Monitoring Carbapenem-Resistant Enterobacterales in the Environment to Assess the Spread in the Community
Abstract
:1. Introduction
2. Results
2.1. Prevalence of CRE
2.2. Confirmation of Meropenem Resistance
2.3. Identification of Species of the Meropenem-Resistant Isolates
2.4. Carbapenemase-Producing Genes Carried by the Isolates
3. Discussion
3.1. Species Monitorable from Environmental Samples
3.2. Carbapenemase Carried by Environmental Isolates
3.3. Effect of Isolation on the Detected Type of Carbapenemase
4. Materials and Methods
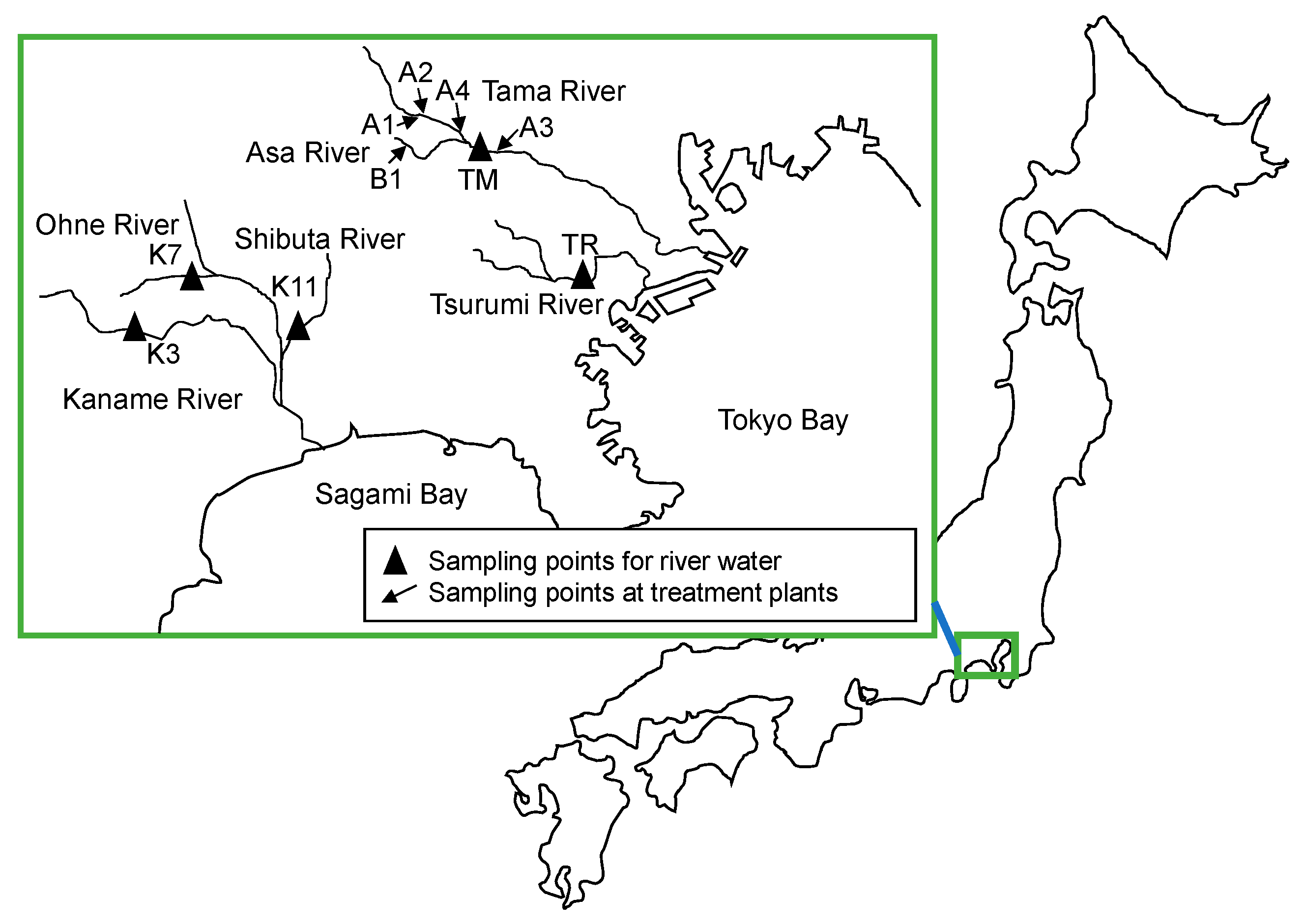
4.1. Sample Origins
4.2. Isolation of CRE
4.3. Confirmation of Resistance for Presumptive CRE
4.4. Identification of Species
4.5. Genotyping of Carbapenemase Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kitajima, M.; Ahmed, W.; Bibby, K.; Carducci, A.; Gerba, C.P.; Hamilton, K.A.; Haramoto, E.; Rose, J.B. SARS-CoV-2 in wastewater: State of the knowledge and research needs. Sci. Total Environ. 2020, 739, 139076. [Google Scholar] [CrossRef] [PubMed]
- Peccia, J.; Zulli, A.; Brackney, D.E.; Grubaugh, N.D.; Kaplan, E.H.; Casanovas-Massana, A.; Ko, A.; Malik, A.A.; Wang, D.; Wang, M.; et al. Measurement of SARS-CoV-2 RNA in wastewater tracks community infection dynamics. Nat. Biotechnol. 2020, 38, 1164–1167. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities. Available online: https://apps.who.int/iris/bitstream/handle/10665/259462/9789241550178-eng.pdf (accessed on 26 May 2022).
- WHO Publishes List of Bacteria for which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news/item/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 6 May 2022).
- Nishiyama, M.; Praise, S.; Tsurumaki, K.; Baba, H.; Kanamori, H.; Watanabe, T. Prevalence of antibiotic-resistant bacteria ESKAPE among healthy people estimated by monitoring of municipal wastewater. Antibiotics 2021, 10, 495. [Google Scholar] [CrossRef] [PubMed]
- Cahill, N.; O’Connor, L.; Mahon, B.; Varley, A.; McGrath, E.; Ryan, P.; Cormican, M.; Brehony, C.; Jolley, K.A.; Maiden, M.C.; et al. Hospital effluent: A reservoir for carbapenemase-producing Enterobacterales? Sci. Total Environ. 2019, 672, 618–624. [Google Scholar] [CrossRef]
- Mills, M.C.; Lee, J. The threat of carbapenem-resistant bacteria in the environment, Evidence of widespread contamination of reservoirs at a global scale. Environ. Pollut. 2019, 255, 113143. [Google Scholar] [CrossRef]
