Investigating the Antituberculosis Activity of Selected Commercial Essential Oils and Identification of Active Constituents Using a Biochemometrics Approach and In Silico Modeling
Abstract
1. Introduction
2. Results
2.1. Antimycobacterial Activity of Essential Oils
2.2. Chemical Profiles of the Essential Oils
2.3. Identification of Bioactive Compounds Using Biochemometrics
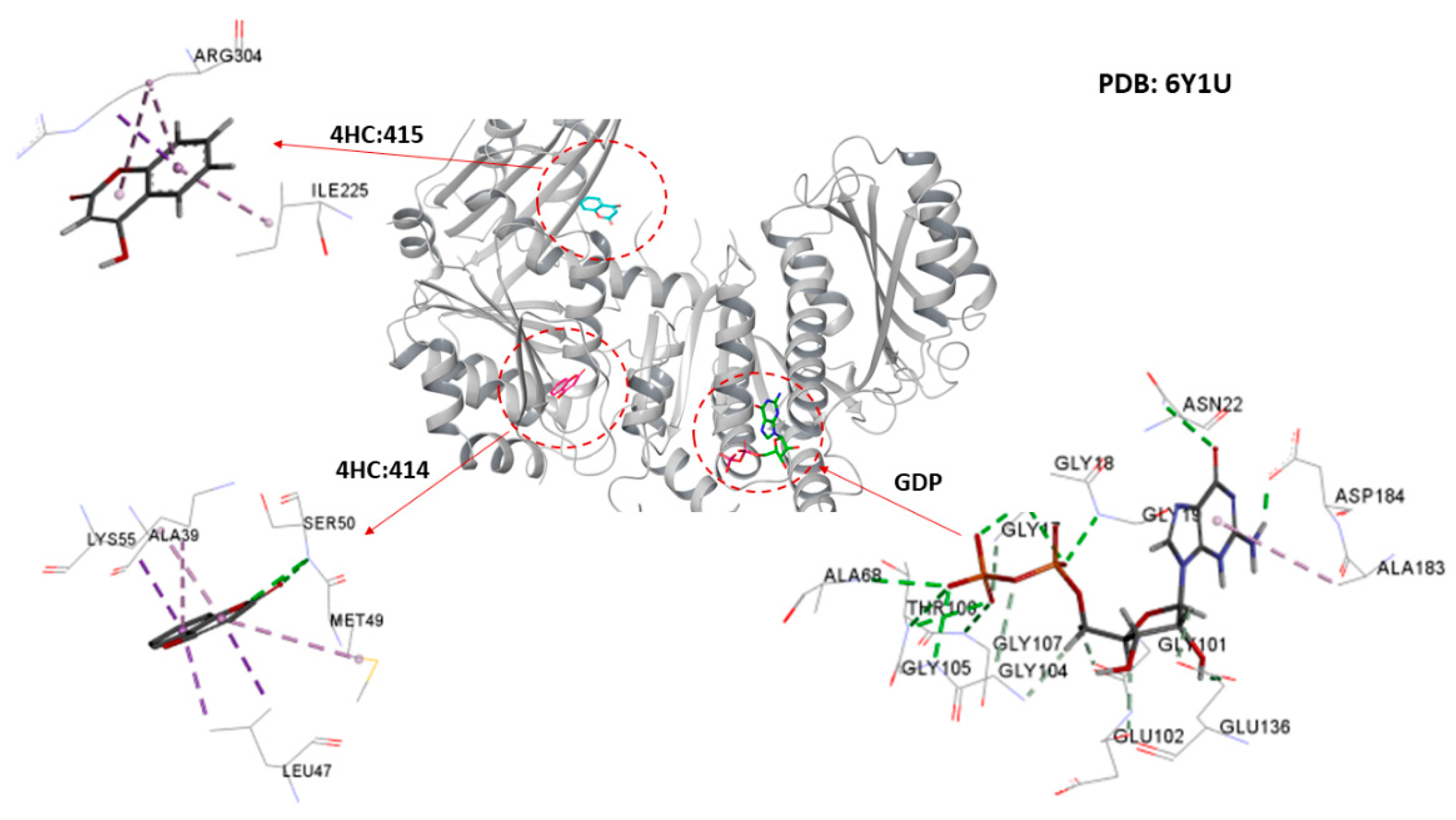
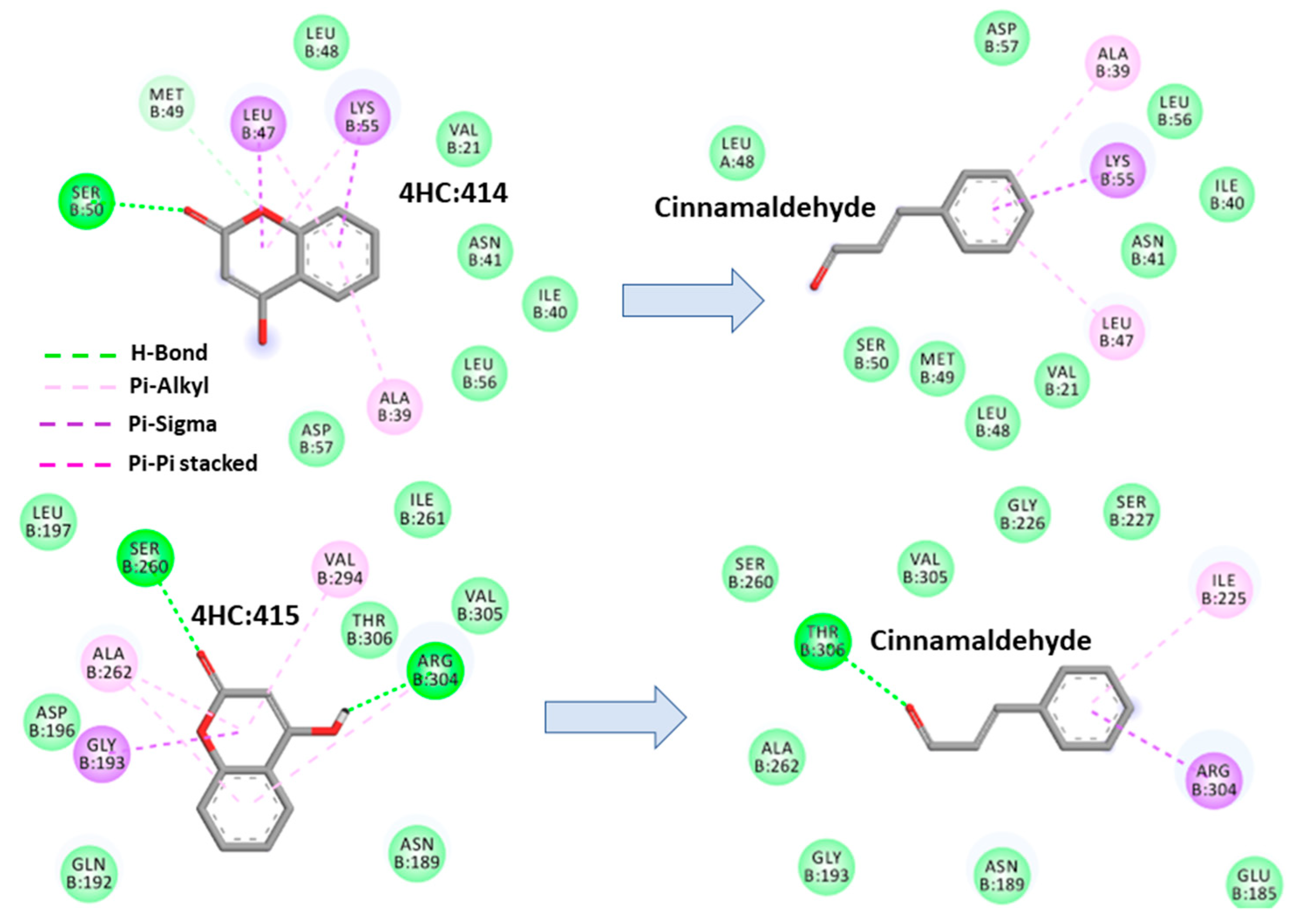
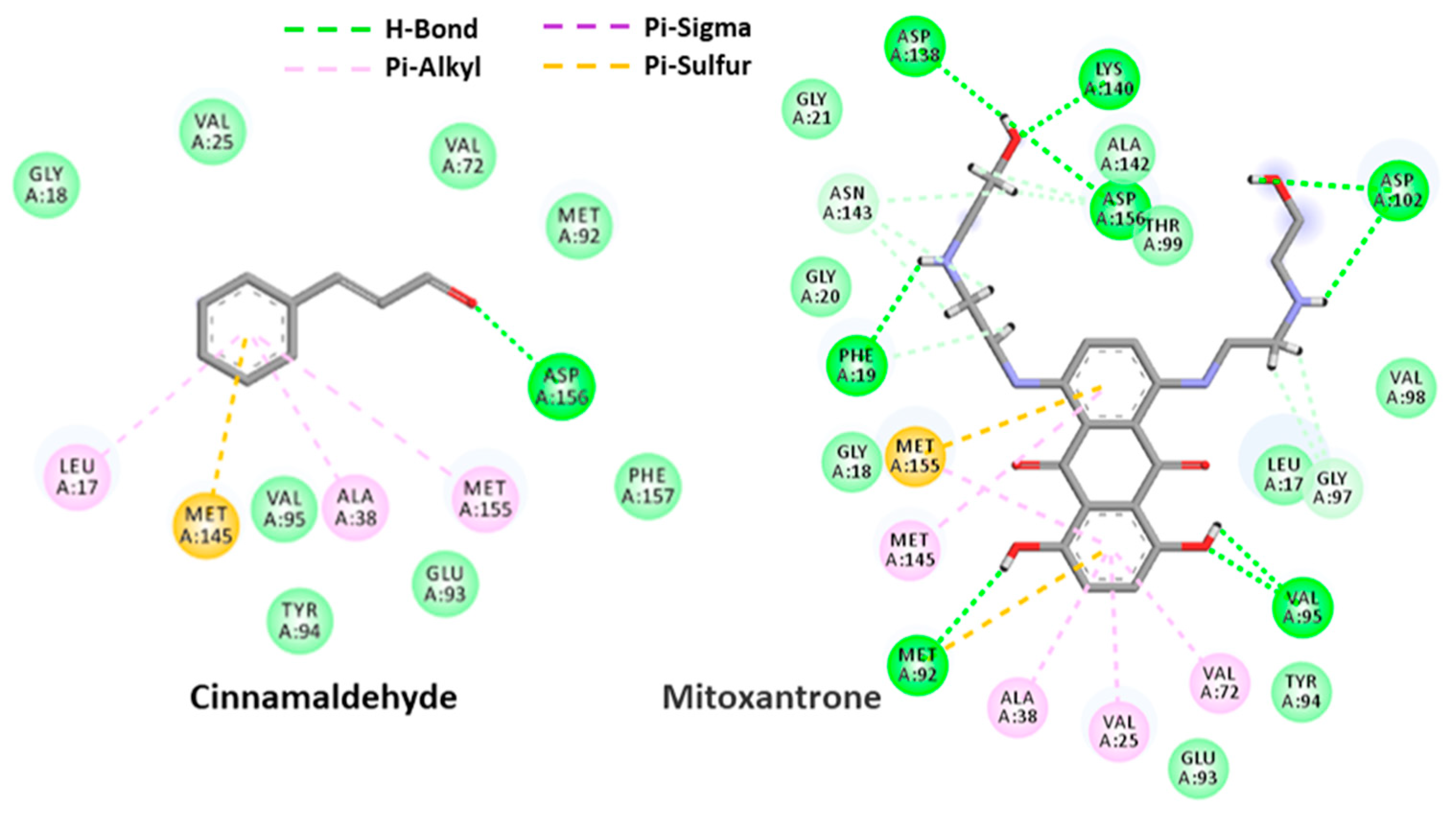
2.4. Molecular Docking Analysis
2.4.1. Cinnamaldehyde-FtsZ and Binding Modes Analysis
2.4.2. Cinnamaldehyde-PknB Binding Mode Analysis
3. Discussion
4. Materials and Methods
4.1. Essential Oils, Chemical Reagents and Solutions
4.2. Mycobacterial Strains, Media and Culture Conditions
4.3. Antimycobacterial Activity Determination
4.3.1. The Microdilution Assay
4.3.2. The Alamar Blue Assay
4.4. GC-MS Analysis of the Essential Oils
4.5. Biochemometrics Analysis
4.6. Antimycobacterial Validation Studies for Identified Active Compounds
4.7. Molecular Docking
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- MedicineNet. Tuberculosis (TB). 2019. Available online: https://www.medicinenet.com/tuberculosis_tb_facts/article.htm (accessed on 22 May 2019).
- National Health Services (NHS UK). 2019. Available online: https://www.nhs.uk/conditions/tuberculosis-tb/ (accessed on 15 April 2020).
