (C2′), 44.94, 44.87 (C5″), 33.1, 32.1, 31.9, 30.8, 30.7, 30.6, 30.5, 26.9, 23.7 (C2*-C9*, C6′), 14.5 (C10*); HRMS ESI+ calcd. for C28H44N5O12+ (M + H)+ 642.2942 found 642.2979.
3.2. Enzyme Assays
The inhibitory activity of the synthesized compounds 1–5 was determined as described in reference [41,42] except that compounds were tested at a final concentration of 10% DMSO. The compounds were evaluated on His-tagged MraY transferase purified from Aquifex aeolicus (MraYAA) prepared as previously described by Chung et al. [37]. The assays were performed as previously described by Stachyra et al. [53].
3.3. Antibacterial Activity
Antibacterial activity was determined as previously described [41,42], but molecules were solubilized in 100% DMSO (cell culture grade) at 1 mg/mL concentration and 10-fold diluted in MHB just before utilization. The MHB-diluted solutions were then serially diluted in 5% DMSO-MHB, at final concentrations ranging from 50 mg/mL to 0.005 mg/mL for compounds and 6.25 to 5% for DMSO. Growth controls for strains indicated that DMSO does not inhibit growth at the highest concentration used in the test.
3.4. Docking
Docking experiments were performed according to the protocol previously described [41,42].
4. Conclusions
We report the synthesis of new inhibitors of the bacterial MraYAA transferase displaying an aminoribosyl uridine scaffold substituted in 5′ position by an oxadiazole linker bearing various hydrophobic substituents. Their straightforward synthesis relies on the microwave-assisted O-acylation of an amidoxime by various acyl chlorides followed by cyclization into the oxadiazole. Their biological activity was evaluated in vitro on purified MraYAA and compared to that of a reference compound with a N-triazole as another heterocyclic linker. Nine out of the ten synthetized compounds revealed MraY inhibition with IC50 ranging from 0.77 to 27.48 µM, the most active compound with a C10 alkyl chain being slightly more active than the N-triazole reference compound. The binding mode of the inhibitors has been studied by docking experiments. The in cellulo evaluation of the synthetized inhibitors on different Gram-positive and Gram-negative bacterial strains only led to the poor activity of the two compounds on Gram-positive bacteria, showing that the further optimization of the lipophilic side chain is still required to improve the antibacterial activity.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/antibiotics11091189/s1, 1H and 13C spectra of all compounds. Table S1: Antibacterial activity of compounds 12a–12i and reference compounds.
Author Contributions
Conceptualization, H.W., R.B.O., L.L.C., S.C.-V., M.B. and C.G.-P.; data curation, H.W., R.B.O., L.L.C., M.P., S.C.-V., M.B. and C.G.-P.; methodology, H.W., R.B.O., L.L.C., M.P., A.A., B.J., S.C.-V., M.B. and C.G.-P.; investigation, H.W., R.B.O., L.L.C., M.P., M.O., T.T., R.A., M.B. and A.A.; writing—original draft preparation, H.W., R.B.O., L.L.C., A.A., S.C.-V., M.B. and C.G.-P.; writing—review and editing, H.W., R.B.O., L.L.C., A.A., B.J., S.C.-V., M.B. and C.G.-P.; supervision, S.C.-V., M.B. and C.G.-P.; project administration, C.G.-P. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the “Centre National de la Recherche Scientifique” and the “Ministère de l’Enseignement Supérieur et de la Recherche”. H. W. thanks the Chinese Scholarship Council for the financial support of her PhD (201909505005).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in Supplementary Material.
