Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects
Abstract
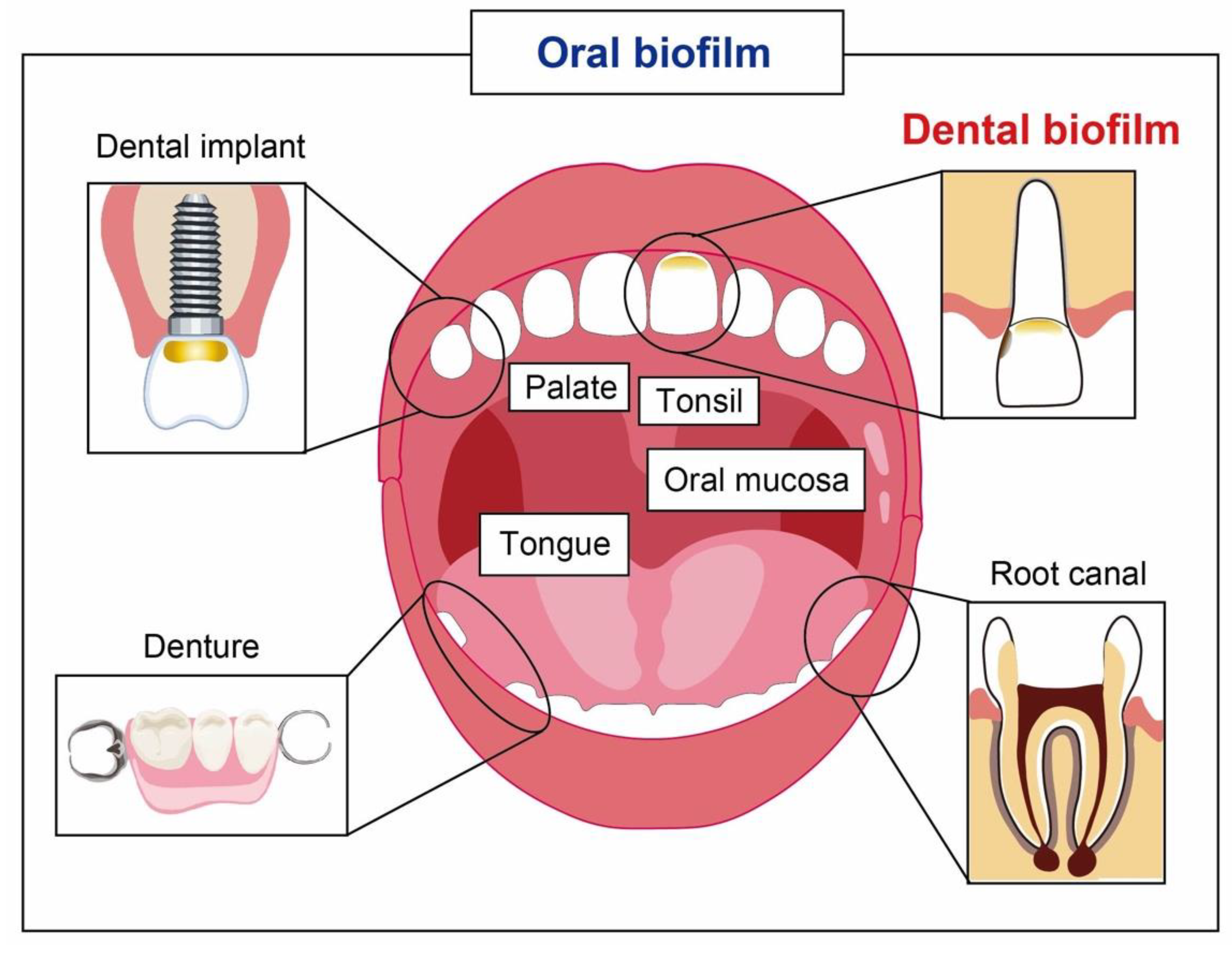
1. Introduction
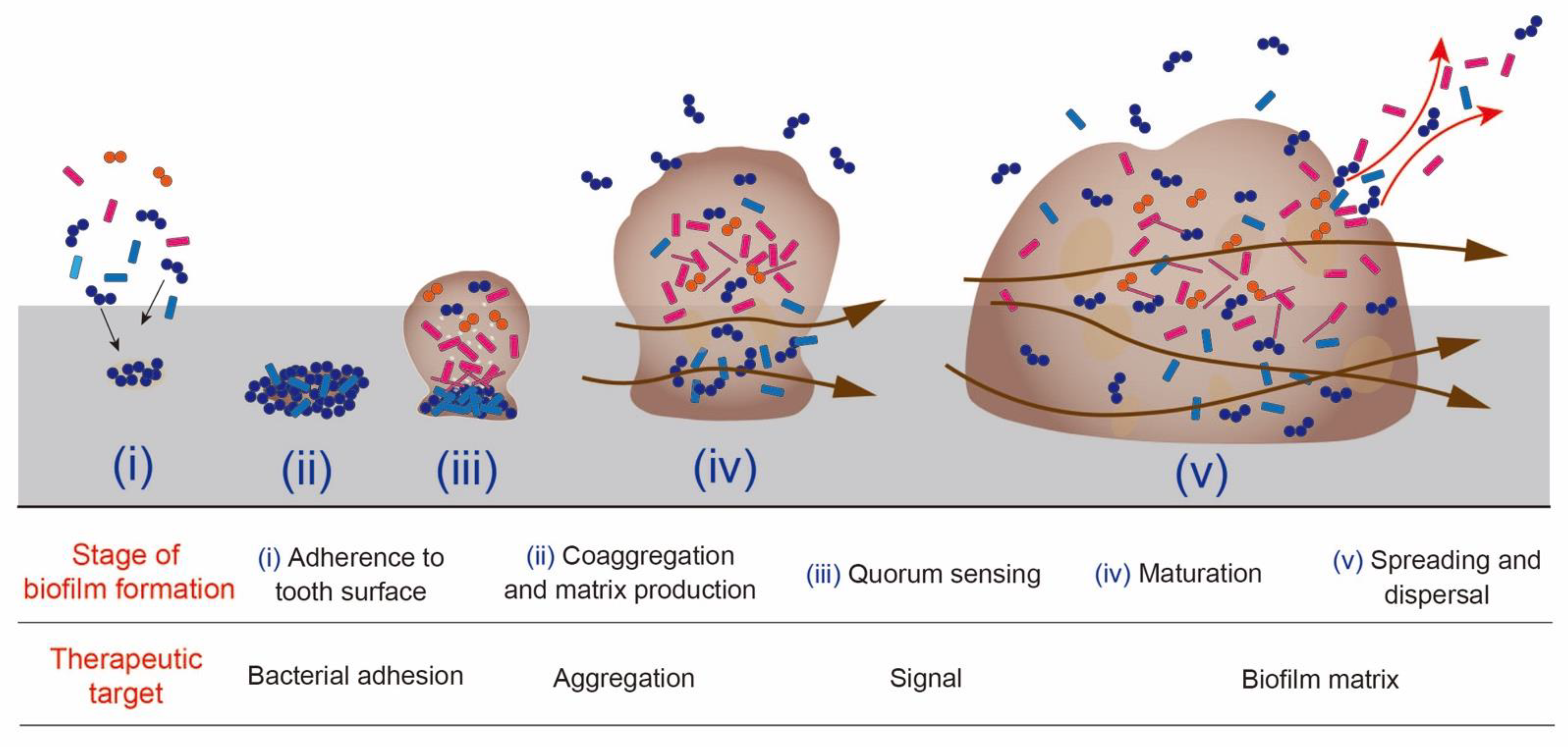
2. The Process of Biofilm Formation and Anti-Biofilm Strategy
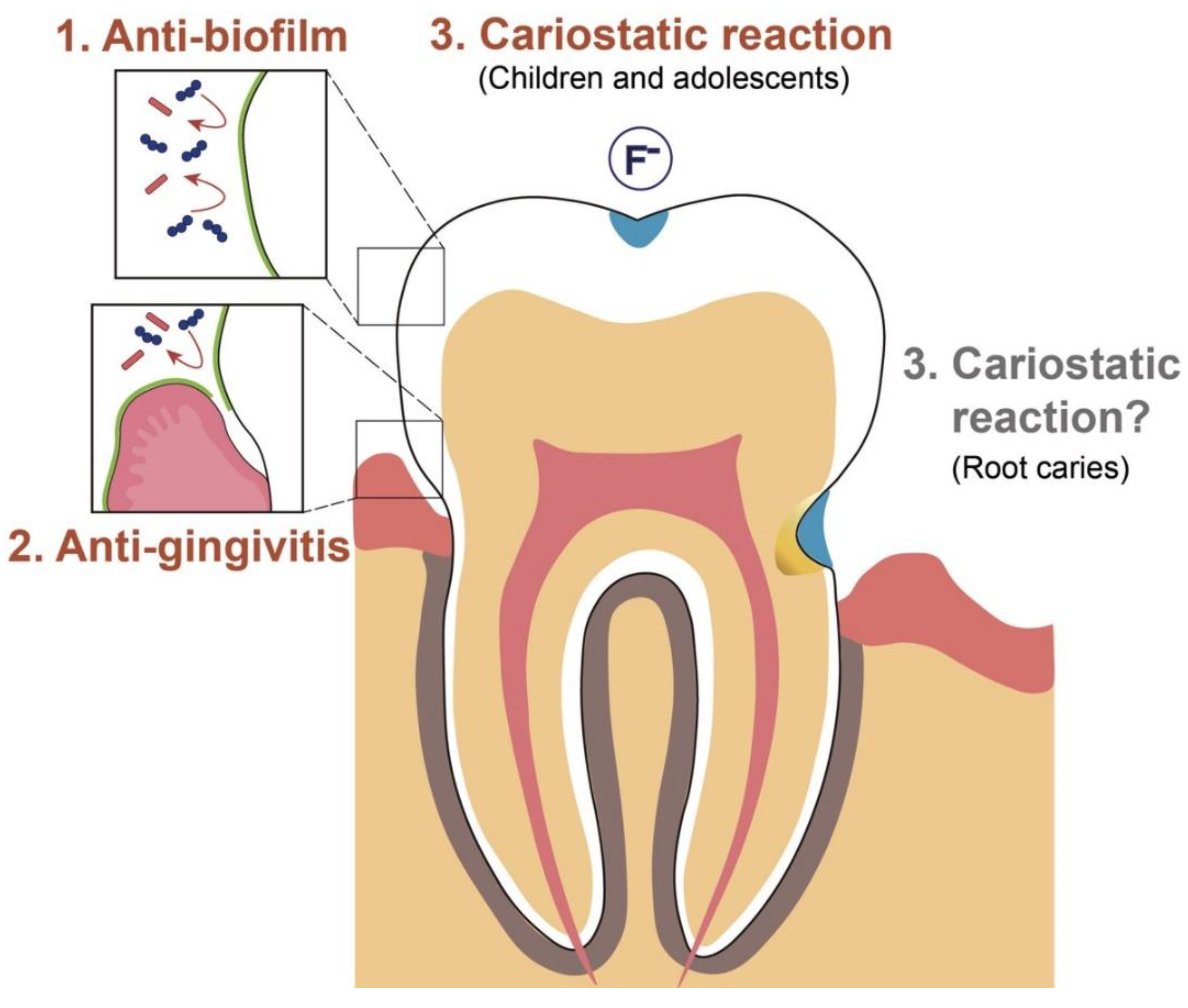
3. Intended Effects of Mouthwash on Biofilm Control
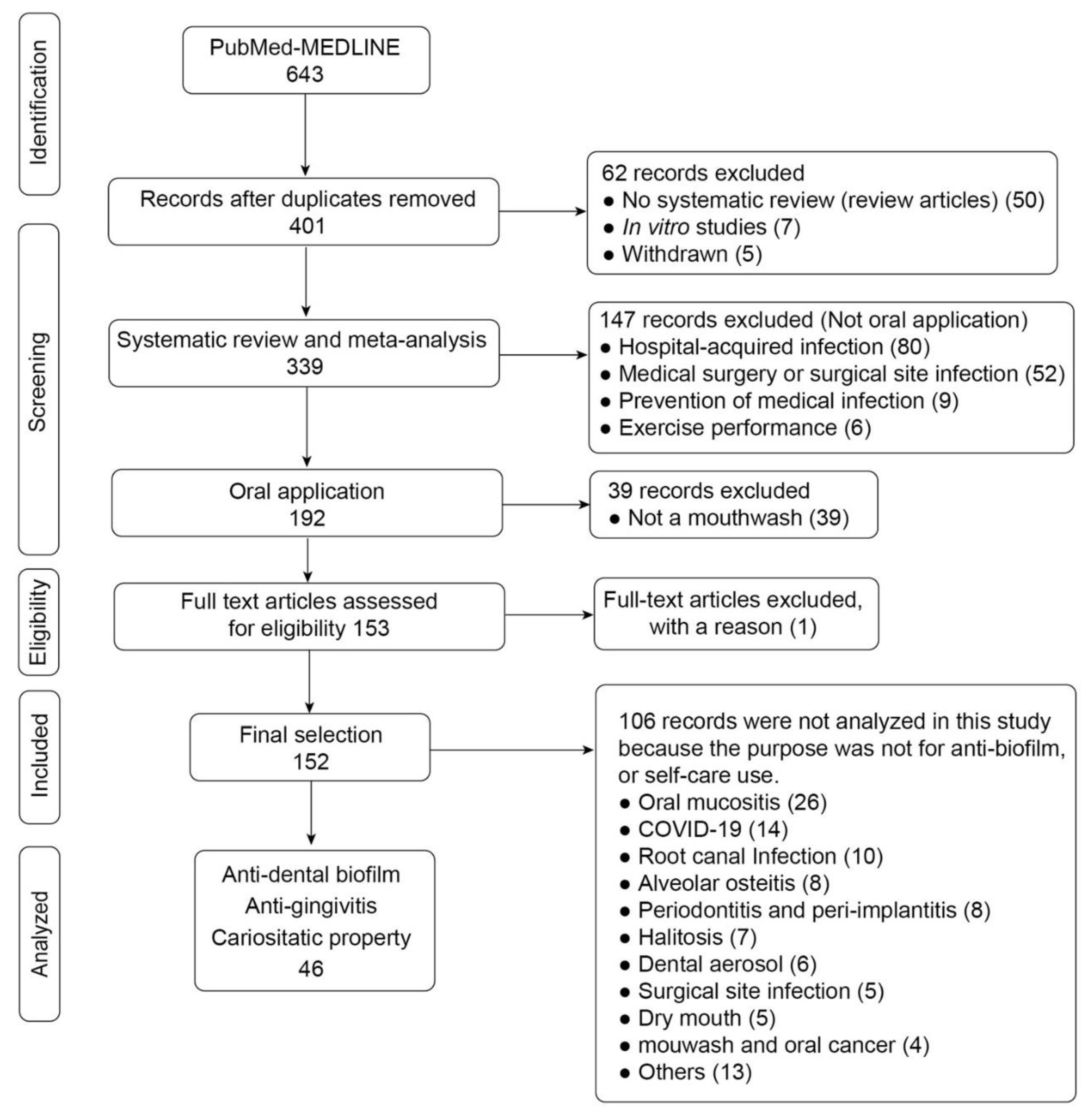
3.1. Study Selection and Data Collection
3.2. Current Evidence on the Effects of Mouthwash
3.2.1. CHG
3.2.2. EO
3.2.3. CPC
3.2.4. Other Chemical Compounds
3.2.5. Natural Products
3.2.6. Sodium Hypochlorite (NaOCl)
3.2.7. Chlorine Dioxide (ClO2)
3.2.8. Cariostatic Property: Children and Adolescents
3.2.9. Cariostatic Property: Root Caries
4. Side Effects and Potential Adverse Reactions to Mouthwashes
4.1. Side Effects (Patients’ Complaints)
4.2. Cytotoxic Effects on Human Cells
4.3. Antimicrobial Resistance
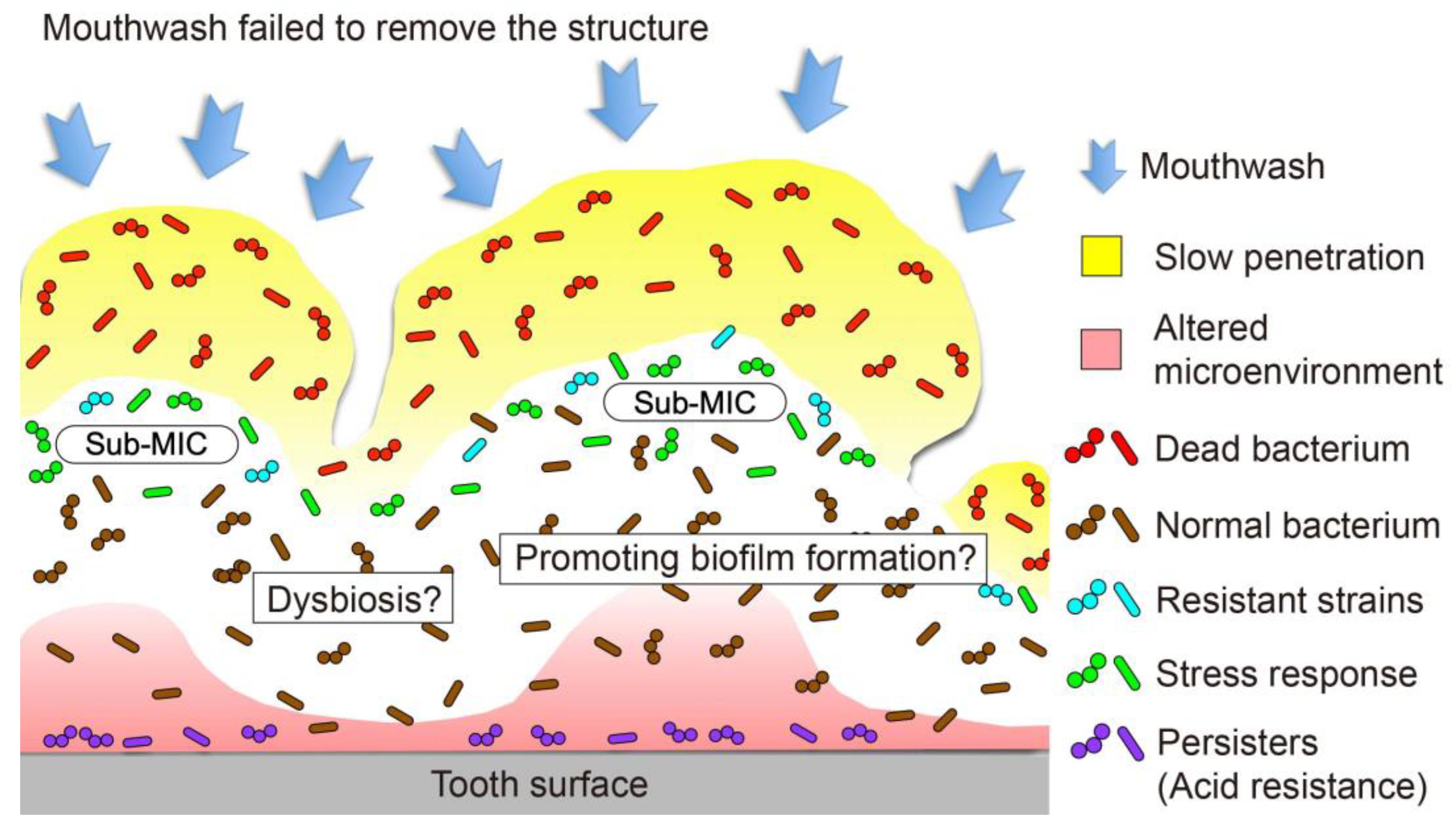
4.4. Residual Structure
4.5. Retarded Penetration into Biofilm and Promotion of Biofilm Development
4.6. Ecological Changes in the Oral Biofilm Microbiota
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.R.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The Human Oral Microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [PubMed]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the Normal Bacterial Flora of the Oral Cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental Plaque as a Biofilm and a Microbial Community-Implications for Health and Disease. BMC Oral Health 2006, 6 (Suppl. S1). [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental Caries. Nat. Rev. Dis. Primers. 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. The Role of Bacteria in the Caries Process: Ecological Perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Noiri, Y.; Li, L.; Ebisu, S. The Localization of Periodontal-Disease-Associated Bacteria in Human Periodontal Pockets. J. Dent. Res. 2001, 80, 1930–1934. [Google Scholar] [CrossRef] [PubMed]
- Kuboniwa, M.; Lamont, R.J. Subgingival Biofilm Formation. Periodontol. 2000 2010, 52, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Noguchi, N.; Noiri, Y.; Narimatsu, M.; Ebisu, S. Identification and Localization of Extraradicular Biofilm-Forming Bacteria Associated with Refractory Endodontic Pathogens. Appl. Environ. Microbiol. 2005, 71, 8738–8743. [Google Scholar] [CrossRef]
- Noiri, Y.; Ehara, A.; Kawahara, T.; Takemura, N.; Ebisu, S. Participation of Bacterial Biofilms in Refractory and Chronic Periapical Periodontitis. J. Endod. 2002, 28, 679–683. [Google Scholar] [CrossRef]
- Roberts, F.A.; Darveau, R.P. Microbial Protection and Virulence in Periodontal Tissue as a Function of Polymicrobial Communities: Symbiosis and Dysbiosis. Periodontol. 2000 2015, 69, 18–27. [Google Scholar] [CrossRef]
- Kilian, M. The Oral Microbiome-Friend or Foe? Eur. J. Oral Sci. 2018, 126 (Suppl. S1), 5–12. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.; Deng, D.; Buskermolen, J.K.; Janus, M.M.; Krom, B.P.; Roffel, S.; Waaijman, T.; van Loveren, C.; Crielaard, W.; Gibbs, S. Multi-Species Oral Biofilm Promotes Reconstructed Human Gingiva Epithelial Barrier Function. Sci. Rep. 2018, 8, 1–12. [Google Scholar]
- Shang, L.; Deng, D.; Buskermolen, J.K.; Roffel, S.; Janus, M.M.; Krom, B.P.; Crielaard, W.; Gibbs, S. Commensal and Pathogenic Biofilms Alter Toll-Like Receptor Signaling in Reconstructed Human Gingiva. Front. Cell. Infect. Microbiol. 2019, 9, 282. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Ohshima, H.; Ohsumi, T.; Okiji, T. Current and Future Strategies for the Control of Mature Oral Biofilms- Shift from a Bacteria-Targeting to a Matrix-Targeting Approach. J. Oral Biosci. 2012, 54, 173–179. [Google Scholar] [CrossRef]
- Van der Weijden, F.; Slot, D.E. Oral Hygiene in the Prevention of Periodontal Diseases: The Evidence. Periodontol. 2000 2011, 55, 104–123. [Google Scholar] [CrossRef] [PubMed]
- Serrano, J.; Escribano, M.; Roldán, S.; Martín, C.; Herrera, D. Efficacy of Adjunctive Antiplaque Chemical Agents in Managing Gingivitis: A Systematic Review and Meta-Analysis. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S106–S138. [Google Scholar] [CrossRef]
- Almerich, J.M.; Cabedo, B.; Ortolá, J.C.; Poblet, J. Influence of Alcohol in Mouthwashes Containing Triclosan and Zinc: An Experimental Gingivitis Study. J. Clin. Periodontol. 2005, 32, 539–544. [Google Scholar] [CrossRef]
