Grafted Chitosan-Hyaluronic Acid (CS-g-poly (MA-co-AN) HA) Complex Inhibits Fluconazole-Resistant Candida albicans Biofilm Formation
Abstract
:1. Introduction
2. Results and Discussion
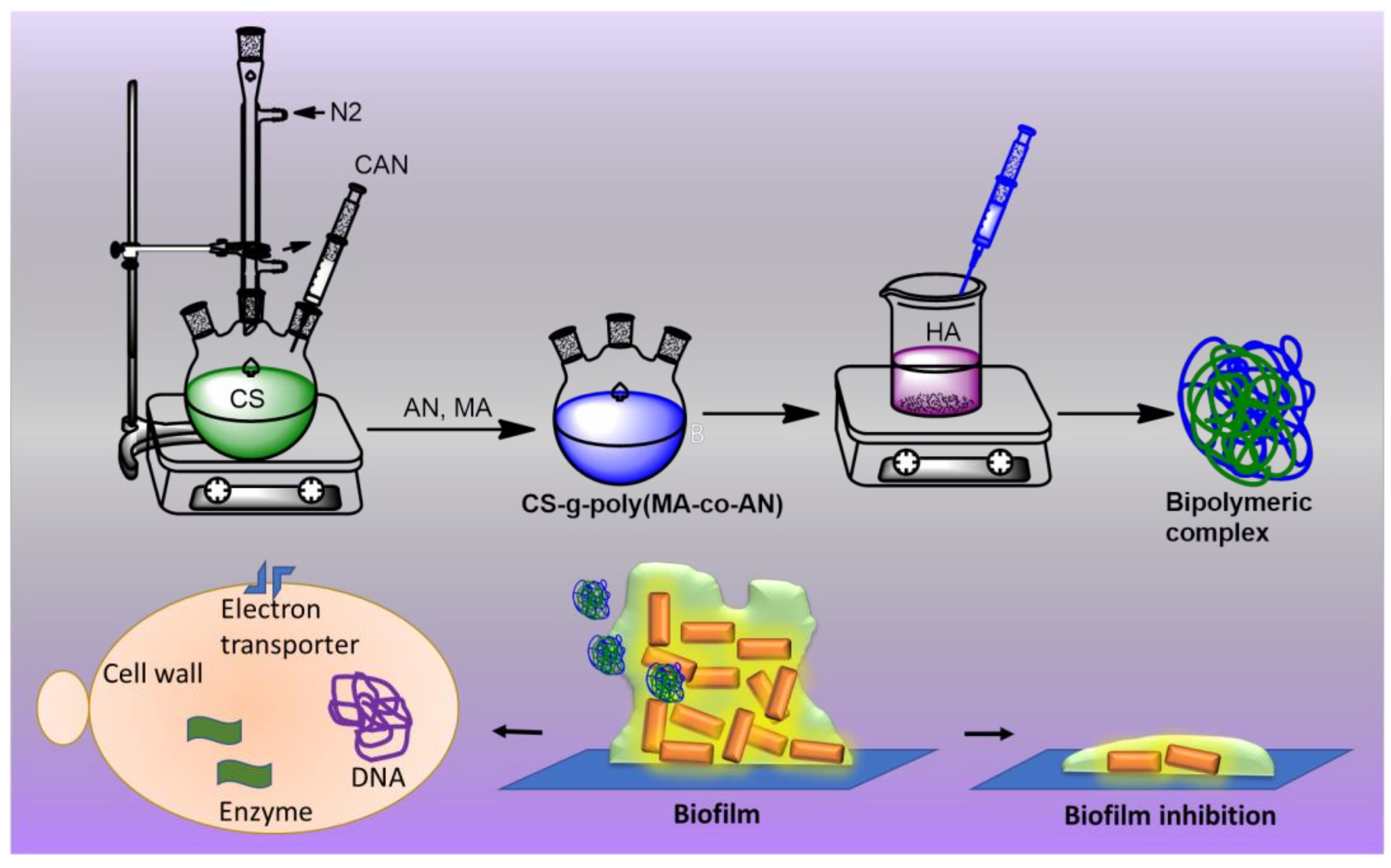
2.1. Characterization of the CS-g-poly (MA-co-AN)-HA Complex
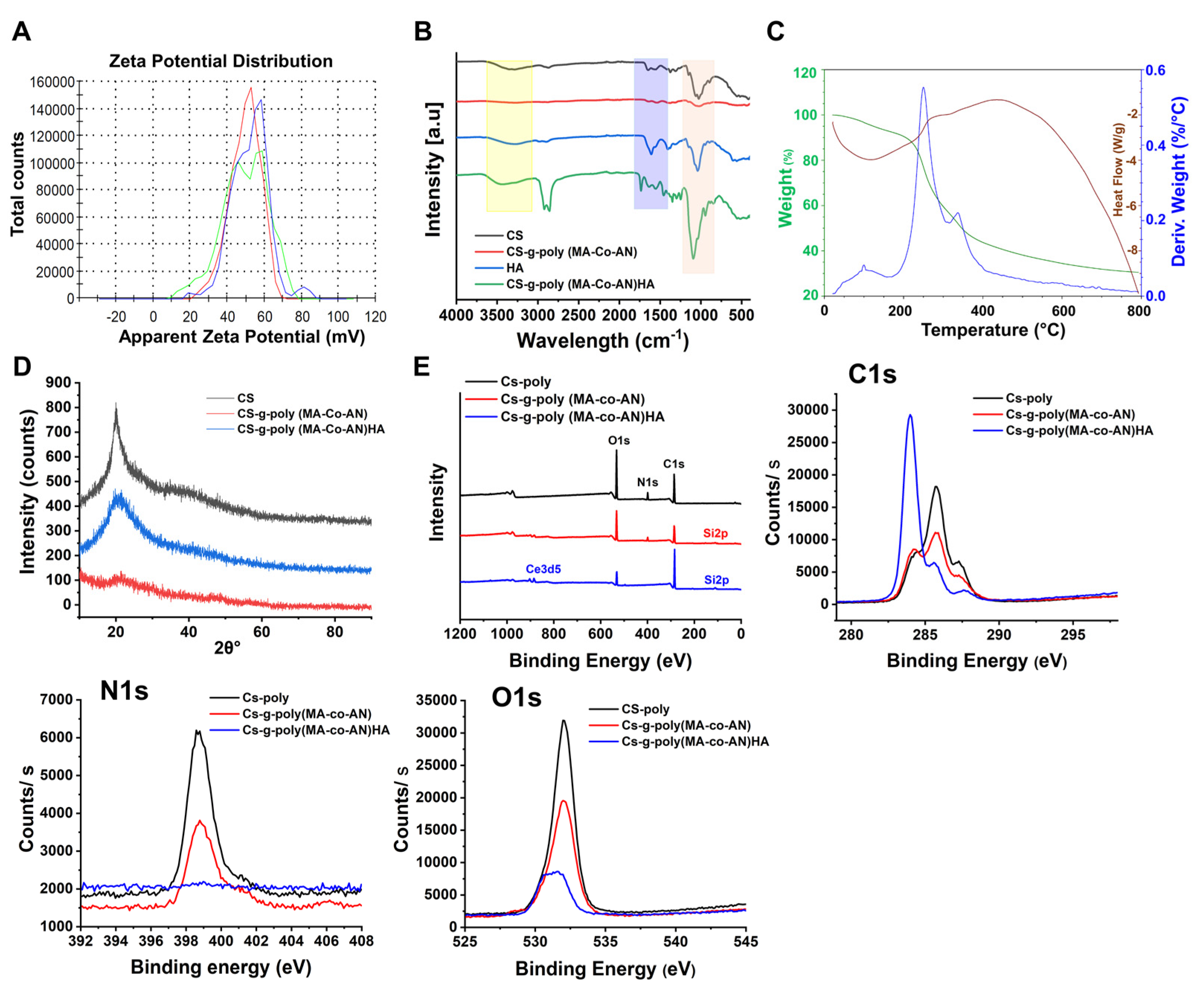
2.1.1. Zeta Potentials
2.1.2. FTIR and XRD Analysis of CS-g-poly (MA-co-AN) HA Complex
2.1.3. DSC–TGA of CS-g-poly (MA-co-AN) HA
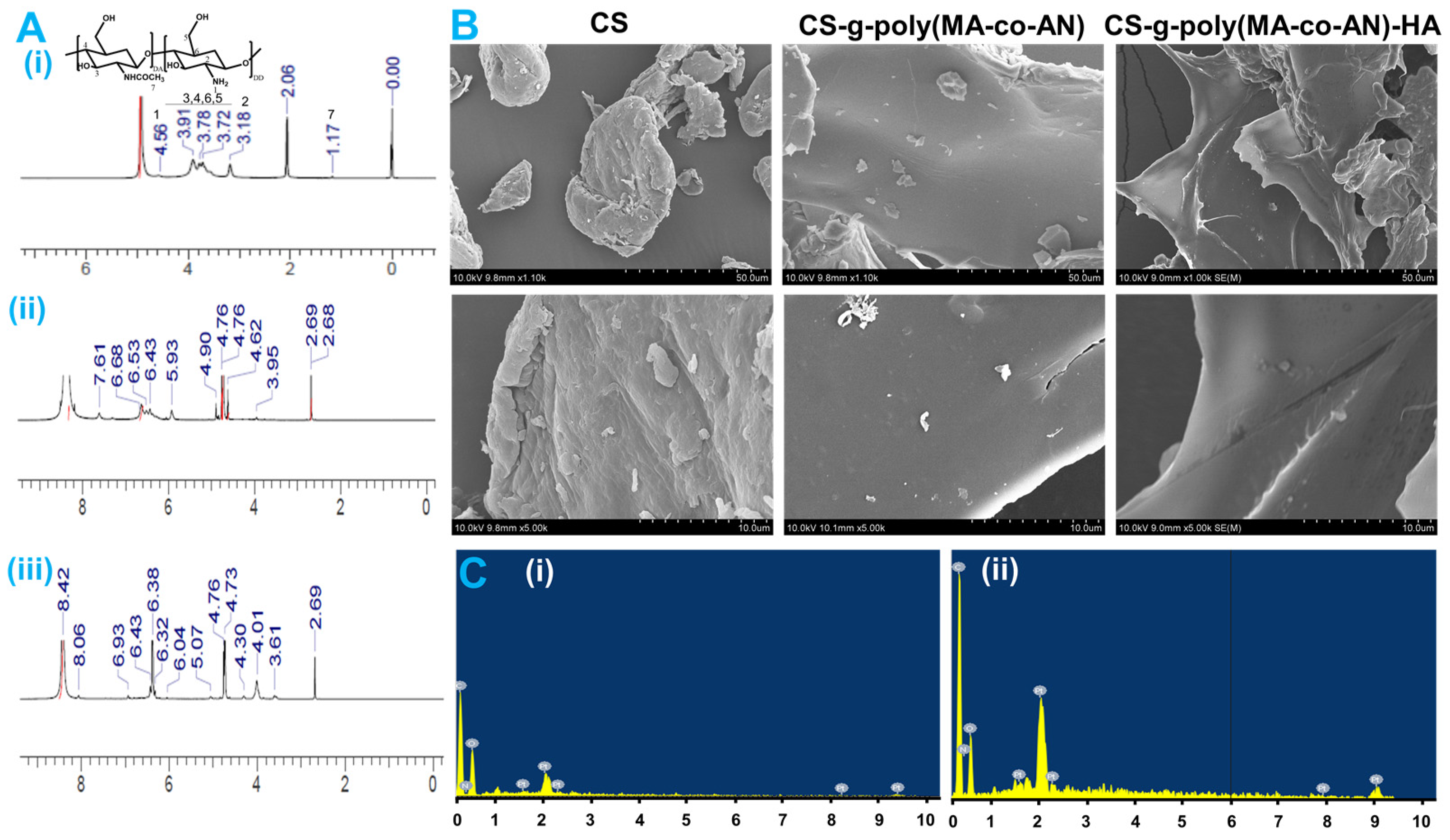
2.1.4. XPS and NMR Analysis
2.1.5. SEM and EDX Analysis of Modified CS
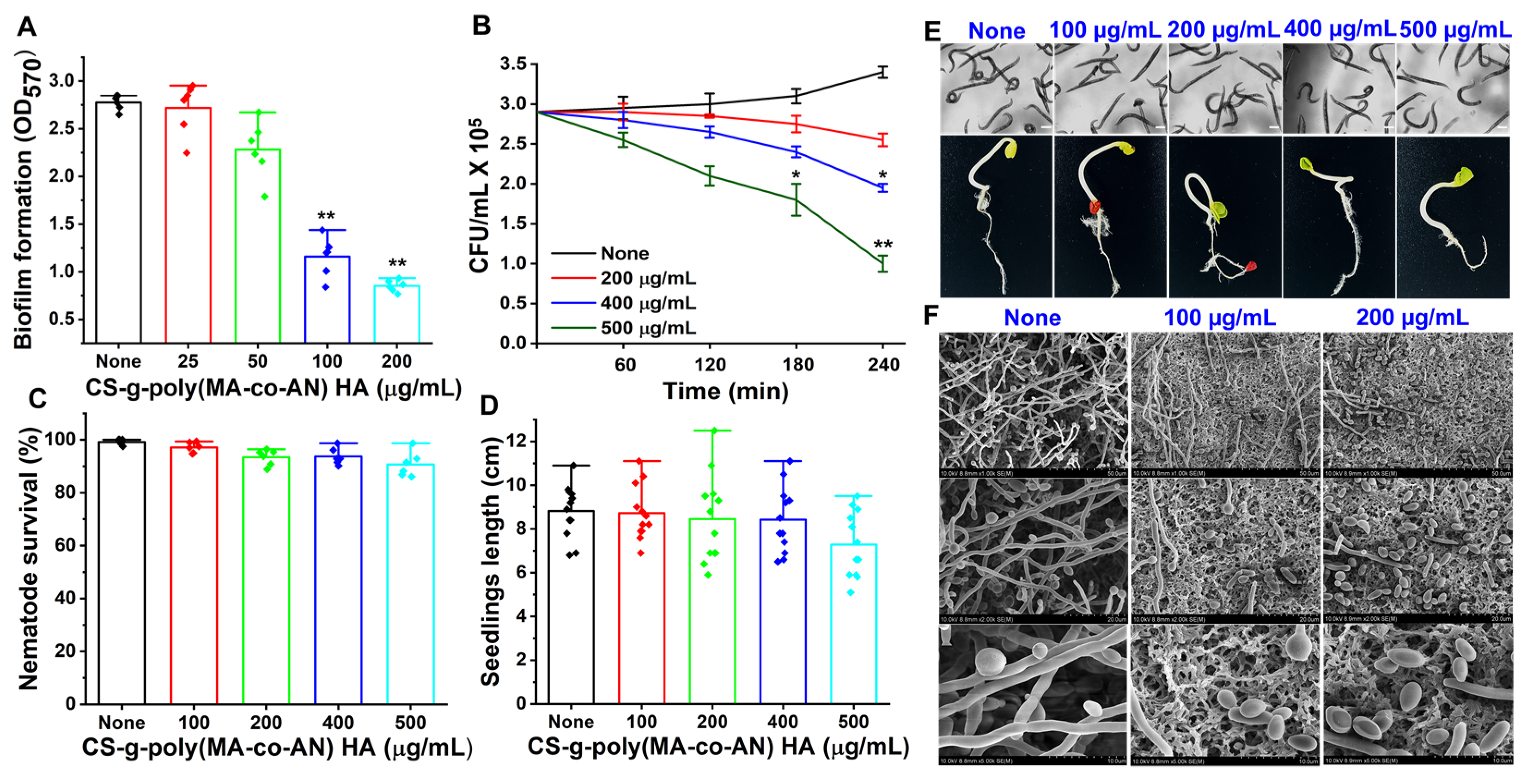
2.2. Antibiofilm Potency of CS-g-poly (MA-co-AN)-HA against C. albicans
2.3. Rapid Killing Activity of CS-g-poly (MA-co-AN) HA
2.4. SEM Analysis of CS-g-poly (MA-Co-AN) HA Treated C. albicans
2.5. Safety Profile of CS-g-poly (MA-co-AN) HA as Determined by C. elegans Viability and Seed Germination Rates
3. Materials and Methods
3.1. Materials and Microbial Stains
3.2. Synthesis of CS-g-poly (MA-co-AN)
3.3. Fabrication of CS-g-poly (MA-co-AN) HA Complex
3.4. Characterization of CS-g-poly (MA-co-AN) HA
3.4.1. Zeta Potential Measurements
3.4.2. FTIR Spectroscopy CS-g-poly (MA-co-AN) HA
3.4.3. X-ray Diffraction (XRD) Analysis of CS-g-poly (MA-co-AN) HA
3.4.4. Simultaneous DSC–TGA Analysis of CS-g-poly (MA-co-AN) HA