Acknowledgments
We warmly thank Dr Ahmed Bouhss (Université Paris-Saclay, INSERM U1204, Univ Evry) and Dr D. Padovani (Université Paris Cité, CNRS) for their interest in this work and for helpful discussions. The assistance of P. Gerardo (Université Paris Cité) for low-resolution and high-resolution mass spectra analyses is gratefully acknowledged. We acknowledge the Macromolecular Modelling Platform and the NMR platform core facilities of the BioTechMed facilities INSERM US36 ׀ CNRS UMS2009 ׀ Université Paris Cité for docking and MD simulation and NMR experiments, respectively. H. W. thanks the Chinese Scholarship Council (201909505005) for the financial support of her PhD thesis.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sherry, N.; Howden, B. Emerging Gram negative resistance to last-line antimicrobial agents fosfomycin, colistin and ceftazidime-avibactam—Epidemiology, laboratory detection and treatment implications. Expert Rev. Anti Infect. Ther. 2018, 16, 289–306. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.; Czaplewski, L.; Piddock, L.J.V. Discovery and development of new antibacterial drugs: Learning from experience? J. Antimicrob. Chemother. 2018, 73, 1452–1459. [Google Scholar] [CrossRef]
- Van Duijkeren, E.; Schink, A.K.; Roberts, M.C.; Wang, Y.; Schwarz, S. Mechanisms of bacterial resistance to antimicrobial agents. Microbiol. Spectr. 2018, 6. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.J.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, T.M.; Barnsbee, L.; Lee, X.J.; Pacella, R.E. Using the best available data to estimate the cost of antimicrobial resistance: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 26. [Google Scholar] [CrossRef] [PubMed]
- Xuemei, Z.; Lundborg, C.S.; Sun, X.; Hu, X.; Dong, H. Economic burden of antibiotic resistance in ESKAPE organisms: A systematic review. Antimicrob. Resist. Infect. Control 2019, 8, 137. [Google Scholar]
- Innes, G.K.; Randad, P.R.; Korinek, A.; Davis, M.F.; Price, L.B.; So, A.D.; Heaney, C.D. External societal costs of antimicrobial resistance in humans attributable to antimicrobial use in livestock. Annu. Rev. Public Health 2020, 41, 141–157. [Google Scholar] [CrossRef]
- Zhen, X.; Li, Y.; Chen, Y.; Dong, P.; Liu, S.; Dong, H. Effect of multiple drug resistance on total medical costs among patients with intra-abdominal infections in China. PLoS ONE 2018, 13, e0193977/1–e0193977/12. [Google Scholar] [CrossRef]
- Barreteau, H.; Kovač, A.; Boniface, A.; Sova, M.; Gobec, S.; Blanot, D. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 168–207. [Google Scholar] [CrossRef]
- Bouhss, A.; Trunkfield, A.E.; Bugg, T.D.H.; Mengin-Lecreulx, D. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol. Rev. 2008, 32, 208–233. [Google Scholar] [CrossRef] [Green Version]
- Bugg, T.D.H.; Lloyd, A.J.; Roper, D.I. Phospho-MurNAc-pentapeptide translocase (MraY) as a target for antibacterial agents and antibacterial proteins. Infect. Dis. Drug Targets 2006, 6, 85–106. [Google Scholar] [CrossRef]
- Bouhss, A.; Mengin-Lecreulx, D.; Le Beller, D.; van Heijenoort, J. Topological analysis of the MraY protein catalysing the first membrane step of peptidoglycan synthesis. Mol. Microbiol. 1999, 34, 576–585. [Google Scholar] [CrossRef]
- Bouhss, A.; Crouvoisier, M.; Blanot, D.; Mengin-Lecreulx, D. Purification and characterization of the bacterial MraY translocase catalyzing the first membrane step of peptidoglycan biosynthesis. J. Biol. Chem. 2004, 279, 29974–29980. [Google Scholar] [CrossRef]
