Diagnosis and Treatment of Invasive Candidiasis
Abstract
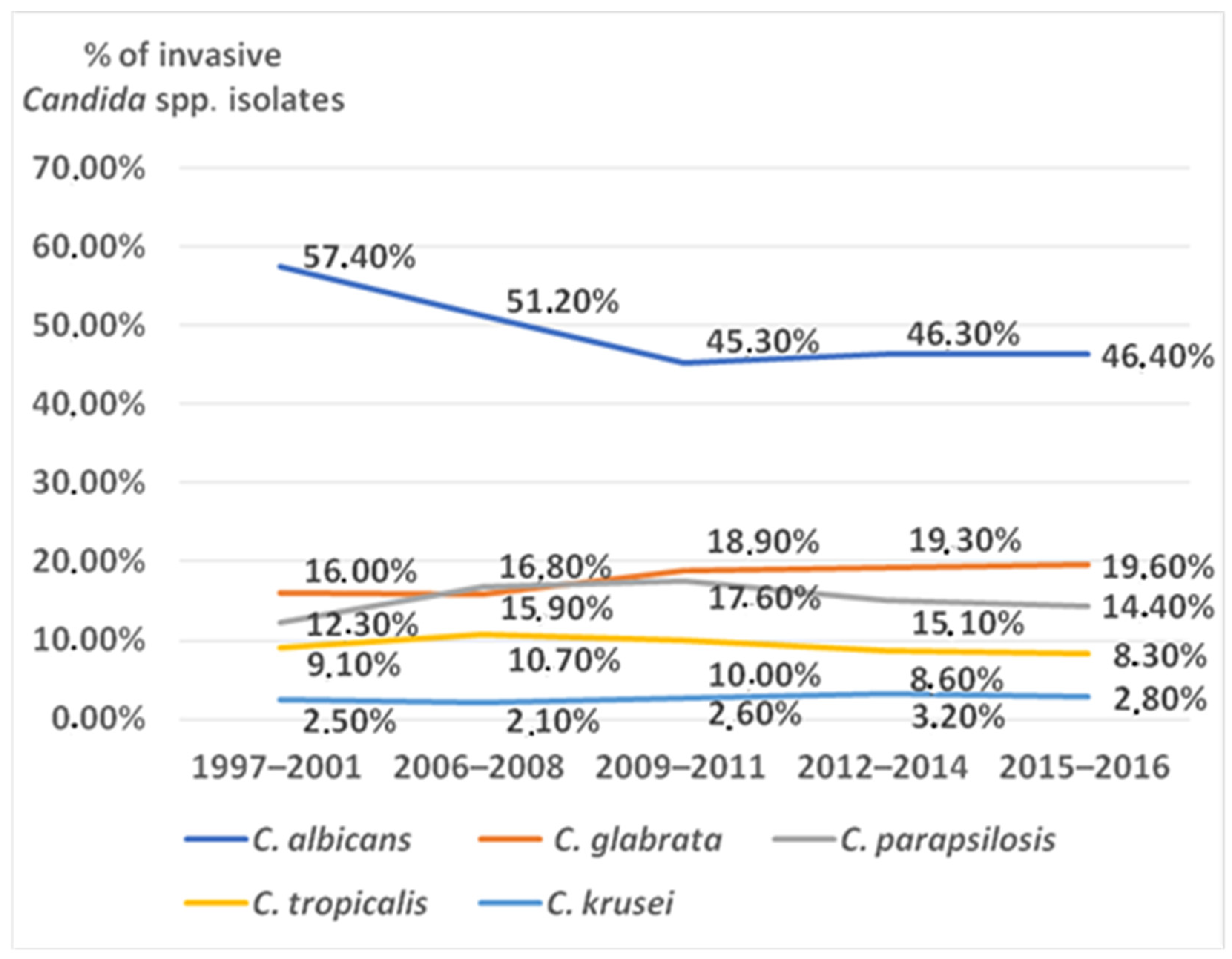
1. Introduction
2. Diagnosis of Invasive Candidiasis
2.1. Clinical Manifestations of Invasive Candidiasis
2.2. “Gold Standard” Methods for the Diagnosis of Invasive Candidiasis
2.3. Identification of Candida Species
2.4. Non-Cultural Laboratory Techniques
2.5. Antifungal Susceptibility Testing
3. Treatment of Invasive Candidiasis
3.1. Principles of Therapy
3.2. Antifungal Preparations
3.3. Choice of Antifungal Preparations and Duration of Treatment
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Romo, J.A.; Kumamoto, C.A. On commensalism of Candida. J. Fungi 2020, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Kumamoto, C.A.; Gresnigt, M.S.; Hube, B. The gut, the bad and the harmless: Candida albicans as a commensal and opportunistic pathogen in the intestine. Curr. Opin. Microbiol. 2020, 56, 7–15. [Google Scholar] [CrossRef]
- Pérez, J.C. Fungi of the human gut microbiota: Roles and significance. Int. J. Med. Microbiol. 2021, 311, 151490. [Google Scholar] [CrossRef] [PubMed]
- Bacali, C.; Vulturar, R.; Buduru, S.; Cozma, A.; Fodor, A.; Chis, A.; Lucaciu, O.; Damian, L.; Moldovan, M.L. Oral microbiome: Metting to now and befriend neighbours, a biological approach. Biomedicines 2022, 10, 671. [Google Scholar] [CrossRef] [PubMed]
- Hameed, S.; Hans, S.; Monasky, R.; Thangamani, S.; Fatima, Z. Understanding human microbiota offers novel and promising therapeutic options against Candida infections. Pathogens 2021, 10, 183. [Google Scholar] [CrossRef] [PubMed]
- Boxberger, M.; Cenizo, V.; Cassir, N.; La Scola, B. Challenges in exploring and manipulating the human skin microbiome. Microbiome 2021, 9, 125. [Google Scholar] [CrossRef]
- Tortelli, B.A.; Lewis, W.G.; Allsworth, J.E.; Member-Meneh, N.; Foster, L.R.; Reno, H.E.; Peipert, J.F.; Fay, J.C.; Lewis, A.L. Associations between the vaginal microbiome and Candida colonization in women of reproductive age. Am. J. Obstet. Gynecol. 2020, 222, 471.e1–471.e9. [Google Scholar] [CrossRef]
- Chee, W.J.Y.; Chew, S.Y.; Than, L.T.L. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb. Cell Fact. 2020, 19, 203. [Google Scholar] [CrossRef]
- Kalia, N.; Singh, J.; Kaur, M. Microbiota in vaginal health and pathogenesis of recurrent vulvovaginal infections: A critical review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 5. [Google Scholar] [CrossRef]
- Pellon, A.; Sadeghi Nasab, S.D.; Moyes, D.L. New insights in Candida albicans innate immunity at the mucosa: Toxins, epithelium, metabolism, and beyond. Front. Cell. Infect. Microbiol. 2020, 10, 81. [Google Scholar] [CrossRef]
- Lanternier, F.; Cypowyj, S.; Picard, C.; Bustamante, J.; Lortholary, O.; Casanova, J.L.; Puel, A. Primary immunodeficiencies underlying fungal infections. Curr. Opin. Pediatrics 2013, 25, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Abd Elaziz, D.; Abd El-Ghany, M.; Meshaal, S.; El Hawary, R.; Lotfy, S.; Galal, N.; Ouf, S.A.; Elmarsafy, A. Fungal infections in primary immunodeficiency diseases. Clin. Immunol. 2020, 219, 108553. [Google Scholar] [CrossRef] [PubMed]
