A Proof of Concept of the Usefulness of a TDM-Guided Strategy for Optimizing Pharmacokinetic/Pharmacodynamic Target of Continuous Infusion Ampicillin-Based Regimens in a Case Series of Patients with Enterococcal Bloodstream Infections and/or Endocarditis
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; Laplante, K.L. A Review of Combination Antimicrobial Therapy for Enterococcus faecalis Bloodstream Infections and Infective Endocarditis. Clin. Infect. Dis. 2018, 67, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Duprè, I.; Zanetti, S.; Schito, A.M.; Fadda, G.; Sechi, L.A. Incidence of virulence determinants in clinical Enterococcus faecium and Enterococcus faecalis isolates collected in Sardinia (Italy). J. Med Microbiol. 2003, 52, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Habib, G.; Lancellotti, P.; Antunes, M.J.; Bongiorni, M.G.; Casalta, J.-P.; Del Zotti, F.; Dulgheru, R.; El Khoury, G.; Erba, P.A.; Iung, B.; et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the Management of Infective Endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM). Eur. Heart J. 2015, 36, 3075–3128. [Google Scholar] [CrossRef]
- Fernández-Hidalgo, N.; Almirante, B.; Gavaldà, J.; Gurgui, M.; Peña, C.; de Alarcón, A.; Ruiz, J.; Vilacosta, I.; Montejo, M.; Vallejo, N.; et al. Ampicillin Plus Ceftriaxone Is as Effective as Ampicillin Plus Gentamicin for Treating Enterococcus faecalis Infective Endocarditis. Clin. Infect. Dis. 2013, 56, 1261–1268. [Google Scholar] [CrossRef] [Green Version]
- Gavaldà, J.; Len, O.; Miró, J.M.; Muñoz, P.; Montejo, M.; Alarcón, A.; de la Torre-Cisneros, J.; Peña, C.; Martínez-Lacasa, X.; Sarria, C.; et al. Brief Communication: Treatment of Enterococcus Faecalis Endocarditis with Ampicillin plus Ceftriaxone. Ann. Intern. Med. 2007, 146, 574–579. [Google Scholar] [CrossRef]
- Roberts, J.A.; Abdul-Aziz, M.-H.; Lipman, J.; Mouton, J.W.; Vinks, A.A.; Felton, T.W.; Hope, W.W.; Farkas, A.; Neely, M.N.; Schentag, J.J.; et al. Individualised antibiotic dosing for patients who are critically ill: Challenges and potential solutions. Lancet Infect. Dis. 2014, 14, 498–509. [Google Scholar] [CrossRef] [Green Version]
- Gatti, M.; Pea, F. Continuous versus intermittent infusion of antibiotics in Gram-negative multidrug-resistant infections. Curr. Opin. Infect. Dis. 2021, 34, 737–747. [Google Scholar] [CrossRef]
- Abdul-Aziz, M.-H.; Alffenaar, J.-W.C.; Bassetti, M.; Bracht, H.; Dimopoulos, G.; Marriott, D.; Neely, M.N.; Paiva, J.-A.; Pea, F.; Sjovall, F.; et al. Antimicrobial therapeutic drug monitoring in critically ill adult patients: A Position Paper#. Intensive Care Med. 2020, 46, 1127–1153. [Google Scholar] [CrossRef]