- Al-Dabbagh, B.; Olatunji, S.; Crouvoisier, M.; El Ghachi, M.; Blanot, D.; Mengin-Lecreulx, D.; Bouhss, A. Catalytic mechanism of MraY and WecA, two paralogues of the polyprenyl-phosphate N-acetylhexosamine 1-phosphate transferase superfamily. Biochimie 2016, 127, 249–257. [Google Scholar] [CrossRef]
- Ubukata, M.; Isono, K.; Kimura, K.; Nelson, C.C.; McCloskey, J.A. The structure of liposidomycin B, an inhibitor of bacterial peptidoglycan synthesis. J. Am. Chem. Soc. 1998, 110, 4416–4417. [Google Scholar] [CrossRef]
- Ubukata, M.; Isono, K.; Kimura, K.; Nelson, C.C.; Gregson, J.M.; McCloskey, J.A. Structure elucidation of liposidomycins, a class of complex lipid nucleoside antibiotics. J. Org. Chem. 1992, 57, 6392–6403. [Google Scholar] [CrossRef]
- Brandish, P.E.; Kimura, K.I.; Inukai, M.; Southgate, R.; Lonsdale, J.T.; Bugg, T.D.H. Modes of action of tunicamycin, liposidomycin B, and mureidomycin A: Inhibition of phospho-N-acetylmuramyl-pentapeptide translocase from Escherichia coli. Antimicrob. Agents Chemother. 1996, 40, 1640–1644. [Google Scholar] [CrossRef]
- McDonald, L.A.; Barbieri, L.R.; Carter, G.T.; Lenoy, E.; Lotvin, J.; Petersen, P.J.; Siegel, M.M.; Singh, G.; Williamson, R.T. Structures of the Muraymycins, novel peptidoglycan biosynthesis inhibitors. J. Am. Chem. Soc. 2002, 124, 10260–10261. [Google Scholar] [CrossRef]
- Igarashi, M.; Nakagawa, N.; Doi, S.; Hattori, N.; Naganawa, H.; Hamada, M. Caprazamycin B, a novel anti-tuberculosis antibiotic, from Streptomyces sp. J. Antibiot. 2003, 56, 580–583. [Google Scholar] [CrossRef]
- Igarashi, M.; Takahashi, Y.; Shitara, T.; Nakamura, H.; Naganawa, H.; Miyake, T.; Akamatsu, Y. Caprazamycins, novel lipo-nucleoside antibiotics, from Streptomyces sp. J. Antibiot. 2005, 58, 327–337. [Google Scholar] [CrossRef] [Green Version]
- Dini, C.; Collette, P.; Drochon, N.; Guillot, J.C.; Lemoine, G.; Mauvais, P.; Aszodi, J. Synthesis of the nucleoside moiety of liposidomycins: Elucidation of the pharmacophore of this family of MraY inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 1839–1843. [Google Scholar] [CrossRef]
- Dini, C.; Drochon, N.; Feteanu, S.; Guillot, J.C.; Peixoto, C.; Aszodi, J. Synthesis of analogues of the O-.beta.-D-ribofuranosyl nucleoside moiety of liposidomycins. Part 1: Contribution of the amino group and the uracil moiety upon the inhibition of MraY. Bioorg. Med. Chem. Lett. 2001, 11, 529–531. [Google Scholar] [CrossRef]
- Dini, C.; Drochon, N.; Guillot, J.C.; Mauvais, P.; Walter, P.; Aszodi, J. Synthesis of analogues of the O-β-D-ribofuranosyl nucleoside moiety of liposidomycins. Part 2: Role of the hydroxyl groups upon the inhibition of MraY. Bioorg. Med. Chem. Lett. 2001, 11, 533–536. [Google Scholar] [CrossRef]
- Patel, B.; Ryan, P.; Makwana, V.; Zunk, M.; Rudrawar, S.; Grant, G. Caprazamycins: Promising lead structures acting on a novel antibacterial target MraY. Eur. J. Med. Chem. 2019, 171, 462–474. [Google Scholar] [CrossRef]
- Fer, M.J.; Le Corre, L.; Pietrancosta, N.; Evrard-Todeschi, N.; Olatunji, S.; Bouhss, A.; Calvet-Vitale, S.; Gravier-Pelletier, C. Bacterial transferase MraY, a source of inspiration towards new antibiotics. Curr. Med. Chem. 2018, 25, 6013–6025. [Google Scholar] [CrossRef]
