Prevalence of Staphylococcus aureus and mec-A Cassette in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy
Abstract
1. Introduction
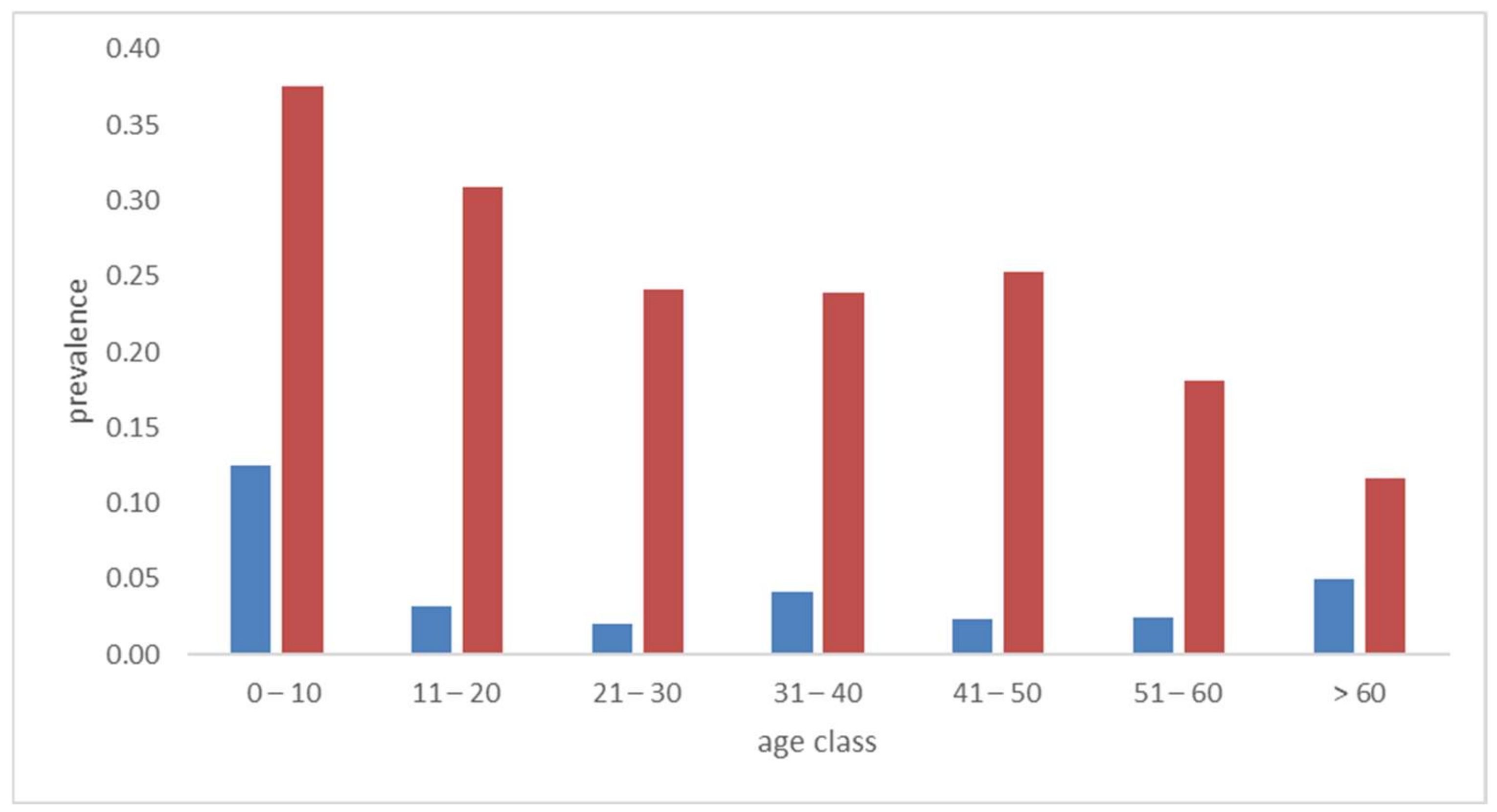
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Molecular Analysis
4.3. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Senn, L.; Basset, P.; Nahimana, I.; Zanetti, G.; Blanc, D.S. Which anatomical sites should be sampled for screening of methicillin-resistant Staphylococcus aureus carriage by culture or by rapid PCR test? Clin. Microbiol. Infect. 2012, 18, E31–E33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wertheim, H.F.; Melles, D.C.; Vos, M.C.; van Leeuwen, W.; van Belkum, A.; Verbrugh, H.A.; Nouwen, J.L. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect. Dis. 2005, 5, 751–762. [Google Scholar] [CrossRef]
- Sollid, J.U.; Furberg, A.S.; Hanssen, A.M.; Johannessen, M. Staphylococcus aureus: Determinants of human carriage. Infect. Genet. Evol. 2014, 21, 531–541. [Google Scholar] [CrossRef]
- Von Eiff, C.; Becker, K.; Machka, K.; Stammer, H.; Peters, G. Nasal carriage as a source of Staphylococcus aureus bacteremia. Study Group. N. Engl. J. Med. 2001, 344, 11–16. [Google Scholar] [CrossRef]
- Klein, E.; Smith, D.L.; Laxminarayan, R. Hospitalizations and deaths caused by methicillin-resistant Staphylococcus aureus, United States, 1999–2005. Emerg. Infect. Dis. 2007, 13, 1840–1846. [Google Scholar] [CrossRef]
- Hanssen, A.M.; Ericson Sollid, J.U. SCCmec in staphylococci: Genes on the move. FEMS Immunol. Med. Microbiol. 2006, 46, 8–20. [Google Scholar] [CrossRef]
- Couto, I.; de Lencastre, H.; Severina, E.; Kloos, W.; Webster, J.A.; Hubner, R.J.; Sanches, I.S.; Tomasz, A. Ubiquitous presence of a mecA homologue in natural isolates of Staphylococcus sciuri. Microb. Drug Resist. 1996, 2, 377–391. [Google Scholar] [CrossRef]
- Archer, G.L.; Niemeyer, D.M. Origin and evolution of DNA associated with resistance to methicillin in staphylococci. Trends Microbiol. 1994, 2, 343–347. [Google Scholar] [CrossRef]
- Chambers, H.F.; Deleo, F.R. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat. Rev. Microbiol. 2009, 7, 629–641. [Google Scholar] [CrossRef]
- Grundmann, H.; Aires-de-Sousa, M.; Boyce, J.; Tiemersma, E. Emergence and resurgence of meticillin-resistant Staphylococcus aureus as a public-health threat. Lancet 2006, 368, 874–885. [Google Scholar] [CrossRef]
- Hischebeth, G.T.; Randau, T.M.; Ploeger, M.M.; Friedrich, M.J.; Kaup, E.; Jacobs, C.; Molitor, E.; Hoerauf, A.; Gravius, S.; Wimmer, M.D. Staphylococcus aureus versus Staphylococcus epidermidis in periprosthetic joint infection-Outcome analysis of methicillin-resistant versus methicillin-susceptible strains. Diagn. Microbiol. Infect. Dis. 2019, 93, 125–130. [Google Scholar] [CrossRef]
