Plants with Antimicrobial Activity Growing in Italy: A Pathogen-Driven Systematic Review for Green Veterinary Pharmacology Applications
Abstract
:1. Introduction
2. Results
3. Discussion
3.1. Plants Active against Gram+
3.2. Plants Active against Gram−
3.3. Pharmacodynamics of Plant Extracts
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Vivo, R.; Zicarelli, L. Influence of carbon fixation on the mitigation of greenhouse gas emissions from livestock activities in Italy and the achievement of carbon neutrality. Transl. Anim. Sci. 2021, 5, txab042. [Google Scholar] [CrossRef] [PubMed]
- Castagna, F.; Piras, C.; Palma, E.; Musolino, V.; Lupia, C.; Bosco, A.; Rinaldi, L.; Cringoli, G.; Musella, V.; Britti, D. Green veterinary pharmacology applied to parasite control: Evaluation of punica granatum, artemisia campestris, salix caprea aqueous macerates against gastrointestinal nematodes of sheep. Vet. Sci. 2021, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.H.; Ejo, M.; Feyera, T.; Regassa, D.; Mummed, B.; Huluka, S.A. In Vitro Anthelmintic Activity of Crude Extracts of Artemisia herba-alba and Punica granatum against Haemonchus contortus. J. Parasitol. Res. 2020, 2020, 4950196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dkhil, M.A. Anti-coccidial, anthelmintic and antioxidant activities of pomegranate (Punica granatum) peel extract. Parasitol. Res. 2013, 112, 2639–2646. [Google Scholar] [CrossRef]
- Conti, B.; Bocchino, R.; Cosci, F.; Ascrizzi, R.; Flamini, G.; Bedini, S. Essential oils against Varroa destructor: A soft way to fight the parasitic mite of Apis mellifera. J. Apic. Res. 2020, 59, 774–782. [Google Scholar] [CrossRef]
- Dahlgren, L.; Johnson, R.M.; Siegfried, R.D.; Ellis, M.D. Comparative toxicity of acaricides to honey bee (Hymenoptera: Apidae) workers and queens. J. Econ. Entomol. 2012, 105, 1895–1902. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Piras, C.; Palma, E.; Cringoli, G.; Musolino, V.; Lupia, C.; Perri, M.R.; Statti, G.; Britti, D.; et al. In vitro evaluation of acute toxicity of five citrus spp. Essential oils towards the parasitic mite varroa destructor. Pathogens 2021, 10, 1182. [Google Scholar] [CrossRef]
- Rinkevich, F.D. Detection of amitraz resistance and reduced treatment efficacy in the Varroa Mite, Varroa destructor, within commercial beekeeping operations. PLoS ONE 2020, 15, e0227264. [Google Scholar] [CrossRef] [Green Version]
- Charlier, J.; Bartley, D.J.; Sotiraki, S.; Martinez-Valladares, M.; Claerebout, E.; von Samson-Himmelstjerna, G.; Thamsborg, S.M.; Hoste, H.; Morgan, E.R.; Rinaldi, L. Anthelmintic resistance in ruminants: Challenges and solutions. Adv. Parasitol. 2022, 115, 171–227. [Google Scholar] [CrossRef]
- Bhullar, K.; Waglechner, N.; Pawlowski, A.; Koteva, K.; Banks, E.D.; Johnston, M.D.; Barton, H.A.; Wright, G.D. Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS ONE 2012, 7, e34953. [Google Scholar] [CrossRef]
- Kashuba, E.; Dmitriev, A.A.; Kamal, S.M.; Melefors, O.; Griva, G.; Römling, U.; Ernberg, I.; Kashuba, V.; Brouchkov, A. Ancient permafrost staphylococci carry antibiotic resistance genes. Microb. Ecol. Health Dis. 2017, 28, 1345574. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.L.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R. Antibiotic resistance is ancient. Nature 2011, 477, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Leporatti, M.L.; Impieri, M. Ethnobotanical notes about some uses of medicinal plants in Alto Tirreno Cosentino area (Calabria, Southern Italy). J. Ethnobiol. Ethnomed. 2007, 3, 34. [Google Scholar] [CrossRef] [Green Version]
- Passalacqua, N.G.; Guarrera, P.M.; De Fine, G. Contribution to the knowledge of the folk plant medicine in Calabria region (Southern Italy). Fitoterapia 2007, 78, 52–68. [Google Scholar] [CrossRef]
- Chassagne, F.; Samarakoon, T.; Porras, G.; Lyles, J.T.; Dettweiler, M.; Marquez, L.; Salam, A.M.; Shabih, S.; Farrokhi, D.R.; Quave, C.L. A systematic review of plants with antibacterial activities: A taxonomic and phylogenetic perspective. Front. Pharmacol. 2021, 11, 586548. [Google Scholar] [CrossRef] [PubMed]
- Badalamenti, N.; Modica, A.; Ilardi, V.; Bruno, M.; Maresca, V.; Zanfardino, A.; Di Napoli, M.; Castagliuolo, G.; Varcamonti, M.; Basile, A. Daucus carota subsp. maximus (Desf.) Ball from Pantelleria, Sicily (Italy): Isolation of essential oils and evaluation of their bioactivity. Nat. Prod. Res. 2021, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Argentieri, M.P.; Madeo, M.; Avato, P.; Iriti, M.; Vitalini, S. Polyphenol content and bioactivity of Achillea moschata from the Italian and Swiss Alps. Z. Fur Naturforsch. Sect. C J. Biosci. 2020, 75, 57–64. [Google Scholar] [CrossRef]
- Garzoli, S.; Turchetti, G.; Giacomello, P.; Tiezzi, A.; Masci, V.L.; Ovidi, E. Liquid and vapour phase of lavandin (Lavandula × intermedia) Essential Oil: Chemical composition and antimicrobial activity. Molecules 2019, 24, 2701. [Google Scholar] [CrossRef] [Green Version]
- Caputo, L.; Nazzaro, F.; Souza, L.F.; Aliberti, L.; De Martino, L.; Fratianni, F.; Coppola, R.; De Feo, V. Laurus nobilis: Composition of essential oil and its biological activities. Molecules 2017, 22, 930. [Google Scholar] [CrossRef]
- Casiglia, S.; Bruno, M.; Senatore, F. Volatile constituents of Dianthus rupicola Biv. from Sicily: Activity against microorganisms affecting cellulosic objects. Nat. Prod. Res. 2014, 28, 1739–1746. [Google Scholar] [CrossRef]
- Fratianni, F.; Coppola, R.; Nazzaro, F. Phenolic composition and antimicrobial and antiquorum sensing activity of an ethanolic extract of peels from the apple cultivar annurca. J. Med. Food 2011, 14, 957–963. [Google Scholar] [CrossRef]