3.4.5. X-ray Photoelectron Spectroscopy (XPS)
3.4.6. 1H-NMR Analysis of CS-g-poly (MA-co-AN) HA
3.4.7. SEM and Energy-Dispersive X-ray Spectroscopy (EDX)
3.5. Antibiofilm Potency of CS-g-poly (MA-co-AN) HA against C. albicans
3.6. Time-Kill Assay
3.7. Seed Germination Toxicity Assay
3.8. In Vivo Toxicity Assessment of CS-g-poly (MA-co-AN) HA Complex against C. elegans
3.9. The Microscopic Architecture of C. albicans Biofilms
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mitcheltree, M.J.; Pisipati, A.; Syroegin, E.A.; Silvestre, K.J.; Klepacki, D.; Mason, J.D.; Terwilliger, D.W.; Testolin, G.; Pote, A.R.; Wu, K.J.Y.; et al. A synthetic antibiotic class overcoming bacterial multidrug resistance. Nature 2021, 599, 507–512. [Google Scholar] [CrossRef] [PubMed]
- Vairappan, C.S.; Suzuki, M.; Abe, T.; Masuda, M. Halogenated metabolites with antibacterial activity from the Okinawan laurencia Species. Phytochemistry 2001, 58, 517–523. [Google Scholar] [CrossRef]
- Hammad, S.G.; El-Gazzar, M.G.; Abutaleb, N.S.; Li, D.; Ramming, I.; Shekhar, A.; Abdel-Halim, M.; Elrazaz, E.Z.; Seleem, M.N.; Bilitewski, U.; et al. Synthesis and antimicrobial evaluation of new halogenated 1,3-thiazolidin-4-ones. Bioorg. Chem. 2020, 95, 103517. [Google Scholar] [CrossRef]