- Santos, A. Evidence-Based Control of Plaque and Gingivitis. J. Clin. Periodontol. 2003, 30 (Suppl. S16), 13–16. [Google Scholar] [CrossRef]
- Brading, M.G.; Marsh, P.D. The Oral Environment: The Challenge for Antimicrobials in Oral Care Products. Int. Dent. J. 2003, 53 (Suppl. S1), 353–362. [Google Scholar] [CrossRef]
- Marsh, P.D. Controlling the Oral Biofilm with Antimicrobials. J. Dent. 2010, 38 (Suppl. S1), S11–S15. [Google Scholar] [CrossRef]
- Marsh, P.D. Contemporary Perspective on Plaque Control. Br. Dent. J. 2012, 212, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Council on Dental Therapeutics. Guidelines for Acceptance of Chemotherapeutic Products for the Control of Supragingival Dental Plaque and Gingivitis. Council on Dental Therapeutics. J. Am. Dent. Assoc. 1986, 112, 529–532. [Google Scholar] [CrossRef]
- Gunsolley, J.C. A Meta-Analysis of Six-Month Studies of Antiplaque and Antigingivitis Agents. J. Am. Dent. Assoc. 2006, 137, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Gunsolley, J.C. Clinical Efficacy of Antimicrobial Mouthrinses. J. Dent. 2010, 38 (Suppl. S1), S6–S10. [Google Scholar] [CrossRef]
- van Leeuwen, M.P.; Slot, D.E.; van der Weijden, G.A. Essential Oils Compared to Chlorhexidine with Respect to Plaque and Parameters of Gingival Inflammation: A Systematic Review. J. Periodontol. 2011, 82, 174–194. [Google Scholar] [CrossRef]
- Stoeken, J.E.; Paraskevas, S.; van der Weijden, G.A. The Long-Term Effect of a Mouthrinse Containing Essential Oils on Dental Plaque and Gingivitis: A Systematic Review. J. Periodontol. 2007, 78, 1218–1228. [Google Scholar] [CrossRef]
- Haps, S.; Slot, D.E.; Berchier, C.E.; van der Weijden, G.A. The Effect of Cetylpyridinium Chloride-Containing Mouth Rinses as Adjuncts to Toothbrushing on Plaque and Parameters of Gingival Inflammation: A Systematic Review. Int. J. Dent. Hyg. 2008, 6, 290–303. [Google Scholar] [CrossRef]
- Sahrmann, P.; Puhan, M.A.; Attin, T.; Schmidlin, P.R. Systematic Review on the Effect of Rinsing with Povidone-Iodine During Nonsurgical Periodontal Therapy. J. Periodont. Res. 2010, 45, 153–164. [Google Scholar] [CrossRef][Green Version]
- Addy, M.; Moran, J.; Newcombe, R.G. Meta-Analysis of Studies of 0.2% Delmopinol Mouth Rinse as an Adjunct to Gingival Health and Plaque Control Measures. J. Clin. Periodontol. 2007, 34, 58–65. [Google Scholar] [CrossRef]
- Afennich, F.; Slot, D.E.; Hossainian, N.; van der Weijden, G.A. The Effect of Hexetidine Mouthwash on the Prevention of Plaque and Gingival Inflammation: A Systematic Review. Int. J. Dent. Hyg. 2011, 9, 182–190. [Google Scholar] [CrossRef]
- Santos, D.S.F.; Peralta-Mamani, M.; Brandão, F.S.; Andrade, F.B.; Cruvinel, T.; Santos, P.S.D.S. Could Polyhexanide and Chlorine Dioxide Be Used as an Alternative to Chlorhexidine? A Systematic Review. Sao Paulo Med. J. 2022, 140, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.M.; van der Weijden, G.A.F.; Slot, D.E. Effect of a Sodium Hypochlorite Mouthwash on Plaque and Clinical Parameters of Periodontal Disease- a Systematic Review. Int. J. Dent. Hyg. 2022, 20, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Kerémi, B.; Márta, K.; Farkas, K.; Czumbel, L.M.; Tóth, B.; Szakács, Z.; Csupor, D.; Czimmer, J.; Rumbus, Z.; Révész, P.; et al. Effect of Chlorine Dioxide on Oral Hygiene- a Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2020, 26, 3015–3025. [Google Scholar] [CrossRef]
- Chen, Y.; Wong, R.W.K.; McGrath, C.; Hagg, U.; Seneviratne, C.J. Natural Compounds Containing Mouthrinses in the Management of Dental Plaque and Gingivitis: A Systematic Review. Clin. Oral Investig. 2014, 18, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Hossainian, N.; Slot, D.E.; Afennich, F.; van der Weijden, G.A. The Effect of Hydrogen Peroxide Mouthwashes on the Prevention of Plaque and Gingival Inflammation: A Systematic Review. Int. J. Dent. Hyg. 2011, 9, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Guidelines for Infection Control in Dental Health-Care settings-2003. Morb. Mortal. Wkly. Rep. 2003, 52, 32. [Google Scholar]
- Marui, V.C.; Souto, M.L.S.; Rovai, E.S.; Romito, G.A.; Chambrone, L.; Pannuti, C.M. Efficacy of Preprocedural Mouthrinses in the Reduction of Microorganisms in Aerosol: A Systematic Review. J. Am. Dent. Assoc. 2019, 150, 1015–1026. [Google Scholar] [CrossRef]