- Festekjian, A.; Neely, M. Incidence and predictors of invasive candidiasis associated with candidaemia in children. Mycoses 2011, 54, 146–153. [Google Scholar] [CrossRef]
- St-Germain, G.; Laverdière, M.; Pelletier, R.; Bourgault, A.M.; Libman, M.; Lemieux, C.; Noël, G. Prevalence and antifungal susceptibility of 442 Candida isolates from blood and other normally sterile sites: Results of a 2-year (1996 to 1998) multicenter surveillance study in Quebec, Canada. J. Clin. Microbiol. 2001, 39, 949–953. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pappas, P.G.; Alexander, B.D.; Andes, D.R.; Hadley, S.; Kauffman, C.A.; Freifeld, A.; Anaissie, E.J.; Brumble, L.M.; Herwaldt, L.; Ito, J.; et al. Invasive fungal infections among organ transplant recipients: Results of the Transplant-Associated Infection Surveillance Network (TRANSNET). Clin. Infect. Dis. 2010, 50, 1101–1111. [Google Scholar] [CrossRef]
- Spampinato, C.; Leonardi, D. Candida infections, causes, targets, and resistance mechanisms: Traditional and alternative antifungal agents. Biomed Res. Int. 2013, 2013, 204237. [Google Scholar] [CrossRef]
- Ghanem-Zoubi, N.; Khoury, J.; Arnon, M.; Zorbavel, D.; Geffen, Y.; Paul, M. Risk factors for non-albicans candidemia focusing on prior antifungal and immunosuppressive therapy. Isr. Med. Assoc. J. 2019, 21, 303–307. [Google Scholar]
- Yapar, N. Epidemiology and risk factors for invasive candidiasis. Ther. Clin. Risk Manag. 2014, 10, 95–105. [Google Scholar] [CrossRef]
- Thomas-Rüddel, D.O.; Schlattmann, P.; Pletz, M.; Kurzai, O.; Bloos, F. Risk factors for invasive Candida infection in critically ill patients: A systematic review and meta-analysis. Chest 2022, 161, 345–355. [Google Scholar] [CrossRef]
- Pyrgos, V.; Ratanavanich, K.; Donegan, N.; Veis, J.; Walsh, T.J.; Shoham, S. Candida bloodstream infections in hemodialysis recipients. Med. Mycol. 2009, 47, 463–467. [Google Scholar] [CrossRef][Green Version]
- Girmenia, C.; Finolezzi, E.; Federico, V.; Santopietro, M.; Perrone, S. Invasive Candida infections in patients with haematological malignancies and hematopoietic stem cell transplant recipients: Current epidemiology and therapeutic options. Mediterr. J. Hematol. Infect. Dis. 2011, 3, e2011013. [Google Scholar] [CrossRef][Green Version]
- Lin, Y.-L.; Chen, I.-C.; Yen, J.-H.; Lai, C.-S.; Tsai, Y.-C.; Lu, C.-T.; Wu, C.-Y.; Lin, W.-S.; Lin, C.-H.; Huang, Y.-C. Invasive Candidiasis in Hospitalized Patients with Major Burns. J. Pers. Med. 2022, 12, 47. [Google Scholar] [CrossRef] [PubMed]
- McCarty, T.P.; White, C.M.; Pappas, P.G. Candidemia and Invasive Candidiasis. Infect Dis. Clin. 2021, 35, 389–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, R.; Luan, Z.; Ma, X. Risk of invasive candidiasis with prolonged duration of ICU stay: A systematic review and meta-analysis. BMJ Open 2020, 10, e036452. [Google Scholar] [CrossRef] [PubMed]
- Delaloye, J.; Calandra, T. Invasive candidiasis as a cause of sepsis in the critically ill patient. Virulence 2014, 5, 161–169. [Google Scholar] [CrossRef]