- Menozzi, G.; Merello, L.; Fossa, P.; Schenone, S.; Ranise, A.; Mosti, L.; Bondavalli, F.; Loddo, R.; Murgioni, C.; Mascia, V.; et al. Synthesis, antimicrobial activity and molecular modeling studies of halogenated 4-[1h-imidazol-1-yl(phenyl)methyl]-1,5- diphenyl-1h-pyrazoles. Bioorg. Med. Chem. 2004, 12, 5465–5483. [Google Scholar] [CrossRef]
- Revie, N.M.; Iyer, K.R.; Robbins, N.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Kanafani, Z.A.; Perfect, J.R. Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raj, V.; Raorane, C.J.; Lee, J.-H.; Lee, J. Appraisal of chitosan-gum arabic-coated bipolymeric nanocarriers for efficient dye removal and eradication of the plant pathogen Botrytis cinerea. ACS Appl. Mater. Interfaces 2021, 13, 47354–47370. [Google Scholar] [CrossRef]
- Raj, V.; Kim, Y.; Kim, Y.-G.; Lee, J.-H.; Lee, J. Chitosan-gum arabic embedded alizarin nanocarriers inhibit biofilm formation of multispecies microorganisms. Carbohydr. Polym. 2021, 284, 118959. [Google Scholar] [CrossRef]
- Jiang, Z.; Dou, G. Preparation and characterization of chitosan grafting hydrogel for mine-fire fighting. ACS Omega 2020, 5, 2303–2309. [Google Scholar] [CrossRef] [Green Version]
- Cheung, R.C.F.; Ng, T.B.; Wong, J.H.; Chan, W.Y. Chitosan: An Update on Potential Biomedical and Pharmaceutical Applications. Mar Drugs 2015, 13, 5156–5186. [Google Scholar] [CrossRef]