- La Vecchia, A.; Ippolito, G.; Taccani, V.; Gatti, E.; Bono, P.; Bettocchi, S.; Pinzani, R.; Tagliabue, C.; Bosis, S.; Marchisio, P.; et al. Epidemiology and antimicrobial susceptibility of Staphylococcus aureus in children in a tertiary care pediatric hospital in Milan, Italy, 2017–2021. Ital. J. Pediatr. 2022, 48, 67. [Google Scholar] [CrossRef]
- Volgenant, C.M.C.; Hoogenkamp, M.A.; Dahlen, G.; Kalfas, S.; Petti, S.; De Soet, J.J. Low prevalence of multi-resistant bacteria in undergraduate dental students; an observational case-control multi-centre study in Europe. J. Oral Microbiol. 2021, 13, 1889898. [Google Scholar] [CrossRef]
- Monaco, M.; Pedroni, P.; Sanchini, A.; Bonomini, A.; Indelicato, A.; Pantosti, A. Livestock-associated methicillin-resistant Staphylococcus aureus responsible for human colonization and infection in an area of Italy with high density of pig farming. BMC Infect. Dis. 2013, 13, 258. [Google Scholar] [CrossRef]
- Abdelmalek, S.M.A.; Qinna, M.W.; Al-Ejielat, R.; Collier, P.J. Methicillin-Resistant Staphylococci (MRS): Carriage and Antibiotic Resistance Patterns in College Students. J. Community Health 2022, 47, 416–424. [Google Scholar] [CrossRef]
- Wielders, C.L.; Vriens, M.R.; Brisse, S.; de Graaf-Miltenburg, L.A.; Troelstra, A.; Fleer, A.; Schmitz, F.J.; Verhoef, J.; Fluit, A.C. In-vivo transfer of mecA DNA to Staphylococcus aureus [corrected]. Lancet 2001, 357, 1674–1675. [Google Scholar] [CrossRef]
- Zanelli, G.; Sansoni, A.; Zanchi, A.; Cresti, S.; Pollini, S.; Rossolini, G.M.; Cellesi, C. Staphylococcus aureus nasal carriage in the community: A survey from central Italy. Epidemiol. Infect. 2002, 129, 417–420. [Google Scholar] [CrossRef]
- Gorwitz, R.J.; Kruszon-Moran, D.; McAllister, S.K.; McQuillan, G.; McDougal, L.K.; Fosheim, G.E.; Jensen, B.J.; Killgore, G.; Tenover, F.C.; Kuehnert, M.J. Changes in the prevalence of nasal colonization with Staphylococcus aureus in the United States, 2001–2004. J. Infect. Dis. 2008, 197, 1226–1234. [Google Scholar] [CrossRef]
- Anwar, M.S.; Jaffery, G.; Rehman Bhatti, K.U.; Tayyib, M.; Bokhari, S.R. Staphylococcus aureus and MRSA nasal carriage in general population. J. Coll. Physicians Surg. Pak. 2004, 14, 661–664. [Google Scholar]
- Orlando, V.; Monetti, V.M.; Moreno Juste, A.; Russo, V.; Mucherino, S.; Trama, U.; Guida, A.; Menditto, E. Drug Utilization Pattern of Antibiotics: The Role of Age, Sex and Municipalities in Determining Variation. Risk Manag. Healthc. Policy 2020, 13, 63–71. [Google Scholar] [CrossRef]
- Palmieri, A.; Lauritano, D.; Pellati, A.; Scapoli, L.; Arcuri, C.; Baggi, L.; Gatto, R.; Carinci, F. Prevalence of Human Papillomavirus in the Oropharynx of Healthy Individuals in an Italian Population. J. Clin. Med. 2022, 11, 1935. [Google Scholar] [CrossRef]
- Ye, J.; Coulouris, G.; Zaretskaya, I.; Cutcutache, I.; Rozen, S.; Madden, T.L. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform. 2012, 13, 134. [Google Scholar] [CrossRef]