- Hoelle, J.; Johnson, J.; Johnston, B.; Kinkle, B.; Boczek, L.; Ryu, H.; Hayes, S. Survey of US wastewater for carbapenem-resistant Enterobacteriaceae. J. Water Health 2019, 17, 219–226. [Google Scholar] [CrossRef]
- Livorsi, D.J.; Chorazy, M.L.; Schweizer, M.L.; Balkenende, E.C.; Blevins, A.E.; Nair, R.; Samore, M.H.; Nelson, R.E.; Khader, K.; Perencevich, E.N. A systematic review of the epidemiology of carbapenem-resistant Enterobacteriaceae in the United States. Antimicrob. Resist. Infect. Control 2018, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Reinke, R.A.; Quach-Cu, J.; Allison, N.; Lynch, B.; Crisostomo, C.; Padilla, M. A method to quantify viable carbapenem resistant gram-negative bacteria in treated and untreated wastewater. J. Microbiol. Methods 2020, 179, 106070. [Google Scholar] [CrossRef]
- Rizzo, L.; Manaia, C.; Merlin, C.; Schwartz, T.; Dagot, C.; Ploy, M.C.; Michael, I.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for antibiotic resistant bacteria and genes spread into the environment: A review. Sci. Total Environ. 2013, 447, 345–360. [Google Scholar] [CrossRef] [Green Version]
- Teban-Man, A.; Szekeres, E.; Fang, P.; Klümper, U.; Hegedus, A.; Baricz, A.; Berendonk, T.U.; Pârvu, M.; Coman, C. Municipal wastewaters carry important carbapenemase genes independent of hospital input and can mirror clinical resistance patterns. Microbiol. Spectr. 2022, 10, e0271121. [Google Scholar] [CrossRef]
- Cherak, Z.; Loucif, L.; Moussi, A.; Rolain, J.M. Carbapenemase-producing Gram-negative bacteria in aquatic environments: A review. J. Glob. Antimicrob. Resist. 2021, 25, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Gomi, R.; Matsuda, T.; Yamamoto, M.; Chou, P.H.; Tanaka, M.; Ichiyama, S.; Yoneda, M.; Matsumura, Y. Characteristics of carbapenemase-producing Enterobacteriaceae in wastewater revealed by genomic analysis. Antimicrob. Agents Chemother. 2018, 62, e02501-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pazda, M.; Kumirska, J.; Stepnowski, P.; Mulkiewicz, E. Antibiotic resistance genes identified in wastewater treatment plant systems—A review. Sci. Total Environ. 2019, 697, 134023. [Google Scholar] [CrossRef] [PubMed]
- Hansen, G.T. Continuous evolution: Perspective on the epidemiology of carbapenemase resistance among Enterobacterales and other Gram-negative bacteria. Infect. Dis. Ther. 2021, 10, 75–92. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, C.; Zacharias, N.; Essert, S.M.; Wasser, F.; Müller, H.; Sib, E.; Precht, T.; Parcina, M.; Bierbaum, G.; Schmithausen, R.M.; et al. Clinically relevant antibiotic-resistant bacteria in aquatic environments—An optimized culture-based approach. Sci. Total Environ. 2021, 750, 142265. [Google Scholar] [CrossRef]
