mcr-1-Mediated Colistin Resistance and Genomic Characterization of Antimicrobial Resistance in ESBL-Producing Salmonella Infantis Strains from a Broiler Meat Production Chain in Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection and Isolate Identification
2.2. Colistin Susceptibility Testing
2.3. Multiplex PCR Analysis for mcr Genes
2.4. Whole-Genome Sequencing
3. Results
3.1. Colistin Susceptibility Test
3.2. Molecular Characterization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Benedict, R.G.; Langlykke, A.F. Antibiotic Activity of Bacillus Polymyxa. J. Bacteriol. 1947, 54, 24. [Google Scholar]
- Poirel, L.; Jayol, A.; Nordmann, P. Polymyxins: Antibacterial Activity, Susceptibility Testing, and Resistance Mechanisms Encoded by Plasmids or Chromosomes. Clin. Microbiol. Rev. 2017, 30, 557–596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempf, I.; Jouy, E.; Chauvin, C. Colistin Use and Colistin Resistance in Bacteria from Animals. Int. J. Antimicrob. Agents 2016, 48, 598–606. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, N.; Aires-de-Sousa, M.; Nordmann, P.; Poirel, L. High Rate of MCR-1-Producing Escherichia coli and Klebsiella pneumoniae among Pigs, Portugal. Emerg. Infect. Dis. 2017, 23, 2023–2029. [Google Scholar] [CrossRef] [Green Version]
- Rhouma, M.; Beaudry, F.; Thériault, W.; Letellier, A. Colistin in Pig Production: Chemistry, Mechanism of Antibacterial Action, Microbial Resistance Emergence, and One Health Perspectives. Front. Microbiol. 2016, 7, 1789. [Google Scholar] [CrossRef]
- Kumar, H.; Chen, B.-H.; Kuca, K.; Nepovimova, E.; Kaushal, A.; Nagraik, R.; Bhatia, S.K.; Dhanjal, D.S.; Kumar, V.; Kumar, A.; et al. Understanding of Colistin Usage in Food Animals and Available Detection Techniques: A Review. Animals 2020, 10, 1892. [Google Scholar] [CrossRef]
- Catry, B.; Cavaleri, M.; Baptiste, K.; Grave, K.; Grein, K.; Holm, A.; Jukes, H.; Liebana, E.; Lopez Navas, A.; Mackay, D.; et al. Use of Colistin-Containing Products within the European Union and European Economic Area (EU/EEA): Development of Resistance in Animals and Possible Impact on Human and Animal Health. Int. J. Antimicrob. Agents 2015, 46, 297–306. [Google Scholar] [CrossRef]
- World Health Organization. GLASS: The Detection and Reporting of Colistin Resistance; World Health Organization: Geneva, Switzerland, 2021; ISBN 978-92-4-001904-1. [Google Scholar]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union Summary Report on Trends and Sources of Zoonoses, Zoonotic Agents and Food-Borne Outbreaks in 2017. EFSA J. 2018, 16, e05500. [CrossRef]
- Aviv, G.; Tsyba, K.; Steck, N.; Salmon-Divon, M.; Cornelius, A.; Rahav, G.; Grassl, G.A.; Gal-Mor, O. A Unique Megaplasmid Contributes to Stress Tolerance and Pathogenicity of an Emergent Salmonella Enterica Serovar Infantis Strain. Environ. Microbiol. 2014, 16, 977–994. [Google Scholar] [CrossRef]