- Sadeghi, Z.; Yang, J.L.; Venditti, A.; Moridi Farimani, M. A review of the phytochemistry, ethnopharmacology and biological activities of Teucrium genus (Germander). Nat. Prod. Res. 2021, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Carlo Tenore, G.; Troisi, J.; Di Fiore, R.; Basile, A.; Novellino, E. Chemical composition, antioxidant and antimicrobial properties of Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) seed meal, a promising protein source of Campania region (southern Italy) horticultural germplasm. J. Sci. Food Agric. 2012, 92, 1716–1724. [Google Scholar] [CrossRef] [PubMed]
- Fratianni, F.; Riccardi, R.; Spigno, P.; Ombra, M.N.; Cozzolino, A.; Tremonte, P.; Coppola, R.; Nazzaro, F. Biochemical Characterization and Antimicrobial and Antifungal Activity of Two Endemic Varieties of Garlic (Allium sativum L.) of the Campania Region, Southern Italy. J. Med. Food 2016, 19, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Garzoli, S.; Masci, V.L.; Franceschi, S.; Tiezzi, A.; Giacomello, P.; Ovidi, E. Headspace/gc–ms analysis and investigation of antibacterial, antioxidant and cytotoxic activity of essential oils and hydrolates from rosmarinus officinalis l. And lavandula angustifolia miller. Foods 2021, 10, 1768. [Google Scholar] [CrossRef] [PubMed]
- Hemeg, H.A.; Moussa, I.M.; Ibrahim, S.; Dawoud, T.M.; Alhaji, J.H.; Mubarak, A.S.; Kabli, S.A.; Alsubki, R.A.; Tawfik, A.M.; Marouf, S.A. Antimicrobial effect of different herbal plant extracts against different microbial population. Saudi J. Biol. Sci. 2020, 27, 3221–3227. [Google Scholar] [CrossRef]
- Covino, S.; D’ellena, E.; Tirillini, B.; Angeles, G.; Arcangeli, A.; Bistocchi, G.; Venanzoni, R.; Angelini, P. Characterization of biological activities of methanol extract of Fuscoporia torulosa (Basidiomycetes) from Italy. Int. J. Med. Mushrooms 2019, 21, 1051–1063. [Google Scholar] [CrossRef]
- Mancini, E.; Senatore, F.; Del Monte, D.; De Martino, L.; Grulova, D.; Scognamiglio, M.; Snoussi, M.; De Feo, V. Studies on chemical composition, antimicrobial and antioxidant activities of five Thymus vulgaris L. essential oils. Molecules 2015, 20, 12016–12028. [Google Scholar] [CrossRef] [Green Version]
- Cerulli, A.; Lauro, G.; Masullo, M.; Cantone, V.; Olas, B.; Kontek, B.; Nazzaro, F.; Bifulco, G.; Piacente, S. Cyclic Diarylheptanoids from Corylus avellana Green Leafy Covers: Determination of Their Absolute Configurations and Evaluation of Their Antioxidant and Antimicrobial Activities. J. Nat. Prod. 2017, 80, 1703–1713. [Google Scholar] [CrossRef]
- Della Pepa, T.; Elshafie, H.S.; Capasso, R.; De Feo, V.; Camele, I.; Nazzaro, F.; Scognamiglio, M.R.; Caputo, L. Antimicrobial and phytotoxic activity of origanum heracleoticum and O. Majorana essential oils growing in cilento (Southern Italy). Molecules 2019, 24, 2576. [Google Scholar] [CrossRef] [Green Version]
- Nostro, A.; Filocamo, A.; Giovannini, A.; Catania, S.; Costa, C.; Marino, A.; Bisignano, G. Antimicrobial activity and phenolic content of natural site and micropropagated Limonium avei (De Not.) Brullo & Erben plant extracts. Nat. Prod. Res. 2012, 26, 2132–2136. [Google Scholar] [CrossRef] [PubMed]
- Turchetti, G.; Garzoli, S.; Masci, V.L.; Sabia, C.; Iseppi, R.; Giacomello, P.; Tiezzi, A.; Ovidi, E. Antimicrobial testing of schinus molle (l.) leaf extracts and fractions followed by gc-ms investigation of biological active fractions. Molecules 2020, 25, 1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mykhailenko, O.; Bezruk, I.; Ivanauskas, L.; Georgiyants, V. Comparative analysis of apocarotenoids and phenolic constituents of Crocus sativus stigmas from 11 countries: Ecological impact. Arch. Pharm. 2022, 355, e2100468. [Google Scholar] [CrossRef] [PubMed]
- Astaf’eva, O.V.; Sukhenko, L.T. Comparative analysis of antibacterial properties and chemical composition of Glycyrrhiza glabra L. from Astrakhan region (Russia) and Calabria region (Italy). Bull. Exp. Biol. Med. 2014, 156, 829–832. [Google Scholar] [CrossRef] [PubMed]
- Caprari, C.; Fantasma, F.; Divino, F.; Bucci, A.; Iorizzi, M.; Naclerio, G.; Ranalli, G.; Saviano, G. Chemical Profile, In Vitro Biological Activity and Comparison of Essential Oils from Fresh and Dried Flowers of Lavandula angustifolia L. Molecules 2021, 26, 5317. [Google Scholar] [CrossRef] [PubMed]
- Maggi, F.; Cecchini, C.; Cresci, A.; Coman, M.M.; Tirillini, B.; Sagratini, G.; Papa, F.; Vittori, S. Chemical composition and antimicrobial activity of the essential oils from several Hypericum taxa (Guttiferae) growing in central Italy (Appennino Umbro-Marchigiano). Chem. Biodivers. 2010, 7, 447–466. [Google Scholar] [CrossRef] [PubMed]
- Casiglia, S.; Bruno, M.; Senatore, F.; Senatore, F. Chemical composition of the essential oil of bupleurum fontanesii (Apiaceae) growing wild in sicily and its activity on microorganisms affecting historical art crafts. Nat. Prod. Commun. 2016, 11, 105–108. [Google Scholar] [CrossRef] [Green Version]
- Flamini, G.; Mastrorilli, E.; Cioni, P.L.; Morelli, I. Essential oil from crithmum maritimum grown in liguria (Italy): Seasonal variation and antimicrobial activity. J. Essent. Oil Res. 1999, 11, 788–792. [Google Scholar] [CrossRef]
- Maggi, F.; Tirillini, B.; Papa, F.; Sagratini, G.; Vittori, S.; Cresci, A.; Coman, M.M.; Cecchini, C. Chemical composition and antimicrobial activity of the essential oil of Ferulago campestris (Besser) Grecescu growing in central Italy. Flavour Fragr. J. 2009, 24, 309–315. [Google Scholar] [CrossRef]
- Biondi, D.; Cianci, P.; Geraci, C.; Ruberto, G.; Piattelli, M. Antimicrobial activity and chemical composition of essential oils from sicilian aromatic plants. Flavour Fragr. J. 1993, 8, 331–337. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ricci, D. Essential oil composition and antimicrobial activity of Angelica archangelica L. (Apiaceae) roots. J. Med. Food 2014, 17, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Fraternale, D.; Genovese, S.; Ricci, D. Essential oil composition and antimicrobial activity of aerial parts and ripe fruits of Echinophora spinosa (Apiaceae) from Italy. Nat. Prod. Commun. 2013, 8, 527–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Oliveira, T.L.C.; de Araújo Soares, R.; Ramos, E.M.; das Graças Cardoso, M.; Alves, E.; Piccoli, R.H. Antimicrobial activity of Satureja montana L. essential oil against Clostridium perfringens type A inoculated in mortadella-type sausages formulated with different levels of sodium nitrite. Int. J. Food Microbiol. 2011, 144, 546–555. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maisetta, G.; Batoni, G.; Caboni, P.; Esin, S.; Rinaldi, A.C.; Zucca, P. Tannin profile, antioxidant properties, and antimicrobial activity of extracts from two Mediterranean species of parasitic plant Cytinus. BMC Complement. Altern. Med. 2019, 19, 82. [Google Scholar] [CrossRef] [Green Version]