- World Health Organization (WHO). Global Tuberculosis Report 2019; World Health Organization: Geneva, Switzerland, 2019; ISBN 978-92-4-156571-4. [Google Scholar]
- Petersen, E.; Rao, M.; Ippolito, G.; Gualano, G.; Chakaya, J.; Ntoumi, F.; Moore, D.; Allen, R.; Gaskell, K.; Öhd, J.N.; et al. Editorial: World Tuberculosis Day March 24th; 2019; Theme: “It’s TIME”—International Journal of Infectious Diseases Tuberculosis Theme Series. Int. J. Infect. Dis. 2019, 80, S1–S5. [Google Scholar] [CrossRef] [PubMed]
- Brenner, G.M.; Stevens, C.W. ‘Chemotherapy’ in Pharmacology, 4th ed.; Elsevier Saunders: Philadelphia, PA, USA, 2006; pp. 386–391, 424–428. [Google Scholar]
- Mishra, S.K.; Tripathi, G.; Kishore, N.; Singh, R.K.; Singh, A.; Tiwari, V.K. Drug development against tuberculosis: Impact of alkaloids. Eur. J. Med. Chem. 2017, 137, 504–544. [Google Scholar] [CrossRef] [PubMed]
- Velayati, A.A.; Farnia, P.; Farahbod, A.M. Overview of drug-resistant tuberculosis worldwide. Int. J. Mycobacteriol. 2016, 5, S161. [Google Scholar] [CrossRef] [PubMed]
- Raut, J.S.; Karuppayil, S.M. A status review on the medicinal properties of essential oils. Ind. Crops Prod. 2014, 62, 250–264. [Google Scholar] [CrossRef]
- Pandey, A.K.; Kumar, P.; Singh, P.; Tripathi, N.N.; Bajpai, V.K. Essential oils: Sources of antimicrobials and food preservatives: Mini-review. Front. Microbiol. 2017, 7, 02161. [Google Scholar] [CrossRef]
- Puressentiel. A Brief History of Aromatherapy. 2019. Available online: https://bit.ly/33mh0LC (accessed on 22 April 2019).
- Chávez-González, M.L.; Rodríguez-Herrera, R.; Aguilar, C.N. Essential oils: A natural alternative to combat antibiotics resistance. In Antibiotic Resistance, Mechanisms and New Antimicrobial Approaches; Kon, K., Rai, M., Eds.; Elsevier Academic Press: London, UK, 2016; pp. 227–237. [Google Scholar]
- Crandall, P.G.; Ricke, S.C.; O’Bryan, C.A.; Parrish, N.M. In vitro effects of citrus oils against Mycobacterium tuberculosis and non-tuberculous Mycobacteria of clinical importance. J. Environ. Sci. Health–B 2012, 47, 736–741. [Google Scholar] [CrossRef]
- Bernuci, K.Z.; Iwanaga, C.C.; Fernandez-Andrade, C.M.M.; Lorenzetti, F.B.; Torres-Santos, E.C.; Faiões, V.D.S.; Gonçalves, J.E.; Do Amaral, W.; Deschamps, C.; Scodro, R.B.D.L.; et al. Evaluation of chemical composition and antileishmanial and antituberculosis activities of essential oils of Piper species. Molecules 2016, 21, 1698. [Google Scholar] [CrossRef]
- Baldin, V.P.; de Lima Scodro, R.B.; Lopes-Ortiz, M.A.; de Almeida, A.L.; Gazim, Z.C.; Ferarrese, L.; dos Santos Faiões, V.; Torres-Santos, E.C.; Pires, C.T.A.; Caleffi-Ferracioli, K.R.; et al. Anti-mycobacterium tuberculosis activity of essential oil and 6,7-dehydroroyleanone isolated from leaves of Tetradenia riparia (Hochst.) Codd (Lamiaceae). Phytomedicine 2018, 47, 34–39. [Google Scholar] [CrossRef]
- Caesar, L.K.; Kellogg, J.J.; Kvalheim, O.M.; Cech, N.B. Opportunities and limitations for untargeted mass spectrometry metabolomics to identify biologically active constituents in complex natural product mixtures. J. Nat. Prod. 2019, 82, 469–484. [Google Scholar] [CrossRef]
- Kumar, K.; Awasthi, D.; Berger, W.T.; Tonge, P.J.; Slayden, R.A.; Ojima, I. Discovery of anti-TB agents that target the cell-division protein FtsZ. Future Med. Chem. 2010, 2, 1305–1323. [Google Scholar] [CrossRef]