- Martin-Loeches, I.; Antonelli, M.; Cuenca-Estrella, M.; Dimopoulos, G.; Einav, S.; De Waele, J.J.; Garnacho-Montero, J.; Kanj, S.S.; Machado, F.R.; Montravers, P.; et al. ESICM/ESCMID task force on practical management of invasive candidiasis in critically ill patients. Intensive Care Med. 2019, 45, 789–805. [Google Scholar] [CrossRef]
- Pappas, P.; Lionakis, M.; Arendrup, M.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat. Rev. Dis. Prim. 2018, 4, 18026. [Google Scholar] [CrossRef]
- Turner, S.A.; Butler, G. The Candida pathogenic species complex. Cold Spring Harb. Perspect. Med. 2014, 4, a019778. [Google Scholar] [CrossRef]
- Papon, N.; Courdavault, V.; Clastre, M.; Bennett, R.J. Emerging and emerged pathogenic Candida species: Beyond the Candida albicans paradigm. PLoS Pathog. 2013, 9, e1003550. [Google Scholar] [CrossRef]
- Singh, D.K.; Tóth, R.; Gácser, A. Mechanisms of pathogenic Candida species to evade the host complement attack. Front. Cell. Infect. Microbiol. 2020, 10, 94. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, Q.; Zhu, F.; An, Y. Epidemiology, species distribution, antifungal susceptibility and mortality risk factors of candidemia among critically ill patients: A retrospective study from 2011 to 2017 in a teaching hospital in China. Antimicrob. Resist. Infect. Control 2019, 8, 89. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G. Antifungal clinical trials and guidelines: What we know and do not know. Cold Spring Harb. Perspect. Med. 2014, 4, a019745. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef] [PubMed]
- Rossato, L.; Colombo, A.L. Candida auris: What have we learned about its mechanisms of pathogenicity? Front. Microbiol. 2018, 9, 3081. [Google Scholar] [CrossRef]
- Du, H.; Bing, J.; Hu, T.; Ennis, C.L.; Nobile, C.J.; Huang, G. Candida auris: Epidemiology, biology, antifungal resistance, and virulence. PLoS Pathog. 2020, 16, e1008921. [Google Scholar] [CrossRef]
- Murphy, S.E.; Bicanic, T. Drug resistance and novel therapeutic approaches in invasive candidiasis. Front. Cell. Infect. Microbiol. 2021, 11, 759408. [Google Scholar] [CrossRef]
- Keighley, C.; Garnham, K.; Harch, S.A.J.; Robertson, M.; Chaw, K.; Teng, J.C.; Chen, S.C.-A. Candida auris: Diagnostic challenges and emerging opportunities for the clinical microbiology laboratory. Curr. Fungal Infect. Rep. 2021, 15, 116–126. [Google Scholar] [CrossRef]
- Lone, S.A.; Ahmad, A. Candida auris—The growing menace to global health. Mycoses 2019, 62, 620–637. [Google Scholar] [CrossRef]
- Garcia-Bustos, V.; Cabanero -Navalon, M.D.; Ruiz-Saurí, A.; Ruiz-Gaitán, A.C.; Salavert, M.; Tormo, M.Á.; Pemán, J. What do we know about Candida auris? State of the art, knowledge gaps, and future directions. Microorganisms 2021, 9, 2177. [Google Scholar] [CrossRef]
- Chatzimoschou, A.; Giampani, A.; Meis, J.F.; Roilides, E. Activities of nine antifungal agents against Candida auris biofilms. Mycoses 2021, 64, 381–384. [Google Scholar] [CrossRef]