- Franco, A.; Leekitcharoenphon, P.; Feltrin, F.; Alba, P.; Cordaro, G.; Iurescia, M.; Tolli, R.; D’Incau, M.; Staffolani, M.; Di Giannatale, E.; et al. Emergence of a Clonal Lineage of Multidrug-Resistant ESBL-Producing Salmonella Infantis Transmitted from Broilers and Broiler Meat to Humans in Italy between 2011 and 2014. PLoS ONE 2015, 10, e0144802. [Google Scholar] [CrossRef] [Green Version]
- Szmolka, A.; Szabó, M.; Kiss, J.; Pászti, J.; Adrián, E.; Olasz, F.; Nagy, B. Molecular Epidemiology of the Endemic Multiresistance Plasmid PSI54/04 of Salmonella Infantis in Broiler and Human Population in Hungary. Food Microbiol. 2018, 71, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Alba, P.; Leekitcharoenphon, P.; Carfora, V.; Amoruso, R.; Cordaro, G.; Di Matteo, P.; Ianzano, A.; Iurescia, M.; Diaconu, E.L.; Study Group, E.-E.-A.N.; et al. Molecular Epidemiology of Salmonella Infantis in Europe: Insights into the Success of the Bacterial Host and Its Parasitic PESI-like Megaplasmid. Microb. Genom. 2020, 6. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of Plasmid-Mediated Colistin Resistance Mechanism MCR-1 in Animals and Human Beings in China: A Microbiological and Molecular Biological Study. Lancet Infect. Dis. 2016, 16, 161–168. [Google Scholar] [CrossRef]
- Sun, J.; Fang, L.-X.; Wu, Z.; Deng, H.; Yang, R.-S.; Li, X.-P.; Li, S.-M.; Liao, X.-P.; Feng, Y.; Liu, Y.-H. Genetic Analysis of the IncX4 Plasmids: Implications for a Unique Pattern in the Mcr-1 Acquisition. Sci. Rep. 2017, 7, 424. [Google Scholar] [CrossRef]
- Carattoli, A. Plasmid-Mediated Antimicrobial Resistance in Salmonella Enterica. Curr. Issues Mol. Biol. 2003, 5, 113–122. [Google Scholar]
- Yang, Q.-E.; Sun, J.; Li, L.; Deng, H.; Liu, B.-T.; Fang, L.-X.; Liao, X.-P.; Liu, Y.-H. IncF Plasmid Diversity in Multi-Drug Resistant Escherichia coli Strains from Animals in China. Front. Microbiol. 2015, 6, 964. [Google Scholar] [CrossRef]
- Casagrande Proietti, P.; Stefanetti, V.; Musa, L.; Zicavo, A.; Dionisi, A.M.; Bellucci, S.; Mensa, A.L.; Menchetti, L.; Branciari, R.; Ortenzi, R.; et al. Genetic Profiles and Antimicrobial Resistance Patterns of Salmonella Infantis Strains Isolated in Italy in the Food Chain of Broiler Meat Production. Antibiotics 2020, 9, 814. [Google Scholar] [CrossRef]
- ISO 6579-1:2017/AMD 1:2020 ISO 6579-1:2017/Amd 1:2020. Available online: https://www.iso.org/cms/render/live/en/sites/isoorg/contents/data/standard/07/66/76671.html (accessed on 19 March 2022).
- Grimont, P.A.D.; Weill, F.-X. Antigenic Formulae of the Salmonella Serovars; WHO: Geneva, Switzerland, 2007. [Google Scholar]
- EUCAST. European Committee on Antimicrobial Susceptibility Testing. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_10.0_Breakpoint (accessed on 19 March 2022).