- Kumar, K.; Awasthi, D.; Lee, S.-Y.; Zanardi, I.; Ruzsicska, B.; Knudson, S.; Tonge, P.J.; Slayden, R.A.; Ojima, I. Novel trisubstituted benzimidazoles; targeting Mtb FtsZ; as a new class of antitubercular agents. J. Med. Chem. 2011, 54, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhu, N.; Han, Y.; Jiang, J.; Si, S. Identification of anti-tuberculosis agents that target the cell-division protein FtsZ. J. Antibiot. 2010, 67, 671–676. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ur Rahman, M.; Wang, P.; Wang, N.; Chen, Y. A key bacterial cytoskeletal cell division protein FtsZ as a novel therapeutic antibacterial drug target. Bosn. J. Basic Med. Sci. 2020, 20, 310–318. [Google Scholar] [CrossRef]
- Silber, N.; Matos de Opitz, C.L.; Mayer, C.; Sass, P. Cell division protein FtsZ: From structure and mechanism to antibiotic target. Future Microbiol. 2020, 15, 801–831. [Google Scholar] [CrossRef]
- Oh, S.; Trifonov, L.; Yadav, V.D.; Barry, C.E.; Boshoff, H.I. Tuberculosis drug discovery: A decade of hit assessment for defined targets. Front. Cell. Infect. Microbiol. 2011, 11, 611304. [Google Scholar] [CrossRef]
- Wehenkel, A.; Fernandez, P.; Bellinzoni, M.; Catherinot, V.; Barilone, N.; Labesse, G.; Jackson, M.; Alzari, P.M. The structure of PknB in complex with mitoxantrone, an ATP-competitive inhibitor, suggests a mode of protein kinase regulation in mycobacteria. FEBS Lett. 2006, 580, 3018–3022. [Google Scholar] [CrossRef]
- Jani, C.; Eoh, H.; Lee, J.J.; Hamasha, K.; Sahana, M.B.; Han, J.S.; Nyayapathy, S.; Lee, J.Y.; Suh, J.W.; Lee, S.H.; et al. Regulation of polar peptidoglycan biosynthesis by Wag31 phosphorylation in mycobacteria. BMC Microbiol. 2010, 10, 327. [Google Scholar] [CrossRef]
- Chawla, Y.; Upadhyay, S.; Khan, S.; Nagarajan, S.N.; Forti, F.; Nandicoori, V.K. Protein kinase B (PknB) of Mycobacterium tuberculosis is essential for growth of the pathogen in vitro as well as for survival within the host. J. Biol. Chem. 2014, 289, 13858–13875. [Google Scholar] [CrossRef]
- Kongkham, B.; Prabakaran, D.; Puttaswamy, H. Opportunities and challenges in managing antibiotic resistance in bacteria using plant secondary metabolites. Fitoterapia 2020, 147, 104762. [Google Scholar] [CrossRef]
- Alnami, A.; Norton, R.S.; Pena, H.P.; Haider, S.; Kozielski, F. Conformational flexibility of a highly conserved helix controls cryptic pocket formation in FtsZ. J. Mol. Biol. 2021, 433, 167061. [Google Scholar] [CrossRef]
- Valero, M.; Giner, M.J. Effects of antimicrobial components of essential oils on growth of Bacillus cereus INRA L2104 in and the sensory qualities of carrot broth. Int. J. Food Microbiol. 2006, 106, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Leuschner, R.G.; Ielsch, V. Antimicrobial effects of garlic; clove and red-hot chilli on Listeria monocytogenes in broth model systems and soft cheese. Int. J. Food Sci. Nutr. 2003, 54, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Oussalah, M.; Caillet, S.; Saucier, L.; Lacroix, M. Inhibitory effects of selected plant essential oils on the growth of four pathogenic bacteria: E. coli O157: H7, Salmonella typhimurium, Staphylococcus aureus and Listeria monocytogenes. Food Control 2007, 18, 414–420. [Google Scholar] [CrossRef]
- Azizkhani, M.; Elizaquível, P.; Sánchez, G.; Selma, M.V.; Aznar, R. Comparative efficacy of Zataria multiflora Boiss.; Origanum compactum and Eugenia caryophyllus essential oils against E. coli O157: H7; feline calicivirus and endogenous microbiota in commercial baby-leaf salads. Int. J. Food Microbiol. 2013, 166, 249–255. [Google Scholar] [CrossRef] [PubMed]
- Maree, J.; Kamatou, G.; Gibbons, S.; Viljoen, A.; van Vuuren, S. The application of GC-MS combined with chemometrics for the identification of antimicrobial compounds from selected commercial essential oils. Chemometr. Intell. Lab. Syst. 2014, 130, 172–181. [Google Scholar] [CrossRef]