- Herdiana, Y.; Wathoni, N.; Shamsuddin, S.; Muchtaridi, M. Drug release study of the chitosan-based nanoparticles. Heliyon 2022, 8, e08674. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic acid and controlled release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef] [PubMed]
- Dovedytis, M.; Liu, Z.J.; Bartlett, S. Hyaluronic acid and its biomedical applications: A Review. Eng. Regen. 2020, 1, 102–113. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Naushad, M.; Al-Misned, F.A.; El-Serehy, H.A.; Ghfar, A.A.; Rai Sharma, K.; Si, C.; Stadler, F.J. Graft copolymerization of acrylonitrile and ethyl acrylate onto Pinus roxburghii wood surface enhanced physicochemical properties and antibacterial activity. J. Chem. 2020. [Google Scholar] [CrossRef]
- Kabir, A.; Dunlop, M.J.; Acharya, B.; Bissessur, R.; Ahmed, M. Water recycling efficacies of extremely hygroscopic, antifouling hydrogels. RSC Adv. 2018, 8, 38100–38107. [Google Scholar] [CrossRef] [Green Version]
- Chiesa, E.; Dorati, R.; Conti, B.; Modena, T.; Cova, E.; Meloni, F.; Genta, I. Hyaluronic acid-decorated chitosan nanoparticles for CD44-targeted delivery of evero-limus. Int. J. Mol. Sci. 2018, 19, 2310. [Google Scholar] [CrossRef] [Green Version]