- Hrenovic, J.; Ivankovic, T.; Ivekovic, D.; Repec, S.; Stipanicev, D.; Ganjto, M. The fate of carbapenem-resistant bacteria in a wastewater treatment plant. Water Res. 2017, 126, 232–239. [Google Scholar] [CrossRef]
- Rodríguez, E.A.; Garzón, L.M.; Gómez, I.D.; Jiménez, J.N. Multidrug resistance and diversity of resistance profiles in carbapenem-resistant Gram-negative bacilli throughout a wastewater treatment plant in Colombia. J. Glob. Antimicrob. Resist. 2020, 22, 358–366. [Google Scholar] [CrossRef]
- Urase, T.; Okazaki, M.; Tsutsui, H. Prevalence of ESBL-producing Escherichia coli and carbapenem-resistant Enterobacteriaceae in treated wastewater: A comparison with nosocomial infection surveillance. J. Water Health 2020, 18, 899–910. [Google Scholar] [CrossRef]
- Azuma, T.; Uchiyama, T.; Zhang, D.; Usui, M.; Hayashi, T. Distribution and characteristics of carbapenem-resistant and extended-spectrum β-lactamase (ESBL) producing Escherichia coli in hospital effluents, sewage treatment plants, and river water in an urban area of Japan. Sci. Total Environ. 2022, 24, 156232. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kawahara, R.; Akeda, Y.; Shanmugakani, R.K.; Yoshida, H.; Hagiya, H.; Hara, N.; Nishi, I.; Yukawa, S.; Asada, R.; et al. Development of selective medium for IMP-type carbapenemase-producing Enterobacteriaceae in stool specimens. BMC Infect. Dis. 2017, 17, 229. [Google Scholar] [CrossRef] [Green Version]
- Davin-Regli, A.; Lavigne, J.P.; Pagès, J.M. Enterobacter spp.: Update on taxonomy, clinical aspects, and emerging antimicrobial resistance. Clin. Microbiol. Rev. 2019, 32, e00002-19. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Feng, Y.; Zong, Z. Precise species identification for Enterobacter: A genome sequence-based study with reporting of two novel species, Enterobacter quasiroggenkampii sp. nov. and Enterobacter quasimori sp. nov. mSystems 2020, 5, e00527-20. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Medina, N.; Barrios-Camacho, H.; Duran-Bedolla, J.; Garza-Ramos, U. Klebsiella variicola: An emerging pathogen in humans. Emerg. Microbes Infect. 2019, 8, 973–988. [Google Scholar] [CrossRef] [Green Version]
- Surveillance on Carbapenem-Resistant Enterobacteriaceae: Collected in 2019 by National Institute of Infectious Diseases. Available online: https://www.niid.go.jp/niid/ja/cre-m/cre-iasrd/10462-496d01.html (accessed on 24 May 2022).
