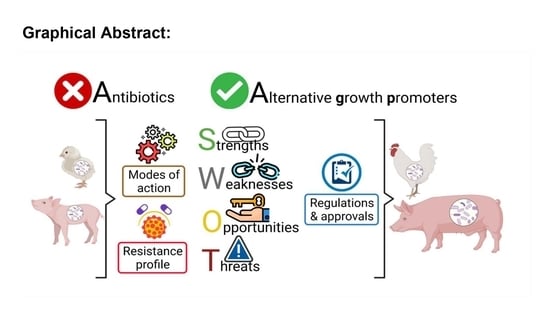
Insights in the Development and Uses of Alternatives to Antibiotic Growth Promoters in Poultry and Swine Production
Abstract
1. Introduction
2. Modes of Action of Antibiotics to Promote Animal Growth
3. Bacterial Resistance: Cross-Resistance and Co-Resistance
4. Alternatives to Antibiotics as Growth Promoters
4.1. Phytochemicals
4.1.1. Modes of Action
4.1.2. Bacterial Resistance to Phytochemicals
4.1.3. Strengths and Weaknesses
4.2. Acidifiers
4.2.1. Modes of Action
4.2.2. Bacterial Resistance to Acidifiers
4.2.3. Strengths and Weaknesses of Acidifiers
4.3. Enzymes
4.3.1. Modes of Action
4.3.2. Bacterial Resistance to Enzymes
4.3.3. Strengths and Weaknesses of Enzymes
4.4. Probiotics and Direct-Fed Microbials (DFM)
4.4.1. Modes of Action
4.4.2. Bacterial Resistance to Probiotics
4.4.3. Strengths and Weaknesses of Probiotics
4.5. Prebiotics
4.5.1. Modes of Action
4.5.2. Bacterial Resistance to Prebiotics
4.5.3. Strengths and Weaknesses of Prebiotics
4.6. Synbiotics
Modes of Action
4.7. Bacteriophages
4.7.1. Modes of Action
4.7.2. Bacterial Resistance to Bacteriophages
4.7.3. Strengths and Weaknesses of Bacteriophages
4.8. Antimicrobial Peptides
4.8.1. Modes of Action
4.8.2. Bacterial Resistance to Antimicrobial Peptides
4.8.3. Strengths and Weaknesses of Antimicrobial Peptides
5. Regulation and Approval of Alternatives to Antibiotics for Use in Animals
6. Discussion and Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef] [PubMed]
- FAO. The Future of Food and Agriculture—Trends and Challenges. 2017. Available online: https://www.fao.org/publications/card/en/c/d24d2507-41d9-4ec2-a3f8-88a489bfe1ad/ (accessed on 19 January 2022).
- Thornton, P.K. Livestock production: Recent trends, future prospects. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 2853–2867. [Google Scholar] [CrossRef] [PubMed]
- Temple, D.; Manteca, X. Animal Welfare in Extensive Production Systems Is Still an Area of Concern. Front. Sustain. Food Syst. 2020, 4, 545902. [Google Scholar] [CrossRef]
- Lam, Y.; Fry, J.P.; Nachman, K.E. Applying an environmental public health lens to the industrialization of food animal production in ten low- and middle-income countries. Glob. Health 2019, 15, 40. [Google Scholar] [CrossRef]
- Gilchrist, M.J.; Greko, C.; Wallinga, D.B.; Beran, G.W.; Riley, D.G.; Thorne, P.S. The Potential Role of Concentrated Animal Feeding Operations in Infectious Disease Epidemics and Antibiotic Resistance. Environ. Health Perspect. 2007, 115, 313–316. [Google Scholar] [CrossRef]
- Farrell, M.J.; Davies, T.J. Disease mortality in domesticated animals is predicted by host evolutionary relationships. Proc. Natl. Acad. Sci. USA 2019, 116, 7911–7915. [Google Scholar] [CrossRef]
- Manyi-Loh, C.; Mamphweli, S.; Meyer, E.; Okoh, A. Antibiotic Use in Agriculture and Its Consequential Resistance in Environmental Sources: Potential Public Health Implications. Molecules 2018, 23, 795. [Google Scholar] [CrossRef]
- Pokharel, S.; Raut, S.; Adhikari, B. Tackling antimicrobial resistance in low-income and middle-income countries. BMJ Glob. Health 2019, 4, e002104. [Google Scholar] [CrossRef]
- Helm, E.T.; Curry, S.; Trachsel, J.M.; Schroyen, M.; Gabler, N.K. Evaluating nursery pig responses to in-feed sub-therapeutic antibiotics. PLoS ONE 2019, 14, e0216070. [Google Scholar] [CrossRef]
- Hou, L.; Cao, S.; Qiu, Y.; Xiong, Y.; Xiao, H.; Wen, X.; Yang, X.; Gao, K.; Wang, L.; Jiang, Z. Effects of early sub-therapeutic antibiotic administration on body tissue deposition, gut microbiota and metabolite profiles of weaned piglets. J. Sci. Food Agric. 2022. [Google Scholar] [CrossRef]
- Low, C.; Tan, L.; Ab Mutalib, N.-S.; Pusparajah, P.; Goh, B.-H.; Chan, K.-G.; Letchumanan, V.; Lee, L.-H. Unveiling the Impact of Antibiotics and Alternative Methods for Animal Husbandry: A Review. Antibiotics 2021, 10, 578. [Google Scholar] [CrossRef] [PubMed]
- Zuidhof, M.J.; Schneider, B.L.; Carney, V.L.; Korver, D.; Robinson, F.E. Growth, efficiency, and yield of commercial broilers from 1957, 1978, and 2005. Poult. Sci. 2014, 93, 2970–2982. [Google Scholar] [CrossRef]
- Rathnayaka, S.D.; Selvanathan, S.; Selvanathan, E.A. Demand for animal-derived food in selected Asian countries: A system-wide analysis. Agric. Econ. 2021, 52, 97–122. [Google Scholar] [CrossRef]
- Chattopadhyay, M.K. Use of antibiotics as feed additives: A burning question. Front. Microbiol. 2014, 5, 334. [Google Scholar] [CrossRef]
- Scott, H.M.; Acuff, G.; Bergeron, G.; Bourassa, M.W.; Gill, J.; Graham, D.W.; Kahn, L.H.; Morley, P.S.; Salois, M.J.; Simjee, S.; et al. Critically important antibiotics: Criteria and approaches for measuring and reducing their use in food animal agriculture. Ann. N. Y. Acad. Sci. 2019, 1441, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Brower, C.; Gilbert, M.; Grenfell, B.T.; Levin, S.A.; Robinson, T.P.; Teillant, A.; Laxminarayan, R. Global trends in antimicrobial use in food animals. Proc. Natl. Acad. Sci. USA 2015, 112, 5649–5654. [Google Scholar] [CrossRef]
- Schoenmakers, K. How China is getting its farmers to kick their antibiotics habit. Nature 2020, 586, S60–S62. [Google Scholar] [CrossRef]
- Aroeira, C.N.; Feddern, V.; Gressler, V.; Contreras-Castillo, C.J.; Hopkins, D.L. A review on growth promoters still allowed in cattle and pig production. Livest. Sci. 2021, 247, 104464. [Google Scholar] [CrossRef]
- Tian, M.; He, X.; Feng, Y.; Wang, W.; Chen, H.; Gong, M.; Liu, D.; Clarke, J.; van Eerde, A. Pollution by Antibiotics and Antimicrobial Resistance in LiveStock and Poultry Manure in China, and Countermeasures. Antibiotics 2021, 10, 539. [Google Scholar] [CrossRef]
- Nunan, C. Farm Antibiotics and Trade Deals—Could UK Standards Be Undermined? 2020. The Alliance to Save Our Antibiotics. Available online: http://www.saveourantibiotics.org (accessed on 17 May 2022).
- Australian Government Department of Health. Review of Published and Grey Literature on The Presence of Antimicrobial Resistance in Food in Australia and New Zealand. 2018. Available online: https://www.amr.gov.au/file/1402/download?token=Yga43nsT (accessed on 1 March 2022).
- Government of Canada. Responsible Use of Medically Important Antimicrobials in Animals; Government of Canada: Ottawa, ON, Canada, 2018.
- Bosman, A.L.; Deckert, A.E.; Carson, C.A.; Poljak, Z.; Reid-Smith, R.J.; McEwen, S.A. Antimicrobial use in lactating sows, piglets, nursery, and grower-finisher pigs on swine farms in Ontario, Canada during 2017 and 2018. Porc. Health Manag. 2022, 8, 17. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Agriculture and Rural Affairs of the People’s Republic of China. Announcement No. 194 of the Ministry of Agriculture and Rural Affairs of the People’s Republic of China. 2019. Available online: http://www.xmsyj.moa.gov.cn/zcjd/201907/t20190710_6320678.htm (accessed on 29 May 2022).