- Forsberg, K.; Woodworth, K.; Walters, M.; Berkow, E.L.; Jackson, B.; Chiller, T.; Vallabhaneni, S. Candida auris: The recent emergence of a multidrug-resistant fungal pathogen. Med. Mycol. 2019, 57, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, S.R.; Etienne, K.A.; Vallabhaneni, S.; Farooqi, J.; Chowdhary, A.; Govender, N.P.; Colombo, A.L.; Calvo, B.; Cuomo, C.; Desjardins, C.A.; et al. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin. Infect. Dis. 2017, 64, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Horton, M.V.; Nett, J.E. Candida auris infection and biofilm formation: Going beyond the surface. Curr. Clin. Microbiol. Rep. 2020, 7, 51–56. [Google Scholar] [CrossRef]
- Lara, H.H.; Ixtepan-Turrent, L.; Jose Yacaman, M.; Lopez-Ribot, J. Inhibition of Candida auris biofilm formation on medical and environmental surfaces by silver nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 21183–21191. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.S.; Gazara, R.K.; Passarelli-Araujo, H.; Valengo, A.E.; Pontes, P.V.M.; Nunes-da-Fonseca, R.; de Souza, R.F.; Venancio, T.M.; Dalla-Costa, L.M. First genome sequences of two multidrug-resistant Candida haemulonii var. vulnera isolates from pediatric patients with candidemia. Front. Microbiol. 2020, 11, 1535. [Google Scholar] [CrossRef]
- Belkin, A.; Gazit, Z.; Keller, N.; Ben-Ami, R.; Wieder-Finesod, A.; Novikov, A.; Rahav, G.; Brosh-Nissimov, T. Candida auris infection leading to nosocomial transmission, Israel, 2017. Emerg. Infect. Dis. 2018, 24, 801–804. [Google Scholar] [CrossRef]
- Cuervo, G.; Garcia-Vidal, C.; Puig-Asensio, M.; Merino, P.; Vena, A.; Martín-Peña, A.; Montejo, J.M.; Ruiz, A.; Lázaro-Perona, F.; Fortún, J.; et al. Usefulness of guideline recommendations for prognosis in patients with candidemia. Med. Mycol. 2019, 57, 659–667. [Google Scholar] [CrossRef]
- Liu, F.; Zhong, L.; Zhou, F.; Zheng, C.; Zhang, K.; Cai, J.; Zhou, H.; Tang, K.; Dong, Z.; Cui, W.; et al. Clinical features, strain distribution, antifungal resistance and prognosis of patients with non-albicans candidemia: A retrospective observational study. Infect. Drug Resist. 2021, 14, 3233–3246. [Google Scholar] [CrossRef]
- Díez, A.; Carrano, G.; Bregón-Villahoz, M.; Cuétara, M.S.; García-Ruiz, J.C.; Fernandez-de-Larrinoa, I.; Moragues, M.D. Biomarkers for the diagnosis of invasive candidiasis in immunocompetent and immunocompromised patients. Diagn. Microbiol. Infect. Dis. 2021, 101, 115509. [Google Scholar] [CrossRef]
- Ahmad, S.; Khan, Z. Invasive candidiasis: A review of nonculture-based laboratory diagnostic methods. Indian J. Med. Microbiol. 2012, 30, 264–269. [Google Scholar] [CrossRef]
- Monday, L.M.; Parraga Acosta, T.; Alangaden, G. T2Candida for the diagnosis and management of invasive Candida infections. J. Fungi 2021, 7, 178. [Google Scholar] [CrossRef] [PubMed]
- Sakai, D.; Matsumiya, W.; Kusuhara, S.; Nakamura, M. Factors associated with the development of ocular candidiasis and ocular prognosis with echinocandin therapy for candidemia. J. Ophthal. Inflamm. Infect. 2021, 11, 17. [Google Scholar] [CrossRef] [PubMed]