- Wiegmann, D.; Koppermann, S.; Wirth, M.; Niro, G.; Leyerer, K.; Ducho, C. Muraymycin nucleoside-peptide antibiotics: Uridine-derived natural products as lead structures for the development of novel antibacterial agents. Beilstein J. Org. Chem. 2016, 12, 769–795. [Google Scholar] [CrossRef]
- Ichikawa, S.; Yamaguchi, M.; Matsuda, A. Antibacterial nucleoside natural products inhibiting phospho-MurNAc-pentapeptide translocase; chemistry and structure-activity relationship. Curr. Med. Chem. 2015, 22, 3951–3979. [Google Scholar] [CrossRef]
- Tanino, T.; Ichikawa, S.; Al-Dabbagh, B.; Bouhss, A.; Oyama, H.; Matsuda, A. Synthesis and biological evaluation of muraymycin analogues active against anti-drug-resistant bacteria. ACS Med. Chem. Lett. 2010, 1, 258–262. [Google Scholar] [CrossRef]
- Ichikawa, S.; Yamaguchi, M.; Shang Hsuan, L.; Kato, Y.; Matsuda, A. Carbacaprazamycins: Chemically stable analogues of the caprazamycin nucleoside antibiotics. ACS Infect. Dis. 2015, 1, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Wiegmann, D.; Koppermann, S.; Ducho, C. Aminoribosylated analogues of muraymycin nucleoside antibiotics. Molecules 2018, 23, 3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leyerer, K.; Koppermann, S.; Ducho, C. Solid phase-supported synthesis of muraymycin analogues. Eur. J. Org. Chem. 2019, 45, 7420–7431. [Google Scholar] [CrossRef]
- Patel, B.; Kerr, R.V.; Malde, A.K.; Zunk, M.; Bugg, T.D.H.; Grant, G.; Rudrawar, S. Simplified novel Muraymycin analogues; using a serine template strategy for linking key pharmacophores. ChemMedChem 2020, 15, 1429–1438. [Google Scholar] [CrossRef] [PubMed]
- Kwak, S.H.; Lim, W.Y.; Hao, A.; Mashalidis, E.H.; Kwon, D.Y.; Jeong, P.; Kim, M.J.; Lee, S.Y.; Hong, J. Synthesis and evaluation of cyclopentane-based muraymycin analogs targeting MraY. Eur. J. Med. Chem. 2021, 215, 113272. [Google Scholar] [CrossRef]
- Okamoto, K.; Ishikawa, A.; Okawa, R.; Yamamoto, K.; Sato, T.; Yokota, S.-I.; Chiba, K.; Ichikawa, S. Design, synthesis and biological evaluation of simplified analogues of MraY inhibitory natural product with rigid scaffold. Biorg. Med. Chem. 2022, 55, 116556. [Google Scholar] [CrossRef] [PubMed]
- Kusaka, S.; Yamamoto, K.; Shinohara, M.; Minato, Y.; Ichikawa, S. Design, synthesis and conformation-activity relationship analysis of LNA/BNA-type 5-O-aminoribosyluridine as MraY inhibitors. Biorg. Med. Chem. 2022, 65, 116744. [Google Scholar] [CrossRef]
- Chung, B.C.; Mashalidis, E.H.; Tanino, T.; Kim, M.; Matsuda, A.; Hong, J.; Ichikawa, S.; Lee, S.Y. Structural insights into inhibition of lipid I production in bacterial cell wall synthesis. Nature 2016, 533, 557–560. [Google Scholar] [CrossRef]
- Mashalidis, E.H.; Kaeser, B.; Terasawa, Y.; Katsuyama, A.; Kwon, D.Y.; Lee, K.; Hong, J.; Ichikawa, S.; Lee, S.Y. Chemical logic of MraY inhibition by antibacterial nucleoside natural products. Nat. Commun. 2019, 10, 2917–2928. [Google Scholar] [CrossRef]
- Fer, M.J.; Olatunji, S.; Bouhss, A.; Calvet-Vitale, S.; Gravier-Pelletier, C. Toward analogues of MraY natural inhibitors: Syn-thesis of 5-triazole-substitute-aminoribosyl uridines through a Cu-catalyzed azide-alkyne cycloaddition. J. Org. Chem. 2013, 78, 10088–10105. [Google Scholar] [CrossRef]
- Fer, M.J.; Bouhss, A.; Patrão, M.; Le Corre, L.; Pietrancosta, N.; Amoroso, A.; Joris, B.; Mengin-Lecreulx, D.; Calvet-Vitale, S.; Gravier-Pelletier, C. 5′-Methylene-triazole-substituted-aminoribosyl uridines as MraY inhibitors: Synthesis, biological evaluation and molecular modeling. Org. Biomol. Chem. 2015, 13, 7193–7222. [Google Scholar] [CrossRef]