- Costerton, J.W.; Stewart, P.S. Battling Biofilms. Sci. Am. 2001, 285, 74–81. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial Biofilms: From the Natural Environment to Infectious Diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Yin, W.; Wang, Y.; Liu, L.; He, J. Biofilms: The Microbial “Protective Clothing” in Extreme Environments. Int. J. Mol. Sci. 2019, 20, 3423. [Google Scholar] [CrossRef]
- Miquel, S.; Lagrafeuille, R.; Souweine, B.; Forestier, C. Anti-biofilm Activity as a Health Issue. Front. Microbiol. 2016, 7, 592. [Google Scholar] [CrossRef]
- Lamont, R.J.; Koo, H.; Hajishengallis, G. The Oral Microbiota: Dynamic Communities and Host Interactions. Nat. Rev. Microbiol. 2018, 16, 745–759. [Google Scholar] [CrossRef] [PubMed]
- Nyvad, B.; Takahashi, N. Integrated Hypothesis of Dental Caries and Periodontal Diseases. J. Oral Microbiol. 2020, 12, 1710953. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G.; Lamont, R.J. Polymicrobial Communities in Periodontal Disease: Their Quasi-organismal Nature and Dialogue with the Host. Periodontol. 2000 2021, 86, 210–230. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.G. Chlorhexidine: Is It Still the Gold Standard? Periodontol. 2000 1997, 15, 55–62. [Google Scholar] [CrossRef]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine Mouthrinse as an Adjunctive Treatment for Gingival Health. Cochrane Database Syst. Rev. 2017, 3, CD008676. [Google Scholar] [CrossRef]
- Escribano, M.; Figuero, E.; Martín, C.; Tobías, A.; Serrano, J.; Roldán, S.; Herrera, D. Efficacy of Adjunctive Anti-Plaque Chemical Agents: A Systematic Review and Network Meta-Analyses of the Turesky Modification of the Quigley and Hein Plaque Undex. J. Clin. Periodontol. 2016, 43, 1059–1073. [Google Scholar] [CrossRef]
- Boyle, P.; Koechlin, A.; Autier, P. Mouthwash Use and the Prevention of Plaque, Gingivitis and Caries. Oral Dis. 2014, 20 (Suppl. S1), 1–68. [Google Scholar] [CrossRef]
- Van Strydonck, D.A.; Slot, D.E.; van der Velden, U.; van der Weijden, F. Effect of a Chlorhexidine Mouthrinse on Plaque, Gingival Inflammation and Staining in Gingivitis Patients: A Systematic Review. J. Clin. Periodontol. 2012, 39, 1042–1055. [Google Scholar] [CrossRef]
- Figuero, E.; Herrera, D.; Tobías, A.; Serrano, J.; Roldán, S.; Escribano, M.; Martín, C. Efficacy of Adjunctive Anti-Plaque Chemical Agents in Managing Gingivitis: A Systematic Review and Network Meta-Analyses. J. Clin. Periodontol. 2019, 46, 723–739. [Google Scholar] [CrossRef]
- Haas, A.N.; Wagner, T.P.; Muniz, F.W.M.G.; Fiorini, T.; Cavagni, J.; Celeste, R.K. Essential Oils-Containing Mouthwashes for Gingivitis and Plaque: Meta-Analyses and Meta-Regression. J. Dent. 2016, 55, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Van Leeuwen, M.P.; Slot, D.E.; van der Weijden, G.A. The Effect of an Essential-Oils Mouthrinse as Compared to a Solution on Plaque and Gingival Inflammation: A Systematic Review and Meta-Analysis. Int. J. Dent. Hyg. 2014, 12, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Van der Weijden, F.; Van der Sluijs, E.; Ciancio, S.G.; Slot, D.E. Can Chemical Mouthwash Agents Achieve Plaque/Gingivitis Control? Dent. Clin. North Am. 2015, 59, 799–829. [Google Scholar] [CrossRef] [PubMed]
- Langa, G.P.J.; Muniz, F.W.M.G.; Costa, R.D.S.A.; da Silveira, T.M.; Rösing, C.K. The Effect of Cetylpyridinium Chloride Mouthrinse as Adjunct to Toothbrushing Compared to Placebo on Interproximal Plaque and Gingival Inflammation-a Systematic Review with Meta-Analyses. Clin. Oral Investig. 2021, 25, 745–757. [Google Scholar] [CrossRef]
- Lim, K.S.; Kam, P.C.A. Chlorhexidine-Pharmacology and Clinical Applications. Anaesth. Intensive Care 2008, 36, 502–512. [Google Scholar] [CrossRef]
- Osso, D.; Kanani, N. Antiseptic Mouth Rinses: An Update on Comparative Effectiveness, Risks and Recommendations. J. Dent. Hyg. 2013, 87, 10–18. [Google Scholar]
- Janakiram, C.; Venkitachalam, R.; Fontelo, P.; Iafolla, T.J.; Dye, B.A. Effectiveness of Herbal Oral Care Products in Reducing Dental Plaque & Gingivitis-a Systematic Review and Meta-Analysis. BMC Complement. Med. Ther. 2020, 20, 43. [Google Scholar] [CrossRef]
- Manipal, S.; Hussain, S.; Wadgave, U.; Duraiswamy, P.; Ravi, K. The Mouthwash War-Chlorhexidine vs. Herbal Mouth Rinses: A Meta-Analysis. J. Clin. Diagn. Res. 2016, 10, 81–83. [Google Scholar] [CrossRef]