- Wang, K.; Li, H.; Xu, Y.; Shao, Q.; Yi, J.; Wang, R.; Cai, W.; Hang, X.; Zhang, C.; Cai, H.; et al. MFEprimer-3.0: Quality control for PCR primers. Nucleic Acids Res. 2019, 47, W610–W613. [Google Scholar] [CrossRef]

| No | Yes | |||
|---|---|---|---|---|
| Male | 527 | 61% | 334 | 39% |
| Smoking | 568 | 66% | 293 | 34% |
| Alcohol | 537 | 62% | 235 | 37% |
| mec-A | Total | |||
|---|---|---|---|---|
| (−) | (+) | |||
| S. aureus | (−) | 650 | 12 | 662 |
| (+) | 184 | 15 | 199 | |
| Total | 834 | 27 | 861 | |
| S. aureus | p Value | OR (95% C.I.) | |||
|---|---|---|---|---|---|
| (−) | (+) | ||||
| Sex | Male | 257 | 77 | 0.97 | 1.0 (0.73–1.4) |
| Female | 405 | 122 | |||
| Smoking | (−) | 449 | 119 | 0.04 | 1.4 (1.0–2.0) |
| (+) | 213 | 80 | |||
| Alcohol | (−) | 425 | 112 | <0.05 | 1.4 (1.0–1.9) |
| (+) | 238 | 87 | |||
| mec-A | p value | OR (95% C.I.) | |||
| (−) | (+) | ||||
| Sex | Male | 323 | 11 | 0.83 | 0.92 (0.42–2.0) |
| Female | 511 | 16 | |||
| Smoking | (−) | 548 | 20 | 0.37 | 0.67 (0.28–1.6) |
| (+) | 286 | 7 | |||
| Alcohol | (−) | 520 | 17 | 0.94 | 0.97 (0.44–2.1) |
| (+) | 315 | 10 | |||
| Primer (5′–3′) | Probe (5′–3′) | |
|---|---|---|
| S. aureus nuc | F-cacctgaaacaaagcatcctaaa | CY5-tggtcctgaagcaagtgcatttacgaaa |
| R-gacctttgtcaaactcgacttca | ||
| SCCmec/mec-A | F-agttagattgggatcatagcgtca | JOE-ccaggaatgcagaaagaccaaagcataca |
| R-gccaattccacattgtttcg | ||
| S. epidermidis | F-gaaccttaccaaatcttgacatcctc | FAM-ccctctagagatagagttttccccttcggg |
| R-tgcaccacctgtcactctgtc | ||
| Total bacteria | F-acgcgargaccttacchr | FAM-cacgagctgacgacarccatgca |
| R-gsacttaasccracatctca |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scapoli, L.; Palmieri, A.; Pellati, A.; Carinci, F.; Lauritano, D.; Arcuri, C.; Baggi, L.; Gatto, R.; Martinelli, M. Prevalence of Staphylococcus aureus and mec-A Cassette in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy. Antibiotics 2022, 11, 949. https://doi.org/10.3390/antibiotics11070949
Scapoli L, Palmieri A, Pellati A, Carinci F, Lauritano D, Arcuri C, Baggi L, Gatto R, Martinelli M. Prevalence of Staphylococcus aureus and mec-A Cassette in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy. Antibiotics. 2022; 11(7):949. https://doi.org/10.3390/antibiotics11070949
Chicago/Turabian StyleScapoli, Luca, Annalisa Palmieri, Agnese Pellati, Francesco Carinci, Dorina Lauritano, Claudio Arcuri, Luigi Baggi, Roberto Gatto, and Marcella Martinelli. 2022. "Prevalence of Staphylococcus aureus and mec-A Cassette in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy" Antibiotics 11, no. 7: 949. https://doi.org/10.3390/antibiotics11070949
APA StyleScapoli, L., Palmieri, A., Pellati, A., Carinci, F., Lauritano, D., Arcuri, C., Baggi, L., Gatto, R., & Martinelli, M. (2022). Prevalence of Staphylococcus aureus and mec-A Cassette in the Throat of Non-Hospitalized Individuals Randomly Selected in Central Italy. Antibiotics, 11(7), 949. https://doi.org/10.3390/antibiotics11070949