- Annual Open Report 2020 (All Facilities) by Japan Nosocomial Infections Surveillance. Available online: https://janis.mhlw.go.jp/english/report/open_report/2020/3/1/ken_Open_Report_Eng_202000_clsi2012.pdf (accessed on 24 May 2022).
- Annual Open Report 2020 (Outpatients) by Japan Nosocomial Infections Surveillance. Available online: https://janis.mhlw.go.jp/report/open_report/2020/3/1/ken_Open_Report_202000_Outpatient.pdf (accessed on 24 May 2022). (In Japanese)
- Oka, K.; Matsumoto, A.; Tetsuka, N.; Morioka, H.; Iguchi, M.; Ishiguro, N.; Nagamori, T.; Takahashi, S.; Saito, N.; Tokuda, K.; et al. Clinical characteristics and treatment outcomes of carbapenem-resistant Enterobacterales infections in Japan. J. Glob. Antimicrob. Resist. 2022, 29, 247–252. [Google Scholar] [CrossRef] [PubMed]
- White, L.; Hopkins, K.L.; Meunier, D.; Perry, C.L.; Pike, R.; Wilkinson, P.; Pickup, R.W.; Cheesbrough, J.; Woodford, N. Carbapenemase-producing Enterobacteriaceae in hospital wastewater: A reservoir that may be unrelated to clinical isolates. J. Hosp. Infect. 2016, 93, 145–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araújo, S.; Sousa, M.; Tacão, M.; Baraúna, R.A.; Silva, A.; Ramos, R.; Alves, A.; Manaia, C.M.; Henriques, I. Carbapenem-resistant bacteria over a wastewater treatment process: Carbapenem-resistant Enterobacteriaceae in untreated wastewater and intrinsically-resistant bacteria in final effluent. Sci. Total Environ. 2021, 782, 146892. [Google Scholar] [CrossRef]
- Umeda, K.; Nakamura, H.; Fukuda, A.; Matsumoto, Y.; Motooka, D.; Nakamura, S.; Yasui, Y.; Yoshida, H.; Kawahara, R. Genomic characterization of clinical Enterobacter roggenkampii co-harbouring blaIMP-1- and blaGES-5-encoding IncP6 and mcr-9-encoding IncHI2 plasmids isolated in Japan. J. Glob. Antimicrob. Resist. 2021, 24, 220–227. [Google Scholar] [CrossRef]
- Liu, Q.; Liu, L.; Li, Y.; Chen, X.; Yan, Q.; Liu, W. Fecal carriage and epidemiology of carbapenem-resistant Enterobacteriaceae among hospitalized patients in a university hospital. Infect. Drug Resist. 2019, 12, 3935–3942. [Google Scholar] [CrossRef] [Green Version]
- Wei, J.; Zou, C.; Wang, D.; Huang, A.; Niuc, S. Genetic diversity and in vitro activity of ceftazidime/avibactam and aztreonam/avibactam against imipenem-resistant Enterobacteriaceae isolates in Southwest China: A single-centre study. J. Glob. Antimicrob. Resist. 2020, 22, 448–451. [Google Scholar] [CrossRef]
- Han, R.; Shi, Q.; Wu, S.; Yin, D.; Peng, M.; Dong, D.; Zheng, Y.; Guo, Y.; Zhang, R.; Hu, F. China antimicrobial surveillance network (CHINET) study group. Dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant Enterobacteriaceae isolated from adult and children patients in China. Front. Cell Infect. Microbiol. 2020, 10, 314. [Google Scholar] [CrossRef]