- Van Boeckel, T.P.; Glennon, E.E.; Chen, D.; Gilbert, M.; Robinson, T.P.; Grenfell, B.T.; Levin, S.A.; Bonhoeffer, S.; Laxminarayan, R. Reducing antimicrobial use in food animals. Science 2017, 357, 1350–1352. [Google Scholar] [CrossRef] [PubMed]
- Ministry for Primary Industries. Antibiotics and Resistance- Learn about Bacteria Developing Resistance to Antibiotics, and How Antibiotic Resistance Can Be Managed. 2017. Available online: https://www.mpi.govt.nz/food-safety-home/safe-levels-of-chemicals-in-food/fertilisers-pesticides-hormones-and-medicines-in-food/antibiotics-and-resistance/ (accessed on 2 February 2022).
- Bengtsson, B.; Wierup, M. Antimicrobial Resistance in Scandinavia after a Ban of Antimicrobial Growth Promoters. Anim. Biotechnol. 2006, 17, 147–156. [Google Scholar] [CrossRef]
- Arsand, J.B.; Hoff, R.B.; Jank, L.; Bussamara, R.; Dallegrave, A.; Bento, F.M.; Kmetzsch, L.; Falção, D.A.; do Carmo Ruaro Peralba, M.; de Araujo Gomes, A.; et al. Presence of antibiotic resistance genes and its association with antibiotic occurrence in Dilúvio River in southern Brazil. Sci. Total Environ. 2020, 738, 139781. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, F.; Li, H.; Wu, H.; Zhao, H.; Cheng, X.; Ba, Y.; Huang, H.; Chen, S.; Zhu, J. Contribution of environmental factors on the distribution of antibiotic resistance genes in agricultural soil. Eur. J. Soil Biol. 2021, 102, 103269. [Google Scholar] [CrossRef]
- Abadi, A.T.B.; Rizvanov, A.A.; Haertlé, T.; Blatt, N.L. World Health Organization Report: Current Crisis of Antibiotic Resistance. BioNanoScience 2019, 9, 778–788. [Google Scholar] [CrossRef]
- Church, N.A.; McKillip, J.L. Antibiotic resistance crisis: Challenges and imperatives. Biologia 2021, 76, 1535–1550. [Google Scholar] [CrossRef]
- Djordjevic, S.P.; Morgan, B.S. A One Health genomic approach to antimicrobial resistance is essential for generating relevant data for a holistic assessment of the biggest threat to public health. Microbiol. Aust. 2019, 40, 73–76. [Google Scholar] [CrossRef]
- Tang, K.L.; Caffrey, N.P.; Nóbrega, D.; Cork, S.C.; Ronksley, P.E.; Barkema, H.; Polachek, A.J.; Ganshorn, H.; Sharma, N.; Kellner, J.; et al. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: A systematic review and meta-analysis. Lancet Planet. Health 2017, 1, e316–e327. [Google Scholar] [CrossRef]
- Van Boeckel, T.P.; Pires, J.; Silvester, R.; Zhao, C.; Song, J.; Criscuolo, N.G.; Gilbert, M.; Bonhoeffer, S.; Laxminarayan, R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science 2019, 365, eaaw1944. [Google Scholar] [CrossRef]
- Chang, Q.; Wang, W.; Regev-Yochay, G.; Lipsitch, M.; Hanage, W.P. Antibiotics in agriculture and the risk to human health: How worried should we be? Evol. Appl. 2015, 8, 240–247. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.L.; Saier, M.H., Jr. The Causal Relationship between Eating Animals and Viral Epidemics. Microb. Physiol. 2020, 30, 2–8. [Google Scholar] [CrossRef] [PubMed]
- Salyer, S.J.; Silver, R.; Simone, K.; Behravesh, C.B. Prioritizing Zoonoses for Global Health Capacity Building—Themes from One Health Zoonotic Disease Workshops in 7 Countries, 2014–2016. Emerg. Infect. Dis. 2017, 23, S55–S64. [Google Scholar] [CrossRef] [PubMed]
- AbuOun, M.; Jones, H.; Stubberfield, E.; Gilson, D.; Shaw, L.P.; Hubbard, A.T.M.; Chau, K.K.; Sebra, R.; Peto, T.E.A.; Crook, D.W.; et al. A genomic epidemiological study shows that prevalence of antimicrobial resistance in Enterobacterales is associated with the livestock host, as well as antimicrobial usage. Microb. Genom. 2021, 7, 000630. [Google Scholar] [CrossRef]
- Rahman, T.; Sobur, A.; Islam, S.; Ievy, S.; Hossain, J.; El Zowalaty, M.E.; Rahman, A.T.; Ashour, H.M. Zoonotic Diseases: Etiology, Impact, and Control. Microorganisms 2020, 8, 1405. [Google Scholar] [CrossRef]
- Ana, K.M.S.; Madriaga, J.; Espino, M.P. β-Lactam antibiotics and antibiotic resistance in Asian lakes and rivers: An overview of contamination, sources and detection methods. Environ. Pollut. 2021, 275, 116624. [Google Scholar] [CrossRef]
- You, X.; Xu, N.; Yang, X.; Sun, W. Pollutants affect algae-bacteria interactions: A critical review. Environ. Pollut. 2021, 276, 116723. [Google Scholar] [CrossRef]
- Gaballah, M.S.; Guo, J.; Sun, H.; Aboagye, D.; Sobhi, M.; Muhmood, A.; Dong, R. A review targeting veterinary antibiotics removal from livestock manure management systems and future outlook. Bioresour. Technol. 2021, 333, 125069. [Google Scholar] [CrossRef]
- Wang, B.; Xie, K.; Lee, K. Veterinary Drug Residues in Animal-Derived Foods: Sample Preparation and Analytical Methods. Foods 2021, 10, 555. [Google Scholar] [CrossRef]
- Kumar, H.; Bhardwaj, K.; Kaur, T.; Nepovimova, E.; Kuča, K.; Kumar, V.; Bhatia, S.K.; Dhanjal, D.S.; Chopra, C.; Singh, R.; et al. Detection of Bacterial Pathogens and Antibiotic Residues in Chicken Meat: A Review. Foods 2020, 9, 1504. [Google Scholar] [CrossRef]
- National Farm Animal Care Council. Code of Practice for the Care and Handling of Hatching Eggs, Breeders, Chicken and Turkeys; National Farm Animal Care Council: Lacombe, AB, Canada, 2016. [Google Scholar]
- Celi, P.; Cowieson, A.; Fru-Nji, F.; Steinert, R.; Kluenter, A.-M.; Verlhac, V. Gastrointestinal functionality in animal nutrition and health: New opportunities for sustainable animal production. Anim. Feed Sci. Technol. 2017, 234, 88–100. [Google Scholar] [CrossRef]
- Cobo-Angel, C.; LeBlanc, S.J.; Roche, S.M.; Ritter, C. A Focus Group Study of Canadian Dairy Farmers’ Attitudes and Social Referents on Antimicrobial Use and Antimicrobial Resistance. Front. Vet. Sci. 2021, 8, 645221. [Google Scholar] [CrossRef] [PubMed]
- Gadde, U.; Kim, W.H.; Oh, S.T.; Lillehoj, H.S. Alternatives to antibiotics for maximizing growth performance and feed efficiency in poultry: A review. Anim. Health Res. Rev. 2017, 18, 26–45. [Google Scholar] [CrossRef] [PubMed]
- Butaye, P.; Devriese, L.A.; Haesebrouck, F. Antimicrobial Growth Promoters Used in Animal Feed: Effects of Less Well Known Antibiotics on Gram-Positive Bacteria. Clin. Microbiol. Rev. 2003, 16, 175–188. [Google Scholar] [CrossRef]
- Broom, L.J. The sub-inhibitory theory for antibiotic growth promoters. Poult. Sci. 2017, 96, 3104–3108. [Google Scholar] [CrossRef] [PubMed]
- Niewold, T.A. The Nonantibiotic Anti-Inflammatory Effect of Antimicrobial Growth Promoters, the Real Mode of Action? A Hypothesis. Poult. Sci. 2007, 86, 605–609. [Google Scholar] [CrossRef]
- Langdon, A.; Crook, N.; Dantas, G. The effects of antibiotics on the microbiome throughout development and alternative approaches for therapeutic modulation. Genome Med. 2016, 8, 39. [Google Scholar] [CrossRef]