- Brooks, R.G. Prospective study of Candida endophthalmitis in hospitalized patients with candidemia. Arch. Intern. Med. 1989, 149, 2226–2228. [Google Scholar] [CrossRef] [PubMed]
- Arshad, H.; Garcia, S.; Khaja, M. Case report of invasive, disseminated candidiasis with peripheral nodular cavitary lesions in the lung. Respir. Med. Case Rep. 2016, 20, 34–37. [Google Scholar] [CrossRef][Green Version]
- Chen, C.Y.; Cheng, A.; Tien, F.M.; Lee, P.C.; Tien, H.F.; Sheng, W.H.; Chen, Y.C. Chronic disseminated candidiasis manifesting as hepatosplenic abscesses among patients with hematological malignancies. BMC Infect. Dis. 2019, 19, 635. [Google Scholar] [CrossRef]
- Boussen, I.; Lisan, Q.; Raffoux, E.; Di Blasi, R.; Boissel, N.; Oksenhendler, E.; Adès, L.; Xhaard, A.; Bretagne, S.; Alanio, A.; et al. Hepatosplenic candidiasis in patients with hematological malignancies: A 13-year retrospective cohort study. Open Forum Infect. Dis. 2022, 9, 88. [Google Scholar] [CrossRef]
- Mikulska, M.; Calandra, T.; Sanguinetti, M.; Poulain, D.; Viscoli, C.; Third European Conference on Infections in Leukemia Group. The use of mannan antigen and anti-mannan antibodies in the diagnosis of invasive candidiasis: Recommendations from the Third European Conference on Infections in Leukemia. Crit. Care 2010, 14, R222. [Google Scholar] [CrossRef]
- Xiao, X.F.; Wu, J.X.; Xu, Y.C. Treatment of invasive fungal disease: A case report. World J. Clin. Cases 2019, 7, 2374–2383. [Google Scholar] [CrossRef]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef]
- Roberto, A.; Xavier, D.E.; Vidal, E.E.; Vidal, C.; Neves, R.P.; Lima-Neto, R.G. Rapid detection of echinocandins resistance by MALDI-TOF MS in Candida parapsilosis complex. Microorganisms 2020, 8, 109. [Google Scholar] [CrossRef]
- De Pauw, B.; Walsh, T.J.; Donnelly, J.P.; Stevens, D.A.; Edwards, J.E.; Calandra, T.; Pappas, P.G.; Maertens, J.; Lortholary, O.; Kauffman, C.A. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 2008, 46, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Azoulay, E.; Kullberg, B.J.; Ruhnke, M.; Shoham, S.; Vazquez, J.; Giacobbe, D.R.; Calandra, T. EORTC/MSGERC Definitions of invasive fungal diseases: Summary of activities of the Intensive Care Unit Working Group. Clin. Infect. Dis. 2021, 72, S121–S127. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L.; Shoham, S.; Vazquez, J.; Reboli, A.; Betts, R.; Barron, M.A.; Schuster, M.; Judson, M.A.; Revankar, S.G.; Caeiro, J.P.; et al. MSG-01: A randomized, double-blind, placebo-controlled trial of caspofungin prophylaxis followed by preemptive therapy for invasive candidiasis in high-risk adults in the critical care setting. Clin. Infect. Dis. 2014, 58, 1219–1226. [Google Scholar] [CrossRef]
- Nieto, M.; Robles, J.C.; Causse, M.; Gutiérrez, L.; Cruz Perez, M.; Ferrer, R.; Xercavins, M.; Herrero, E.; Sirvent, E.; Fernández, C.; et al. Polymerase chain reaction versus blood culture to detect Candida species in high-risk patients with suspected invasive candidiasis: The MICAFEM Study. Infect. Dis. Ther. 2019, 8, 429–444. [Google Scholar] [CrossRef] [PubMed]