- Akeda, Y. Current situation of carbapenem-resistant Enterobacteriaceae and Acinetobacter in Japan and Southeast Asia. Microbiol. Immunol. 2021, 65, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Sekizuka, T.; Inamine, Y.; Segawa, T.; Kuroda, M. Characterization of NDM-5- and CTX-M-55-coproducing Escherichia coli GSH8M-2 isolated from the effluent of a wastewater treatment plant in Tokyo Bay. Infect. Drug Resist. 2019, 12, 2243–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, F.; Tian, D.; Wang, B.; Zhao, W.; Qin, H.; Zhang, T.; Zhang, H. Fecal carriage and molecular epidemiology of carbapenem-resistant Enterobacteriaceae from outpatient children in Shanghai. BMC Infect. 2019, 19, 678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savin, M.; Bierbaum, G.; Mutters, N.T.; Schmithausen, R.M.; Kreyenschmidt, J.; García-Meniño, I.; Schmoger, S.; Käsbohrer, A.; Hammerl, J.A. Genetic characterization of carbapenem-resistant Klebsiella spp. from municipal and slaughterhouse wastewater. Antibiotics 2022, 11, 435. [Google Scholar] [CrossRef]
- Carlsen, L.; Büttner, H.; Christner, M.; Franke, G.; Indenbirken, D.; Knobling, B.; Lütgehetmann, M.; Knobloch, J. High burden and diversity of carbapenemase-producing Enterobacterales observed in wastewater of a tertiary care hospital in Germany. Int. J. Hyg. Environ. Health 2022, 242, 113968. [Google Scholar] [CrossRef]
- Tafoukt, T.; Touati, A.; Leangapichart, T.; Bakour, S.; Rolain, J.M. Characterization of OXA-48-like-producing Enterobacteriaceae isolated from river water in Algeria. Water Res. 2017, 120, 185–189. [Google Scholar] [CrossRef]
- Bleichenbacher, S.; Stevens, M.J.A.; Zurfluh, K.; Perreten, V.; Endimiani, A.; Stephan, R.; Nüesch-Inderbinen, M. Environmental dissemination of carbapenemase-producing Enterobacteriaceae in rivers in Switzerland. Environ. Pollut. 2020, 265 Pt B, 115081. [Google Scholar] [CrossRef]
- Ho, B.S.W.; Tam, T.Y. Enumeration of E. coli in environmental waters and wastewater using a chromogenic medium. Water Sci. Technol. 1997, 35, 409–413. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). M100-S22 Performance Standard for Antimicrobial Susceptibility Testing. 2012. Available online: https://m.ibric.org/miniboard/down.php?Board=exp_qna&filename=CLSI%20-%20M100%20S22E.pdf&id=531983&fidx=1 (accessed on 31 May 2022).
- EUCAST (European Committee on Antimicrobial Susceptibility Testing). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 12.0, Valid from 1 January 2022. Available online: http://www.eucast.org/clinical_breakpoints/ (accessed on 10 May 2022).