- Geng, W.; Lin, J. Bacterial bile salt hydrolase: An intestinal microbiome target for enhanced animal health. Anim. Health Res. Rev. 2016, 17, 148–158. [Google Scholar] [CrossRef]
- Che, L.; Hu, Q.; Wang, R.; Zhang, D.; Liu, C.; Zhang, Y.; Xin, G.; Fang, Z.; Lin, Y.; Xu, S.; et al. Inter-correlated gut microbiota and SCFAs changes upon antibiotics exposure links with rapid body-mass gain in weaned piglet model. J. Nutr. Biochem. 2019, 74, 108246. [Google Scholar] [CrossRef]
- Zarrinpar, A.; Chaix, A.; Xu, Z.Z.; Chang, M.W.; Marotz, C.A.; Saghatelian, A.; Knight, R.; Panda, S. Antibiotic-induced microbiome depletion alters metabolic homeostasis by affecting gut signaling and colonic metabolism. Nat. Commun. 2018, 9, 2872. [Google Scholar] [CrossRef]
- Humam, A.M.; Loh, T.C.; Foo, H.L.; Samsudin, A.A.; Mustapha, N.M.; Zulkifli, I.; Izuddin, W.I. Effects of Feeding Different Postbiotics Produced by Lactobacillus plantarum on Growth Performance, Carcass Yield, Intestinal Morphology, Gut Microbiota Composition, Immune Status, and Growth Gene Expression in Broilers under Heat Stress. Animals 2019, 9, 644. [Google Scholar] [CrossRef] [PubMed]
- Neveling, D.P.; Dicks, L.M. Probiotics: An Antibiotic Replacement Strategy for Healthy Broilers and Productive Rearing. Probiotics Antimicrob. Proteins 2021, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sinurat, A.P.; Pasaribu, T.; Purwadaria, T.; Haryati, T.; Wina, E.; Wardhani, T. Biological Evaluation of Some Plant Bioactives as Feed Additives to Replace Antibiotic Growth Promoters in Broiler Feeds. J. Ilmu Ternak Vet. 2020, 25, 81–90. [Google Scholar] [CrossRef]
- Thema, K.; Mlambo, V.; Snyman, N.; Mnisi, C.M. Evaluating Alternatives to Zinc-Bacitracin Antibiotic Growth Promoter in Broilers: Physiological and Meat Quality Responses. Animals 2019, 9, 1160. [Google Scholar] [CrossRef] [PubMed]
- He, T.; Long, S.; Mahfuz, S.; Wu, D.; Wang, X.; Wei, X.; Piao, X. Effects of Probiotics as Antibiotics Substitutes on Growth Performance, Serum Biochemical Parameters, Intestinal Morphology, and Barrier Function of Broilers. Animals 2019, 9, 958. [Google Scholar] [CrossRef] [PubMed]
- Lowell, J.; Bohrer, B.; Wilson, K.; Overholt, M.; Harsh, B.; Stein, H.; Dilger, A.; Boler, D. Growth performance, carcass quality, fresh belly characteristics, and commercial bacon slicing yields of growing-finishing pigs fed a subtherapeutic dose of an antibiotic, a natural antimicrobial, or not fed an antibiotic or antimicrobial. Meat Sci. 2018, 136, 93–103. [Google Scholar] [CrossRef]
- Petrujkić, B.T.; Beier, R.C.; He, H.; Genovese, K.J.; Swaggerty, C.L.; Hume, M.E.; Crippen, T.L.; Harvey, R.B.; Anderson, R.C.; Nisbet, D.J. Nigella sativa L. as an alternative antibiotic feed supplement and effect on growth performance in weanling pigs. J. Sci. Food Agric. 2018, 98, 3175–3181. [Google Scholar] [CrossRef]
- Braundmeier-Fleming, A.G.; Skenandore, C.S.; Gil, L.; Jacobsen, V.; Cregger, M.; Badger, T.; Karr, M.; Wu, G.; Smith, S.B.; Newell-Fugate, A.E. Dietary substitution of soybean oil with coconut oil in the absence of dietary antibiotics supports growth performance and immune function in nursery and grower pigs. J. Anim. Sci. Biotechnol. 2020, 11, 27. [Google Scholar] [CrossRef]
- Lei, X.J.; Zhang, W.L.; Cheong, J.Y.; Lee, S.I.; Kim, I.H. Effect of antibiotics and synbiotic on growth performance, nutrient digestibility, and faecal microbial shedding in growing-finishing pigs. J. Appl. Anim. Res. 2018, 46, 1202–1206. [Google Scholar] [CrossRef]
- Perron, K.; Caille, O.; Rossier, C.; van Delden, C.; Dumas, J.-L.; Köhler, T. CzcR-CzcS, a Two-component System Involved in Heavy Metal and Carbapenem Resistance in Pseudomonas aeruginosa. J. Biol. Chem. 2004, 279, 8761–8768. [Google Scholar] [CrossRef]
- Pietsch, F.; O’Neill, A.; Ivask, A.; Jenssen, H.; Inkinen, J.; Kahru, A.; Ahonen, M.; Schreiber, F. Selection of resistance by antimicrobial coatings in the healthcare setting. J. Hosp. Infect. 2020, 106, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Baker-Austin, C.; Wright, M.S.; Stepanauskas, R.; McArthur, J. Co-selection of antibiotic and metal resistance. Trends Microbiol. 2006, 14, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, K.B.; Muller-Pebody, B.; Smieszek, T.; Hopkins, S.; Robotham, J.V. Selection and co-selection of antibiotic resistances among Escherichia coli by antibiotic use in primary care: An ecological analysis. PLoS ONE 2019, 14, e0218134. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef] [PubMed]
- Süntar, I.; Yakıncı, Ö.F. Chapter Six—Potential Risks of Phytonutrients Associated with High-Dose or Long-Term Use. In Phytonutrients in Food; Nabavi, S.M., Suntar, I., Barreca, D., Khan, H., Eds.; Woodhead Publishing: Sawston, UK, 2020; pp. 137–155. [Google Scholar]
- WHO. WHO Traditional Medicine Strategy: 2014–2023. WHO Website. Available online: https://www.who.int/medicines/publications/traditional/trm_strategy14_23/en/2013 (accessed on 27 June 2020).
- Research, P.M. Phytochemicals Market Is Expected to Reach ~US$9.0Bn by 2029—PMR. Available online: https://www.globenewswire.com/news-release/2019/08/12/1900582/0/en/Phytochemicals-Market-is-Expected-to-Reach-US-9-0-Bn-by-2029-PMR.html2019 (accessed on 27 June 2020).
- Mahfuz, S.; Shang, Q.; Piao, X. Phenolic compounds as natural feed additives in poultry and swine diets: A review. J. Anim. Sci. Biotechnol. 2021, 12, 48. [Google Scholar] [CrossRef] [PubMed]
- Saracila, M.; Panaite, T.; Papuc, C.; Criste, R. Heat Stress in Broiler Chickens and the Effect of Dietary Polyphenols, with Special Reference to Willow (Salix spp.) Bark Supplements—A Review. Antioxidants 2021, 10, 686. [Google Scholar] [CrossRef]
- Deminicis, R.G.D.S.; Meneghetti, C.; de Oliveira, E.B.; Júnior, A.A.P.G.; Filho, R.V.F.; Deminicis, B.B. Systematic review of the use of phytobiotics in broiler nutrition. Rev. Cienc. Agrovet. 2021, 20, 098–106. [Google Scholar] [CrossRef]
- Mohammadi, F. Effect of different levels of clove (Syzygium aromaticum L.) essential oil on growth performance and oxidative/nitrosative stress biomarkers in broilers under heat stress. Trop. Anim. Health Prod. 2021, 53, 84. [Google Scholar] [CrossRef]
- Omonijo, F.A.; Ni, L.; Gong, J.; Wang, Q.; Lahaye, L.; Yang, C. Essential oils as alternatives to antibiotics in swine production. Anim. Nutr. 2018, 4, 126–136. [Google Scholar] [CrossRef]
- National Archives and Records Administration. Substances Generally Recognized as Safe. 2019. Available online: https://www.ecfr.gov/cgi-bin/text-idx?SID=e956d645a8b4e6b3e34e4e5d1b690209&mc=true&node=pt21.3.182&rgn=div5#se21.3.182_11073 (accessed on 10 March 2020).