- Oliver, M.; Le Corre, L.; Poinsot, M.; Corio, A.; Madegard, L.; Bosco, M.; Amoroso, A.; Joris, B.; Auger, R.; Touzé, T.; et al. Synthesis, biological evaluation and molecular modeling of urea-containing MraY inhibitors. Org. Biomol. Chem. 2021, 19, 5844–5866. [Google Scholar] [CrossRef] [PubMed]
- Oliver, M.; Le Corre, L.; Poinsot, M.; Bosco, M.; Wan, H.; Amoroso, A.; Joris, B.; Bouhss, A.; Calvet-Vitale, S.; Gravier-Pelletier, C. A sub-micromolar MraYAA Inhibitor with an aminoribosyl uridine structure and a (S,S)-tartaric diamide: Synthesis, biological evaluation and molecular modeling. Molecules 2022, 27, 1769. [Google Scholar] [CrossRef] [PubMed]
- Hirano, S.; Ichikawa, S.; Matsuda, A. Total synthesis of caprazol, a core structure of the caprazamycin antituberculosis antibiotics. Angew. Chem. Int. Ed. 2005, 44, 1854–1856. [Google Scholar] [CrossRef]
- Fer, M.J.; Doan, P.; Prangé, T.; Calvet-Vitale, S.; Gravier-Pelletier, C. A diastereoselective synthesis of 5’-substituted-uridine derivatives. J. Org. Chem. 2014, 79, 7758–7765. [Google Scholar] [CrossRef]
- Paz, N.R.; Santana, A.G.; Francisco, C.G.; Suárez, E.; González, C.C. Synthesis of tetrazole-fused glycosides by a tandem fragmentation-cyclization reaction. Org. Lett. 2012, 14, 3388–3392. [Google Scholar] [CrossRef]
- Dini, C.; Didier-Laurent, S.; Drochon, N.; Feteanu, S.; Guillot, J.C.; Monti, F.; Uridat, E.; Zhang, J.; Aszodi, J. Synthesis of sub-micromolar inhibitors of MraY by exploring the region originally occupied by the diazepanone ring in the liposidomycin structure. Biorg. Med. Chem. Lett. 2002, 12, 1209–1213. [Google Scholar] [CrossRef]
- Kourra, C.; Klotter, F.; Sladojevich, F.; Dixon, D.J. Alkali base-initiated michael addition/alkyne carbocyclization cascades. Org. Lett. 2012, 14, 1016–1019. [Google Scholar] [CrossRef]
- Wçlk, C.; Drescher, S.; Meister, A.; Blume, A.; Langner, A.; Dobner, B. General synthesis and physicochemical characterisation of a series of peptide-mimic lysine-based amino-functionalised lipids. Chem. Eur. J. 2013, 19, 12824–12838. [Google Scholar]
- Santucci, P.; Dedaki, C.; Athanasoulis, A.; Gallorini, L.; Munoz, A.; Canaan, S.; Cavalier, J.F.; Magrioti, V. Synthesis of long-chain -lactones and their antibacterial activities against pathogenic Mycobacteria. ChemMedChem 2019, 14, 349–358. [Google Scholar] [CrossRef]
- Ehsan, M.; Du, Y.; Scull, N.J.; Tikhonova, E.; Tarrasch, J.; Mortensen, J.S.; Loland, C.J.; Skiniotis, G.; Guan, L.; Byrne, B.; et al. Highly branched pentasaccharide-bearing amphiphiles for membrane protein studies. J. Am. Chem. Soc. 2016, 138, 3789–3796. [Google Scholar] [CrossRef]
- Chung, B.C.; Zhao, J.; Gillespie, R.A.; Kwon, D.Y.; Guan, Z.; Hong, J.; Zhou, P.; Lee, S.Y. Crystal structure of MraY, an essential membrane enzyme for bacterial cell wall synthesis. Science 2013, 341, 1012–1016. [Google Scholar] [CrossRef]
- Wu, G.S.; Robertson, D.H.; Brooks, C.L.; Vieth, M. Detailed analysis of grid-based molecular docking: A case study of CDOCKER-A CHARMm-based MD docking algorithm. J. Comput. Chem. 2003, 24, 1549–1562. [Google Scholar] [CrossRef]
- Stachyra, T.; Dini, C.; Ferrari, P.; Bouhss, A.; van Heijenoort, J.; Mengin-Lecreulx, D.; Blanot, D.; Bitton, J.; Le Beller, D. Fluorescence detection-based functional assay for high-throughput screening for MraY. Antimicrob. Agents Chemother. 2004, 48, 897–902. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).