- Paulshus, E.; Kühn, I.; Moellby, R.; Colque, P.; O’Sullivan, K.; Midtvedt, T.; Lingaas, E.; Holmstad, R.; Sørum, H. Diversity and antibiotic resistance among Escherichia coli populations in hospital and community wastewater compared to wastewater at the receiving urban treatment plant. Water Res. 2019, 161, 232–241. [Google Scholar] [CrossRef]
- Sakanashi, D.; Miyazaki, N.; Kawamoto, Y.; Ohno, T.; Yamada, A.; Koita, I.; Suematsu, H.; Hagihara, M.; Asai, N.; Koizumi, Y.; et al. A novel disk-based detection method with superior sensitivity for β-lactamase production in third-generation cephalosporin-resistant Enterobacteriaceae. J. Infect. Chemother. 2019, 25, 330–336. [Google Scholar] [CrossRef]
- Kim, M.; Oh, H.S.; Park, S.C.; Chun, J. Towards a taxonomic coherence between average nucleotide identity and 16S rRNA gene sequence similarity for species demarcation of prokaryotes. Int. J. Syst. Evol. Microbiol. 2014, 64 Pt 2, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Oho, M.; Funashima, Y.; Nagasawa, Z.; Miyamoto, H.; Sueoka, E. Rapid detection method of carbapenemase-producing Enterobacteriaceae by MALDI-TOF MS with imipenem/cilastatin (KB) disc and zinc sulfate solution. J. Infect. Chemother. 2021, 27, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kayama, S.; Yano, R.; Yamasaki, K.; Fukuda, C.; Nishimura, K.; Miyamoto, H.; Ohge, H.; Sugai, M. Rapid identification of carbapenemase-type blaGES and ESBL-type blaGES using multiplex PCR. J. Microbiol. Methods 2018, 148, 117–119. [Google Scholar] [CrossRef]

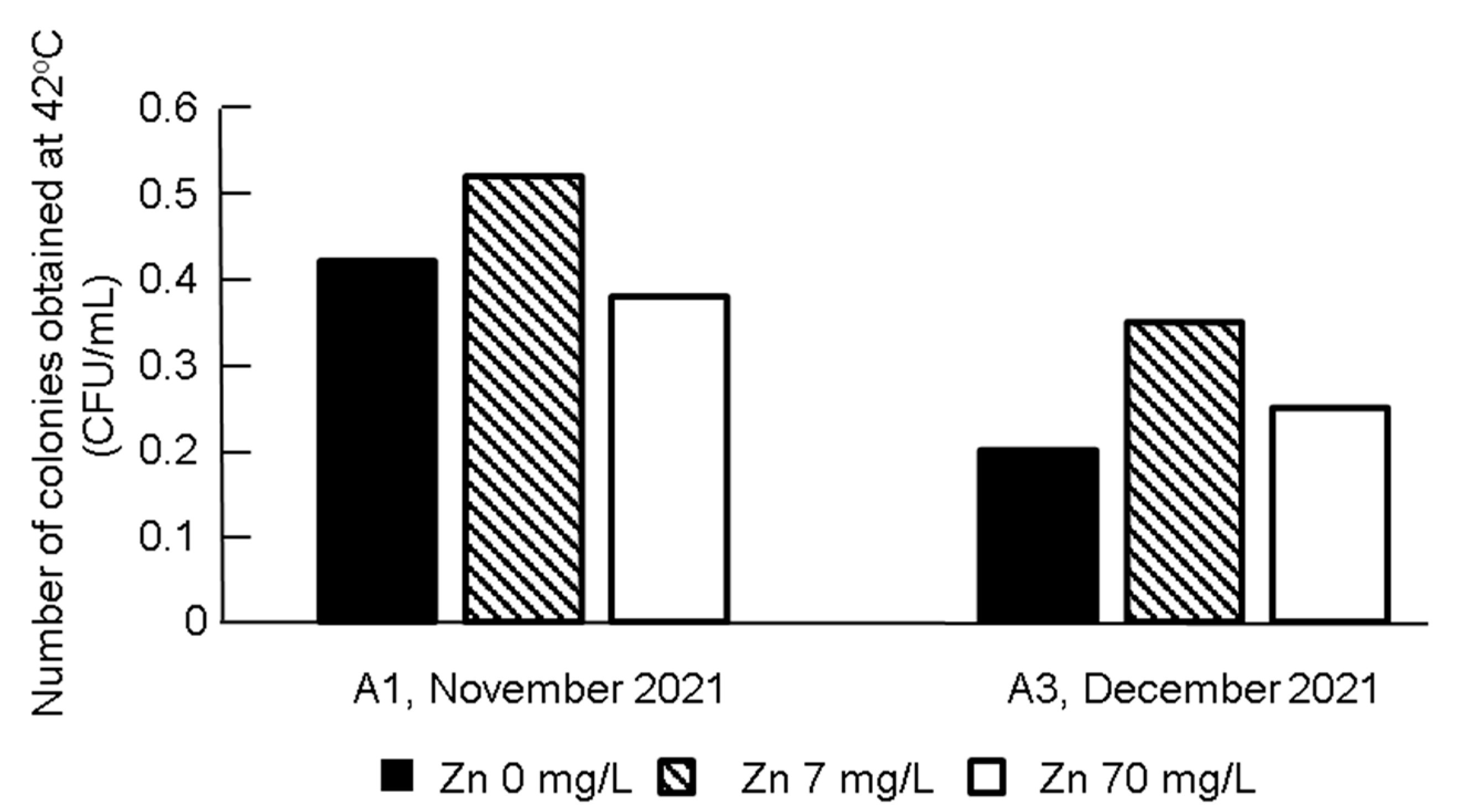



| Isolates from 37 °C Plates | Isolates from 42 °C Plates | ||||||
|---|---|---|---|---|---|---|---|
| Examined | MPM-R * | MBL-P ** | Examined | MPM-R * | MBL-P ** | ||
| A1 | 20 August 2019 | 30 | 16 | 11 | |||
| 25 November 2019 | 52 | 24 | 21 | 59 | 39 | 9 | |
| 19 April 2021 | 30 | 13 | 13 | 7 | 2 | 0 | |