- Ogawa, T.; Kasahara, K.; Ikawa, K.; Shigeta, J.; Komatsu, Y.; Kuruno, N.; Uno, K.; Maeda, K.; Mikasa, K. Continuous ampicillin infusion as an alternative to intermittent infusion for adult inpatients: A case series. J. Infect. Chemother. 2014, 20, 653–655. [Google Scholar] [CrossRef]
- Lewis, P.O.; Jones, A.; Amodei, R.J.; Youssef, D. Continuous Infusion Ampicillin for the Outpatient Management of Enterococcal Endocarditis: A Case Report and Literature Review. J. Pharm. Pract. 2020, 33, 392–394. [Google Scholar] [CrossRef]
- Parsonson, F.; Legg, M.A.; Halford, M.M.; McCarthy, K. Contemporaneous management of ampicillin infusions in the outpatient setting through the use of therapeutic drug monitoring. Int. J. Antimicrob. Agents 2020, 55, 105975. [Google Scholar] [CrossRef] [PubMed]
- Li, J.S.; Sexton, D.J.; Mick, N.; Nettles, R.; Fowler, V.G., Jr.; Ryan, T.; Bashore, T.; Corey, G.R. Proposed Modifications to the Duke Criteria for the Diagnosis of Infective Endocarditis. Clin. Infect. Dis. 2000, 30, 633–638. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Pascale, R.; Tonetti, T.; Laici, C.; Dell’Olio, A.; Siniscalchi, A.; Giannella, M.; Viale, P.; Pea, F. Assessment of a PK/PD Target of Continuous Infusion Beta-Lactams Useful for Preventing Microbiological Failure and/or Resistance Development in Critically Ill Patients Affected by Documented Gram-Negative Infections. Antibiotics 2021, 10, 1311. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Voulgaris, G.L.; Maliaros, A.; Samonis, G.; Falagas, M.E. Prolonged versus short-term intravenous infusion of antipseudomonal β-lactams for patients with sepsis: A systematic review and meta-analysis of randomised trials. Lancet Infect. Dis. 2018, 18, 108–120. [Google Scholar] [CrossRef]
- Thauvin, C.; Eliopoulos, G.M.; Willey, S.; Wennersten, C.; Moellering, R.C. Continuous-infusion ampicillin therapy of enterococcal endocarditis in rats. Antimicrob. Agents Chemother. 1987, 31, 139–143. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, T.; Sato, M.; Yonekawa, S.; Nakagawa, C.; Uno, K.; Kasahara, K.; Maeda, K.; Konishi, M.; Mikasa, K. Infective Endocarditis Caused by Enterococcus faecalis treated with Continuous Infusion of Ampicillin without Adjunctive Aminoglycosides. Intern. Med. 2013, 52, 1131–1135. [Google Scholar] [CrossRef] [Green Version]
- Lupia, T.; Roberto, G.; Scaglione, L.; Shbaklo, N.; De Benedetto, I.; Scabini, S.; Pinna, S.M.; Curtoni, A.; Cavallo, R.; De Rosa, F.G.; et al. Clinical and microbiological characteristics of bloodstream infections caused by Enterococcus spp. within internal medicine wards: A two-year single-centre experience. Intern. Emerg. Med. 2022, 17, 1129–1137. [Google Scholar] [CrossRef]