- Bottoni, M.; Milani, F.; Mozzo, M.; Kolloffel, D.A.R.; Papini, A.; Fratini, F.; Maggi, F.; Santagostini, L. Sub-tissue localization of phytochemicals in Cinnamomum camphora (L.) j. presl. growing in northern Italy. Plants 2021, 10, 1008. [Google Scholar] [CrossRef]
- Nabavizadeh, M.; Abbaszadegan, A.; Gholami, A.; Sheikhiani, R.; Shokouhi, M.; Shams, M.S.; Ghasemi, Y. Chemical constituent and antimicrobial effect of essential oil from Myrtus communis leaves on microorganisms involved in persistent endodontic infection compared to two common endodontic irrigants: An in vitro study. J. Conserv. Dent. JCD 2014, 17, 449. [Google Scholar]
- Kivçak, B.; Mert, T.; Denizci, A.A. Antimicrobial activity of Arbutus unedo L. Fabad J. Pharm. Sci. 2001, 26, 125–128. [Google Scholar]
- Najar, B.; Pistelli, L.; Mancini, S.; Fratini, F. Chemical composition and in vitro antibacterial activity of essential oils from different species of Juniperus (section Juniperus). Flavour Fragr. J. 2020, 35, 623–638. [Google Scholar] [CrossRef]
- Aissani, N.; Coroneo, V.; Fattouch, S.; Caboni, P. Inhibitory effect of carob (Ceratonia siliqua) leaves methanolic extract on Listeria monocytogenes. J. Agric. Food Chem. 2012, 60, 9954–9958. [Google Scholar] [CrossRef]
- El Menyiy, N.; Guaouguaou, F.E.; El Baaboua, A.; El Omari, N.; Taha, D.; Salhi, N.; Shariati, M.A.; Aanniz, T.; Benali, T.; Zengin, G.; et al. Phytochemical properties, biological activities and medicinal use of Centaurium erythraea Rafn. J. Ethnopharmacol. 2021, 276, 114171. [Google Scholar] [CrossRef]
- Tardugno, R.; Serio, A.; Purgatorio, C.; Savini, V.; Paparella, A.; Benvenuti, S. Thymus vulgarisL. essential oils from Emilia Romagna Apennines (Italy): Phytochemical composition and antimicrobial activity on food-borne pathogens. Nat. Prod. Res. 2022, 36, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Marini, E.; Magi, G.; Ferretti, G.; Bacchetti, T.; Giuliani, A.; Pugnaloni, A.; Rippo, M.R.; Facinelli, B. Attenuation of Listeria monocytogenes virulence by Cannabis sativa L. Essential oil. Front. Cell. Infect. Microbiol. 2018, 8, 293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardugno, R.; Serio, A.; Pellati, F.; D’Amato, S.; Chaves López, C.; Bellardi, M.G.; Di Vito, M.; Savini, V.; Paparella, A.; Benvenuti, S. Lavandula x intermedia and Lavandula angustifolia essential oils: Phytochemical composition and antimicrobial activity against foodborne pathogens. Nat. Prod. Res. 2019, 33, 3330–3335. [Google Scholar] [CrossRef] [PubMed]
- Caputo, L.; Amato, G.; Fratianni, F.; Coppola, R.; Candido, V.; De Feo, V.; Nazzaro, F. Chemical characterization and antibiofilm activities of bulbs and leaves of two aglione (Allium ampeloprasum var. holmense asch. et Graebn.) landraces grown in southern italy. Molecules 2020, 25, 5486. [Google Scholar] [CrossRef]
- Fratianni, F.; Cozzolino, A.; de Feo, V.; Coppola, R.; Ombra, M.N.; Nazzaro, F. Polyphenols, Antioxidant, Antibacterial, and Biofilm Inhibitory Activities of Peel and Pulp of Citrus medica L., Citrus bergamia, and Citrus medica cv. Salò cultivated in southern Italy. Molecules 2019, 24, 4577. [Google Scholar] [CrossRef] [Green Version]
- Di Napoli, M.; Varcamonti, M.; Basile, A.; Bruno, M.; Maggi, F.; Zanfardino, A. Anti-Pseudomonas aeruginosa activity of hemlock (Conium maculatum, Apiaceae) essential oil. Nat. Prod. Res. 2019, 33, 3436–3440. [Google Scholar] [CrossRef]
- Aliberti, L.; Caputo, L.; De Feo, V.; De Martino, L.; Nazzaro, F.; Souza, L.F. Chemical composition and in vitro antimicrobial, cytotoxic, and central nervous system activities of the essential oils of Citrus medica L. cv. ‘Liscia’ and C. medica cv. ‘Rugosa’ cultivated in Southern Italy. Molecules 2016, 21, 1244. [Google Scholar] [CrossRef] [Green Version]
- Said, M.E.-A.E.; Militello, M.; Saia, S.; Settanni, L.; Aleo, A.; Mammina, C.; Bombarda, I.; Vanloot, P.; Roussel, C.; Dupuy, N.; et al. Artemisia arborescens Essential Oil Composition, Enantiomeric Distribution, and Antimicrobial Activity from Different Wild Populations from the Mediterranean Area. Chem. Biodivers. 2016, 13, 1095–1102. [Google Scholar] [CrossRef]
- Ferrazzano, G.F.; Scioscia, E.; Sateriale, D.; Pastore, G.; Colicchio, R.; Pagliuca, C.; Cantile, T.; Alcidi, B.; Coda, M.; Ingenito, A.; et al. In vitro antibacterial activity of pomegranate juice and peel extracts on cariogenic bacteria. Biomed Res. Int. 2017, 2017, 2152749. [Google Scholar] [CrossRef] [Green Version]
- Sharifi-Rad, J.; Dey, A.; Koirala, N.; Shaheen, S.; El Omari, N.; Salehi, B.; Goloshvili, T.; Cirone Silva, N.C.; Bouyahya, A.; Vitalini, S.; et al. Cinnamomum Species: Bridging Phytochemistry Knowledge, Pharmacological Properties and Toxicological Safety for Health Benefits. Front. Pharmacol. 2021, 12, 600139. [Google Scholar] [CrossRef]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Juliano, C.; Usai, M. Analytical Profiling of Phenolic Compounds in Extracts of Three Cistus Species from Sardinia and Their Potential Antimicrobial and Antioxidant Activity. Chem. Biodivers. 2021, 18, e2100053. [Google Scholar] [CrossRef]