- Pitarch, A.; Nombela, C.; Gil, C. Diagnosis of invasive candidiasis: From gold standard methods to promising leading-edge technologies. Curr. Top. Med. Chem. 2018, 18, 1375–1392. [Google Scholar] [CrossRef] [PubMed]
- Clancy, C.J.; Nguyen, M.H. Non-Culture Diagnostics for Invasive Candidiasis: Promise and Unintended Consequences. J. Fungi 2018, 4, 27. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Diagnosing Invasive Candidiasis. J. Clin. Microbiol. 2018, 56, e01909-17. [Google Scholar] [CrossRef]
- Clancy, C.J.; Nguyen, M.H. Finding the “missing 50%” of invasive candidiasis: How nonculture diagnostics will improve understanding of disease spectrum and transform patient care. Clin. Infect. Dis. 2013, 56, 1284–1292. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Bille, J.; Dannaoui, E.; Ruhnke, M.; Heussel, C.P.; Kibbler, C. ECIL-3 classical diagnostic procedures for the diagnosis of invasive fungal diseases in patients with leukaemia. Bone Marrow Transplant. 2012, 47, 1030–1045. [Google Scholar] [CrossRef]
- Avni, T.; Leibovici, L.; Paul, M. PCR diagnosis of invasive candidiasis: Systematic review and meta-analysis. J. Clin. Microbiol. 2011, 49, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Taira, C.L.; Okay, T.S.; Delgado, A.F.; Ceccon, M.E.; de Almeida, M.T.; Del Negro, G.M. A multiplex nested PCR for the detection and identification of Candida species in blood samples of critically ill paediatric patients. BMC Infect. Dis. 2014, 14, 406. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.L.; Davies, H.D.; Barton, M.; O’Brien, K.; Simpson, K.; Asztalos, E.; Synnes, A.; Rubin, E.; Le Saux, N.; Hui, C.; et al. Characteristics and outcome of infants with candiduria in neonatal intensive care—A Paediatric Investigators Collaborative Network on Infections in Canada (PICNIC) study. BMC Infect. Dis. 2009, 9, 183. [Google Scholar] [CrossRef] [PubMed]
- Katragkou, A.; Fisher, B.T.; Groll, A.H.; Roilides, E.; Walsh, T.J. Diagnostic imaging and invasive fungal diseases in children. J. Pediatric Infect. Dis. Soc. 2017, 6, S22–S31. [Google Scholar] [CrossRef]
- Adhya, A.K. Grocott methenamine silver positivity in neutrophils. J. Cytol. 2019, 36, 184. [Google Scholar] [CrossRef]
- Shalin, S.C.; Ferringer, T.; Cassarino, D.S. PAS and GMS utility in dermatopathology: Review of the current medical literature. J. Cutan. Pathol. 2020, 47, 1096–1102. [Google Scholar] [CrossRef]
- Song, G.; Liang, G.; Liu, W. Fungal co-infections associated with global COVID-19 pandemic: A clinical and diagnostic perspective from China. Mycopathologia 2020, 185, 599–606. [Google Scholar] [CrossRef]
- Wright, A.M.; Mody, D.R.; Anton, R.C.; Schwartz, M.R. Aberrant staining with Grocott’s methenamine silver: Utility beyond fungal organisms. J. Am. Soc. Cytopathol. 2017, 6, 223–227. [Google Scholar] [CrossRef]