- Cozic, C.; Picton, L.; Garda, M.-R.; Marlhoux, F.; Le Cerf, D. Analysis of arabic gum: Study of degradation and water desorption processes. Food Hydrocoll. 2009, 23, 1930–1934. [Google Scholar] [CrossRef]
- Yu, Q.; Wu, Z.; Chen, H. Dual-function antibacterial surfaces for biomedical applications. Acta Biomat. 2015, 16, 1–13. [Google Scholar] [CrossRef]
- Rivera Aguayo, P.; Bruna Larenas, T.; Alarcón Godoy, C.; Cayupe Rivas, B.; Gonzá-lez-Casanova, J.; Rojas-Gómez, D.; Caro Fuentes, N. Antimicrobial and anti-biofilm capacity of chitosan nanoparticles against wild type strain of Pseudomonas sp. isolated from milk of cows diagnosed with bovine mastitis. Antibiotics 2020, 9, 551. [Google Scholar] [CrossRef]
- Pedroso-Santana, S.; Fleitas-Salazar, N. Ionotropic gelation method in the synthesis of nanoparticles/microparticles for biomedical purposes. Poly. Inter. 2020, 69, 443–447. [Google Scholar] [CrossRef]
- Manoharan, R.K.; Lee, J.; Lee, J. Efficacy of 7-benzyloxyindole and other halogenated indoles to inhibit Candida albicans biofilm and hyphal formation. Microb Biotechnol. 2018, 11, 1060–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.-G.; Lee, J.-H.; Park, J.G.; Lee, J. Inhibition of Candida albicans and Staphylococcus aureus biofilms by centipede oil and linoleic acid. Biofouling 2020, 36, 126–137. [Google Scholar] [CrossRef] [PubMed]