- Crass, R.L.; Rodvold, K.A.; Mueller, B.A.; Pai, M.P. Renal Dosing of Antibiotics: Are We Jumping the Gun? Clin. Infect. Dis. 2019, 68, 1596–1602. [Google Scholar] [CrossRef]
- Vardakas, K.Z.; Kalimeris, G.D.; Triarides, N.A.; Falagas, M.E. An update on adverse drug reactions related to β-lactam antibiotics. Expert Opin. Drug Saf. 2018, 17, 499–508. [Google Scholar] [CrossRef]
- Roger, C.; Louart, B. Beta-Lactams Toxicity in the Intensive Care Unit: An Underestimated Collateral Damage? Microorganisms 2021, 9, 1505. [Google Scholar] [CrossRef]
- Pea, F.; Viale, P. Bench-to-bedside review: Appropriate antibiotic therapy in severe sepsis and septic shock—Does the dose matter? Crit. Care 2009, 13, 214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bussini, L.; Del Turco, E.R.; Pasquini, Z.; Scolz, K.; Amedeo, A.; Beci, G.; Giglia, M.; Tedeschi, S.; Pascale, R.; Ambretti, S.; et al. Risk factors for persistent enterococcal bacteraemia: A multicentre retrospective study. J. Glob. Antimicrob. Resist. 2022, 29, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Sumi, C.D.; Heffernan, A.J.; Lipman, J.; Roberts, J.A.; Sime, F.B. What Antibiotic Exposures Are Required to Suppress the Emergence of Resistance for Gram-Negative Bacteria? A Systematic Review. Clin. Pharmacokinet. 2019, 58, 1407–1443. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, M.; Tedeschi, S.; Scudeller, L.; Pascale, R.; Rosselli Del Turco, E.; Trapani, F.; Tumietto, F.; Virgili, G.; Marconi, L.; Ianniruberto, S.; et al. Impact on mortality of a bundle for the management of enterococcal bloodstream infection. Open Forum Infect. Dis. 2019, 6, ofz473. [Google Scholar] [CrossRef] [PubMed]
- López-Cortés, L.E.; Del Toro, M.D.; Gálvez-Acebal, J.; Bereciartua-Bastarrica, E.; Fariñas, M.C.; Sanz-Franco, M.; Natera, C.; Corzo, J.E.; Lomas, J.M.; Pasquau, J.; et al. Impact of an Evidence-Based Bundle Intervention in the Quality-of-Care Management and Outcome of Staphylococcus aureus Bacteremia. Clin. Infect. Dis. 2013, 57, 1225–1233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paladino, J.A.; Poretz, D. Outpatient Parenteral Antimicrobial Therapy Today. Clin. Infect. Dis. 2010, 51 (Suppl. S2), S198–S208. [Google Scholar] [CrossRef] [Green Version]
- Barza, M.; Weinstein, L. Pharmacokinetics of the Penicillins in Man. Clin. Pharmacokinet. 1976, 1, 297–308. [Google Scholar] [CrossRef]