- Qian, W.; Sun, Z.; Wang, T.; Yang, M.; Liu, M.; Zhang, J.; Li, Y. Antimicrobial activity of eugenol against carbapenem-resistant Klebsiella pneumoniae and its effect on biofilms. Microb. Pathog. 2020, 139, 103924–103932. [Google Scholar] [CrossRef] [PubMed]
- Vitali, L.A.; Beghelli, D.; Nya, P.C.B.; Bistoni, O.; Cappellacci, L.; Damiano, S.; Lupidi, G.; Maggi, F.; Orsomando, G.; Papa, F.; et al. Diverse biological effects of the essential oil from Iranian Trachyspermum ammi. Arab. J. Chem. 2016, 9, 775–786. [Google Scholar] [CrossRef]
- Baran, A.I.; Jahanghiri, F.; Hajipour, N.; Sparagano, O.A.E.; Norouzi, R.; Moharramnejad, S. In vitro acaricidal activity of essential oil and alcoholic extract of Trachyspermum ammi against Dermanyssus gallinae. Vet. Parasitol. 2020, 278, 109030. [Google Scholar] [CrossRef]
- Yanishlieva, N.V.; Marinova, E.M.; Gordon, M.H.; Raneva, V.G. Antioxidant activity and mechanism of action of thymol and carvacrol in two lipid systems. Food Chem. 1999, 64, 59–66. [Google Scholar] [CrossRef]
- Haeseler, G.; Maue, D.; Grosskreutz, J.; Bufler, J.; Nentwig, B.; Piepenbrock, S.; Dengler, R.; Leuwer, M. Voltage-dependent block of neuronal and skeletal muscle sodium channels by thymol and menthol. Eur. J. Anaesthesiol. 2000, 19, 571–579. [Google Scholar] [CrossRef]
- Magyar, J.; Szentandrássy, N.; Bányász, T.; Fülöp, L.; Varró, A.; Nánási, P.P. Effects of thymol on calcium and potassium currents in canine and human ventricular cardiomyocytes. Br. J. Pharmacol. 2002, 136, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Nostro, A.; Blanco, A.R.; Cannatelli, M.A.; Enea, V.; Flamini, G.; Morelli, I.; Sudano Roccaro, A.; Alonzo, V. Susceptibility of methicillin-resistant staphylococci to oregano essential oil; carvacrol and thymol. FEMS Microbiol. Lett. 2004, 230, 191–195. [Google Scholar] [CrossRef]
- Braga, P.C.; Dal Sasso, M.; Culici, M.; Bianchi, T.; Bordoni, L.; Marabini, L. Anti-inflammatory activity of thymol: Inhibitory effect on the release of human neutrophil elastase. Pharmacology 2006, 77, 130–136. [Google Scholar] [CrossRef]
- Rota, M.C.; Herrera, A.; Martínez, R.M.; Sotomayor, J.A.; Jordán, M.J. Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control 2008, 19, 681–687. [Google Scholar] [CrossRef]
- Mendes, S.S.; Bomfim, R.R.; Jesus, H.C.R.; Alves, P.B.; Blank, A.F.; Estevam, C.S.; Antoniolli, A.R.; Thomazzi, S.M. Evaluation of the analgesic and anti-inflammatory effects of the essential oil of Lippia gracilis leaves. J. Ethnopharmacol. 2010, 129, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Peixoto-Neves, D.; Silva-Alves, K.S.; Gomes, M.D.M.; Lima, F.C.; Lahlou, S.; Magalhães, P.J.C.; Ceccatto, V.M.; Coelho-de-Souza, A.N.; Leal-Cardoso, J.H. Vasorelaxant effects of the monoterpenic phenol isomers; carvacrol and thymol; on rat isolated aorta. Fundam. Clin. Pharmacol. 2010, 24, 341–350. [Google Scholar] [CrossRef] [PubMed]
- De Castro, R.D.; de Souza, T.M.P.A.; Bezerra, L.M.D.; Ferreira, G.L.S.; de Brito Costa, E.M.M.; Cavalcanti, A.L. Antifungal activity and mode of action of thymol and its synergism with nystatin against Candida species involved with infections in the oral cavity: An in vitro study. BMC Complement. Alt. Med. 2015, 15, 417. [Google Scholar] [CrossRef]
- Kang, S.H.; Kim, Y.S.; Kim, E.K.; Hwang, J.W.; Jeong, J.H.; Dong, X.; Lee, J.W.; Moon, S.H.; Jeon, B.T.; Park, P.J. Anticancer effect of thymol on AGS human gastric carcinoma cells. J. Microbiol. Biotechnol. 2016, 26, 28–37. [Google Scholar] [CrossRef]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef]