- Rebelo, A.R.; Bortolaia, V.; Kjeldgaard, J.S.; Pedersen, S.K.; Leekitcharoenphon, P.; Hansen, I.M.; Guerra, B.; Malorny, B.; Borowiak, M.; Hammerl, J.A.; et al. Multiplex PCR for Detection of Plasmid-Mediated Colistin Resistance Determinants, Mcr-1, Mcr-2, Mcr-3, Mcr-4 and Mcr-5 for Surveillance Purposes. Eurosurveillance 2018, 23, 17-00672. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [Green Version]
- Gurevich, A.; Saveliev, V.; Vyahhi, N.; Tesler, G. QUAST: Quality assessment tool for genome assemblies. Bioinformatics 2013, 29, 1072–1075. [Google Scholar] [CrossRef]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, Y.; Xiao, Y. Prevalence and Transmission of Mobilized Colistin Resistance (Mcr) Gene in Bacteria Common to Animals and Humans. Biosaf. Health 2020, 2, 71–78. [Google Scholar] [CrossRef]
- Carfora, V.; Alba, P.; Leekitcharoenphon, P.; Ballarò, D.; Cordaro, G.; Di Matteo, P.; Donati, V.; Ianzano, A.; Iurescia, M.; Stravino, F.; et al. Corrigendum: Colistin Resistance Mediated by Mcr-1 in ESBL-Producing, Multidrug Resistant Salmonella Infantis in Broiler Chicken Industry, Italy (2016–2017). Front. Microbiol. 2018, 9, 2395. [Google Scholar] [CrossRef] [Green Version]
- Vázquez, X.; García, V.; Fernández, J.; Bances, M.; de Toro, M.; Ladero, V.; Rodicio, R.; Rodicio, M.R. Colistin Resistance in Monophasic Isolates of Salmonella Enterica ST34 Collected from Meat-Derived Products in Spain, with or without CMY-2 Co-Production. Front. Microbiol. 2021, 12, 735364. [Google Scholar] [CrossRef]
- Maron, D.F.; Smith, T.J.S.; Nachman, K.E. Restrictions on Antimicrobial Use in Food Animal Production: An International Regulatory and Economic Survey. Glob. Health 2013, 9, 48. [Google Scholar] [CrossRef] [Green Version]
- Moulin, G.; Catry, B.; Baptiste, K.; Cavaco, L.; Jukes, H.; Kluytmans, J. Updated Advice on the Use of Colistin Products in Animals within the European Union: Development of Resistance and Possible Impact on Human and Animal Health. Eur. Med. Agency 2016, 44, 56. [Google Scholar]