- Valenzuela-Grijalva, N.V.; Pinelli-Saavedra, A.; Muhlia-Almazan, A.; Domínguez-Díaz, D.; González-Ríos, H. Dietary inclusion effects of phytochemicals as growth promoters in animal production. J. Anim. Sci. Technol. 2017, 59, 8. [Google Scholar] [CrossRef]
- Hashemipour, H.; Kermanshahi, H.; Golian, A.; Veldkamp, T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poult. Sci. 2013, 92, 2059–2069. [Google Scholar] [CrossRef] [PubMed]
- Navarro, M.; Stanley, R.; Cusack, A.; Sultanbawa, Y. Combinations of plant-derived compounds against Campylobacter in vitro. J. Appl. Poult. Res. 2015, 24, 352–363. [Google Scholar] [CrossRef]
- Mohebodini, H.; Jazi, V.; Bakhshalinejad, R.; Shabani, A.; Ashayerizadeh, A. Effect of dietary resveratrol supplementation on growth performance, immune response, serum biochemical indices, cecal microflora, and intestinal morphology of broiler chickens challenged with Escherichia coli. Livest. Sci. 2019, 229, 13–21. [Google Scholar] [CrossRef]
- Chamorro, S.; Viveros, A.; Centeno, C.; Romero, C.; Arija, I.; Brenes, A. Effects of dietary grape seed extract on growth performance, amino acid digestibility and plasma lipids and mineral content in broiler chicks. Animal 2013, 7, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Galassi, G.; Mason, F.; Rapetti, L.; Crovetto, G.M.; Spanghero, M. Digestibility and metabolic utilisation of diets containing chestnut tannins and their effects on growth and slaughter traits of heavy pigs. Ital. J. Anim. Sci. 2019, 18, 746–753. [Google Scholar] [CrossRef]
- Muurinen, J.; Richert, J.; Wickware, C.L.; Richert, B.; Johnson, T.A. Swine growth promotion with antibiotics or alternatives can increase antibiotic resistance gene mobility potential. Sci. Rep. 2021, 11, 5485. [Google Scholar] [CrossRef]
- Willing, B.P.; Pepin, D.M.; Marcolla, C.S.; Forgie, A.J.; Diether, N.E.; Bourrie, B.C.T. Bacterial resistance to antibiotic alternatives: A wolf in sheep’s clothing? Anim. Front. 2018, 8, 39–47. [Google Scholar] [CrossRef]
- Alves-Santos, A.M.; Sugizaki, C.S.A.; Lima, G.C.; Naves, M.M.V. Prebiotic effect of dietary polyphenols: A systematic review. J. Funct. Foods 2020, 74, 104169. [Google Scholar] [CrossRef]
- Chakraborty, S.B.; Horn, P.; Hancz, C. Application of phytochemicals as growth-promoters and endocrine modulators in fish culture. Rev. Aquac. 2014, 6, 1–19. [Google Scholar] [CrossRef]
- Pearlin, B.V.; Muthuvel, S.; Govidasamy, P.; Villavan, M.; Alagawany, M.; Farag, M.R.; Dhama, K.; Gopi, M. Role of acidifiers in livestock nutrition and health: A review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 558–569. [Google Scholar] [CrossRef]
- Ferronato, G.; Prandini, A. Dietary Supplementation of Inorganic, Organic, and Fatty Acids in Pig: A Review. Animals 2020, 10, 1740. [Google Scholar] [CrossRef] [PubMed]
- Tugnoli, B.; Giovagnoni, G.; Piva, A.; Grilli, E. From Acidifiers to Intestinal Health Enhancers: How Organic Acids Can Improve Growth Efficiency of Pigs. Animals 2020, 10, 134. [Google Scholar] [CrossRef] [PubMed]
- FDA; Scogs, U. Select Committee on GRAS Substances. 2019. Available online: https://www.accessdata.fda.gov/scripts/fdcc/index.cfm?set=SCOGS&sort=Sortsubstance&order=ASC&startrow=1&type=basic&search= (accessed on 10 March 2020).
- Guo, Y.; Wang, Z.; Wang, Y.; Chen, B.; Huang, Y.; Li, P.; Tan, Q.; Zhang, H.; Chen, W. Impact of drinking water supplemented 2-hydroxy-4-methylthiobutyric acid in combination with acidifier on performance, intestinal development, and microflora in broilers. Poult. Sci. 2022, 101, 101661. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.-L.; Liu, C.; Mo, X.-J.; Chen, M.; Zhao, X.-L.; Liu, M.-Z.; Wang, S.-B.; Zhou, B.; Zhao, C.-X. Drinking Water Supplemented with Acidifiers Improves the Growth Performance of Weaned Pigs and Potentially Regulates Antioxidant Capacity, Immunity, and Gastrointestinal Microbiota Diversity. Antioxidants 2022, 11, 809. [Google Scholar] [CrossRef]
- Research, M. Feed Acidifiers Market by Type (Propionic Acid, Formic Acid, Lactic Acid, Citric Acid, Sorbic Acid, Malic Acid), Form (Dry, Liquid), Compound (Blended, Single), Livestock; Poultry, Ruminants, Aquaculture), and Region—Global Forecast to 2023. Available online: https://www.marketsandmarkets.com/Market-Reports/feed-acidifiers-market-163262152.html (accessed on 2 December 2021).
- Khan, S.H.; Iqbal, J. Recent advances in the role of organic acids in poultry nutrition. J. Appl. Anim. Res. 2016, 44, 359–369. [Google Scholar] [CrossRef]
- Xu, Y.; Lahaye, L.; He, Z.; Zhang, J.; Yang, C.; Piao, X. Micro-encapsulated essential oils and organic acids combination improves intestinal barrier function, inflammatory responses and microbiota of weaned piglets challenged with enterotoxigenic Escherichia coli F4 (K88+). Anim. Nutr. 2020, 6, 269–277. [Google Scholar] [CrossRef]
- Markazi, A.D.; Luoma, A.; Shanmugasundaram, R.; Murugesan, R.; Mohnl, M.; Selvaraj, R. Effect of Acidifier Product Supplementation in Laying Hens Challenged with Salmonella. J. Appl. Poult. Res. 2019, 28, 919–929. [Google Scholar] [CrossRef]
- Fernández-Rubio, C.; Ordóñez, C.; González, J.A.; García-Gallego, A.; Honrubia, M.P.; Mallo, J.J.; Balana-Fouce, R. Butyric acid-based feed additives help protect broiler chickens from Salmonella Enteritidis infection. Poult. Sci. 2009, 88, 943–948. [Google Scholar] [CrossRef]
- Aljumaah, M.R.; Alkhulaifi, M.M.; Abudabos, A.M.; Alabdullatifb, A.; El-Mubarak, A.H.; Al Suliman, A.R.; Stanley, D. Organic acid blend supplementation increases butyrate and acetate production in Salmonella enterica serovar Typhimurium challenged broilers. PLoS ONE 2020, 15, e0232831. [Google Scholar] [CrossRef]
- Suiryanrayna, M.V.A.N.; Ramana, J.V. A review of the effects of dietary organic acids fed to swine. J. Anim. Sci. Biotechnol. 2015, 6, 45. [Google Scholar] [CrossRef]
- Xie, J.; Li, L.; Dai, T.; Qi, X.; Wang, Y.; Zheng, T.; Gao, X.; Zhang, Y.; Ai, Y.; Ma, L.; et al. Short-Chain Fatty Acids Produced by Ruminococcaceae Mediate α-Linolenic Acid Promote Intestinal Stem Cells Proliferation. Mol. Nutr. Food Res. 2022, 66, 2100408. [Google Scholar] [CrossRef]
- Kaczmarek, B. Tannic Acid with Antiviral and Antibacterial Activity as A Promising Component of Biomaterials—A Minireview. Materials 2020, 13, 3224. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xin, H.; Yang, C.; Yang, X. Impact of essential oils and organic acids on the growth performance, digestive functions and immunity of broiler chickens. Anim. Nutr. 2018, 4, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Emami, N.K.; Naeini, S.Z.; Ruiz-Feria, C. Growth performance, digestibility, immune response and intestinal morphology of male broilers fed phosphorus deficient diets supplemented with microbial phytase and organic acids. Livest. Sci. 2013, 157, 506–513. [Google Scholar] [CrossRef]
- Capcarova, M.; Kalafova, A.; Hrncar, C.; Kopecky, J.; Weis, J. Comparative analysis of acetic and citric acid on internal milieu of broiler chickens. Potravin. Slovak J. Food Sci. 2014, 8, 190–195. [Google Scholar] [CrossRef]