- Andrade-Ochoa, S.; Nevárez-Moorillón, G.V.; Sánchez-Torres, L.E.; Villanueva-García, M.; Sánchez-Ramírez, B.E.; Rodríguez-Valdez, L.M.; Rivera-Chavira, B.E. Quantitative structure-activity relationship of molecules constituent of different essential oils with antimycobacterial activity against Mycobacterium tuberculosis and Mycobacterium bovis. BMC Complement. Altern. Med. 2015, 23, 332. [Google Scholar] [CrossRef]
- Tariq, S.; Wani, S.; Rasool, W.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 2019, 134, 103580–103599. [Google Scholar] [CrossRef] [PubMed]
- Unlu, M.; Ergene, E.; Unlu, G.V.; Zeytinoglu, H.S.; Vural, N. Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem. Toxicol. 2010, 48, 3274–3280. [Google Scholar] [CrossRef] [PubMed]
- Cardoso-Ugarte, G.A.; López-Malo, A.; Sosa-Morales, M.E. Cinnamon (Cinnamomum zeylanicum) essential oils. In Essential Oils in Food Preservation, Flavour and Safety; Preedy, V.R., Ed.; Elsevier Academic Press: London, UK, 2016; pp. 339–347. [Google Scholar]
- Vasconcelos, N.G.; Croda, J.; Simionatto, S. Antibacterial mechanisms of cinnamon and its constituents: A review. Microb. Pathog. 2018, 120, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Juven, B.J.; Kanner, J.; Schved, F.; Weisslowicz, H. Factors that interact with the antibacterial action of thyme essential oil and its active constituents. J. Appl. Bacteriol. 1994, 76, 626–631. [Google Scholar] [CrossRef]
- Helander, I.M.; Alakomi, H.L.; Latva-Kala, K.; Mattila-Sandholm, T.; Pol, I.; Smid, E.J.; Gorris, L.G.; von Wright, A. Characterization of the action of selected essential oil components on Gram-negative bacteria. J. Agric. Food Chem. 1998, 46, 3590–3595. [Google Scholar] [CrossRef]
- Löwe, J.; Amos, L.A. Crystal structure of the bacterial cell-division protein FtsZ. Nature 1998, 391, 203–206. [Google Scholar] [CrossRef]
- Domadia, P.; Swarup, S.; Bhunia, A.; Sivaraman, J.; Dasgupta, D. Inhibition of bacterial cell division protein FtsZ by cinnamaldehyde. Biochem. Pharmacol. 2007, 74, 831–840. [Google Scholar] [CrossRef]
- Brackman, G.; Celen, S.; Hillaert, U.; Van Calenbergh, S.; Cos, P.; Maes, L.; Nelis, H.J.; Coenye, T. Structure-activity relationship of cinnamaldehyde analogs as inhibitors of AI-2 based quorum sensing and their effect on virulence of Vibrio spp. PLoS ONE 2011, 6, e16084. [Google Scholar] [CrossRef]
- Amalaradjou, M.A.R.; Venkitanarayanan, K. Effect of trans-cinnamaldehyde on inhibition and inactivation of Cronobacter sakazakii biofilm on abiotic surfaces. J. Food Prot. 2011, 74, 200–208. [Google Scholar] [CrossRef]
- Doyle, A.A.; Stephens, J.C. A review of cinnamaldehyde and its derivatives as antibacterial agents. Fitoterapia 2019, 139, 104405–104422. [Google Scholar] [CrossRef]
- Plaza, A.; Keffer, J.L.; Bifulco, G.; Lloyd, J.R.; Bewley, C.A. Chrysophaentins A−H, antibacterial bisdiarylbutene macrocycles that inhibit the bacterial cell division protein FtsZ. J. Am. Chem. Soc. 2010, 132, 9069–9077. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Jindal, B.; Surolia, A.; Panda, D. A rhodanine derivative CCR-11 inhibits bacterial proliferation by inhibiting the assembly and GTPase activity of FtsZ. Biochemistry 2012, 51, 5434–5442. [Google Scholar] [CrossRef]
- Awasthi, D.; Kumar, K.; Knudson, S.E.; Slayden, R.A.; Ojima, I. SAR studies on trisubstituted benzimidazoles as inhibitors of Mtb FtsZ for the development of novel antitubercular agents. J. Med. Chem. 2013, 56, 9756–9770. [Google Scholar] [CrossRef] [PubMed]