| 28 November 2021 | 43 | 5 | 1 | 19 | 3 | 0 | |
| A2 | 25 November 2019 | 2 | 2 | 2 | 3 | 1 | 1 |
| 6 July 2020 | 53 | 51 | 48 | 21 | 20 | 16 | |
| A3 | 21 June 2021 | 35 | 21 | 14 | 20 | 7 | 2 |
| 28 July 2021 | 8 | 3 | 1 | 10 | 5 | 4 | |
| 18 October 2021 | 14 | 12 | 7 | 7 | 5 | 5 | |
| 9 December 2021 | 21 | 12 | 7 | 5 | 5 | 4 | |
| A4 | 12 July 2021 | 6 | 6 | 5 | 3 | 2 | 2 |
| 6 December 2021 | 0 | 0 | 0 | 1 | 1 | 0 | |
| B1 | 26 October 2020 | 52 | 48 | 46 | 5 | 4 | 1 |
| TR | 24 September 2019 | 39 | 11 | 13 | |||
| TM | 21 June 2021 | 5 | 2 | 2 | 1 | 1 | 1 |
| K3 | 24 September 2019 | 1 | 1 | 1 | |||
| K7 | 28 October 2021 | 1 | 1 | 1 | 5 | 5 | 3 |
| K11 | 28 October 2021 | 45 | 43 | 38 | 2 | 0 | 0 |
| Total | 367 | 243 | 206 | 238 | 128 | 73 | |
| Enterobacterales | Others | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ent. | Kleb. | Esch. | Others | % | Sten. | Pseu. | Aer. | Others | % | Total | |
| River water | 0 | 1 | 0 | 0 | 6 | 8 | 6 | 1 | 0 | 94 | 16 |
| Raw wastewater | 0 | 0 | 5 | 0 | 16 | 20 | 4 | 0 | 2 | 16 | 31 |
| Treated wastewater | 58 | 46 | 4 | 12 | 60 | 41 | 16 | 22 | 1 | 40 | 200 |
| Total | 58 | 47 | 9 | 12 | 51 | 69 | 26 | 23 | 3 | 49 | 247 |
| Enterobacterales | Others | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ent. | Kleb. | Esch. | Others | % | Sten. | Pseu. | Aer. | Others | % | Total | |
| 37 °C without Zn | 21 | 13 | 4 | 6 | 32 | 58 | 12 | 22 | 0 | 68 | 136 |
| 37 °C with Zn | 1 | 5 | 0 | 1 | 64 | 3 | 0 | 1 | 0 | 36 | 11 |
| 42 °C without Zn | 33 | 18 | 5 | 3 | 70 | 8 | 14 | 0 | 3 | 30 | 84 |
| 42 °C with Zn | 3 | 11 | 0 | 2 | 100 | 0 | 0 | 0 | 0 | 0 | 16 |
| Total | 58 | 47 | 9 | 12 | 51 | 69 | 26 | 23 | 3 | 49 | 247 |
| Total | IMP-1 | IMP-6 | NDM | GES-Type Carbapenemase | GES (ESBL-Like Activity) | % Metallo * | |
|---|---|---|---|---|---|---|---|
| 37 °C without Zn | 44 | 4 | 0 | 4 | 27 | 0 | 6/44 (14%) |