- Gatti, M.; Cojutti, P.G.; Bartoletti, M.; Tonetti, T.; Bianchini, A.; Ramirez, S.; Pizzilli, G.; Ambretti, S.; Giannella, M.; Mancini, R.; et al. Expert clinical pharmacological advice may make an antimicrobial TDM program for emerging candidates more clinically useful in tailoring therapy of critically ill patients. Crit. Care 2022, 26, 178. [Google Scholar] [CrossRef] [PubMed]
| ID Cases | Age/Sex | Ward | Isolates | Ampicillin MIC (mg/L) | Initial Ampicillin Dosage | Average CLCr (mL/min/1.73m2) | Average fCss (mg/L) | Average fCss/MIC | Combination Therapy | Dosing Adjustment | Treatment Duration (Days) | Persistent BSI | 90-Day Mortality |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Endocarditis | |||||||||||||
| #1 | 81/M | Internal medicine | E. faecalis | 2 | 2 g LD 2 g q6h CI | 53 | 30.1 | 15.6 | Ceftriaxone 2 g q12h | Reduction | 22 | No | No |
| #2 | 64/M | Cardiosurgery | No isolate | NA | 2 g LD 3 g q6h CI | 11 | 160.8 | NA | Daptomycin 700 mg q48h | Reduction | 33 | NA | No |
| #3 | 77/M | Infectious Disease Unit | E. faecalis | 1 | 2 g LD 1.5 g q6h CI | 31 | 29.84 | 29.84 | Ceftriaxone 2 g q12h | Reduction | 13 | No | No |
| #4 | 67/M | Internal medicine | No isolate | NA | 2 g LD 1.5 g q6h CI | 13 | 107 | NA | Ceftriaxone 2 g q12h | Reduction | 10 | NA | Yes |
| #5 | 62/F | Cardiosurgery | E. faecalis | 1 | 2 g LD 1.5 g q6h CI | 18 | 67.3 | 67.3 | Ceftriaxone 2 g q12h | Reduction | 29 | No | Yes |
| Bloodstream infections | |||||||||||||
| #6 | 68/M | Cardiac ICU | E. casseliflavus | 1 | 2 g LD 3 g q6h CI | 47 | 70.6 | 70.6 | None | Reduction | 21 | No | No |
| #7 | 72/M | Internal medicine | E. faecalis | 1 | 2 g LD 3 g q6h CI | 59 | 21 | 21 | Ceftriaxone 2 g q12h | Reduction | 10 | No | No |
| #8 | 70/M | Urology | E. faecalis | 1 | 2 g LD 2 g q6h CI | 61 | 17.8 | 17.8 | Ceftriaxone 2 g q12h | Confirmation | 10 | No | No |
| #9 | 56/F | Cardiosurgery | E. faecalis | 1 | 2 g LD 3 g q6h CI | 75 | 35.4 | 35.4 | Ceftriaxone 2 g q12h | Reduction | 14 | No | No |
| #10 | 61/F | COVID ICU | E. faecalis | 4 | 2 g LD 3 g q6h CI | CVVHDF | 64 | 16 | None | Reduction | 10 | No | Yes |
| #11 | 83/M | Internal medicine | E. faecalis | 1 | 2 g LD 3 g q6h CI | 33 | 148 | 148 | Ceftriaxone 2 g q12h | Reduction | 7 | No | No |
| #12 | 71/F | Internal medicine | E. faecalis | 1 | 2 g LD 2 g q6h CI | 114 | 81 | 81 | Ceftriaxone 2 g q12h | Reduction | 14 | No | No |
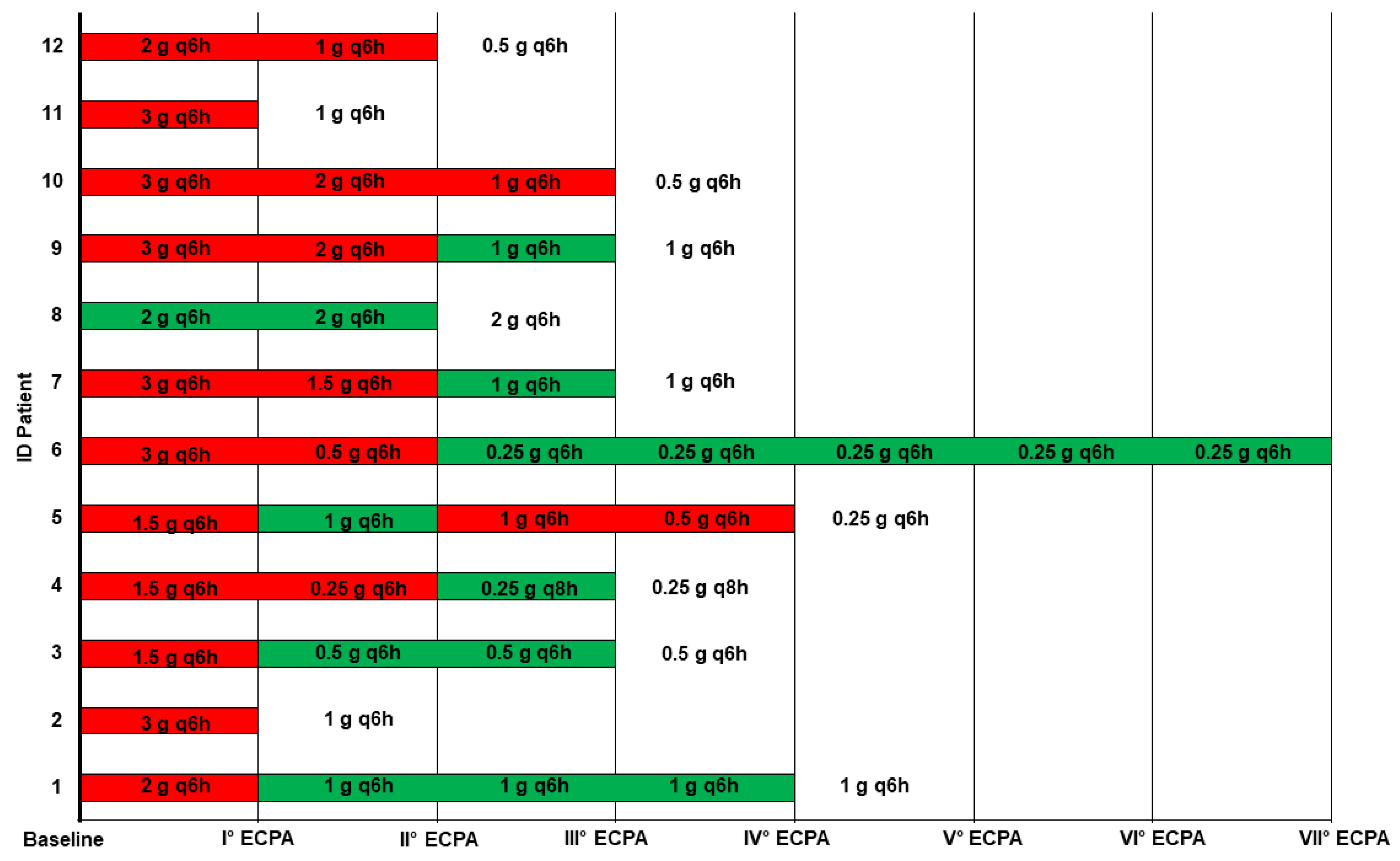
| ID Case | Day | Ampicillin | |
|---|---|---|---|
| Dosage | fCss/MIC | ||
| #1 | 3 | 2 g q6h | 21.2 |
| 7 | 1 g q6h | 11.8 | |
| 11 | 1 g q6h | 9.1 | |
| 13 | 1 g q6h | 11.4 | |
| #2 | 3 | 3 g q6h | 40.2 |
| #3 | 3 | 1.5 g q6h | 48.9 |
| 6 | 0.5 g q6h | 24.6 | |