- Cai, H.; Chen, J.; Panagodage Perera, N.K.; Liang, X. Effects of Herbal Mouthwashes on Plaque and Inflammation Control for Patients with Gingivitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid. Based Complement. Alternat. Med. 2020, 2020, 2829854. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Alhajj, M.N.; Deshisha, E.A.; Alshafei, A.K.; Ahmed, A.I.; Almudayfi, N.O.; Alshammari, S.A.; Alsharif, A.; Kassim, S. Curcumin Mouthwashes Versus Chlorhexidine in Controlling Plaque and Gingivitis: A Systematic Review and Meta-Analysis. Int. J. Dent. Hyg. 2022, 20, 53–61. [Google Scholar] [CrossRef]
- Hwu, Y.J.; Lin, F.Y. Effectiveness of Propolis on Oral Health: A Meta-Analysis. J. Nurs. Res. 2014, 22, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Jassoma, E.; Baeesa, L.; Sabbagh, H. The Antiplaque/Anticariogenic Efficacy of Salvadora persica (Miswak) Mouthrinse in Comparison to That of Chlorhexidine: A Systematic Review and Meta-Analysis. BMC Oral Health 2019, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Mathur, A.; Gopalakrishnan, D.; Mehta, V.; Rizwan, S.A.; Shetiya, S.H.; Bagwe, S. Efficacy of Green Tea-Based Mouthwashes on Dental Plaque and Gingival Inflammation: A Systematic Review and Meta-Analysis. Indian J. Dent. Res. 2018, 29, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Sharifi-Rad, J.; Rayess, Y.E.; Rizk, A.A.; Sadaka, C.; Zgheib, R.; Zam, W.; Sestito, S.; Rapposelli, S.; Neffe-Skocińska, K.; Zielińska, D.; et al. Turmeric and Its Major Compound Curcumin on Health: Bioactive Effects and Safety Profiles for Food, Pharmaceutical, Biotechnological and Medicinal Applications. Front. Pharmacol. 2020, 11, 01021. [Google Scholar] [CrossRef]
- AlJameel, A.H.; Almalki, S.A. Effect of Triphala Mouthrinse on Plaque and Gingival Inflammation: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Int. J. Dent. Hyg. 2020, 18, 344–351. [Google Scholar] [CrossRef]
- Shanbhag, V.K.L. Triphala in Prevention of Dental Caries and as an Antimicrobial in Oral Cavity-a Review. Infect. Disord. Drug Targets 2015, 15, 89–97. [Google Scholar] [CrossRef]
- Mohammadi, Z. Sodium Hypochlorite in Endodontics: An Update Review. Int. Dent. J. 2008, 58, 329–341. [Google Scholar] [CrossRef]
- Paraskevas, S.; Rosema, N.A.M.; Versteeg, P.; Van der Velden, U.; Van der Weijden, G.A. Chlorine Dioxide and Chlorhexidine Mouthrinses Compared in a 3-Day Plaque Accumulation Model. J. Periodontol. 2008, 79, 1395–1400. [Google Scholar] [CrossRef]
- Marinho, V.C.C.; Chong, L.Y.; Worthington, H.V.; Walsh, T. Fluoride Mouthrinses for Preventing Dental Caries in Children and Adolescents. Cochrane Database Syst. Rev. 2016, 7, CD002284. [Google Scholar] [CrossRef]
- O’Mullane, D.M.; Baez, R.J.; Jones, S.; Lennon, M.A.; Petersen, P.E.; Rugg-Gunn, A.J.; Whelton, H.; Whitford, G.M. Fluoride and Oral Health. Commun. Dent. Health 2016, 33, 69–99. [Google Scholar]
- Marinho, V.C.; Higgins, J.P.; Sheiham, A.; Logan, S. Combinations of Topical Fluoride (Toothpastes, Mouthrinses, Gels, Varnishes) Versus Single Topical Fluoride for Preventing Dental Caries in Children and Adolescents. Cochrane Database Syst. Rev. 2004, 1, CD002781. [Google Scholar] [CrossRef] [PubMed]
- Twetman, S.; Petersson, L.; Axelsson, S.; Dahlgren, H.; Holm, A.K.; Källestål, C.; Lagerlöf, F.; Lingström, P.; Mejàre, I.; Nordenram, G.; et al. Caries-Preventive Effect of Sodium Fluoride Mouthrinses: A Systematic Review of Controlled Clinical Trials. Acta Odontol. Scand. 2004, 62, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.; Duckworth, R.M.; Marsh, P.; Mutti, B.; Parnell, C.; Zero, D. Post-Brushing Rinsing for the Control of Dental Caries: Exploration of the Available Evidence to Establish What Advice We Should Give Our Patients. Br. Dent. J. 2012, 212, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Zanatta, R.F.; Caneppele, T.M.F.; Scaramucci, T.; El Dib, R.E.; Maia, L.C.; Ferreira, D.M.T.P.; Borges, A.B. Protective Effect of Fluorides on Erosion and Erosion/Abrasion in Enamel: A Systematic Review and Meta-Analysis of Randomized In Situ Trials. Arch. Oral Biol. 2020, 120, 104945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Sardana, D.; Li, K.Y.; Leung, K.C.M.; Lo, E.C.M. Topical Fluoride to Prevent Root Caries: Systematic Review with Network Meta-Analysis. J. Dent. Res. 2020, 99, 506–513. [Google Scholar] [CrossRef]
- Wierichs, R.J.; Meyer-Lueckel, H. Systematic Review on Non-Invasive Treatment of Root Caries Lesions. J. Dent. Res. 2015, 94, 261–271. [Google Scholar] [CrossRef]
- Schwendicke, F.; Göstemeyer, G. Cost-Effectiveness of Root Caries Preventive Treatments. J. Dent. 2017, 56, 58–64. [Google Scholar] [CrossRef]