- Walsh, T.R.; Wu, Y. China Bans Colistin as a Feed Additive for Animals. Lancet Infect. Dis. 2016, 16, 1102–1103. [Google Scholar] [CrossRef]
- Uddin, M.B.; Hossain, S.M.B.; Hasan, M.; Alam, M.N.; Debnath, M.; Begum, R.; Roy, S.; Harun-Al-Rashid, A.; Chowdhury, M.S.R.; Rahman, M.M.; et al. Multidrug Antimicrobial Resistance and Molecular Detection of Mcr-1 Gene in Salmonella Species Isolated from Chicken. Animals 2021, 11, 206. [Google Scholar] [CrossRef] [PubMed]
- Agersø, Y.; Torpdahl, M.; Zachariasen, C.; Seyfarth, A.; Hammerum, A.M.; Nielsen, E.M. Tentative Colistin Epidemiological Cut-off Value for Salmonella spp. Foodborne Pathog. Dis. 2012, 9, 367–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simoni, S.; Morroni, G.; Brenciani, A.; Vincenzi, C.; Cirioni, O.; Castelletti, S.; Varaldo, P.E.; Giovanetti, E.; Mingoia, M. Spread of Colistin Resistance Gene Mcr-1 in Italy: Characterization of the Mcr-1.2 Allelic Variant in a Colistin-Resistant Blood Isolate of Escherichia Coli. Diagn. Microbiol. Infect. Dis. 2018, 91, 66–68. [Google Scholar] [CrossRef]
- Di Pilato, V.; Arena, F.; Tascini, C.; Cannatelli, A.; Henrici De Angelis, L.; Fortunato, S.; Giani, T.; Menichetti, F.; Rossolini, G.M. Mcr-1.2, a New Mcr Variant Carried on a Transferable Plasmid from a Colistin-Resistant KPC Carbapenemase-Producing Klebsiella Pneumoniae Strain of Sequence Type 512. Antimicrob. Agents Chemother. 2016, 60, 5612–5615. [Google Scholar] [CrossRef] [Green Version]
- Alba, P.; Leekitcharoenphon, P.; Franco, A.; Feltrin, F.; Ianzano, A.; Caprioli, A.; Stravino, F.; Hendriksen, R.S.; Bortolaia, V.; Battisti, A. Molecular Epidemiology of Mcr-Encoded Colistin Resistance in Enterobacteriaceae from Food-Producing Animals in Italy Revealed through the EU Harmonized Antimicrobial Resistance Monitoring. Front. Microbiol. 2018, 9, 1217. [Google Scholar] [CrossRef]