- Raorane, C.J.; Lee, J.-H.; Lee, J. Rapid killing and biofilm inhibition of multidrug- resistant Acinetobacter baumannii strains and other microbes by iodoindoles. Biomolecules 2020, 10, 1186. [Google Scholar] [CrossRef] [PubMed]
- Abouelhassan, Y.; Zhang, P.; Ding, Y.; Huigens, R.W. Rapid kill assessment of an N- Arylated NH125 analogue against drug-resistant microorganisms. MedChemComm 2019, 10, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Raorane, C.J.; Raj, V.; Lee, J.-H.; Lee, J. Antifungal activities of fluoroindoles against the postharvest pathogen Botrytis cinerea: In vitro and in silico approaches. Int. J. Food Microb. 2022, 362, 109492. [Google Scholar] [CrossRef] [PubMed]
- Rajasekharan, S.K.; Raorane, C.J.; Lee, J. A Facile and modified scheme for synchronization and isolation of nematode eggs. Agriculture 2021, 11, 676. [Google Scholar] [CrossRef]
- Rajasekharan, S.K.; Raorane, C.J.; Lee, J. LED Based Real-Time Survival Bioassays for Nematode Research. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Raorane, C.J.; Lee, J.H.; Kim, Y.G.; Rajasekharan, S.K.; García-Contreras, R.; Lee, J. Antibiofilm and antivirulence efficacies of flavonoids and curcumin against Acinetobacter baumannii. Front. Microb. 