- Deus, F.P.; Ouanounou, A. Chlorhexidine in Dentistry: Pharmacology, Uses, and Adverse Effects. Int. Dent. J. 2022, 72, 269–277. [Google Scholar] [CrossRef]
- Overholser, C.D., Jr. Longitudinal Clinical Studies with Antimicrobial Mouthrinses. J. Clin. Periodontol. 1988, 15, 517–519. [Google Scholar] [CrossRef]
- Preus, H.R.; Koldsland, O.C.; Aass, A.M.; Sandvik, L.; Hansen, B.F. The Plaque-and Gingivitis-inhibiting Capacity of a Commercially Available Essential Oil Product. A Parallel, Split-mouth, Single Blind, Randomized, Placebo-controlled Clinical Study. Acta Odontol. Scand. 2013, 71, 1613–1619. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Tadakamadla, S.K.; Connelly, S.T.; Sforza, C.; Martín, C. Adverse Events Associated with Home Use of Mouthrinses: A Systematic Review. Ther. Adv. Drug Saf. 2019, 10, 2042098619854881. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, E.; Mummolo, S.; Mattia, J.D.; Casalena, F.; Martino, S.D.; Mattei, A.; Marzo, G. Efficacy of Essential Oil Mouthwash with and without Alcohol: A 3-day Plaque Accumulation Model. Trials 2011, 12, 262. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, E.; Tecco, S.; Caterini, E.; Casalena, F.; Quinzi, V.; Mattei, A.; Marzo, G. Alcohol-free Essential Oils Containing Mouthrinse Efficacy on Three-day Supragingival Plaque Regrowth: A Randomized Crossover Clinical Trial. Trials 2017, 18, 154. [Google Scholar] [CrossRef] [PubMed]
- Cosyn, J.; Princen, K.; Miremadi, R.; Decat, E.; Vaneechoutte, M.; Bruyn, H.D. A Double-blind Randomized Placebo-controlled Study on the Clinical and Microbial Effects of an Essential Oil Mouth Rinse used by Patients in Supportive Periodontal Care. Int. J. Dent. Hyg. 2013, 11, 53–61. [Google Scholar] [CrossRef]
- Albert-Kiszely, A.; Pjetursson, B.E.; Salvi, G.E.; Witt, J.; Hamilton, A.; Persson, G.R.; Lang, N.P. Comparison of the Effects of Cetylpyridinium Chloride with an Essential Oil Mouth Rinse on Dental Plaque and Gingivitis-a Six-month Randomized Controlled Clinical Trial. J. Clin. Periodontol. 2007, 34, 658–667. [Google Scholar] [CrossRef]
- Tartaglia, G.M.; Manoharan, V.; Fareed, N.; Battur, H.; Khanagar, S.; Praveena, J. Effectiveness of Mouthrinses in Prevention and Treatment of Radiation Induced Mucositis: A Systematic Review. J. Cancer Res. Ther. 2020, 16, S1–S10. [Google Scholar] [CrossRef]
- Kőhidai, Z.; Takács, A.; Lajkó, E.; Géczi, Z.; Pállinger, É.; Láng, O.; Kőhidai, L. The Effects of Mouthwashes in Human Gingiva Epithelial Progenitor (HGEPp) Cells. Clin. Oral Investig. 2022; Online ahead of print. [Google Scholar] [CrossRef]
- Müller, H.D.; Eick, S.; Moritz, A.; Lussi, A.; Gruber, R. Cytotoxicity and Antimicrobial Activity of Oral Rnses In Vitro. BioMed Res. Int. 2017, 2017, 4019723. [Google Scholar] [CrossRef]
- Tsourounakis, I.; Palaiologou-Gallis, A.A.; Stoute, D.; Maney, P.; Lallier, T.E. Effect of Essential Oil and Chlorhexidine Mouthwashes on Gingival Fibroblast Survival and Migration. J. Periodontol. 2013, 84, 1211–1220. [Google Scholar] [CrossRef]
- Xu, C.; Wang, A.; Hoskin, E.R.; Cugini, C.; Markowitz, K.; Chang, T.L.; Fine, D.H. Differential Effects of Antiseptic Mouth Rinses on SARS-CoV-2 Infectivity In Vitro. Pathogens 2021, 10, 272. [Google Scholar] [CrossRef]
- Brookes, Z.L.S.; Bescos, R.; Belfield, L.A.; Ali, K.; Roberts, A. Current Uses of Chlorhexidine for Management of Oral Disease: A Narrative Review. J. Dent. 2020, 103, 103497. [Google Scholar] [CrossRef]
- Cieplik, F.; Jakubovics, N.S.; Buchalla, W.; Maisch, T.; Hellwig, E.; Al-Ahmad, A. Resistance Toward Chlorhexidine in Oral Bacteria- Is There Cause for Concern? Front. Microbiol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.G.; Seers, C.A.; Sabri, A.N.; Reynolds, E.C. Dental Plaque Bacteria with Reduced Susceptibility to Chlorhexidine Are Multidrug Resistant. BMC Microbiol. 2016, 16, 214. [Google Scholar] [CrossRef] [PubMed]
- Muehler, D.; Mao, X.; Czemmel, S.; Geiβert, J.; Engesser, C.; Hiller, K.A.; Widbiller, M.; Maisch, T.; Buchalla, W.; Al-Ahmad, A.; et al. Transcriptomic Stress Response in Streptococcus mutans following Treatment with a Sublethal Concentration of Chlorhexidine Digluconate. Microorganisms 2022, 10, 561. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, S.; Trivedi, H.M.; Corbin, A.; Pitts, B.; Stewart, P.S. Direct Visualization of Spatial and Temporal Patterns of Antimicrobial Action Within Model Oral Biofilms. Appl. Environ. Microbiol. 2008, 74, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Corbin, A.; Pitts, B.; Parker, A.; Stewart, P.S. Antimicrobial Penetration and Efficacy in an In Vitro Oral Biofilm Model. Antimicrob. Agents Chemother. 2011, 55, 3338–3344. [Google Scholar] [CrossRef] [PubMed]