- Galli, G.M.; Aniecevski, E.; Petrolli, T.G.; da Rosa, G.; Boiago, M.M.; Simões, C.A.; Wagner, R.; Copetti, P.M.; Morsch, V.M.; Araujo, D.N.; et al. Growth performance and meat quality of broilers fed with microencapsulated organic acids. Anim. Feed Sci. Technol. 2021, 271, 114706. [Google Scholar] [CrossRef]
- Gómez-García, M.; Sol, C.; De Nova, P.J.G.; Puyalto, M.; Mesas, L.; Puente, H.; Mencía-Ares, Ó.; Miranda, R.; Argüello, H.; Rubio, P.; et al. Antimicrobial activity of a selection of organic acids, their salts and essential oils against swine enteropathogenic bacteria. Porc. Health Manag. 2019, 5, 32. [Google Scholar] [CrossRef]
- Wegl, G.; Rolinec, M.; Nagl, V.; Gierus, M.; Klose, V. Effect of oxytetracycline as well as an acid-based feed supplement on the prevalence and abundance of selected antibiotic resistance genes in weaned piglets. Anim. Husb. Dairy Vet. Sci. 2017, 1, 1–6. [Google Scholar] [CrossRef]
- Roth, N.; Mayrhofer, S.; Gierus, M.; Weingut, C.; Schwarz, C.; Doupovec, B.; Berrios, R.; Domig, K.J. Effect of an organic acids based feed additive and enrofloxacin on the prevalence of antibiotic-resistant E. coli in cecum of broilers. Poult. Sci. 2017, 96, 4053–4060. [Google Scholar] [CrossRef]
- Ngoc, T.T.B.; Oanh, D.T.; Pineda, L.; Ayudhya, S.; De Groot, N.; Han, Y. The effects of synergistic blend of organic acid or antibiotic growth promoter on performance and antimicrobial resistance of bacteria in grow–finish pigs. Transl. Anim. Sci. 2020, 4, txaa211. [Google Scholar] [CrossRef]
- Kim, Y.Y.; Kil, D.Y.; Oh, H.K.; Han, I.K. Acidifier as an Alternative Material to Antibiotics in Animal Feed. Asian-Australas. J. Anim. Sci. 2005, 18, 1048–1060. [Google Scholar] [CrossRef]
- Liu, Y.; Espinosa, C.D.; Abelilla, J.J.; Casas, G.A.; Lagos, L.V.; Lee, S.A.; Kwon, W.B.; Mathai, J.K.; Navarro, D.M.D.L.; Jaworski, N.W.; et al. Non-antibiotic feed additives in diets for pigs: A review. Anim. Nutr. 2018, 4, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Nowak, P.; Zaworska-Zakrzewska, A.; Frankiewicz, A.; Kasprowicz-Potocka, M. The effects and mechanisms of acids on the health of piglets and weaners—A review. Ann. Anim. Sci. 2021, 21, 433–455. [Google Scholar] [CrossRef]
- Raveendran, S.; Parameswaran, B.; Ummalyma, S.B.; Abraham, A.; Mathew, A.K.; Madhavan, A.; Rebello, S.; Pandey, A. Applications of Microbial Enzymes in Food Industry. Food Technol. Biotechnol. 2018, 56, 16–30. [Google Scholar] [CrossRef] [PubMed]
- Cowieson, A.; Kluenter, A. Contribution of exogenous enzymes to potentiate the removal of antibiotic growth promoters in poultry production. Anim. Feed Sci. Technol. 2019, 250, 81–92. [Google Scholar] [CrossRef]
- Rhouma, M.; Fairbrother, J.M.; Beaudry, F.; Letellier, A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017, 59, 31. [Google Scholar] [CrossRef]
- Vangroenweghe, F.; Poulsen, K.; Thas, O. Supplementation of a β-mannanase enzyme reduces post-weaning diarrhea and antibiotic use in piglets on an alternative diet with additional soybean meal. Porc. Health Manag. 2021, 7, 8. [Google Scholar] [CrossRef]
- Jang, J.; Kim, K.; Kim, D.; Jang, S.; Hong, J.; Heo, P.; Kim, Y. Effects of increasing levels of palm kernel meal containing β-mannanase to growing-finishing pig diets on growth performance, nutrient digestibility, and pork quality. Livest. Sci. 2020, 238, 104041. [Google Scholar] [CrossRef]
- Da Silva, I.; Broch, J.; Wachholz, L.; De Souza, C.; Dalolio, F.; Teixeira, L.; Eyng, C.; Nunes, R. Dry Residue of Cassava Associated with Carbohydrases in Diets for Broiler Chickens. J. Appl. Poult. Res. 2019, 28, 1189–1201. [Google Scholar] [CrossRef]
- Roofchaei, A.; Rezaeipour, V.; Vatandour, S.; Zaefarian, F. Influence of dietary carbohydrases, individually or in combination with phytase or an acidifier, on performance, gut morphology and microbial population in broiler chickens fed a wheat-based diet. Anim. Nutr. 2019, 5, 63–67. [Google Scholar] [CrossRef]
- Forecast, M.D. Feed Enzymes Mark. 2019. Available online: https://www.marketdataforecast.com/market-reports/feed-enzymes-market (accessed on 13 January 2022).
- Bedford, M.R.; Cowieson, A.J. Matrix values for exogenous enzymes and their application in the real world. J. Appl. Poult. Res. 2020, 29, 15–22. [Google Scholar] [CrossRef]
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M.R.; Martínez, M.A. Enzymes in Feed and Animal Health. In Nutraceuticals in Veterinary Medicine; Springer: Cham, Switzerland, 2019; pp. 303–313. [Google Scholar] [CrossRef]
- Hassan, Y.I.; Lahaye, L.; Gong, M.M.; Peng, J.; Gong, J.; Liu, S.; Gay, C.G.; Yang, C. Innovative drugs, chemicals, and enzymes within the animal production chain. Vet. Res. 2018, 49, 71. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Kong, J.; Zhang, G.; Zhang, X.; Seviour, R.; Kong, Y. Effects of dietary supplementation with lysozyme on the structure and function of the cecal microbiota in broiler chickens. PLoS ONE 2019, 14, e0216748. [Google Scholar] [CrossRef] [PubMed]
- Torres-Pitarch, A.; Hermans, D.; Manzanilla, E.; Bindelle, J.; Everaert, N.; Beckers, Y.; Torrallardona, D.; Bruggeman, G.; Gardiner, G.; Lawlor, P. Effect of feed enzymes on digestibility and growth in weaned pigs: A systematic review and meta-analysis. Anim. Feed Sci. Technol. 2017, 233, 145–159. [Google Scholar] [CrossRef]
- Alagawany, M.; El-Hack, M.E.A.; Farag, M.R.; Sachan, S.; Karthik, K.; Dhama, K. The use of probiotics as eco-friendly alternatives for antibiotics in poultry nutrition. Environ. Sci. Pollut. Res. 2018, 25, 10611–10618. [Google Scholar] [CrossRef]
- Liao, S.F.; Nyachoti, M. Using probiotics to improve swine gut health and nutrient utilization. Anim. Nutr. 2017, 3, 331–343. [Google Scholar] [CrossRef]
- Zaghari, M.; Zahroojian, N.; Riahi, M.; Parhizkar, S. Effect ofBacillus SubtilisSpore (GalliPro®) Nutrients Equivalency Value on Broiler Chicken Performance. Ital. J. Anim. Sci. 2015, 14, 3555. [Google Scholar] [CrossRef]
- Bajagai, Y.S.; Klieve, A.V.; Dart, P.J.; Bryden, W.L. Probiotics in Animal Nutrition—Production, Impact and Regulation; FAO Animal Production and Health, Paper FAO 2016; FAO: Rome, Italy, 2016. [Google Scholar]
- Angelin, J.; Kavitha, M. Exopolysaccharides from probiotic bacteria and their health potential. Int. J. Biol. Macromol. 2020, 162, 853–865. [Google Scholar] [CrossRef]
- Bikric, S.; Aslim, B.; Dincer, I.; Yuksekdag, Z.; Ulusoy, S.; Yavuz, S. Characterization of Exopolysaccharides (EPSs) Obtained from Ligilactobacillus salivarius Strains and Investigation at the Prebiotic Potential as an Alternative to Plant Prebiotics at Poultry. Probiotics Antimicrob. Proteins 2022, 14, 49–59. [Google Scholar] [CrossRef]
- MarketsandMarkets. Probiotics in Animal Feed Market. 2019. Available online: https://www.marketsandmarkets.com/Market-Reports/probiotics-animal-feed-market-85832335.html (accessed on 17 May 2022).