- Duggirala, S.; Nankar, R.P.; Rajendran, S.; Doble, M. Phytochemicals as inhibitors of bacterial cell division protein FtsZ: Coumarins are promising candidates. Appl. Biochem. Biotechnol. 2014, 174, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Mathew, B.; Hobrath, J.V.; Ross, L.; Connelly, M.C.; Lofton, H.; Rajagopalan, M.; Guy, R.K.; Reynolds, R.C. Screening and development of new inhibitors of FtsZ from M. tuberculosis. PLoS ONE 2016, 11, e0164100. [Google Scholar] [CrossRef]
- Mitra, K.; Chadha, A.; Doble, M. Pharmacophore based approach to screen and evaluate novel Mycobacterium cell division inhibitors targeting FtsZ-A modeling and experimental study. Eur. J. Pharm. Sci. 2019, 1, 103–112. [Google Scholar] [CrossRef]
- Li, X.; Sheng, J.; Huang, G.; Ma, R.; Yin, F.; Song, D.; Zhao, C.; Ma, S. Design, synthesis and antibacterial activity of cinnamaldehyde derivatives as inhibitors of the bacterial cell division protein FtsZ. Eur. J. Med. Chem. 2015, 97, 32–41. [Google Scholar] [CrossRef]
- Malhotra, S.; Vedithi, S.C.; Blundell, T.L. Decoding the similarities and differences among mycobacterial species. PLoS Negl. Trop. Dis. 2017, 11, e0005883. [Google Scholar] [CrossRef]
- van Vuuren, S.F.; Kamatou, G.P.; Viljoen, A.M. Volatile composition and antimicrobial activity of twenty commercial frankincense essential oil samples. S. Afr. J. Bot. 2010, 76, 686–691. [Google Scholar] [CrossRef]
- Sawicki, R.; Sieniawska, E.; Swatko-Ossor, M.; Golus, J.; Ginalska, G. The frequently occurring components of essential oils beta-elemene and R-limonene alter expression of dprE1 and clgR genes of Mycobacterium tuberculosis H37Ra. Food Chem. Toxicol. 2018, 112, 145–149. [Google Scholar] [CrossRef]
- Eloff, J.N. A sensitive and quick microplate method to determine the minimal inhibitory concentration of plant extracts for bacteria. Planta Med. 1998, 64, 711–713. [Google Scholar] [CrossRef] [PubMed]
- De Rapper, S.; Kamatou, G.; Viljoen, A.; van Vuuren, S. The in vitro antimicrobial activity of Lavandula angustifolia essential oil in combination with other aroma-therapeutic oils. Evid. Based Complement. Alternat. Med. 2013, 852049. [Google Scholar] [CrossRef]
- Viljoen, A.; van Vuuren, S.; Ernst, E.; Klepser, M.; Demirci, B.; Başer, H.; Van Wyk, B.E. Osmitopsis asteriscoides (Asteraceae)-the antimicrobial activity and essential oil composition of a Cape-Dutch remedy. J. Ethnopharmacol. 2003, 88, 137–143. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M.; Özek, T.; Başer, K.H.C. Chemical composition of the wood and leaf oils from the “Clan William Cedar” (Widdringtonia cedarbergensis JA Marsh): A critically endangered species. S. Afr. J. Bot. 2010, 76, 652–654. [Google Scholar] [CrossRef]
- Worley, B.; Powers, R. PCA as a practical indicator of OPLS-DA model reliability. Curr. Metab. 2016, 4, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Friesner, R.A.; Banks, J.L.; Murphy, R.B.; Halgren, T.A.; Klicic, J.J.; Mainz, D.T.; Repasky, M.P.; Knoll, E.H.; Shelley, M.; Perry, J.K.; et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004, 47, 1739–1749. [Google Scholar] [CrossRef]
- Friesner, R.A.; Murphy, R.B.; Repasky, M.P.; Frye, L.L.; Greenwood, J.R.; Halgren, T.A.; Sanschagrin, P.C.; Mainz, D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006, 49, 6177–6196. [Google Scholar] [CrossRef] [PubMed]
- Sastry, G.M.; Adzhigirey, M.; Day, T.; Annabhimoju, R.; Sherman, W. Protein and ligand preparation: Parameters, protocols and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013, 27, 221–234. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Tirado-Rives, J. The OPLS [optimized potentials for liquid simulations] potential functions for proteins, energy minimizations for crystals of cyclic peptides and crambin. J. Am. Chem. Soc. 1988, 110, 1657–1666. [Google Scholar] [CrossRef]