| 8 | 0.5 g q6h | 15.8 | |
| #4 | 3 | 1.5 g q6h | 39.8 |
| 6 | 0.25 g q6h | 30.4 | |
| 8 | 0.25 g q8h | 10.0 | |
| #5 | 3 | 1.5 g q6h | 39.2 |
| 8 | 1 g q6h | 45.0 | |
| 11 | 1 g q6h | 57.6 | |
| 14 | 0.5 g q6h | 127.2 | |
| #6 | 3 | 3 g q6h | 292.0 |
| 6 | 0.5 g q6h | 93.6 | |
| 10 | 0.25 g q6h | 9.3 | |
| 13 | 0.25 g q6h | 9.1 | |
| 17 | 0.25 g q6h | 7.9 | |
| 19 | 0.25 g q6h | 10.3 | |
| 21 | 0.25 g q6h | 9.7 | |
| #7 | 2 | 3 g q6h | 29.8 |
| 4 | 1.5 g q6h | 20.4 | |
| 7 | 1 g q6h | 12.8 | |
| #8 | 3 | 2 g q6h | 21.1 |
| 6 | 2 g q6h | 14.6 | |
| #9 | 2 | 3 g q6h | 43.6 |
| 4 | 2 g q6h | 46.7 | |
| 7 | 1 g q6h | 15.7 | |
| #10 | 3 | 3 g q6h | 18.3 |
| 6 | 2 g q6h | 16.0 | |
| 8 | 1 g q6h | 13.7 | |
| #11 | 3 | 3 g q6h | 148.0 |
| #12 | 3 | 2 g q6h | 96.0 |
| 6 | 1 g q6h | 66.0 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gatti, M.; Tedeschi, S.; Trapani, F.; Ramirez, S.; Mancini, R.; Giannella, M.; Viale, P.; Pea, F. A Proof of Concept of the Usefulness of a TDM-Guided Strategy for Optimizing Pharmacokinetic/Pharmacodynamic Target of Continuous Infusion Ampicillin-Based Regimens in a Case Series of Patients with Enterococcal Bloodstream Infections and/or Endocarditis. Antibiotics 2022, 11, 1037. https://doi.org/10.3390/antibiotics11081037
Gatti M, Tedeschi S, Trapani F, Ramirez S, Mancini R, Giannella M, Viale P, Pea F. A Proof of Concept of the Usefulness of a TDM-Guided Strategy for Optimizing Pharmacokinetic/Pharmacodynamic Target of Continuous Infusion Ampicillin-Based Regimens in a Case Series of Patients with Enterococcal Bloodstream Infections and/or Endocarditis. Antibiotics. 2022; 11(8):1037. https://doi.org/10.3390/antibiotics11081037
Chicago/Turabian StyleGatti, Milo, Sara Tedeschi, Filippo Trapani, Stefania Ramirez, Rita Mancini, Maddalena Giannella, Pierluigi Viale, and Federico Pea. 2022. "A Proof of Concept of the Usefulness of a TDM-Guided Strategy for Optimizing Pharmacokinetic/Pharmacodynamic Target of Continuous Infusion Ampicillin-Based Regimens in a Case Series of Patients with Enterococcal Bloodstream Infections and/or Endocarditis" Antibiotics 11, no. 8: 1037. https://doi.org/10.3390/antibiotics11081037
APA StyleGatti, M., Tedeschi, S., Trapani, F., Ramirez, S., Mancini, R., Giannella, M., Viale, P., & Pea, F. (2022). A Proof of Concept of the Usefulness of a TDM-Guided Strategy for Optimizing Pharmacokinetic/Pharmacodynamic Target of Continuous Infusion Ampicillin-Based Regimens in a Case Series of Patients with Enterococcal Bloodstream Infections and/or Endocarditis. Antibiotics, 11(8), 1037. https://doi.org/10.3390/antibiotics11081037