2019, 10, 990. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raorane, C.J.; Shastri, D.; Parveen, A.S.; Haldhar, R.; Raj, V.; Kim, S.-C. Grafted Chitosan-Hyaluronic Acid (CS-g-poly (MA-co-AN) HA) Complex Inhibits Fluconazole-Resistant Candida albicans Biofilm Formation. Antibiotics 2022, 11, 950. https://doi.org/10.3390/antibiotics11070950
Raorane CJ, Shastri D, Parveen AS, Haldhar R, Raj V, Kim S-C. Grafted Chitosan-Hyaluronic Acid (CS-g-poly (MA-co-AN) HA) Complex Inhibits Fluconazole-Resistant Candida albicans Biofilm Formation. Antibiotics. 2022; 11(7):950. https://doi.org/10.3390/antibiotics11070950
Chicago/Turabian StyleRaorane, Chaitany Jayprakash, Divya Shastri, Asrafali Shakila Parveen, Rajesh Haldhar, Vinit Raj, and Seong-Cheol Kim. 2022. "Grafted Chitosan-Hyaluronic Acid (CS-g-poly (MA-co-AN) HA) Complex Inhibits Fluconazole-Resistant Candida albicans Biofilm Formation" Antibiotics 11, no. 7: 950. https://doi.org/10.3390/antibiotics11070950
APA StyleRaorane, C. J., Shastri, D., Parveen, A. S., Haldhar, R., Raj, V., & Kim, S.-C. (2022). Grafted Chitosan-Hyaluronic Acid (CS-g-poly (MA-co-AN) HA) Complex Inhibits Fluconazole-Resistant Candida albicans Biofilm Formation. Antibiotics, 11(7), 950. https://doi.org/10.3390/antibiotics11070950