- Wakamatsu, R.; Takenaka, S.; Ohsumi, T.; Terao, Y.; Ohshima, H.; Okiji, T. Penetration Kinetics of Four Mouthrinses into Streptococcus mutans Biofilms Analyzed by Direct Time-Lapse Visualization. Clin. Oral Investig. 2014, 18, 625–634. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Noiri, Y.; Kuboniwa, M.; Yamamoto, R.; Asahi, Y.; Maezono, H.; Hayashi, M.; Ebisu, S. Porphyromonas gingivalis Biofilms Persist After Chlorhexidine Treatment. Eur. J. Oral Sci. 2013, 121, 162–168. [Google Scholar] [CrossRef]
- Davison, W.M.; Pitts, B.; Stewart, P.S. Spatial and Temporal Patterns of Biocide Action Against Staphylococcus epidermidis Biofilms. Antimicrob. Agents Chemother. 2010, 54, 2920–2927. [Google Scholar] [CrossRef]
- Song, L.; Hou, J.; van der Mei, H.C.; Veeregowda, D.H.; Busscher, H.J.; Sjollema, J. Antimicrobials Influence Bond Stiffness and Detachment of Oral Bacteria. J. Dent. Res. 2016, 95, 793–799. [Google Scholar] [CrossRef]
- Ohsumi, T.; Takenaka, S.; Wakamatsu, R.; Sakaue, Y.; Narisawa, N.; Senpuku, H.; Ohshima, H.; Terao, Y.; Okiji, T. Residual Structure of Streptococcus mutans Biofilm Following Complete Disinfection Favors Secondary Bacterial Adhesion and Biofilm Redevelopment. PLoS ONE 2015, 10, e0116647. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J. The Biofilm Matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gaekwad, J.; Wolfert, M.A.; Boons, G.J. Modulation of Innate Immune Responses with Synthetic Lipid A Derivatives. J. Am. Chem. Soc. 2007, 129, 5200–5216. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Yip, H.K. Supragingival Calculus: Formation and Control. Crit. Rev. Oral Biol. Med. 2002, 13, 426–441. [Google Scholar] [CrossRef] [PubMed]
- Fux, C.A.; Costerton, J.W.; Stewart, P.S.; Stoodley, P. Survival Strategies of Infectious Biofilms. Trends Microbiol. 2005, 13, 34–40. [Google Scholar] [CrossRef]
- Kaplan, J.B.; Izano, E.A.; Gopal, P.; Karwacki, M.T.; Kim, S.; Bose, J.L.; Bayles, K.W.; Horswill, A.R. Low Levels of β-lactam Antibiotics Induce Extracellular DNA Release and Biofilm Formation in Staphylococcus aureus. MBio 2012, 3, e00198-112. [Google Scholar] [CrossRef]
- Suzuki, Y.; Ohsumi, T.; Isono, T.; Nagata, R.; Hasegawa, T.; Takenaka, S.; Terao, Y.; Noiri, Y. Effects of a Sub-Minimum Inhibitory Concentration of Chlorhexidine Gluconate on the Development of In Vitro Multi-Species Biofilms. Biofouling 2020, 36, 146–158. [Google Scholar] [CrossRef]
- Dong, L.; Tong, Z.; Linghu, D.; Lin, Y.; Tao, R.; Liu, J.; Tian, Y.; Ni, L. Effect of Sub-minimum Inhibitory Concemtrations of Antimicrobial Agents on Streptococcus mutans Biofilm Formation. Int. J. Antimicrob. Agents. 2012, 39, 390–395. [Google Scholar] [CrossRef]
- Bedran, T.B.; Grignon, L.; Spolidorio, D.P.; Grenier, D. Subinhibitory Concentrations of Triclosan Promote Streptococcus mutans Biofilm Formation and Adherence to Oral Epithelial Cells. PLoS ONE 2014, 9, e89059. [Google Scholar] [CrossRef]
- Tong, Z.; Huang, L.; Ling, J.; Mao, X.; Ning, Y.; Deng, D. Effects of Intracanal Irrigant MTAD Combined with Nisin at Sub-minimum Inhibitory Concentration Levels on Enterococcus faecalis Growth and the Expression of Pathogenic Genes. PLoS ONE 2014, 9, e90235. [Google Scholar] [CrossRef]
- Mostajo, M.F.Y.; Exterkate, R.A.M.; Buijs, M.J.; Crielaard, W.; Zaura, E. Effect of Mouthwashes on the Composition and Metabolic Activity of Oral Biofilms Grown In Vitro. Clin. Oral Investig. 2017, 21, 1221–1230. [Google Scholar] [CrossRef]
- Chatzigiannidou, I.; Teughels, W.; Van de Wiele, T.; Boon, N. Oral Biofilms Exposure to Chlorhexidine Results in Altered Microbial Composition and Metabolic Profile. NPJ biofilms Microbiomes 2020, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Minah, G.E.; DePaola, L.G.; Overholser, C.D.; Meiller, T.F.; Niehaus, C.; Lamm, R.A.; Ross, N.M.; Dills, S.S. Effects of 6 Months Use of an Antiseptic Mouthrinse on Supragingival Dental Plaque Microflora. J. Clin. Periodontol. 1989, 16, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Filoche, S.K.; Soma, D.; van Bekkum, M.; Sissons, C.H. Plaques from Different Individuals Yield Different Microbiota Responses to Oral-antiseptic Treatment. FEMS Immunol. Med. Microbiol. 2008, 54, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Bescos, R.; Ashworth, A.; Cutler, C.; Brookes, Z.L.; Belfield, L.; Rodiles, A.; Casas-Agustench, P.; Farnham, G.; Liddle, L.; Burleigh, M.; et al. Effects of Chlorhexidine Mouthwash on the Oral Microbiome. Sci. Rep. 2020, 10, 5254. [Google Scholar] [CrossRef] [PubMed]