- Harder, E.; Damm, W.; Maple, J.; Wu, C.; Reboul, M.; Xiang, J.Y.; Wang, L.; Lupyan, D.; Dahlgren, M.K.; Knight, J.L.; et al. OPLS3: A force field providing broad coverage of drug-like small molecules and proteins. J. Chem. Theory Comput. 2016, 12, 281–296. [Google Scholar] [CrossRef]
- Maestro, S. Schrödinger Release 2020-1; LLC: New York, NY, USA, 2020. [Google Scholar]

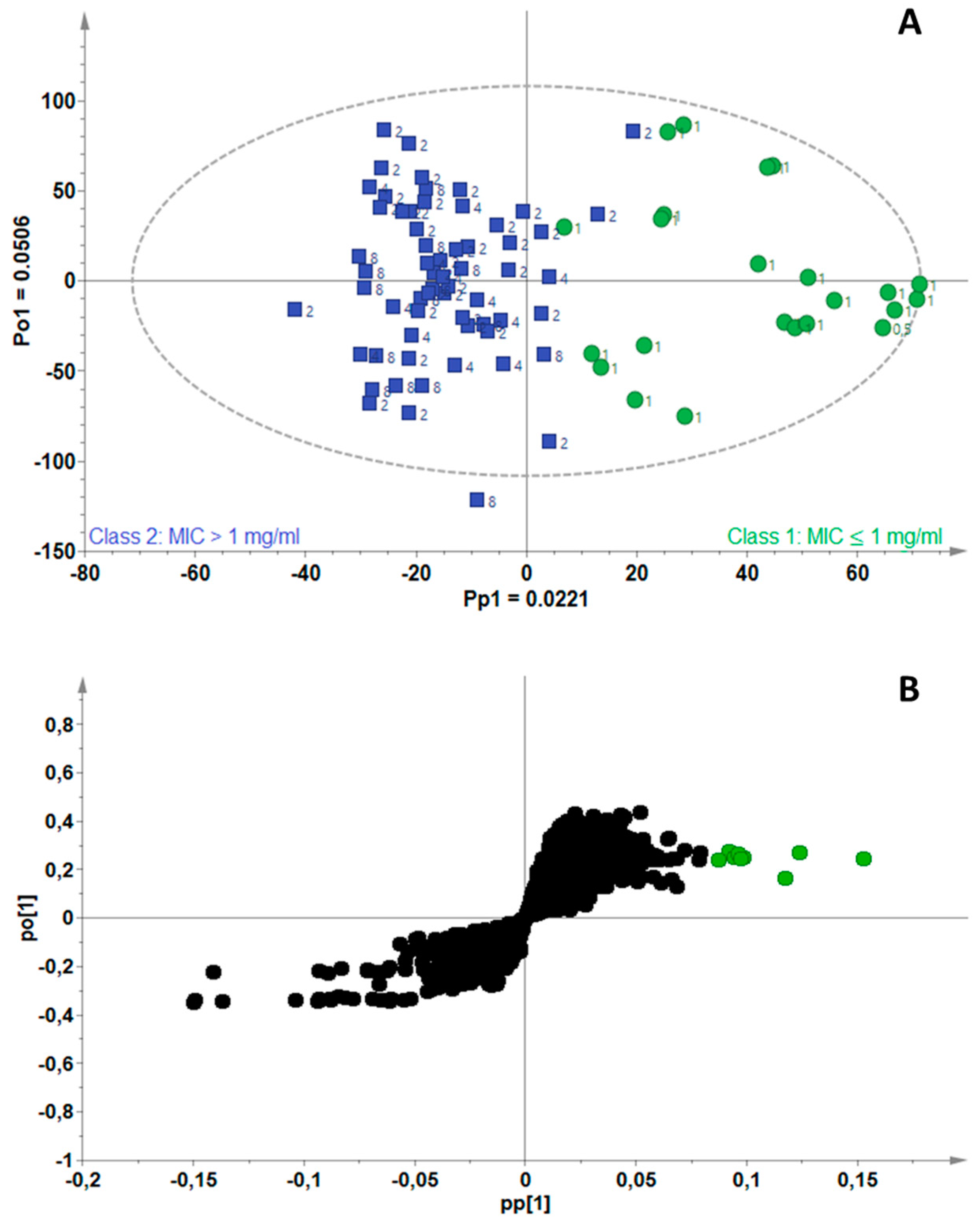
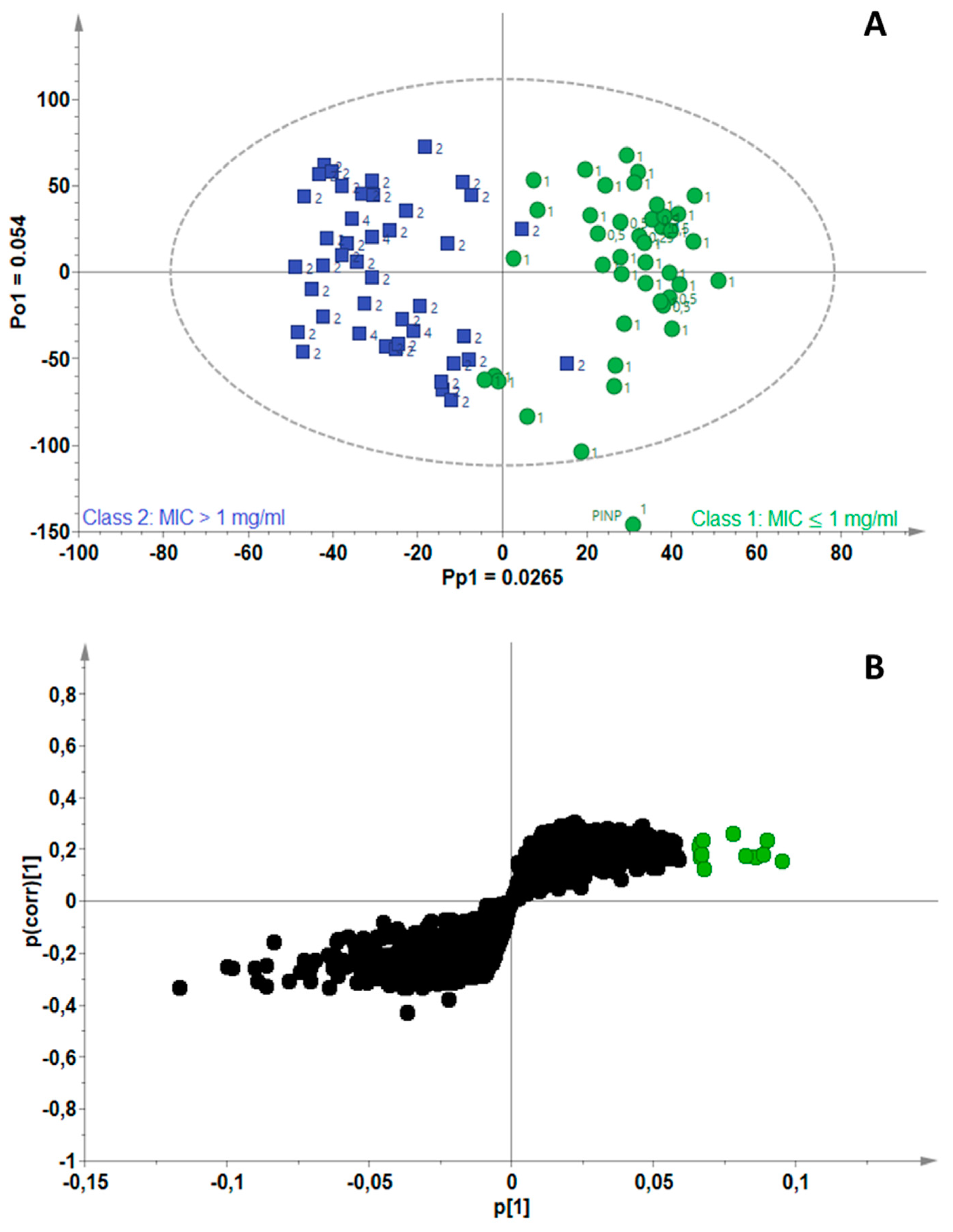
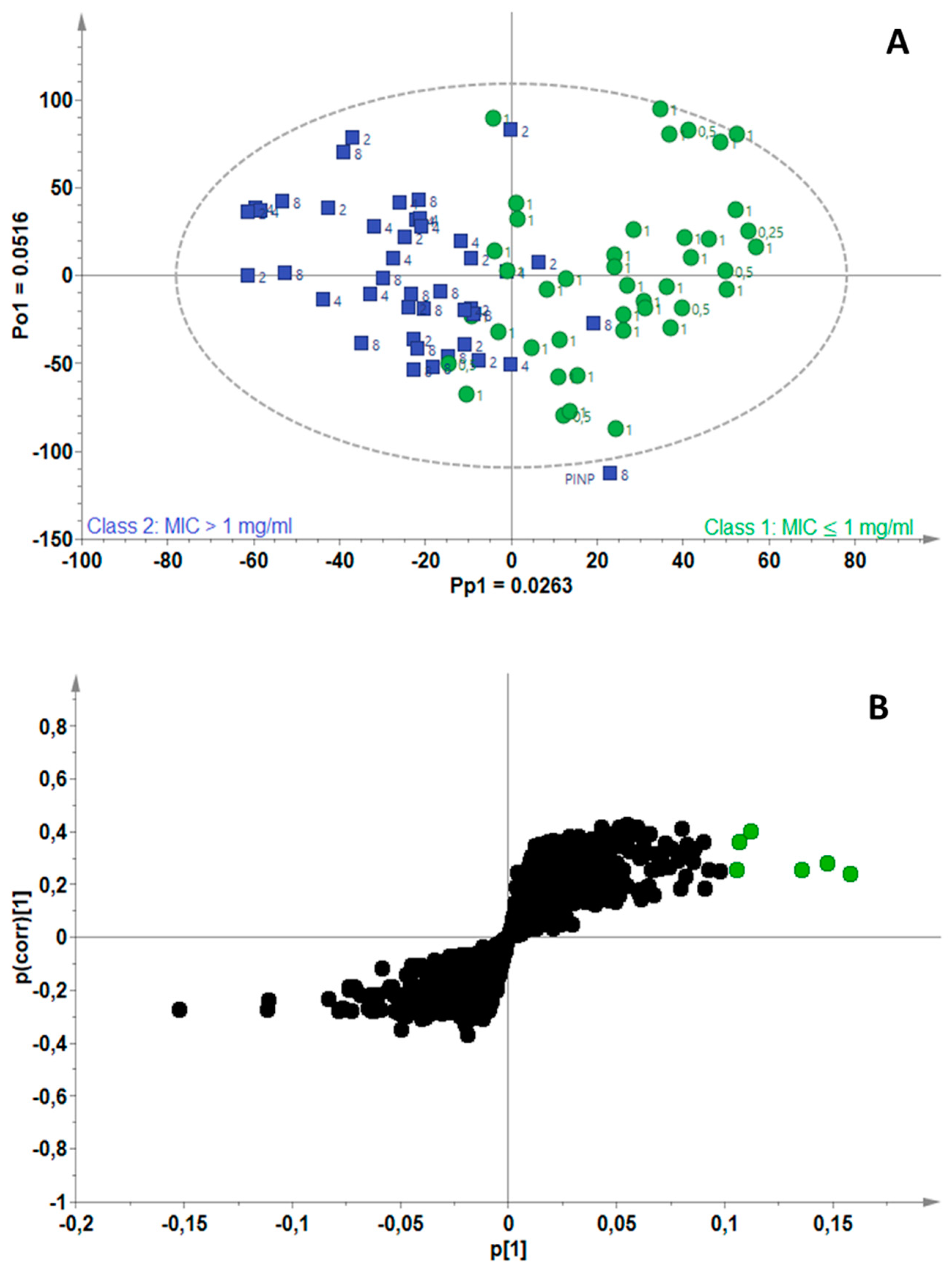
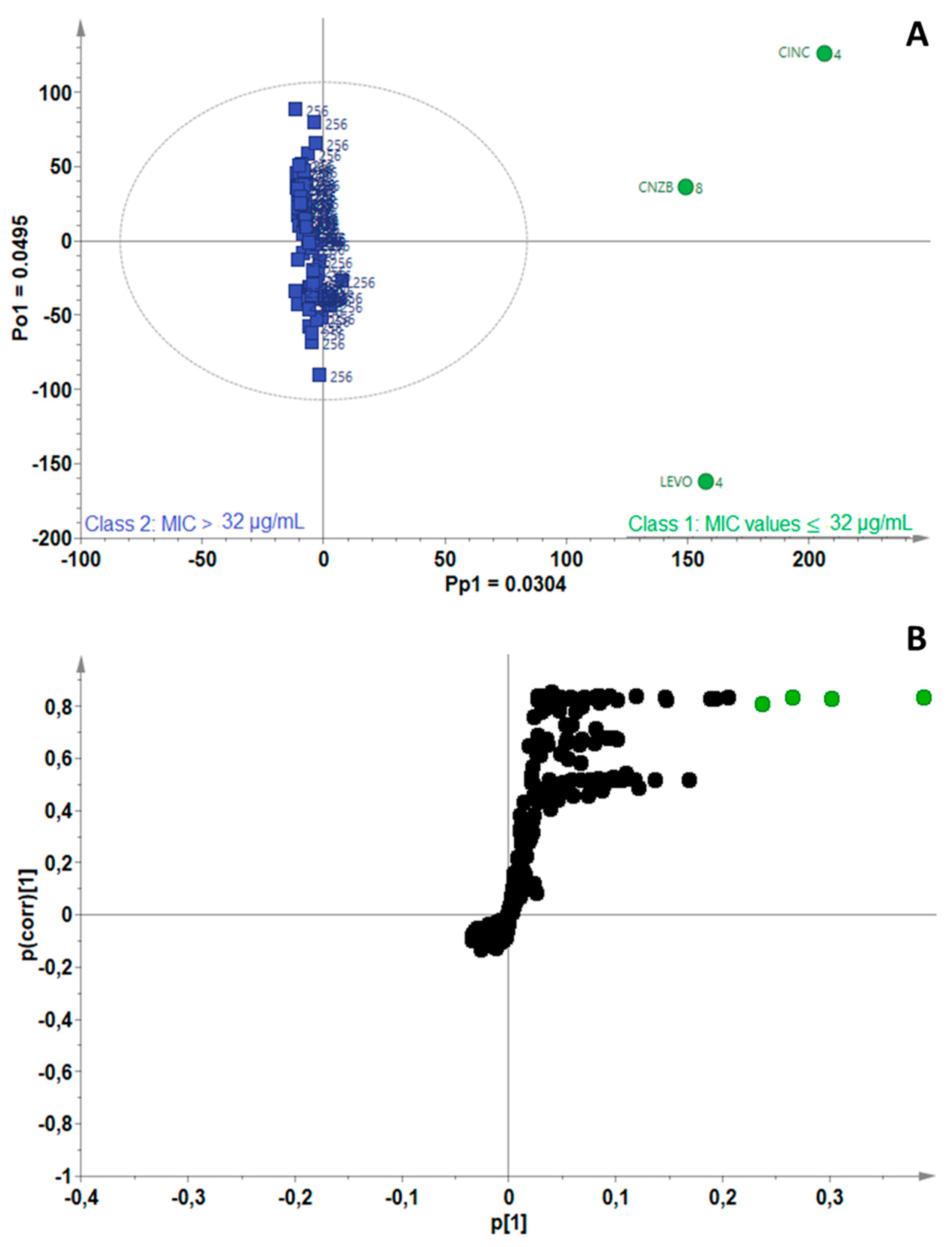


| Mycobacterium Strains | Number of Components (A) | R2Xcum | R2Ycum | Q2cum |
|---|---|---|---|---|
| M. fortuitum | 1 + 2 | 0.117 | 0.792 | 0.372 |
| M. gordonae | 1 + 1 | 0.078 | 0.585 | −0.284 |
| M. smegmatis | 1 + 2 | 0.112 | 0.752 | −0.115 |
| M. tuberculosis | 1 + 1 | 0.0799 | 0.969 | 0.577 |
| Biomarker | M. smegmatis ATCC 19420 | M. fortuitum ATCC 6841 | M. gordonae ATCC 14470 | M. tuberculosis H37Ra ATCC 25177 (µg/mL) |
|---|---|---|---|---|
| Cinnamaldehyde | 0.250 | 0.130 | 0.030 | 8.00 |
| Eugenol | 1.000 | 1.000 | 0.250 | 256 |
| Thymol | 0.500 | 1.000 | 0.190 | 256 |
| Ciprofloxacin (µg/mL) | 0.310 | 0.160 | 0.310 | - |
| Ethambutol (µg/mL) | - | - | - | 2.00 |
| Entry | Compound | Glide Score. kcal mol−1 | Glide Energy. kcal mol−1 | Interacting Binding Site Residues |
|---|---|---|---|---|
| 1 | Co-crystal GDP | −9.04 | −69.51 | Asn-22, Thr-130, Arg-140, Asn-163, Phe-180 |
| 2 | Co-crystal 4HC_414 | −3.90 | −21.37 | Ala-39, Leu-47, Ser-50, Lys-55 |
| 3 | 4wCo-crystal 4HC_415 | −4.21 | −24.06 | Gly-193, Ser-260, Ala-262, Val-294, Arg-304 |
| 4 | Cinnamaldehyde active site | −2.96 | −21.79 | Ala-183, Thr-130 |
| 5 | Cinnamaldehyde_4HC_414 site | −3.04 | −21.88 | Ala-39, Asn-41, Lys-55 |
| 6 | Cinnamaldehyde_4HC_415 site | −3.16 | −21.66 | Ala-183, Thr-130 |
| Entry | Compound | Glide Score. kcal mol−1 | Glide Energy. kcal mol−1 | Interacting Binding Site Residues |
|---|---|---|---|---|
| 1 | Co-crystal mitoxantrone | −11.49 | −64.21 | Asn-22, Thr-130, Arg-140, Asn-163, Phe-180 |
| 2 | Cinnamaldehyde | −5.57 | −20.94 | Ala-39, Leu-47, Ser-50, Lys-55 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boussamba-Digombou, K.J.; Sandasi, M.; Kamatou, G.P.; van Vuuren, S.; Sawicki, R.; Fakhar, Z.; Viljoen, A.M. Investigating the Antituberculosis Activity of Selected Commercial Essential Oils and Identification of Active Constituents Using a Biochemometrics Approach and In Silico Modeling. Antibiotics 2022, 11, 948. https://doi.org/10.3390/antibiotics11070948
Boussamba-Digombou KJ, Sandasi M, Kamatou GP, van Vuuren S, Sawicki R, Fakhar Z, Viljoen AM. Investigating the Antituberculosis Activity of Selected Commercial Essential Oils and Identification of Active Constituents Using a Biochemometrics Approach and In Silico Modeling. Antibiotics. 2022; 11(7):948. https://doi.org/10.3390/antibiotics11070948
Chicago/Turabian StyleBoussamba-Digombou, Katyna J., Maxleene Sandasi, Guy P. Kamatou, Sandy van Vuuren, Rafal Sawicki, Zeynab Fakhar, and Alvaro M. Viljoen. 2022. "Investigating the Antituberculosis Activity of Selected Commercial Essential Oils and Identification of Active Constituents Using a Biochemometrics Approach and In Silico Modeling" Antibiotics 11, no. 7: 948. https://doi.org/10.3390/antibiotics11070948
APA StyleBoussamba-Digombou, K. J., Sandasi, M., Kamatou, G. P., van Vuuren, S., Sawicki, R., Fakhar, Z., & Viljoen, A. M. (2022). Investigating the Antituberculosis Activity of Selected Commercial Essential Oils and Identification of Active Constituents Using a Biochemometrics Approach and In Silico Modeling. Antibiotics, 11(7), 948. https://doi.org/10.3390/antibiotics11070948