- Viñes, J.; Cuscó, A.; Napp, S.; Alvarez, J.; Saez-Llorente, J.L.; Rosàs-Rodoreda, M.; Francino, O.; Migura-Garcia, L. Transmission of Similar Mcr-1 Carrying Plasmids among Different Escherichia coli Lineages Isolated from Livestock and the Farmer. Antibiotics 2021, 10, 313. [Google Scholar] [CrossRef]
- EFSA Panel on Biological Hazards (EFSA BIOHAZ Panel); Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Salmonella Control in Poultry Flocks and Its Public Health Impact. EFSA J. 2019, 17, e05596. [Google Scholar] [CrossRef]
- Hopkins, K.L.; Davies, R.H.; Threlfall, E.J. Mechanisms of Quinolone Resistance in Escherichia coli and Salmonella: Recent Developments. Int. J. Antimicrob. Agents 2005, 25, 358–373. [Google Scholar] [CrossRef]
- Eaves, D.J.; Randall, L.; Gray, D.T.; Buckley, A.; Woodward, M.J.; White, A.P.; Piddock, L.J.V. Prevalence of Mutations within the Quinolone Resistance-Determining Region of GyrA, GyrB, ParC, and ParE and Association with Antibiotic Resistance in Quinolone-Resistant Salmonella Enterica. Antimicrob. Agents Chemother. 2004, 48, 4012–4015. [Google Scholar] [CrossRef] [Green Version]
- Kawai, R.; Yada, S.; Yoshimura, T. Characterization and Solution Properties of Quaternary-Ammonium-Salt-Type Amphiphilic Gemini Ionic Liquids. ACS Omega 2019, 4, 14242–14250. [Google Scholar] [CrossRef]
- Jaglic, Z.; Cervinkova, D. Genetic Basis of Resistance to Quaternary Ammonium Compounds—The Qac Genes and Their Role: A Review. Vet. Med. 2012, 57, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Russell, A.D. Do Biocides Select for Antibiotic Resistance? J. Pharm. Pharmacol. 2000, 52, 227–233. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, Y.; Li, Y.; Zhang, K.; Liu, L.; Wang, H.; Tian, J.; Ying, H.; Shi, L.; Yu, T. Characterization and Horizontal Transfer of QacH-Associated Class 1 Integrons in Escherichia coli Isolated from Retail Meats. Int. J. Food Microbiol. 2017, 258, 12–17. [Google Scholar] [CrossRef]