| Sequence No. | Terms and Strategy (Publication Dates from 2012 to 2022) | Hits |
|---|---|---|
| #1 | (“mouthwashes” [Pharmacological Action] OR “mouthwashes” [MeSH Terms] OR “mouthwashes” [All Fields] OR “mouthwash” [All Fields] OR “mouthwashing” [All Fields] OR “mouthwashings” [All Fields]) AND (“systematic review” [Publication Type] OR “systematic reviews as topic” [MeSH Terms] OR “systematic review” [All Fields]) | 300 |
| #2 | (“mouthwashes” [Pharmacological Action] OR “mouthwashes” [MeSH Terms] OR “mouthwashes” [All Fields] OR “mouthwash” [All Fields] OR “mouthwashing” [All Fields] OR “mouthwashings” [All Fields]) AND (“meta analysis” [Publication Type] OR “meta analysis as topic” [MeSH Terms] OR “meta analysis” [All Fields]) | 246 |
| #3 | (“mouthrinse” [All Fields] OR “mouthrinsed” [All Fields] OR “mouthrinses” [All Fields] OR “mouthrinsing” [All Fields] OR “mouthrinsings” [All Fields]) AND (“systematic review” [Publication Type] OR “systematic reviews as topic” [MeSH Terms] OR “systematic review” [All Fields]) | 57 |
| #4 | (“mouthrinse” [All Fields] OR “mouthrinsed” [All Fields] OR “mouthrinses” [All Fields] OR “mouthrinsing” [All Fields] OR “mouthrinsings” [All Fields]) AND (“meta analysis” [Publication Type] OR “meta analysis as topic” [MeSH Terms] OR “meta analysis” [All Fields]) | 40 |
| Solutions | n | Weighted Mean Difference (95% Cl) | Index | Compared Control | Follow-up Periods | Reference |
|---|---|---|---|---|---|---|
| CHG | 12 | −1.45 (−1.00 to −1.90) | QHI | Baseline | 4 to 6 weeks | [46] |
| 4 | −0.78 (−1.07 to −0.49) | TQHI | Placebo | 6 months | [47] | |
| 3 | −0.640 (−0.756 to −0.524) | TQHI | Placebo | 6 months | [16] | |
| 2 | −0.208 (−0.351 to −0.065) | PI | Placebo | 6 months | [16] | |
| 17 | −0.362 (−0.571 to −0.153) † | QHI or TQHI | Baseline | ≥ 4 weeks | [48] | |
| 5 | −0.39 (−0.70 to −0.08) | PI | Baseline | 6w or 6m | [49] | |
| 10 | −0.67 (−0.82 to −0.52) | QHI | Baseline | 4w to 6m | [49] | |
| EO | 9 | −0.86 (−1.05 to −0.68) | TQHI | Placebo | 6 months | [47] |
| 9 | −0.827 (−1.053 to −0.600) | TQHI | Placebo | 6 months | [16] | |
| 16 | −0.265 (−0.405 to −0.124) † | QHI or TQHI | Baseline | ≥ 4 weeks | [48] | |
| 14 | −0.86 (−1.05 to −0.66) | QHI | Placebo | 6 months | [51] | |
| 4 | −0.39 (−0.3 to −0.47) | QHI | 21.6 or 26.9% hydro-alcohol | 6 months | [52] | |
| CPC (>0.05%) | 6 | −0.41 (−0.65 to −0.17) | TQHI | Placebo | 6 months | [47] |
| 3 | −0.465 (−0.631 to −0.299) | TQHI | Placebo | 6 months | [16] | |
| 8 | −0.112 (−0.273 to 0.029) *,† | TQHI | Baseline | ≥ 4 weeks | [48] | |
| 9 | −0.70 (−0.83 to −0.57) | PI, TQHI, MPI | Placebo | ≥ 6 weeks | [54] | |
| CPC (<0.05%) | 3 | −0.26 (0.07 to −0.55) * | TQHI | Placebo | 6 months | [47] |
| Del | 2 | −0.24 (−0.67 to 0.19) * | TQHI | Placebo | 6 months | [47] |
| 3 | −0.144 (−0.231 to −0.058) | TQHI | Placebo | 6 months | [16] | |
| 4 | −0.173 (−0.853 to 0.507) *,† | TQHI | Baseline | 4 weeks | [48] | |
| AmF/SnF | 2 | −0.079 (−0.260 to 0.101) * | TQHI | Placebo | 6 months | [31] |
| 2 | −0.195 (−0.335 to −0.054) | PI | Placebo | 6 months | [31] | |
| Alexi | 2 | −0.18 (−0.60 to 0.24) * | TQHI | Placebo | 6 months | [47] |
| Tric | 3 | −0.67 (−1.05 to −0.30) | TQHI | Placebo | 6 months | [47] |
| Herb | 6 | −2.93 (−6.43 to 0.58) * | PI or TQHI | CHG | 4 weeks | [57] |
| 6 | 2.61 (4.42 to 0.9) | PI or TQHI | CHG or Placebo | 12 weeks | [57] | |
| 11 | 0.22 (0.20 to 0.24) | PI or QHI or TQHI | CHG | [58] | ||
| 5 | −0.61 (−0.80 to −0.42) | QHI | Placebo | 2w to 3 M | [59] | |
| 5 | 0.08 (−0.19 to 0.34) * | PI | CHG | 10d to 3w | [59] | |
| 5 | 0.00 (−0.04 to 0.04) * | TQHI | CHG | 2 or 4w | [59] | |
| Curcumin | 6 | 0.27 (−0.53 to 1.07) * | PI or TQHI | CHG | 21 or 28 days | [60] |
| Propolis | 3 | −1.24 (−2.51 to 0.04) * | PI | Placebo | 3 or 5 days | [61] |
| Salvadora persica | 12 | −0.46 (−0.29 to −0.63) | PI or TQHI | Placebo | 4d to 2m | [62] |
| Salvadora persica | 18 | 0.19 (0.01 to 0.37) * | PI or TQHI | CHG | 4d to 2m | [62] |