- Zucca, P.; Pintus, M.; Manzo, G.; Nieddu, M.; Steri, D.; Rinaldi, A.C. Antimicrobial, antioxidant and anti-tyrosinase properties of extracts of the Mediterranean parasitic plant Cytinus hypocistis. BMC Res. Notes 2015, 8, 562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanna, C.; Maxia, A.; Fenu, G.; Loi, M.C. So uncommon and so singular, but underexplored: An updated overview on ethnobotanical uses, biological properties and phytoconstituents of sardinian endemic plants. Plants 2020, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Mir, M.A.; Bashir, N.; Alfaify, A.; Oteef, M.D.Y. Gc-ms analysis of myrtus communis extract and its antibacterial activity against gram-positive bacteria. BMC Complement. Med. Ther. 2020, 20, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Souza, E.L.; de Barros, J.C.; de Oliveira, C.E.V.; da Conceição, M.L. Influence of Origanum vulgare L. essential oil on enterotoxin production, membrane permeability and surface characteristics of Staphylococcus aureus. Int. J. Food Microbiol. 2010, 137, 308–311. [Google Scholar] [CrossRef] [PubMed]
- Mezni, F.; Aouadhi, C.; Khouja, M.L.; Khaldi, A.; Maaroufi, A. In vitro antimicrobial activity of Pistacia lentiscus L. edible oil and phenolic extract. Nat. Prod. Res. 2015, 29, 565–570. [Google Scholar] [CrossRef]
- Pulaj, B.; Mustafa, B.; Nelson, K.; Quave, C.L.; Hajdari, A. Chemical composition and in vitro antibacterial activity of Pistacia terebinthus essential oils derived from wild populations in Kosovo. BMC Complement. Altern. Med. 2016, 16, 147. [Google Scholar] [CrossRef] [Green Version]
- Nakagawa, S.; Hillebrand, G.G.; Nunez, G. Rosmarinus officinalis l. (rosemary) extracts containing carnosic acid and carnosol are potent quorum sensing inhibitors of staphylococcus aureus virulence. Antibiotics 2020, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Farahpour, M.R.; Pirkhezr, E.; Ashrafian, A.; Sonboli, A. Accelerated healing by topical administration of Salvia officinalis essential oil on Pseudomonas aeruginosa and Staphylococcus aureus infected wound model. Biomed. Pharmacother. 2020, 128, 110120. [Google Scholar] [CrossRef]
- Juliano, C.; Mattana, A.; Usai, M. Composition and in vitro antimicrobial activity of the essential oil of thymus herba-barona loisel growing wild in sardinia. J. Essent. Oil Res. 2000, 12, 516–522. [Google Scholar] [CrossRef]
- de Carvalho, R.J.; de Souza, G.T.; Honório, V.G.; de Sousa, J.P.; da Conceição, M.L.; Maganani, M.; de Souza, E.L. Comparative inhibitory effects of Thymus vulgaris L. essential oil against Staphylococcus aureus, Listeria monocytogenes and mesophilic starter co-culture in cheese-mimicking models. Food Microbiol. 2015, 52, 59–65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Agostino, G.; Badalamenti, N.; Franco, P.; Bruno, M.; Gallo, G. The chemical composition of the flowers essential oil of Inula crithmoides (Asteraceae) growing in aeolian islands, Sicily (Italy) and its biocide properties on microorganisms affecting historical art crafts. Nat. Prod. Res. 2022, 36, 2993–3001. [Google Scholar] [CrossRef] [PubMed]
- Ouassou, H.; Bouhrim, M.; Kharchoufa, L.; Imtara, H.; Daoudi, N.E.; Benoutman, A.; Bencheikh, N.; Ouahhoud, S.; Elbouzidi, A.; Bnouham, M. Caralluma europaea (Guss) N.E.Br.: A review on ethnomedicinal uses, phytochemistry, pharmacological activities, and toxicology. J. Ethnopharmacol. 2021, 273, 113769. [Google Scholar] [CrossRef] [PubMed]
- Najar, B.; Nardi, V.; Cervelli, C.; Mancianti, F.; Nardoni, S.; Ebani, V.V.; Pistelli, L. Helichrysum araxinum Takht. ex Kirp. grown in Italy: Volatiloma composition and in vitro antimicrobial activity. Z. Fur Naturforsch. Sect. C J. Biosci. 2020, 75, 265–270. [Google Scholar] [CrossRef]
- Zengin, G.; Menghini, L.; Di Sotto, A.; Mancinelli, R.; Sisto, F.; Carradori, S.; Cesa, S.; Fraschetti, C.; Filippi, A.; Angiolella, L.; et al. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. Essential oil: A multidisciplinary study. Molecules 2018, 23, 3266. [Google Scholar] [CrossRef] [Green Version]
- Quave, C.L.; Estévez-Carmona, M.; Compadre, C.M.; Hobby, G.; Hendrickson, H.; Beenken, K.E.; Smeltzer, M.S. Ellagic acid derivatives from Rubus ulmifolius inhibit Staphylococcus aureus biofilm formation and improve response to antibiotics. PLoS ONE 2012, 7, e28737. [Google Scholar] [CrossRef] [Green Version]
- Pellegrini, M.; Ricci, A.; Serio, A.; Chaves-López, C.; Mazzarrino, G.; D’Amato, S.; Lo Sterzo, C.; Paparella, A. Characterization of essential oils obtained from Abruzzo autochthonous plants: Antioxidant and antimicrobial activities assessment for food application. Foods 2018, 7, 19. [Google Scholar] [CrossRef] [Green Version]
- Loy, G.; Cottiglia, F.; Garau, D.; Deidda, D.; Pompei, R.; Bonsignore, L. Chemical composition and cytotoxic and antimicrobial activity of Calycotome villosa (Poiret) Link leaves. FARMACO 2001, 56, 433–436. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Russo, M.T.; Coroneo, V.; Dessí, S.; Cabras, P. Chemical composition of the essential oils of Juniperus from ripe and unripe berries and leaves and their antimicrobial activity. J. Agric. Food Chem. 2003, 51, 3073–3078. [Google Scholar] [CrossRef]
- Mazzanti, G.; Battinelli, L.; Salvatore, G. Antimicrobial properties of the linalol-rich essential oil of Hyssopos officinalis L. var decumbens (Lamiaceae). Flavour Fragr. J. 1998, 13, 289–294. [Google Scholar] [CrossRef]
- Coqueiro, A.; Regasini, L.O.; Stapleton, P.; Da Silva Bolzani, V.; Gibbons, S. In vitro antibacterial activity of prenylated guanidine alkaloids from Pterogyne nitens and synthetic analogues. J. Nat. Prod. 2014, 77, 1972–1975. [Google Scholar] [CrossRef] [PubMed]
- Masi, M.; Roscetto, E.; Cimmino, A.; Catania, M.R.; Surico, G.; Evidente, A. Farnesane-type sesquiterpenoids with antibiotic activity from chiliadenus lopadusanus. Antibiotics 2021, 10, 148. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, S.; Santos, J.; Duarte, A.; Duarte, A.P.; Queiroz, J.A.; Domingues, F.C. Screening of antimicrobial activity of Cistus ladanifer and Arbutus unedo extracts. Nat. Prod. Res. 2012, 26, 1558–1560. [Google Scholar] [CrossRef] [PubMed]