- Deng, W.; Dittoe, D.K.; Pavilidis, H.O.; Chaney, W.E.; Yang, Y.; Ricke, S.C. Current Perspectives and Potential of Probiotics to Limit Foodborne Campylobacter in Poultry. Front. Microbiol. 2020, 11, 583429. [Google Scholar] [CrossRef]
- He, Y.; Liu, X.; Dong, Y.; Lei, J.; Ito, K.; Zhang, B. Enterococcus faecium PNC01 isolated from the intestinal mucosa of chicken as an alternative for antibiotics to reduce feed conversion rate in broiler chickens. Microb. Cell Factories 2021, 20, 122. [Google Scholar] [CrossRef] [PubMed]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Qattan, S.Y.A.; Batiha, G.E.; Khafaga, A.F.; Abdel-Moneim, A.-M.E.; Alagawany, M. Probiotics in poultry feed: A comprehensive review. J. Anim. Physiol. Anim. Nutr. 2020, 104, 1835–1850. [Google Scholar] [CrossRef] [PubMed]
- Hmidet, N.; El-Hadj Ali, N.; Haddar, A.; Kanoun, S.; Alya, S.-K.; Nasri, M. Alkaline proteases and thermostable α-amylase co-produced by Bacillus licheniformis NH1: Characterization and potential application as detergent additive. Biochem. Eng. J. 2009, 47, 71–79. [Google Scholar] [CrossRef]
- Zhang, J.; He, M.; He, Z. Operational and storage stability of neutral β-mannanase from Bacillus licheniformis. Biotechnol. Lett. 2002, 24, 1611–1613. [Google Scholar] [CrossRef]
- Imperial, I.C.V.; Ibana, J.A. Addressing the Antibiotic Resistance Problem with Probiotics: Reducing the Risk of Its Double-Edged Sword Effect. Front. Microbiol. 2016, 7, 1983. [Google Scholar] [CrossRef]
- Bujnakova, D.; Strakova, E.; Kmet, V. In vitro evaluation of the safety and probiotic properties of Lactobacilli isolated from chicken and calves. Anaerobe 2014, 29, 118–127. [Google Scholar] [CrossRef]
- Daniali, M.; Nikfar, S.; Abdollahi, M. Antibiotic resistance propagation through probiotics. Expert Opin. Drug Metab. Toxicol. 2020, 16, 1207–1215. [Google Scholar] [CrossRef]
- Alayande, K.A.; Aiyegoro, O.A.; Ateba, C.N. Probiotics in Animal Husbandry: Applicability and Associated Risk Factors. Sustainability 2020, 12, 1087. [Google Scholar] [CrossRef]
- Abdel-Megeed, R.M. Probiotics: A Promising Generation of Heavy Metal Detoxification. Biol. Trace Elem. Res. 2021, 199, 2406–2413. [Google Scholar] [CrossRef]
- Cheng, G.; Hao, H.; Xie, S.; Wang, X.; Dai, M.; Huang, L.; Yuan, Z.-H. Antibiotic alternatives: The substitution of antibiotics in animal husbandry? Front. Microbiol. 2014, 5, 217. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Choi, Y.P.; Koo, J.W. Mental Disorders Linked to Crosstalk between The Gut Microbiome and The Brain. Exp. Neurobiol. 2020, 29, 403–416. [Google Scholar] [CrossRef] [PubMed]
- Joshi, D.; Roy, S.; Banerjee, S. Chapter 19. Prebiotics: A Functional Food in Health and Disease. In Natural Products and Drug Discovery; Mandal, S.C., Mandal, V., Konishi, T., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 507–523. [Google Scholar]
- Ramnani, P.; Chitarrari, R.; Tuohy, K.; Grant, J.; Hotchkiss, S.; Philp, K.; Campbell, R.; Gill, C.; Rowland, I. In vitro fermentation and prebiotic potential of novel low molecular weight polysaccharides derived from agar and alginate seaweeds. Anaerobe 2012, 18, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Redzwan, S.M.; Jamaluddin, R.; Ahmad, F.N.; Lim, Y.-J. Chapter 27—Probiotics as Potential Adsorbent of Aflatoxin. In Probiotics, Prebiotics, and Synbiotics; Academic Press: Cambridge, MA, USA, 2016; pp. 409–419. [Google Scholar] [CrossRef]
- Markowiak, P.; Śliżewska, K. The role of probiotics, prebiotics and synbiotics in animal nutrition. Gut Pathog. 2018, 10, 21. [Google Scholar] [CrossRef] [PubMed]
- Tzortzis, G.; Goulas, A.K.; Gee, J.M.; Gibson, G.R. A Novel Galactooligosaccharide Mixture Increases the Bifidobacterial Population Numbers in a Continuous In Vitro Fermentation System and in the Proximal Colonic Contents of Pigs In Vivo. J. Nutr. 2005, 135, 1726–1731. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.R.; Hu, C.H.; Xia, M.S.; Zhan, X.A.; Wang, M.Q. Effects of dietary fructooligosaccharide on digestive enzyme activities, intestinal microflora and morphology of male broilers. Poult. Sci. 2003, 82, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Yusrizal, Y.; Chen, T.C. Effect of Adding Chicory Fructans in Feed on Fecal and Intestinal Microflora and Excreta Volatile Ammonia. Int. J. Poult. Sci. 2003, 2, 188–194. [Google Scholar] [CrossRef]
- Kleessen, B.; Elsayed, N.A.A.E.; Loehren, U.; Schroedl, W.; Krueger, M. Jerusalem Artichokes Stimulate Growth of Broiler Chickens and Protect Them against Endotoxins and Potential Cecal Pathogens. J. Food Prot. 2003, 66, 2171–2175. [Google Scholar] [CrossRef]
- Sohail, M.U.; Ijaz, A.; Yousaf, M.S.; Ashraf, K.; Zaneb, H.; Aleem, M.; Rehman, H. Alleviation of cyclic heat stress in broilers by dietary supplementation of mannan-oligosaccharide and Lactobacillus-based probiotic: Dynamics of cortisol, thyroid hormones, cholesterol, C-reactive protein, and humoral immunity. Poult. Sci. 2010, 89, 1934–1938. [Google Scholar] [CrossRef]
- Tavaniello, S.; Maiorano, G.; Stadnicka, K.; Mucci, R.; Bogucka, J.; Bednarczyk, M. Prebiotics offered to broiler chicken exert positive effect on meat quality traits irrespective of delivery route. Poult. Sci. 2018, 97, 2979–2987. [Google Scholar] [CrossRef]
- Corino, C.; Di Giancamillo, A.; Modina, S.; Rossi, R. Prebiotic Effects of Seaweed Polysaccharides in Pigs. Animals 2021, 11, 1573. [Google Scholar] [CrossRef]
- Adhikari, P.A.; Kim, W.K. Overview of Prebiotics and Probiotics: Focus on Performance, Gut Health and Immunity—A Review. Ann. Anim. Sci. 2017, 17, 949–966. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, J.; Zhong, H.; Li, N.; Xu, H.; Zhu, Q.; Liu, Y. Effect of probiotics on the meat flavour and gut microbiota of chicken. Sci. Rep. 2017, 7, 6400. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Ares, I.; Martínez-Larrañaga, M.R.; Martínez, M.A. Prebiotics and Probiotics in Feed and Animal Health. In Nutraceuticals in Veterinary Medicine; Springer: Cham, Switzerland, 2019; pp. 261–285. [Google Scholar] [CrossRef]
- Scott, K.P.; Grimaldi, R.; Cunningham, M.; Sarbini, S.R.; Wijeyesekera, A.; Tang, M.L.; Lee, J.C.; Yau, Y.F.; Ansell, J.; Theis, S.; et al. Developments in understanding and applying prebiotics in research and practice—An ISAPP conference paper. J. Appl. Microbiol. 2020, 128, 934–949. [Google Scholar] [CrossRef] [PubMed]
- El-Hack, M.E.A.; El-Saadony, M.T.; Shafi, M.E.; Alshahrani, O.A.; Saghir, S.A.M.; Al-Wajeeh, A.S.; Al-Shargi, O.Y.; Taha, A.E.; Mesalam, N.M.; Abdel-Moneim, A.-M.E. Prebiotics can restrict Salmonella populations in poultry: A review. Anim. Biotechnol. 2021, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Herfel, T.; Jacobi, S.; Lin, X.; Van Heugten, E.; Fellner, V.; Odle, J. Stabilized rice bran improves weaning pig performance via a prebiotic mechanism. J. Anim. Sci. 2013, 91, 907–913. [Google Scholar] [CrossRef] [PubMed]
- Chacher, M.; Kamran, Z.; Ahsan, U.; Ahmad, S.; Koutoulis, K.; Din, H.Q.U.; Cengiz, Ö. Use of mannan oligosaccharide in broiler diets: An overview of underlying mechanisms. World’s Poult. Sci. J. 2017, 73, 831–844. [Google Scholar] [CrossRef]
- Śliżewska, K.; Chlebicz, A. Synbiotics impact on dominant faecal microbiota and short-chain fatty acids production in sows. FEMS Microbiol. Lett. 2019, 366, fnz157. [Google Scholar] [CrossRef]
- Kridtayopas, C.; Rakangtong, C.; Bunchasak, C.; Loongyai, W. Effect of prebiotic and synbiotic supplementation in diet on growth performance, small intestinal morphology, stress, and bacterial population under high stocking density condition of broiler chickens. Poult. Sci. 2019, 98, 4595–4605. [Google Scholar] [CrossRef]