- Aconiti Mandolini, N.; Perugini, G.; Filippini, G.; Pierucci, P.; Baiguini, A.; Vaccaro, A.; Pelagalli, G.; Marinelli, F.; Capuccella, M. Evaluation of Colistin Consumption in Swine and Poultry of Marche Region during the 2017–2019 Period. Eur. J. Public Health 2020, 30, ckaa166.1305. [Google Scholar] [CrossRef]
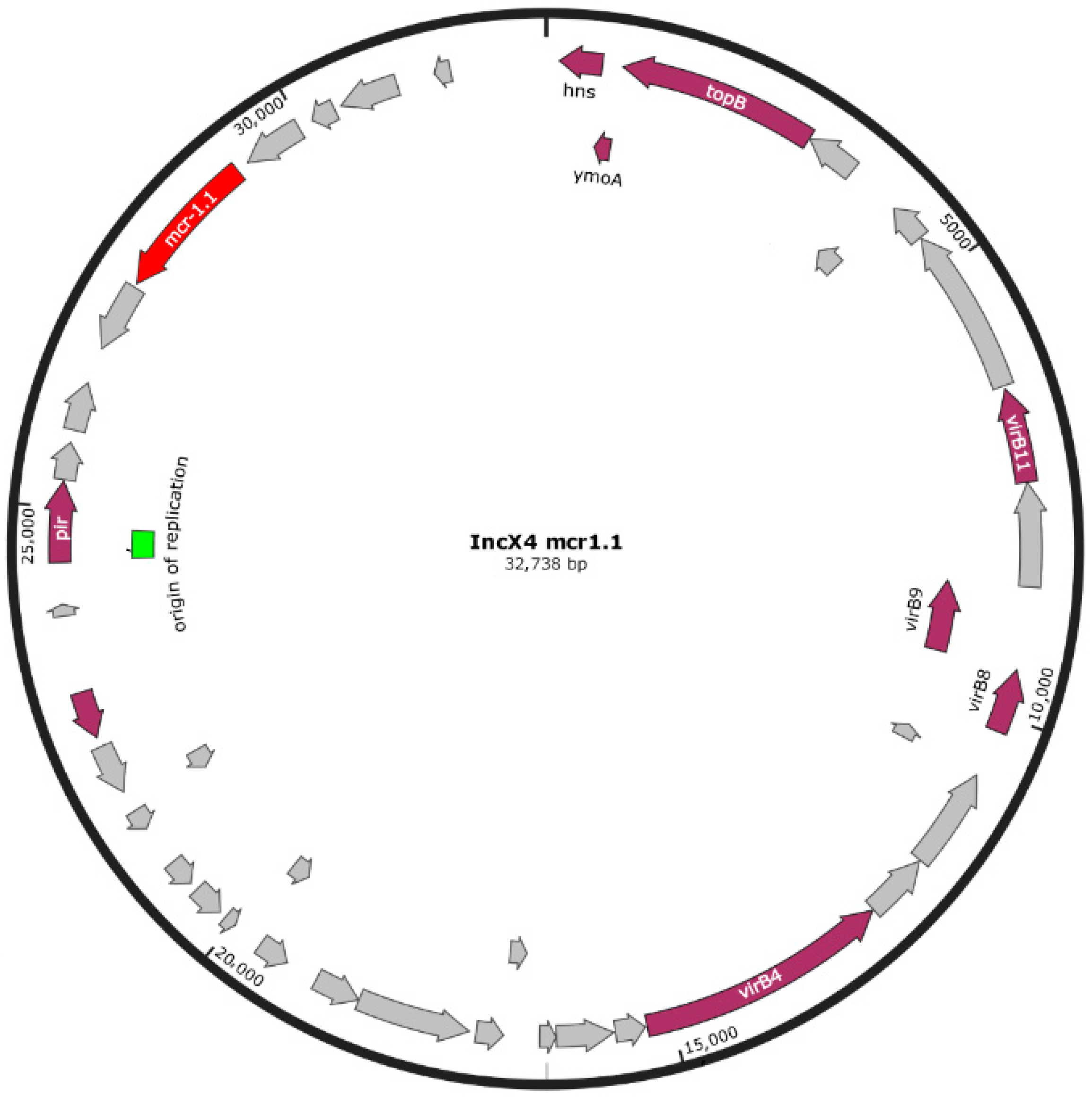
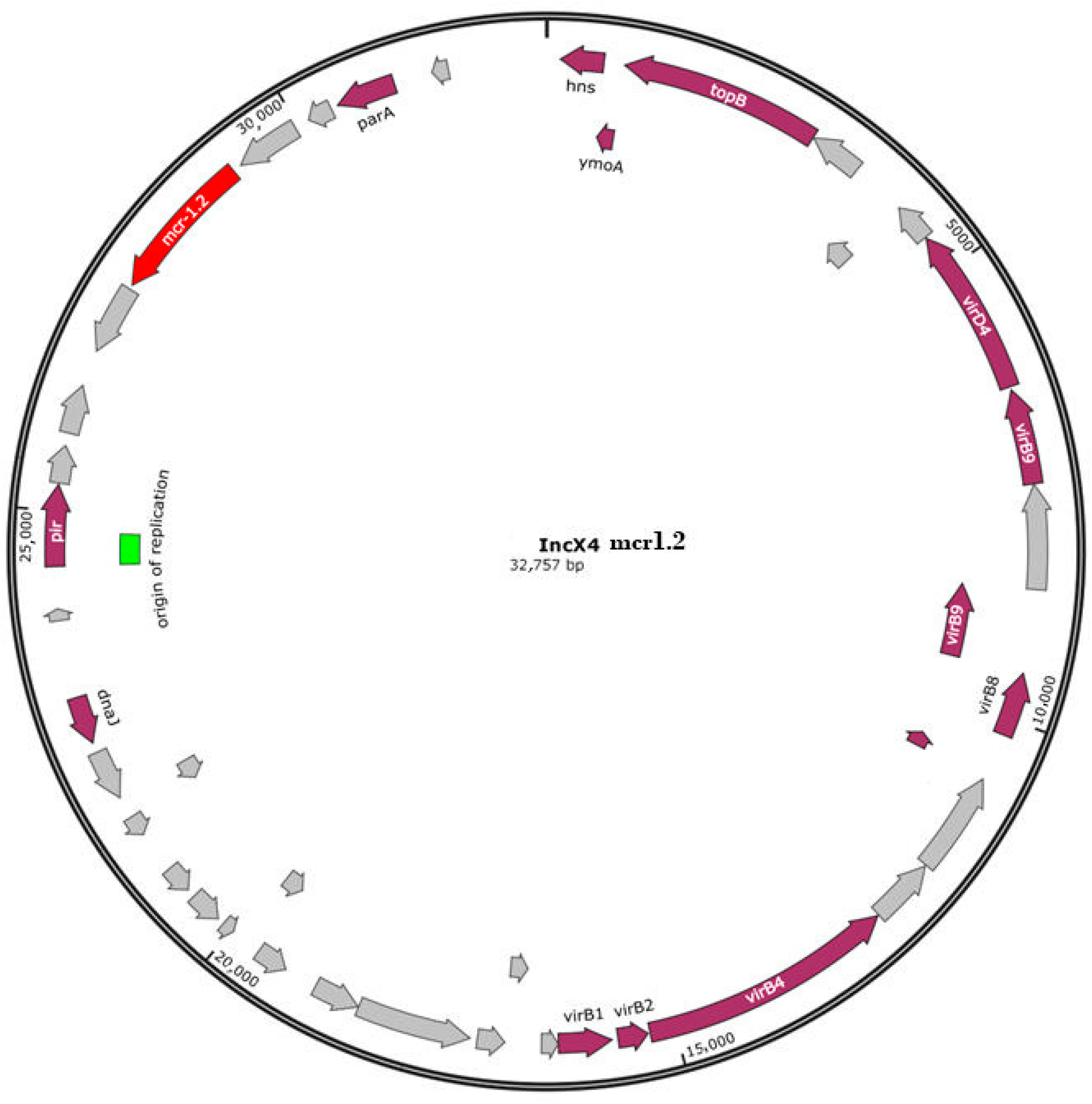
| Isolate | Origin | Source | Year | Topology | AMR Genotype | Phenotypic Profile [18] | PFGE [18] |
|---|---|---|---|---|---|---|---|
| S1 | Northern Italy | Neck Skin | 2019 | Chromosomic | parC (T57S), blaCTX-M-1, aac(6′)-Iaa, gyrA(D87G) | A, Ams, Cl, Ctx, Gm, Na, Sxt, Te | NA |
| Plasmidic pESI-like | sul1, qacE, dfrA1, tet(A) | ||||||
| S2 | Northern Italy | Neck Skin | 2016 | Chromosomic | parC (T57S), blaCTX-M-1, aac(6′)-Iaa, gyrA(D87G) | Ams, Cl, Col, Ctx, Caz Na, Sxt, Te | D |
| Plasmidic pESI-like | sul1, qacE, dfrA1, tet(A) | ||||||
| Pasmidic IncX4 | mcr-1.1 | ||||||
| Plasmidic IncFII | |||||||
| S3 | Northern Italy | Drumstick | 2017 | Chromosomic | parC (T57S), blaCTX-M-1, aac(6′)-Iaa, aph(3′)-Ia, gyrA(D87G) | A, Cl, Col, Ctx, Na, Sxt, Te | XbaI 0126 |
| Plasmidic pESI-like | sul1, qacE, dfrA1, drfA14, tet(A) | ||||||
| Plasmidic IncX4 | mcr-1.2 | ||||||
| S4 | Northern Italy | Neck Skin | 2017 | Chromosomic | parC (T57S), blaCTX-M-1, aac(6′)-Iaa, gyrA(D87G) | A, Ams, Cl, Ctx, Gm, Na, Te | NA |
| Plasmidic pESI-like | sul1, qacE, dfrA1, tet(A) | ||||||
| S5 | Northern Italy | Drumstick | 2017 | Chromosomic | parC (T57S), blaCTX-M-1, aac(6′)-Iaa, aph(3′)-Ia gyrA(D87G) | A, Cl, Col, Ctx, Na, Sxt, Te | XbaI 0126 |
| Plasmidic pESI-like | sul1, qacE, dfrA1, drfA14, tet(A) | ||||||
| Plasmidic IncX4 | mcr-1.2 | ||||||
| S6 | Southern Italy | 2016 | Chromosomic | parC (T57S), blaCTX-M-1, aac(6′)-Iaa, gyrA(D87G) | A, Ctx, Cz, Na, Sxt, Te | XbaI 0126 | |