- Karasuno, T.; Sata, H.; Noda, Y.; Imakita, M.; Yasumi, M. Invasive candidiasis leading to gastric perforation in an immunocompromised patient. IDCases 2019, 18, e00627. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Sae-Tia, S.; Fries, B.C. Candidiasis and mechanisms of antifungal resistance. Antibiotics 2020, 9, 312. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J.; Turnidge, J.D.; Castanheira, M.; Jones, R.N. Twenty years of the SENTRY Antifungal Surveillance Program: Results for Candida species from 1997–2016. Open Forum Infect. Dis. 2019, 6, S79–S94. [Google Scholar] [CrossRef] [PubMed]
- Montes, K.; Ortiz, B.; Galindo, C.; Figueroa, I.; Braham, S.; Fontecha, G. Identification of Candida species from clinical samples in a honduran tertiary hospital. Pathogens 2019, 8, 237. [Google Scholar] [CrossRef] [PubMed]
- Eraso, E.; Moragues, M.D.; Villar-Vidal, M.; Sahand, I.H.; González-Gómez, N.; Pontón, J.; Quindós, G. Evaluation of the new chromogenic medium Candida ID 2 for isolation and identification of Candida albicans and other medically important Candida species. J. Clin. Microbiol. 2006, 44, 3340–3345. [Google Scholar] [CrossRef] [PubMed]
- Ghelardi, E.; Pichierri, G.; Castagna, B.; Barnini, S.; Tavanti, A.; Campa, M. Efficacy of Chromogenic Candida Agar for isolation and presumptive identification of pathogenic yeast species. Clin. Microbiol. Infect. 2008, 14, 141–147. [Google Scholar] [CrossRef]
- Ozcan, K.; Ilkit, M.; Ates, A.; Turac-Bicer, A.; Demirhindi, H. Performance of chromogenic Candida agar and CHROMagar Candida in recovery and presumptive identification of monofungal and polyfungal vaginal isolates. Med. Mycol. 2010, 48, 29–34. [Google Scholar] [CrossRef]
- Mulet Bayona, J.V.; Salvador García, C.; Tormo Palop, N.; Valentín Martín, A.; González Padrón, C.; Colomina Rodríguez, J.; Pemán, J.; Gimeno Cardona, C. Novel chromogenic medium CHROMagarTM Candida Plus for detection of Candida auris and other Candida species from surveillance and environmental samples: A multicenter study. J. Fungi. 2022, 8, 281. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Shin, J.H.; Mok, J.H.; Kim, S.Y.; Song, S.A.; Kim, H.R.; Kook, J.K.; Chang, Y.H.; Bae, I.K.; Lee, K. Misidentification of Candida guilliermondii as C. famata among strains isolated from blood cultures by the VITEK 2 system. BioMed Res. Int. 2014, 2014, 250408. [Google Scholar] [CrossRef]
- Huang, Y.S.; Wang, F.D.; Chen, Y.C.; Huang, Y.T.; Hsieh, M.H.; Hii, I.M.; Lee, Y.L.; Ho, M.W.; Liu, C.E.; Chen, Y.H.; et al. High rates of misidentification of uncommon Candida species causing bloodstream infections using conventional phenotypic methods. J. Formos. Med. Assoc. 2021, 120, 1179–1187. [Google Scholar] [CrossRef]
- Cheng, J.W.; Yu, S.Y.; Xiao, M.; Wang, H.; Kudinha, T.; Kong, F.; Xu, Y.C. Identification and antifungal susceptibility profile of Candida guilliermondii and Candida fermentati from a multicenter study in China. J. Clin. Microbiol. 2016, 54, 2187–2189. [Google Scholar] [CrossRef]
- Arastehfar, A.; Daneshnia, F.; Kord, M.; Roudbary, M.; Zarrinfar, H.; Fang, W.; Hashemi, S.J.; Najafzadeh, M.J.; Khodavaisy, S.; Pan, W.; et al. Comparison of 21-Plex PCR and API 20C AUX, MALDI-TOF MS, and rDNA Sequencing for a wide range of clinically isolated yeast species: Improved identification by combining 21-Plex PCR and API 20C AUX as an alternative strategy for developing countries. Front. Cell. Infect. Microbiol. 2019, 9, 21. [Google Scholar] [CrossRef]