- Roth, N.; Hofacre, C.; Zitz, U.; Mathis, G.F.; Moder, K.; Doupovec, B.; Berghouse, R.; Domig, K.J. Prevalence of antibiotic-resistant E. coli in broilers challenged with a multi-resistant E. coli strain and received ampicillin, an organic acid-based feed additive or a synbiotic preparation. Poult. Sci. 2019, 98, 2598–2607. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Śliżewska, K.; Markowiak-Kopeć, P.; Żbikowski, A.; Szeleszczuk, P. The effect of synbiotic preparations on the intestinal microbiota and her metabolism in broiler chickens. Sci. Rep. 2020, 10, 4281. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Wareth, A.A.; Hammad, S.; Khalaphallah, R.; Salem, W.M.; Lohakare, J. Synbiotic as eco-friendly feed additive in diets of chickens under hot climatic conditions. Poult. Sci. 2019, 98, 4575–4583. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Chousalkar, K.K. Short-term feeding of probiotics and synbiotics modulates caecal microbiota during Salmonella Typhimurium infection but does not reduce shedding and invasion in chickens. Appl. Microbiol. Biotechnol. 2020, 104, 319–334. [Google Scholar] [CrossRef] [PubMed]
- Stefaniak, T.; Madej, J.P.; Graczyk, S.; Siwek, M.; Łukaszewicz, E.; Kowalczyk, A.; Sieńczyk, M.; Bednarczyk, M. Selected prebiotics and synbiotics administered in ovo can modify innate immunity in chicken broilers. BMC Vet. Res. 2019, 15, 105. [Google Scholar] [CrossRef]
- Asahara, T.; Takahashi, A.; Yuki, N.; Kaji, R.; Takahashi, T.; Nomoto, K. Protective Effect of a Synbiotic against Multidrug-Resistant Acinetobacter baumannii in a Murine Infection Model. Antimicrob. Agents Chemother. 2016, 60, 3041–3050. [Google Scholar] [CrossRef]
- Tayeri, V.; Seidavi, A.; Asadpour, L.; Phillips, C.J.C. A comparison of the effects of antibiotics, probiotics, synbiotics and prebiotics on the performance and carcass characteristics of broilers. Vet. Res. Commun. 2018, 42, 195–207. [Google Scholar] [CrossRef]
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, prebiotics and synbiotics—A review. J. Food Sci. Technol. 2015, 52, 7577–7587. [Google Scholar] [CrossRef]
- Krumbeck, J.A.; Walter, J.; Hutkins, R.W. Synbiotics for Improved Human Health: Recent Developments, Challenges, and Opportunities. Annu. Rev. Food Sci. Technol. 2018, 9, 451–479. [Google Scholar] [CrossRef]
- Cobb, L.H.; Park, J.; Swanson, E.A.; Beard, M.C.; McCabe, E.M.; Rourke, A.; Seo, K.S.; Olivier, A.K.; Priddy, L.B. CRISPR-Cas9 modified bacteriophage for treatment of Staphylococcus aureus induced osteomyelitis and soft tissue infection. PLoS ONE 2019, 14, e0220421. [Google Scholar] [CrossRef]
- Kosznik-Kwaśnicka, K.; Topka, G.; Dydecka, A.; Necel, A.; Nejman-Faleńczyk, B.; Bloch, S.; Węgrzyn, G.; Węgrzyn, A. The Use of Bacteriophages in Animal Health and Food Protection. In Phage Therapy: A Practical Approach; Springer: Cham, Switzerland, 2019; pp. 213–256. [Google Scholar] [CrossRef]
- De Paepe, M.; Leclerc, M.; Tinsley, C.R.; Petit, M.-A. Bacteriophages: An underestimated role in human and animal health? Front. Cell. Infect. Microbiol. 2014, 4, 39. [Google Scholar] [CrossRef]
- Niu, Y.; Johnson, R.; Xu, Y.; McAllister, T.; Sharma, R.; Louie, M.; Stanford, K. Host range and lytic capability of four bacteriophages against bovine and clinical human isolates of Shiga toxin-producing Escherichia coli O157:H7. J. Appl. Microbiol. 2009, 107, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Richards, P.J.; Connerton, P.L.; Connerton, I. Phage Biocontrol of Campylobacter jejuni in Chickens Does Not Produce Collateral Effects on the Gut Microbiota. Front. Microbiol. 2019, 10, 476. [Google Scholar] [CrossRef]
- Hong, S.S.J.; Jeong, J.; Lee, J.; Kim, S.; Min, W.; Myung, H. Therapeutic Effects of Bacteriophages Against Salmonella gallinarum Infection in Chickens. J. Microbiol. Biotechnol. 2013, 23, 1478–1483. [Google Scholar] [CrossRef] [PubMed]
- bikowska, K.; Michalczuk, M.; Dolka, B. The Use of Bacteriophages in the Poultry Industry. Animals 2020, 10, 872. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between Bacteriophage, Bacteria, and the Mammalian Immune System. Viruses 2018, 11, 10. [Google Scholar] [CrossRef] [PubMed]
- Popescu, M.; Van Belleghem, J.D.; Khosravi, A.; Bollyky, P.L. Bacteriophages and the Immune System. Annu. Rev. Virol. 2021, 8, 415–435. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Wang, Z.; Zou, T.; Chen, J.; Li, G.; Zheng, L.; Li, S.; You, J. Bacteriophage as an Alternative to Antibiotics Promotes Growth Performance by Regulating Intestinal Inflammation, Intestinal Barrier Function and Gut Microbiota in Weaned Piglets. Front. Vet. Sci. 2021, 8, 623899. [Google Scholar] [CrossRef]
- Calero-Caceres, W.; Ye, M.; Balcázar, J.L. Bacteriophages as Environmental Reservoirs of Antibiotic Resistance. Trends Microbiol. 2019, 27, 570–577. [Google Scholar] [CrossRef]
- Yang, Y.; Xie, X.; Tang, M.; Liu, J.; Tuo, H.; Gu, J.; Tang, Y.; Lei, C.; Wang, H.; Zhang, A. Exploring the profile of antimicrobial resistance genes harboring by bacteriophage in chicken feces. Sci. Total Environ. 2020, 700, 134446. [Google Scholar] [CrossRef]
- Wang, M.; Liu, P.; Zhou, Q.; Tao, W.; Sun, Y.; Zeng, Z. Estimating the contribution of bacteriophage to the dissemination of antibiotic resistance genes in pig feces. Environ. Pollut. 2018, 238, 291–298. [Google Scholar] [CrossRef]
- Lekunberri, I.; Subirats, J.; Borrego, C.; Balcázar, J.L. Exploring the contribution of bacteriophages to antibiotic resistance. Environ. Pollut. 2017, 220, 981–984. [Google Scholar] [CrossRef] [PubMed]
- Rios, A.C.; Moutinho, C.; Pinto, F.C.; Del Fiol, F.; Jozala, A.; Chaud, M.V.; Vila, M.M.; Teixeira, J.; Balcão, V.M. Alternatives to overcoming bacterial resistances: State-of-the-art. Microbiol. Res. 2016, 191, 51–80. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Silvestri, E.; Esposito, S. Advantages and limitations of bacteriophages for the treatment of bacterial infections. Front. Pharmacol. 2019, 10, 513. [Google Scholar] [CrossRef] [PubMed]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef]
- Callaway, T.R.; Edrington, T.S.; Brabban, A.; Kutter, B.; Karriker, L.; Stahl, C.; Wagstrom, E.; Anderson, R.; Poole, T.L.; Genovese, K.; et al. Evaluation of Phage Treatment as a Strategy to ReduceSalmonellaPopulations in Growing Swine. Foodborne Pathog. Dis. 2011, 8, 261–266. [Google Scholar] [CrossRef]
- Silveira, R.F.; Roque-Borda, C.A.; Vicente, E.F. Antimicrobial peptides as a feed additive alternative to animal production, food safety and public health implications: An overview. Anim. Nutr. 2021, 7, 896–904. [Google Scholar] [CrossRef]
- Wang, G.; Song, Q.; Huang, S.; Wang, Y.; Cai, S.; Yu, H.; Ding, X.; Zeng, X.; Zhang, J. Effect of Antimicrobial Peptide Microcin J25 on Growth Performance, Immune Regulation, and Intestinal Microbiota in Broiler Chickens Challenged with Escherichia coli and Salmonella. Animals 2020, 10, 345. [Google Scholar] [CrossRef]
- Xu, B.C.; Fu, J.; Zhu, L.Y.; Li, Z.; Wang, Y.Z.; Jin, M.L. Overall assessment of antimicrobial peptides in piglets: A set of meta-analyses. Animal 2020, 14, 2463–2471. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.-M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Q.; Wen, L.; Wu, C.; Yao, Z.; Yan, Z.; Li, R.; Chen, L.; Chen, F.; Xie, Z.; et al. The Effect of the Antimicrobial Peptide Plectasin on the Growth Performance, Intestinal Health, and Immune Function of Yellow-Feathered Chickens. Front. Vet. Sci. 2021, 8, 688611. [Google Scholar] [CrossRef]
- Raheem, N.; Straus, S.K. Mechanisms of Action for Antimicrobial Peptides with Antibacterial and Antibiofilm Functions. Front. Microbiol. 2019, 10, 2866. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wang, Y.; Zeng, X.; Cai, S.; Wang, G.; Liu, L.; Huang, S.; Li, N.; Liu, H.; Ding, X.; et al. Therapeutic administration of the recombinant antimicrobial peptide microcin J25 effectively enhances host defenses against gut inflammation and epithelial barrier injury induced by enterotoxigenic Escherichia coli infection. FASEB J. 2020, 34, 1018–1037. [Google Scholar] [CrossRef] [PubMed]
- Tai, H.-M.; Huang, H.-N.; Tsai, T.-Y.; You, M.-F.; Wu, H.-Y.; Rajanbabu, V.; Chang, H.-Y.; Pan, C.-Y.; Chen, J.-Y. Dietary supplementation of recombinant antimicrobial peptide Epinephelus lanceolatus piscidin improves growth performance and immune response in Gallus gallus domesticus. PLoS ONE 2020, 15, e0230021. [Google Scholar] [CrossRef]