- Bouamama, H.; Noël, T.; Villard, J.; Benharref, A.; Jana, M. Antimicrobial activities of the leaf extracts of two Moroccan Cistus L. species. J. Ethnopharmacol. 2006, 104, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Zalegh, I.; Akssira, M.; Bourhia, M.; Mellouki, F.; Rhallabi, N.; Salamatullah, A.M.; Alkaltham, M.S.; Khalil Alyahya, H.; Mhand, R.A. A review on cistus sp.: Phytochemical and antimicrobial activities. Plants 2021, 10, 1214. [Google Scholar] [CrossRef]
- Bisio, A.; Schito, A.M.; Ebrahimi, S.N.; Hamburger, M.; Mele, G.; Piatti, G.; Romussi, G.; Dal Piaz, F.; De Tommasi, N. Antibacterial compounds from Salvia adenophora Fernald (Lamiaceae). Phytochemistry 2015, 110, 120–132. [Google Scholar] [CrossRef]
- Khaoukha, G.; Ben Jemia, M.; Amira, S.; Laouer, H.; Bruno, M.; Scandolera, E.; Senatore, F. Characterisation and antimicrobial activity of the volatile components of the flowers of Magydaris tomentosa (Desf.) DC. collected in Sicily and Algeria. Nat. Prod. Res. 2014, 28, 1152–1158. [Google Scholar] [CrossRef]
- Magi, G.; Marini, E.; Facinelli, B. Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci. Front. Microbiol. 2015, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, L.C.D.S.; Sampaio, F.C.; Sampaio, M.C.C.; Pereira, M.D.S.V.; Higino, J.S.; Peixoto, M.H.P. Minimum inhibitory concentration of adherence of Punica granatum Linn (pomegranate) gel against S. mutans, S. mitis and C. albicans. Braz. Dent. J. 2006, 17, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Gulube, Z.; Patel, M. Effect of Punica granatum on the virulence factors of cariogenic bacteria Streptococcus mutans. Microb. Pathog. 2016, 98, 45–49. [Google Scholar] [CrossRef]
- Vahid-Dastjerdi, E.; Monadi, E.; Khalighi, H.R.; Torshabi, M. Down-regulation of glycosyl transferase genes in Streptococcus mutans by Punica granatum L. Flower and Rhus coriaria L. Fruit water extracts. Iran. J. Pharm. Res. 2016, 15, 513–519. [Google Scholar] [PubMed]
- Maggi, F.; Bramucci, M.; Cecchini, C.; MM, C.; Cresci, A.; Cristalli, G.; Lupidi, G.; Papa, F.; Quassinti, L.; Sagratini, G.; et al. Composition and biological activity of essential oil of Achillea ligustica All. (Asteraceae) naturalized in central Italy: Ideal candidate for anti-cariogenic formulations. Fitoterapia 2009, 80, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Carev, I.; Maravić, A.; Ilić, N.; Čulić, V.Č.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS phytochemical analysis of two Croatian cistus species and their biological activity. Life 2020, 10, 112. [Google Scholar] [CrossRef]
- Aleksic Sabo, V.; Svircev, E.; Mimica-Dukic, N.; Orcic, D.; Narancic, J.; Knezevic, P. Anti-Acinetobacter baumannii activity of Rumex crispus L. And Rumex sanguineus L. extracts. Asian Pac. J. Trop. Biomed. 2020, 10, 172–182. [Google Scholar]
- Gaglio, R.; Barbera, M.; Aleo, A.; Lommatzsch, I.; La Mantia, T.; Settanni, L. Inhibitory Activity and Chemical Characterization of Daucus carota subsp. maximus Essential Oils. Chem. Biodivers. 2017, 14, e1600477. [Google Scholar] [CrossRef] [Green Version]
- Pľuchtová, M.; Gervasi, T.; Benameur, Q.; Pellizzeri, V.; Gruľová, D.; Campone, L.; Sedlák, V.; Cicero, N.; Pl’uchtova, M.; Gervasi, T.; et al. Antimicrobial activity of two mentha species essential oil and its dependence on different origin and chemical diversity. Nat. Prod. Commun. 2018, 13, 1051–1054. [Google Scholar]
- Miceli, N.; Cavò, E.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Marino, A.; Cincotta, F.; Condurso, C.; Taviano, M.F. Phytochemical Characterization and Biological Activities of a Hydroalcoholic Extract Obtained from the Aerial Parts of Matthiola incana (L.) R.Br. subsp. incana (Brassicaceae) Growing Wild in Sicily (Italy). Chem. Biodivers. 2019, 16, e1800677. [Google Scholar] [CrossRef] [PubMed]
- Miceli, N.; Filocamo, A.; Ragusa, S.; Cacciola, F.; Dugo, P.; Mondello, L.; Celano, M.; Maggisano, V.; Taviano, M.F. Chemical Characterization and Biological Activities of Phenolic-Rich Fraction from Cauline Leaves of Isatis tinctoria L. (Brassicaceae) Growing in Sicily, Italy. Chem. Biodivers. 2017, 14, e1700073. [Google Scholar] [CrossRef] [PubMed]
- Gelmini, F.; Squillace, P.; Testa, C.; Sparacino, A.C.; Angioletti, S.; Beretta, G. GC-MS characterisation and biological activity of essential oils from different vegetative organs of Plectranthus barbatus and Plectranthus caninus cultivated in north Italy. Nat. Prod. Res. 2015, 29, 993–998. [Google Scholar] [CrossRef]
- Cottiglia, F.; Loy, G.; Garau, D.; Floris, C.; Casu, M.; Pompei, R.; Bonsignore, L. Antimicrobial evaluation of coumarins and flavonoids from the stems of Daphne gnidium L. Phytomedicine 2001, 8, 302–305. [Google Scholar] [CrossRef]
- Tuberoso, C.I.; Kowalczyk, A.; Coroneo, V.; Russo, M.T.; Dessì, S.; Cabras, P. Chemical composition and antioxidant, antimicrobial, and antifungal activities of the essential oil of Achillea ligustica all. J. Agric. Food Chem. 2005, 53, 10148–10153. [Google Scholar]
- Romeo, F.V.; Fabroni, S.; Ballistreri, G.; Muccilli, S.; Spina, A.; Rapisarda, P. Characterization and antimicrobial activity of alkaloid extracts from seeds of different genotypes of Lupinus spp. Sustainability 2018, 10, 788. [Google Scholar] [CrossRef] [Green Version]
- Mandalari, G.; Bisignano, C.; Cirmi, S.; Navarra, M. Effectiveness of Citrus Fruits on Helicobacter pylori. Evid.-Based Complement. Altern. Med. 2017, 2017, 8379262. [Google Scholar] [CrossRef] [Green Version]
- Menghini, L.; Leporini, L.; Tirillini, B.; Epifano, F.; Genovese, S. Chemical composition and inhibitory activity against helicobacter pylori of the essential oil of Apium nodiflorum (Apiaceae). J. Med. Food 2010, 13, 228–230. [Google Scholar] [CrossRef] [PubMed]