| Green Tea | 5 | −0.14(−1.80 to 1.43) * | PI | CHG | 2 to 4w | [63] |
| ClO2 | 5 | −0.720 (−0.487 to −0.952) | PI | Placebo | 7 days to 5w | [33] |
| Solutions | n | Weighted Mean Difference (95% Cl) | Index | Compared Control | Follow-up Periods | Reference |
|---|---|---|---|---|---|---|
| CHG | 3 | −1.20 (−0.23 to −2.16) | GI or MGI | Placebo | 6 months | [50] |
| 10 | −0.21 (−0.11 to −0.31) | GI | Baseline | 4 to 6 weeks | [46] | |
| 4 | −0.185 (−0.285 to −0.086) | GI | Placebo | 6 months | [16] | |
| 19 | −0.223 (−0.412 to −0.034) † | GI or MGI | Baseline | ≥ 4 weeks | [48] | |
| 8 | −0.32 (−0.42 to −0.23) | GI | Baseline | 6w, 3m, 6m | [49] | |
| EO | 9 | −1.44 (−0.82 to −2.06) | GI or MGI | Placebo | 6 months | [50] |
| 2 | −0.133 (−0.194 to −0.072) | GI | Placebo | 6 months | [16] | |
| 8 | −0.537 (−0.764 to −0.311) | MGI | Placebo | 6 months | [16] | |
| 16 | −0.203 (−0.312 to −0.093) † | GI or MGI | Baseline | ≥ 4 weeks | [48] | |
| 2 | −0.36 (−0.26 to −0.62) | GI | 21.6 or 26.9% hydro-alcohol | 6 months | [52] | |
| 11 | −0.52 (−0.67 to −0.37) | MGI | Placebo | 6 months | [51] | |
| 3 | −0.24 (−0.46 to −0.01) | GI | Placebo | 6 months | [51] | |
| CPC (>0.05%) | 2 | −1.04 (0.06 to −2.14) * | GI or MGI | Placebo | 6 months | [50] |
| 5 | −0.70 (−0.83 to −0.57) | GI | Placebo | ≥ 6 weeks | [54] | |
| 3 | −0.344 (−0.627 to −0.062) | GI | Placebo | 6 months | [16] | |
| 2 | −0.357 (−0.483 to −0.231) | MGI | Placebo | 6 months | [16] | |
| 8 | −0.126 (−0.312 to 0.059) *,† | GI or MGI | Baseline | ≥ 4 weeks | [48] | |
| CPC (≤0.05%) | 3 | −0.31 (0.76 to −1.38) * | GI or MGI | Placebo | 6 months | [50] |
| Del | 1 | −0.06 (1.86 to −1.98) * | GI or MGI | Placebo | 6 months | [50] |
| 2 | −0.038 (−0.145 to −0.069) * | MGI | Placebo | 6 months | [16] | |
| 3 | −0.014 (−2.337 to 2.308) †,* | GI or MGI | Baseline | ≥ 4 weeks | [48] | |
| AmF/SnF AmF/SnF | 1 | −0.76 (0.72 to −2.25) * | GI or MGI | Placebo | 6 months | [50] |
| 2 | −0.248 (−0.427 to −0.069) | GI | Placebo | 6 months | [16] | |
| Alexi | 1 | −0.16 (1.77 to −2.09) * | GI or MGI | Placebo | 6 months | [50] |
| Tric | 3 | −1.50 (−0.36 to −2.62) | GI or MGI | Placebo | 6 months | [50] |
| Herb | 6 | −0.15 (−0.32 to 0.01) * | GI | CHG | 4 weeks | [57] |
| 5 | −0.09 (−0.25 to 0.08) * | GI | CHG | 12 weeks | [57] | |
| 3 | −0.28 (−0.51 to −0.06) | GI | Placebo | 3 w or 3 m | [59] | |
| 3 | −0.59 (−1.08 to −0.11) | MGI | Placebo | 2 to 4 weeks | [59] | |
| 5 | −0.07 (−0.22 to 0.07) * | GI | CHG | 2 to 4 w | [59] | |
| Curcumin | 6 | −0.13 (−0.35 to 0.09) * | GI | CHG | 21 or 28 days | [60] |
| Green Tea | 5 | 0.43(−0.63 to 1.49) * | GI | CHG | 2 to 4 w | [63] |
| ClO2 | 4 | −0.712 (−0.457 to −0.967) | GI | Placebo | 7 to 21 days | [33] |
| Biocide | Concentration | Species | Condition of Bacteria | Incubation Time | Upregulated Genes | Reference |
|---|---|---|---|---|---|---|
| NaF, CHG, Tea polyphenol | 1/2 MIC | S. mutans | Planktonic | 24 h | gtfB, gtfC, luxS, comD, comE | [108] |
| NaF, CHG, Tea polyphenol | 1/2 MIC | S. mutans | Biofilm | 24 h | gtfB, gtfC, luxS, comD, comE | [108] |
| Triclosan | 1/2 and 1/4 MIC | S. mutans | Planktonic | 2 h | atlA, gtfB, gtfC, comD, luxS | [109] |
| MTAD, MTADN, MTAN | 1/4 MIC | Porphyromonas gingivalis | Planktonic | 1 h | clpC, clpP (MTAD, MTADN, MTAN), sprE (MTAD, MTADN), ace, clpX, cylB, efaA, gelE (MTAN) | [110] |
| CHG | 1/10 MIC | S. mutans, Streptococcus oralis, Actinomyces naeslundii | Biofilm | 48 h | gtfB, gtfC, gtfD, comD, luxS | [107] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takenaka, S.; Sotozono, M.; Ohkura, N.; Noiri, Y. Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects. Antibiotics 2022, 11, 727. https://doi.org/10.3390/antibiotics11060727
Takenaka S, Sotozono M, Ohkura N, Noiri Y. Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects. Antibiotics. 2022; 11(6):727. https://doi.org/10.3390/antibiotics11060727
Chicago/Turabian StyleTakenaka, Shoji, Maki Sotozono, Naoto Ohkura, and Yuichiro Noiri. 2022. "Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects" Antibiotics 11, no. 6: 727. https://doi.org/10.3390/antibiotics11060727
APA StyleTakenaka, S., Sotozono, M., Ohkura, N., & Noiri, Y. (2022). Evidence on the Use of Mouthwash for the Control of Supragingival Biofilm and Its Potential Adverse Effects. Antibiotics, 11(6), 727. https://doi.org/10.3390/antibiotics11060727