| 37 °C with Zn | 7 | 0 | 4 | 0 | 4 | 0 | 4/7 (57%) |
| 42 °C without Zn | 59 | 7 | 3 | 2 | 24 | 0 | 10/59 (17%) |
| 42 °C with Zn | 16 | 0 | 10 | 1 | 7 | 10 | 10/16 (63%) |
| Total | 126 | 11 | 17 | 7 | 62 | 10 | 30/126 (24%) |
| Location | Sampling Dates | Description |
|---|---|---|
| A1 | 20 August 2019, 25 November 2019, 19 April 2021, 28 November 2021 | Treated wastewater from municipal large-scale WWTPs (81,000–294,000 m3/d) |
| A2 | 25 November 2019, 6 July 2020 | |
| A3 | 21 June 2021, 28 July 2021, 18 October 2021, 9 December 2021 | |
| A4 | 12 July 2021, 6 December 2021 | |
| B1 | 26 October 2020 | Raw municipal wastewater |
| TR | 24 September 2019 | River under the influence of combined sewer overflows and treated wastewater |
| TM | 21 June 2021 | |
| K3 | 24 September 2019 | River under the influence of treated wastewater |
| K7 | 28 October 2021 | River under the influence of livestock farms |
| K11 | 28 October 2021 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urase, T.; Goto, S.; Sato, M. Monitoring Carbapenem-Resistant Enterobacterales in the Environment to Assess the Spread in the Community. Antibiotics 2022, 11, 917. https://doi.org/10.3390/antibiotics11070917
Urase T, Goto S, Sato M. Monitoring Carbapenem-Resistant Enterobacterales in the Environment to Assess the Spread in the Community. Antibiotics. 2022; 11(7):917. https://doi.org/10.3390/antibiotics11070917
Chicago/Turabian StyleUrase, Taro, Saki Goto, and Mio Sato. 2022. "Monitoring Carbapenem-Resistant Enterobacterales in the Environment to Assess the Spread in the Community" Antibiotics 11, no. 7: 917. https://doi.org/10.3390/antibiotics11070917
APA StyleUrase, T., Goto, S., & Sato, M. (2022). Monitoring Carbapenem-Resistant Enterobacterales in the Environment to Assess the Spread in the Community. Antibiotics, 11(7), 917. https://doi.org/10.3390/antibiotics11070917