- Miguel, M.G.; Faleiro, M.L.; Guerreiro, A.C.; Antunes, M.D. Arbutus unedo L.: Chemical and biological properties. Molecules 2014, 19, 15799–15823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanjust, E.; Rinaldi, A.C. Cytinus under the microscope: Disclosing the secrets of a parasitic plant. Plants 2021, 10, 146. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019, 8, 108, Pathogens Erratum in Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Milia, E.; Bullitta, S.M.; Mastandrea, G.; Szotáková, B.; Schoubben, A.; Langhansová, L.; Quartu, M.; Bortone, A.; Eick, S. Leaves and fruits preparations of pistacia lentiscus l.: A review on the ethnopharmacological uses and implications in inflammation and infection. Antibiotics 2021, 10, 425. [Google Scholar] [CrossRef]
- Çoban, E.P.; Biyik, H.H.; Törün, B.; Yaman, F. Evaluation the antimicrobial effects of Pistacia terebinthus L. and Papaver rhoeas L. extracts against some pathogen microorganisms. Indian J. Pharm. Educ. Res. 2017, 51, S377–S380. [Google Scholar] [CrossRef] [Green Version]
- Iannello, C.; Bastida, J.; Bonvicini, F.; Antognoni, F.; Gentilomi, G.A.; Poli, F. Chemical composition, and in vitro antibacterial and antifungal activity of an alkaloid extract from Crinum angustum Steud. Nat. Prod. Res. 2014, 28, 704–710. [Google Scholar]
- Bonvicini, F.; Mandrone, M.; Antognoni, F.; Poli, F.; Gentilomi, G.A. Ethanolic extracts of Tinospora cordifolia and Alstonia scholaris show antimicrobial activity towards clinical isolates of methicillin-resistant and carbapenemase-producing bacteria. Nat. Prod. Res. 2014, 28, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, G.L.; Cicero, N.; Nava, V.; Macrì, A.; Gervasi, C.; Capparucci, F.; Sciortino, M.; Avellone, G.; Benameur, Q.; Santini, A.; et al. Chemical Characterization, Antibacterial Activity, and Embryo Acute Toxicity of Rhus coriaria L. Genotype from Sicily (Italy). Foods 2022, 11, 538. [Google Scholar] [CrossRef] [PubMed]
- Iseppi, R.; Di Cerbo, A.; Aloisi, P.; Manelli, M.; Pellesi, V.; Provenzano, C.; Camellini, S.; Messi, P.; Sabia, C. In vitro activity of essential oils against planktonic and biofilm cells of extended-spectrum β-lactamase (ESBL)/carbapenamase-producing gram-negative bacteria involved in human nosocomial infections. Antibiotics 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, M.; Cacaci, M.; Barbanti, L.; Martini, C.; Sanguinetti, M.; Benvenuti, S.; Tosi, G.; Fiorentini, L.; Scozzoli, M.; Bugli, F.; et al. Origanum vulgare essential oil vs. A commercial mixture of essential oils: In vitro effectiveness on salmonella spp. from poultry and swine intensive livestock. Antibiotics 2020, 9, 763. [Google Scholar] [CrossRef] [PubMed]
- Militaru, D.; Popa, V.; Botus, D.; Stirbu, B.; Caplan, E.M. In vitro evaluation of the potential antibacterial effect of artemisinin on Campylobacter jejuni. Rom. Biotechnol. Lett. 2015, 20, 10221–10227. [Google Scholar]
- Milia, E.; Usai, M.; Szotáková, B.; Elstnerová, M.; Králová, V.; D’hallewin, G.; Spissu, Y.; Barberis, A.; Marchetti, M.; Bortone, A.; et al. The pharmaceutical ability of Pistacia lentiscus L. Leaves essential oil against periodontal bacteria and Candida sp. and its anti-inflammatory potential. Antibiotics 2020, 9, 281. [Google Scholar] [CrossRef]
- Palmieri, S.; Maggio, F.; Pellegrini, M.; Ricci, A.; Serio, A.; Paparella, A.; Lo Sterzo, C. Effect of the Distillation Time on the Chemical Composition, Antioxidant Potential and Antimicrobial Activity of Essential Oils from Different Cannabis sativa L. Cultivars. Molecules 2021, 26, 4770. [Google Scholar] [CrossRef]
- Price, R. O’Neill report on antimicrobial resistance: Funding for antimicrobial specialists should be improved. Eur. J. Hosp. Pharm. 2016, 23, 245–247. [Google Scholar] [CrossRef]
- Magallón, S.; Hilu, K.W. Land plants (embryophyta). In The Timetree of Life; Hedges, S.B., Kumar, S., Eds.; Oxford University Press: New York, NY, USA, 2009; pp. 133–137. [Google Scholar]
- Clarke, J.T.; Warnock, R.C.M.; Donoghue, P.C.J. Establishing a time-scale for plant evolution. New Phytol. 2011, 192, 266–301. [Google Scholar] [CrossRef]
- Owusu-Kwarteng, J.; Wuni, A.; Akabanda, F.; Tano-Debrah, K.; Jespersen, L. Prevalence, virulence factor genes and antibiotic resistance of Bacillus cereus sensu lato isolated from dairy farms and traditional dairy products. BMC Microbiol. 2017, 17, 65. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, M.; Christiansson, A.; Svensson, B. Bacillus cereus spores during housing of dairy cows: Factors affecting contamination of raw milk. J. Dairy Sci. 2007, 90, 2745–2754. [Google Scholar] [CrossRef]
- Otter, A.; Uzal, F.A. Clostridial diseases in farm animals: 2. Histotoxic and neurotoxic diseases. Practice 2020, 42, 279–288. [Google Scholar] [CrossRef]
- Otter, A.; Uzal, F.A. Clostridial diseases in farm animals: 1. Enterotoxaemias and other alimentary tract infections. Practice 2020, 42, 219–232. [Google Scholar] [CrossRef]
- Arias, C.A.; Murray, B.E. Emergence and management of drug-resistant enterococcal infections. Expert Rev. Anti. Infect. Ther. 2008, 6, 637–655. [Google Scholar] [CrossRef]
- Hammerum, A.M. Enterococci of animal origin and their significance for public health. Clin. Microbiol. Infect. 2012, 18, 619–625. [Google Scholar] [CrossRef]
- Torres, C.; Alonso, C.A.; Ruiz-Ripa, L.; León-Sampedro, R.; Del Campo, R.; Coque, T.M. Antimicrobial resistance in Enterococcus spp. of animal origin. Microbiol. Spectr. 2018, 6, 4–6. [Google Scholar] [CrossRef]
- Shepheard, M.A.; Fleming, V.M.; Connor, T.R.; Corander, J.; Feil, E.J.; Fraser, C.; Hanage, W.P. Historical zoonoses and other changes in host tropism of Staphylococcus aureus, identified by phylogenetic analysis of a population dataset. PLoS ONE 2013, 8, e62369. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.C. Livestock-associated Staphylococcus aureus: The United States experience. PLoS Pathog. 2015, 11, e1004564. [Google Scholar] [CrossRef] [PubMed]
- Nocera, F.P.; Attili, A.-R.; De Martino, L. Acinetobacter baumannii: Its clinical significance in human and veterinary medicine. Pathogens 2021, 10, 127. [Google Scholar] [CrossRef]