- Xiao, H.; Shao, F.; Wu, M.; Ren, W.; Xiong, X.; Tan, B.; Yin, J. The application of antimicrobial peptides as growth and health promoters for swine. J. Anim. Sci. Biotechnol. 2015, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dou, X.; Song, J.; Lyu, Y.; Zhu, X.; Xu, L.; Li, W.; Shan, A. Antimicrobial peptides: Promising alternatives in the post feeding antibiotic era. Med. Res. Rev. 2019, 39, 831–859. [Google Scholar] [CrossRef]
- Cutler, S.A.; Lonergan, S.M.; Cornick, N.; Johnson, A.K.; Stahl, C.H. Dietary Inclusion of Colicin E1 Is Effective in Preventing Postweaning Diarrhea Caused by F18-Positive Escherichia coli in Pigs. Antimicrob. Agents Chemother. 2007, 51, 3830–3835. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, F.; Huang, Z.; Liu, H.; Xie, C.; Zhang, J.; Thacker, P.A.; Qiao, S. Effects of the antimicrobial peptide cecropin AD on performance and intestinal health in weaned piglets challenged with Escherichia coli. Peptides 2012, 35, 225–230. [Google Scholar] [CrossRef]
- Assoni, L.; Milani, B.; Carvalho, M.R.; Nepomuceno, L.N.; Waz, N.T.; Guerra, M.E.S.; Converso, T.R.; Darrieux, M. Resistance Mechanisms to Antimicrobial Peptides in Gram-Positive Bacteria. Front. Microbiol. 2020, 11, 593215. [Google Scholar] [CrossRef]
- Joo, H.-S.; Fu, C.-I.; Otto, M. Bacterial strategies of resistance to antimicrobial peptides. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150292. [Google Scholar] [CrossRef]
- de Freire Bastos, M.D.C.; Coelho, M.L.V.; Da Silva Santos, O.C. Resistance to bacteriocins produced by Gram-positive bacteria. Microbiology 2015, 161, 683–700. [Google Scholar] [CrossRef] [PubMed]
- Guinane, C.M.; Cotter, P.D.; Hill, C.; Ross, R.P. Spontaneous resistance in Lactococcus lactis IL1403 to the lantibiotic lacticin 3147. FEMS Microbiol. Lett. 2006, 260, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Konstantinos Papadimitriou, V.A.; Tsakalidou, E. Fighting off Human Infections a New Role for Bacteriocin Molecules. Interact. Probiotics Ed. Enrica Pessione 2014, 22–51. [Google Scholar]
- Kaur, G.; Singh, T.P.; Malik, R.K.; Bhardwaj, A.; De, S. Antibacterial efficacy of nisin, pediocin 34 and enterocin FH99 against L. monocytogenes, E. faecium and E. faecalis and bacteriocin cross resistance and antibiotic susceptibility of their bacteriocin resistant variants. J. Food Sci. Technol. 2014, 51, 233–244. [Google Scholar] [CrossRef][Green Version]
- Carlson, S.A.; Frana, T.S.; Griffith, R.W. Antibiotic Resistance in Salmonella enterica Serovar Typhimurium Exposed to Microcin-Producing Escherichia coli. Appl. Environ. Microbiol. 2001, 67, 3763–3766. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, H.C.; Russell, J.B. Nisin Resistance of Streptococcus bovis. Appl. Environ. Microbiol. 2001, 67, 808–813. [Google Scholar] [CrossRef] [PubMed]
- Madrazo, A.L.; Campos, M.R.S. Review of antimicrobial peptides as promoters of food safety: Limitations and possibilities within the food industry. J. Food Saf. 2020, 40, e12854. [Google Scholar] [CrossRef]
- Udenigwe, C.C.; Fogliano, V. Food matrix interaction and bioavailability of bioactive peptides: Two faces of the same coin? J. Funct. Foods 2017, 35, 9–12. [Google Scholar] [CrossRef]
- Nazeer, N.; Uribe-Diaz, S.; Rodriguez-Lecompte, J.C.; Ahmed, M. Antimicrobial peptides as an alternative to relieve antimicrobial growth promoters in poultry. Br. Poult. Sci. 2021, 62, 672–685. [Google Scholar] [CrossRef]
- Kurt, T.; Wong, N.; Fowler, H.; Gay, C.; Lillehoj, H.; Plummer, P.; Scott, H.M.; Hoelzer, K. Strategic Priorities for Research on Antibiotic Alternatives in Animal Agriculture—Results from an Expert Workshop. Front. Vet. Sci. 2019, 6, 429. [Google Scholar] [CrossRef]
- Ioannou, F.; Burnsteel, C.; Mackay, D.K.; Gay, C. Regulatory pathways to enable the licencing of alternatives to antibiotics. Biologicals 2018, 53, 72–75. [Google Scholar] [CrossRef] [PubMed]
- Bedford, M.R. The evolution and application of enzymes in the animal feed industry: The role of data interpretation. Br. Poult. Sci. 2018, 59, 486–493. [Google Scholar] [CrossRef] [PubMed]
- Von Rosenvinge, E.C.; O’May, G.A.; Macfarlane, S.; Macfarlane, G.T.; Shirtliff, M.E. Microbial biofilms and gastrointestinal diseases. Pathog. Dis. 2013, 67, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Motta, J.-P.; Wallace, J.L.; Buret, A.G.; Deraison, C.; Vergnolle, N. Gastrointestinal biofilms in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 314–334. [Google Scholar] [CrossRef]




| Country | Year | Action |
|---|---|---|
| Australia | 2017 | Antibiotics used in human medicine are not licensed as growth promoters. However, five antibiotics (olaquindox, avilamycin, bambermycin, monensin, and salinomycin) not currently used in human medicine are used as growth promoters in poultry, pigs, cattle, and sheep [23]. |
| Canada | 2020 | Growth promotion claims on medically important antimicrobials (MIAs) (Category I, II, and III antimicrobials) will no longer be permitted. Ionophore and coccidiostat products will be unaffected, as they are not considered MIAs [24,25]. |
| China | 2020 | All antibiotic growth promoters except herbal medicine have been banned [26]. |
| European Union | 2006 2022 | Illegal across the EU The EU will ban the importation of meat and dairy produced using antibiotic growth promoters. However, fluoroquinolones continue to be licensed in the UK and in many EU countries [27]. |
| New Zealand | 2017 | No banning claim found. The Ministry of Health and the Ministry for Primary Industries (MPI) stated in 2017: “If antibiotics used in food-producing animals, MPI must also be satisfied that the antibiotic will not leave residues above the maximum residue level in food from the treated animals”. Compared to EU countries, New Zealand uses more cephalosporins and macrolides, but less quinolones [28]. |
| Sweden | 1986 | First country to ban the use of antibiotics as growth promoters [29]. |
| USA | 2017 | Medically important antimicrobials are banned. However, bacitracin and carbadox, which are classified as medically important by the World Health Organization, are still used as growth promoters in pigs [30]. |
| Strengths | Weaknesses [89] |
|---|---|
|
|
| Opportunities [89,90] | Threats [89,91] |
|
|
| Strengths [116,117,118] | Weaknesses [92,116,119] |
|---|---|
|
|
| Opportunities [118] | Threats |
|
|
| Strengths [129,132] | Weaknesses [121] |
|---|---|
|
|
| Opportunities | Threats |
|
|
| Strengths [134,142] | Weaknesses [140,149] |
|---|---|
|
|
| Opportunities [151,152] | Threats [147,150] |
|
|
| Strengths [163,167] | Weaknesses [149] |
|---|---|
|
|
| Opportunities [90] | Threats [149] |
|
|
| Strengths [197,198] | Weaknesses [149,199] |
|---|---|
|
|
| Opportunities [200] | Threats [149] |
|
|
| Strengths [221] | Weaknesses [210,222] |
|---|---|
|
|
| Opportunities [223] | Threats [210] |
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rahman, M.R.T.; Fliss, I.; Biron, E. Insights in the Development and Uses of Alternatives to Antibiotic Growth Promoters in Poultry and Swine Production. Antibiotics 2022, 11, 766. https://doi.org/10.3390/antibiotics11060766
Rahman MRT, Fliss I, Biron E. Insights in the Development and Uses of Alternatives to Antibiotic Growth Promoters in Poultry and Swine Production. Antibiotics. 2022; 11(6):766. https://doi.org/10.3390/antibiotics11060766
Chicago/Turabian StyleRahman, Md Ramim Tanver, Ismail Fliss, and Eric Biron. 2022. "Insights in the Development and Uses of Alternatives to Antibiotic Growth Promoters in Poultry and Swine Production" Antibiotics 11, no. 6: 766. https://doi.org/10.3390/antibiotics11060766
APA StyleRahman, M. R. T., Fliss, I., & Biron, E. (2022). Insights in the Development and Uses of Alternatives to Antibiotic Growth Promoters in Poultry and Swine Production. Antibiotics, 11(6), 766. https://doi.org/10.3390/antibiotics11060766