| Plasmidic pESI-like | sul1, qacE, dfrA1drfA14, tet(A) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casagrande Proietti, P.; Musa, L.; Stefanetti, V.; Orsini, M.; Toppi, V.; Branciari, R.; Blasi, F.; Magistrali, C.F.; Capomaccio, S.; Kika, T.S.; et al. mcr-1-Mediated Colistin Resistance and Genomic Characterization of Antimicrobial Resistance in ESBL-Producing Salmonella Infantis Strains from a Broiler Meat Production Chain in Italy. Antibiotics 2022, 11, 728. https://doi.org/10.3390/antibiotics11060728
Casagrande Proietti P, Musa L, Stefanetti V, Orsini M, Toppi V, Branciari R, Blasi F, Magistrali CF, Capomaccio S, Kika TS, et al. mcr-1-Mediated Colistin Resistance and Genomic Characterization of Antimicrobial Resistance in ESBL-Producing Salmonella Infantis Strains from a Broiler Meat Production Chain in Italy. Antibiotics. 2022; 11(6):728. https://doi.org/10.3390/antibiotics11060728
Chicago/Turabian StyleCasagrande Proietti, Patrizia, Laura Musa, Valentina Stefanetti, Massimiliano Orsini, Valeria Toppi, Raffaella Branciari, Francesca Blasi, Chiara Francesca Magistrali, Stefano Capomaccio, Tana Shtylla Kika, and et al. 2022. "mcr-1-Mediated Colistin Resistance and Genomic Characterization of Antimicrobial Resistance in ESBL-Producing Salmonella Infantis Strains from a Broiler Meat Production Chain in Italy" Antibiotics 11, no. 6: 728. https://doi.org/10.3390/antibiotics11060728
APA StyleCasagrande Proietti, P., Musa, L., Stefanetti, V., Orsini, M., Toppi, V., Branciari, R., Blasi, F., Magistrali, C. F., Capomaccio, S., Kika, T. S., & Franciosini, M. P. (2022). mcr-1-Mediated Colistin Resistance and Genomic Characterization of Antimicrobial Resistance in ESBL-Producing Salmonella Infantis Strains from a Broiler Meat Production Chain in Italy. Antibiotics, 11(6), 728. https://doi.org/10.3390/antibiotics11060728