- Melo-Nascimento, A.O.D.S.; Treumann, C.; Neves, C.; Andrade, E.; Andrade, A.C.; Edwards, R.; Dinsdale, E.; Bruce, T. Functional characterization of ligninolytic Klebsiella spp. strains associated with soil and freshwater. Arch. Microbiol. 2018, 200, 1267–1278. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.; Zhou, H.; Qin, L.; Pang, Z.; Qin, T.; Ren, H.; Pan, Z.; Zhou, J. Frequency, antimicrobial resistance and genetic diversity of Klebsiella pneumoniae in food samples. PLoS ONE 2016, 11, e0153561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kikuchi, N.; Blakeslee, J.R.; Hiramune, T. Plasmid profiles of Klebsiella pneumoniae isolated from horses. J. Vet. Med. Sci. 1995, 57, 113–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saishu, N.; Ozaki, H.; Murase, T. CTX-M-type extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolated from cases of bovine mastitis in Japan. J. Vet. Med. Sci. 2014, 76, 1153–1156. [Google Scholar] [CrossRef] [Green Version]
- Hertl, J.A.; Schukken, Y.H.; Welcome, F.L.; Tauer, L.W.; Gröhn, Y.T. Pathogen-specific effects on milk yield in repeated clinical mastitis episodes in Holstein dairy cows. J. Dairy Sci. 2014, 97, 1465–1480. [Google Scholar] [CrossRef] [Green Version]
- Piras, C.; Greco, V.; Gugliandolo, E.; Soggiu, A.; Tilocca, B.; Bonizzi, L.; Zecconi, A.; Cramer, R.; Britti, D.; Urbani, A.; et al. Raw cow milk bacterial consortium as bioindicator of circulating anti-microbial resistance (Amr). Animals 2020, 10, 2378. [Google Scholar] [CrossRef]
- Haenni, M.; Bour, M.; Châtre, P.; Madec, J.-Y.; Plésiat, P.; Jeannot, K. Resistance of animal strains of Pseudomonas aeruginosa to carbapenems. Front. Microbiol. 2017, 8, 1847. [Google Scholar] [CrossRef]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Caudullo, G.; Carrara, M. Essential oil phytocomplex activity, a review with a focus on multivariate analysis for a network pharmacology-informed phytogenomic approach. Molecules 2020, 25, 1833. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, B.; Alves, L. Synergy in plant medicines. Curr. Med. Chem. 2003, 10, 13–20. [Google Scholar] [CrossRef]
- Abreu, A.C.; McBain, A.J.; Simoes, M. Plants as sources of new antimicrobials and resistance-modifying agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Abreu, A.C.; Coqueiro, A.; Sultan, A.R.; Lemmens, N.; Kim, H.K.; Verpoorte, R.; Van Wamel, W.J.B.; Simões, M.; Choi, Y.H. Looking to nature for a new concept in antimicrobial treatments: Isoflavonoids from Cytisus striatus as antibiotic adjuvants against MRSA. Sci. Rep. 2017, 7, 3777. [Google Scholar] [CrossRef] [PubMed]
- Dettweiler, M.; Melander, R.J.; Porras, G.; Risener, C.; Marquez, L.; Samarakoon, T.; Melander, C.; Quave, C.L. A clerodane diterpene from Callicarpa americana resensitizes methicillin-resistant Staphylococcus aureus to β-lactam antibiotics. ACS Infect. Dis. 2020, 6, 1667–1673. [Google Scholar] [CrossRef] [PubMed]
- Russell, A.D. Bacterial resistance to disinfectants: Present knowledge and future problems. J. Hosp. Infect. 1999, 43, S57–S68. [Google Scholar] [CrossRef]
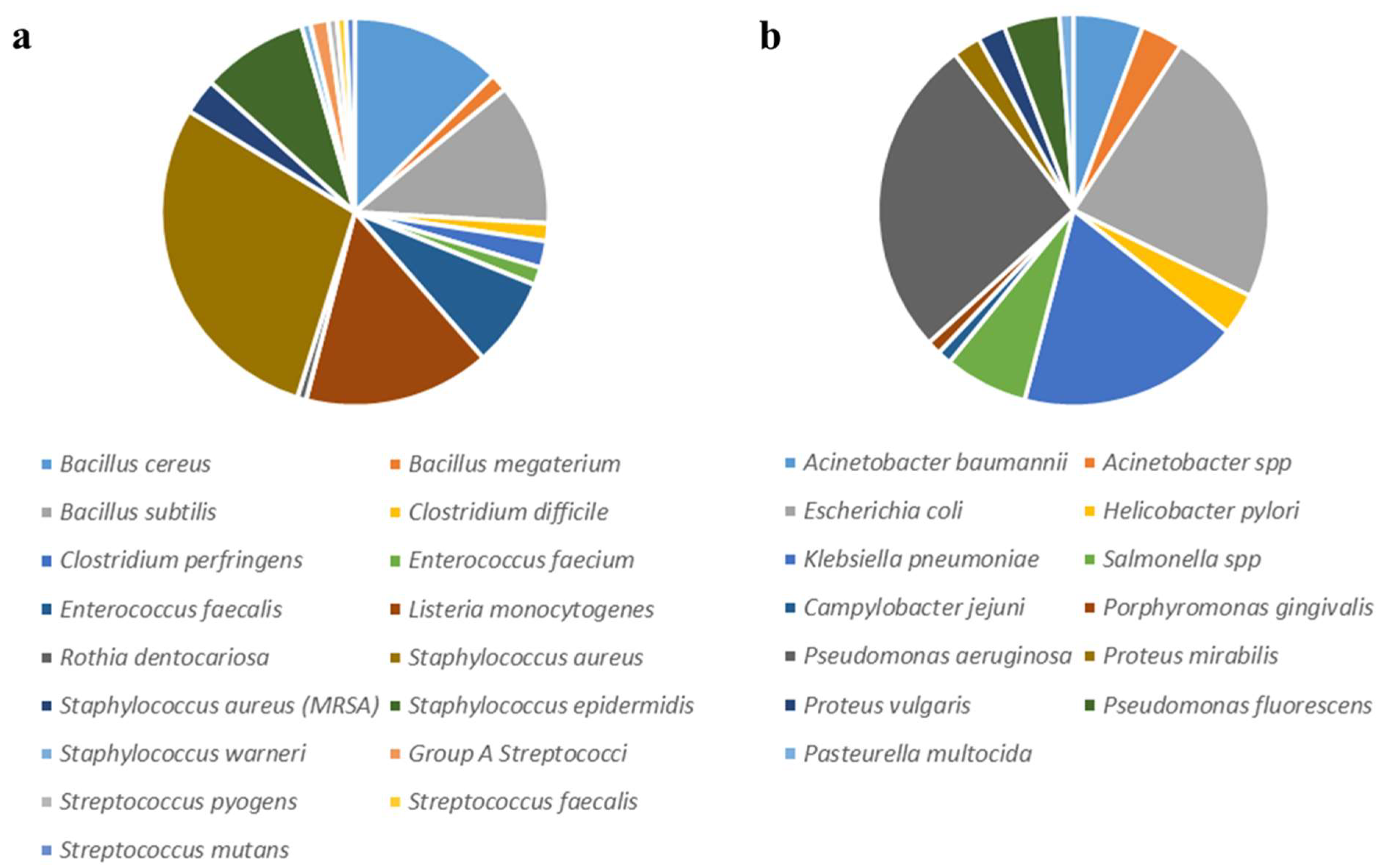
| Bacterium (Gram+) | Number of Plant Species | Plant Name |
|---|---|---|
| Bacillus cereus | 17 | Daucus carota subsp. maximus (Desf.)Ball [16]; Achillea moschata [17]; Lavandula × intermedia [18]; Laurus nobilis [19]; Dianthus rupicola [20]; Malus domestica var. Annurca [21]; Teucrium genus (Germander) [22]; Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [23]; Allium sativum L. [24]; Rosmarinus officinalis L. and Lavandula angustifolia Miller [25]; Guava (Psidium guajava), Sage (Salvia officinalis), Rhamnus (Ziziphusspina Christi), Mulberry (Morusalba L.), and Olive (Oleaeuropaea L.) [26]; Fuscoporia torulosa [27]; Thymus vulgaris [28]; Corylus avellana [29] |
| Bacillus megaterium | 2 | Origanum heracleoticum and O. majorana [30] |
| Bacillus subtilis | 16 | Limonium avei (De Not.) Brullo and Erben [31]; Schinus molle (L.) [32]; Thymus vulgaris [28]; Crocus sativus L. [33]; Glycyrrhiza glabra [34]; Dianthus rupicola [20]; Lavandula angustifolia L. [35]; Hypericum taxa (Guttiferae) [36]; Fuscoporia torulosa (Basidiomycetes) [27]; Fuscoporia torulosa [27]; Thymus vulgaris [28]; Bupleurum fontanesii [37]; Crithmum maritimum [38]; Ferulago campestris [39]; Origanum onites and Thymus capitatus [40] |
| Clostridium difficile | 2 | Angelica archangelica L. [41]; Echinophora spinosa (Apiaceae) [42] |
| Clostridium perfringens | 3 | Angelica archangelica L. (Apiaceae) [41]; Satureja montana L. [43]; Echinophora spinosa (Apiaceae) [42] |
| Enterococcus faecium | 2 | Cytinus hypocistis, Cytinus ruber [44] |
| Enterococcus faecalis | 10 | Schinus molle (L.) [32]; Achillea moschata [17]; Angelica archangelica L. (Apiaceae) [41]; Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [23]; Cinnamomum camphora (L.) [45]; Myrtus communis [46]; Arbutus unedo L. [47]; Echinophora spinosa (Apiaceae) [42]; Ferulago campestris [39]; Juniperus spp. [48] |
| Listeria monocytogenes | 21 | Ceratonia siliqua L. [49]; Daucus carota subsp. maximus (Desf.) Ball [16]; Limonium avei (De Not.) Brullo and Erben [31]; Centaurium erythraea [50]; Thymus vulgaris L. [51]; Cannabis sativa [52]; Lavandula × intermedia and Lavandula angustifolia [53]; Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [23]; Cinnamomum camphora (L.) [45]; Allium ampeloprasum [54]; Citrus taxa-Citrus medica, Citrus bergamia [55]; Conium maculatum, Apiaceae [56]; Allium sativum L. [24]; Schinus molle (L.) [32]; Cytinus [44]; Citrus medica L. [57]; Achillea moschata [17]; Crithmum maritimum [38]; Artemisia arborescens [58] |
| Rothia dentocariosa | 1 | Punica granatum L. [59] |
| Staphylococcus aureus | 39 | Cinnamomum [60]; Cinnamomum camphora (L.) [45]; Cistus monspeliensis L. [61]; Cistus salviifolius L. [61]; Cytinus hypocistis (L.) L. [62]; Limonium morisianum Arrigoni [63]; Myrtus communis L. [64]; Origanum vulgare L. [65]; Pistacia lentiscus L. [66]; Pistacia terebinthus L. [67]; Rosmarinus officinalis L. [68]; Salvia officinalis L. [69]; Thymus herba-barona Loise L. [70]; Thymus vulgaris L. [71]; Inula crithmoides [72]; Caralluma europaea [73]; Crocus sativus [33]; Helichrysum araxinum [74]; Schinus molle (L.) [32]; Cannabis sativa [75]; Centaurium erythraea [50]; Citrus medica L., Citrus bergamia, and Citrus medica [55]; Laurus nobilis [19]; Rubus ulmifolius [76]; Malus domestica var. Annurca [21]; Teucrium genus (Germander) [22]; Daucus carota subsp. maximus (Desf.) [16]; Cytinus [44]; T. vulgaris, S. montana and C. sativum [77]; Garlic (Allium sativum L.) [24]; Thymus vulgaris L. [28]; Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [23]; Calycotome villosa (Poiret) [78]; Juniperus spp. [79]; Hyssopus officinalis [80] |
| Staphylococcus aureus (MRSA) | 4 | Crinum angustum Steud. [81]; Limonium avei (De Not.) Brullo and Erben [31]; Cytinus hypocistis [62]; Chiliadenus lopadusanus [82] |
| Staphylococcus epidermidis | 12 | Arbutus unedo L. [83]; Cistus monspeliensis L. [84]; Cistus salviifolius L. [85]; Cytinus hypocistis (L.) L. [62]; Limonium avei (De Not.) Brullo and Erben [31]; Limonium morisianum Arrigoni [63]; Myrtus communis L. [46]; Pistacia lentiscus L. [66]; Cytinus. [44]; Thymus vulgaris L. [28]; Salvia adenophora [86]; Magydaris tomentosa [87]; |
| Staphylococcus warneri | 1 | Daucus carota subsp. maximus (Desf.) Ball [16] |
| Group A Streptococci | 2 | Origanum and Thymus [88] |
| Streptococcus pyogens | 1 | Teucrium genus [22] |
| Streptococcus faecalis | 1 | Thymus vulgaris L. [28] |
| Streptococcus mutans | 1 | Punica granatum L. [89,90,91]; Achillea ligustica [92] |
| Bacterium (Gram−) | Number of Plant Species | Plant Name |
|---|---|---|
| Acinetobacter baumannii | 5 | Chiliadenus lopadusanus [82]; Cistus creticus (CC) and Cistus salviifolius (CS) [93]; Rumex crispus L. and Rumex sanguineus [94] |
| Acinetobacter spp. | 3 | Daucus carota subsp. maximus [95]; Lavandula × intermedia [18]; Cytinus hypocistis [62] |
| Enterobacter cloacae | 1 | Mentha spp. [96] |
| Escherichia coli | 20 | Daucus carota subsp. Maximus [16]; Cytinus hypocistis [62]; Matthiola incana (L.) R.Br. subsp. incana (Brassicaceae) [97]; Lavandula × intermedia [18]; Laurus nobilis [19]; Glycyrrhiza glabra L. [34]; Malus domestica var. Annurca [21]; Teucrium genus (Germander) [22]; Daucus carota subsp. maximus (Desf.) [16]; Isatis tinctoria L. (Brassicaceae) [98]; Garlic (Allium sativum L.) [24]; Thymus vulgaris L. [28]; Plectranthus barbatus and Plectranthus caninus [99]; Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [23]; Daphne gnidium L. [100]; Calycotome villosa [78]; Hyssopos officinalis L. [80]; Achillea ligustica [101]; Lupinus spp. [102]; |
| Helicobacter pylori | 3 | Citrus spp. [103]; Cannabis sativa L. [75]; Apium nodiflorum (Apiaceae). [104] |
| Klebsiella pneumoniae | 16 | Arbutus unedo [105]; Cistus spp. [93]; Cytinus hypocistis [62,106]; Myrtus comunis [107]; Pistacia lentiscus [108]; Teucrium genus (Germander) [22]; Cytinus. [44]; Thymus vulgaris L. [28]; Pistacia terebinthus [109]; Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [23]; Crinum angustum [110]; Tinospora cordifolia and Alstonia scholaris [111]; Rhus coriaria L. [112]; Calycotome villosa [78]; Melaleuca alternifolia [113]; Mentha spp. [96] |
| Salmonella spp. | 6 | Origanum vulgare [114]; Lavandula × intermedia and Lavandula angustifolia [53]; Thymus vulgaris L. [28]; Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [23]; Mentha spp. [96] |
| Campylobacter jejuni | 1 | Artemisia annua [115] |
| Porphyromonas gingivalis | 1 | Pistacia lentiscus L. [116] |
| Pseudomonas aeruginosa | 23 | Cinnamomum camphora [45]; Allium ampeloprasum var. holmense Asch. et Graebn. [54]; Schinus molle (L.) [32]; Achillea moschata [17]; Citrus medica L., Citrus bergamia, and Citrus medica [55]; Centaurium erythraea [50]; Laurus nobilis [19]; Teucrium genus (Germander) [22]; Cytinus. [44]; Conium maculatum, Apiaceae [56]; Garlic (Allium sativum L.) [24]; Five Thymus vulgaris L. [28]; Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [23]; Lupinus spp. [102]; Calycotome villosa [78]; Juniperus spp. [79]; Allium ampeloprasum [54]; Allium sativum [24]; Melaleuca alternifolia [113]; Conium maculatum [56]; Achillea ligustica [101] |
| Proteus mirabilis | 2 | Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [23]; Hyssopos officinalis L. [80] |
| Proteus vulgaris | 2 | Thymus vulgaris L. [28] Rapa Catozza Napoletana (Brassica rapa L. var. rapa DC.) [23] |
| Pseudomonas fluorescens | 4 | Lavandula × intermedia [18]; Origanum heracleoticum and O. majorana [30]; Cannabis sativa [117] |
| Pasteurella multocida | 1 | Morus alba [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Piras, C.; Tilocca, B.; Castagna, F.; Roncada, P.; Britti, D.; Palma, E. Plants with Antimicrobial Activity Growing in Italy: A Pathogen-Driven Systematic Review for Green Veterinary Pharmacology Applications. Antibiotics 2022, 11, 919. https://doi.org/10.3390/antibiotics11070919
Piras C, Tilocca B, Castagna F, Roncada P, Britti D, Palma E. Plants with Antimicrobial Activity Growing in Italy: A Pathogen-Driven Systematic Review for Green Veterinary Pharmacology Applications. Antibiotics. 2022; 11(7):919. https://doi.org/10.3390/antibiotics11070919
Chicago/Turabian StylePiras, Cristian, Bruno Tilocca, Fabio Castagna, Paola Roncada, Domenico Britti, and Ernesto Palma. 2022. "Plants with Antimicrobial Activity Growing in Italy: A Pathogen-Driven Systematic Review for Green Veterinary Pharmacology Applications" Antibiotics 11, no. 7: 919. https://doi.org/10.3390/antibiotics11070919
APA StylePiras, C., Tilocca, B., Castagna, F., Roncada, P., Britti, D., & Palma, E. (2022). Plants with Antimicrobial Activity Growing in Italy: A Pathogen-Driven Systematic Review for Green Veterinary Pharmacology Applications. Antibiotics, 11(7), 919. https://doi.org/10.3390/antibiotics11070919