- Fasciana, T.; Cortegiani, A.; Ippolito, M.; Giarratano, A.; Di Quattro, O.; Lipari, D.; Graceffa, D.; Giammanco, A. Candida auris: An overview of how to screen, detect, test and control this emerging pathogen. Antibiotics 2020, 9, 778. [Google Scholar] [CrossRef] [PubMed]
- Leaw, S.N.; Chang, H.C.; Sun, H.F.; Barton, R.; Bouchara, J.P.; Chang, T.C. Identification of medically important yeast species by sequence analysis of the internal transcribed spacer regions. J. Clin. Microbiol. 2006, 44, 693–699. [Google Scholar] [CrossRef]
- Colabella, C.; Casagrande Pierantoni, D.; Corte, L.; Roscini, L.; Conti, A.; Bassetti, M.; Tascini, C.; Robert, V.; Cardinali, G. Single strain high-depth NGS reveals high rDNA (ITS-LSU) variability in the four prevalent pathogenic species of the genus Candida. Microorganisms 2021, 9, 302. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, N.P.; Babiker, W.M.; Merz, W.G.; Carroll, K.C.; Zhang, S.X. Evaluation of nucleic acid sequencing of the D1/D2 region of the large subunit of the 28S rDNA and the internal transcribed spacer region using SmartGene IDNS [corrected] software for identification of filamentous fungi in a clinical laboratory. J. Mol. Diagn. 2012, 14, 393–401. [Google Scholar] [CrossRef]
- Crossley, B.M.; Bai, J.; Glaser, A.; Maes, R.; Porter, E.; Killian, M.L.; Clement, T.; Toohey-Kurth, K. Guidelines for Sanger sequencing and molecular assay monitoring. J. Vet. Diagn. Investig. 2020, 32, 767–775. [Google Scholar] [CrossRef]
- De Cario, R.; Kura, A.; Suraci, S.; Magi, A.; Volta, A.; Marcucci, R.; Gori, A.M.; Pepe, G.; Giusti, B.; Sticchi, E. Sanger validation of high-throughput sequencing in genetic diagnosis: Still the best practice? Front. Genet. 2020, 11, 592588. [Google Scholar] [CrossRef]
- Chen, L.; Cai, Y.; Zhou, G.; Shi, X.; Su, J.; Chen, G.; Lin, K. Rapid Sanger sequencing of the 16S rRNA gene for identification of some common pathogens. PLoS ONE 2014, 9, e88886. [Google Scholar] [CrossRef]
- Oviaño, M.; Rodríguez-Sánchez, B. MALDI-TOF mass spectrometry in the 21st century clinical microbiology laboratory. Enferm. Infecc. Y Microbiol. Clin. 2021, 39, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Robert, M.G.; Cornet, M.; Hennebique, A.; Rasamoelina, T.; Caspar, Y.; Pondérand, L.; Bidart, M.; Durand, H.; Jacquet, M.; Garnaud, C.; et al. MALDI-TOF MS in a medical mycology maboratory: On stage and backstage. Microorganisms 2021, 9, 1283. [Google Scholar] [CrossRef]
- Ceballos-Garzón, A.; Cabrera, E.; Cortes-Fraile, G.C.; León, A.; Aguirre-Guataqui, K.; Linares-Linares, M.Y.; Ariza, B.; Valderrama-Beltrán, S.; Parra-Giraldo, C.M. In-house protocol and performance of MALDI-TOF MS in the early diagnosis of bloodstream infections in a fourth-level hospital in Colombia: Jumping to full use of this technology. Int. J. Infect. Dis. 2020, 101, 85–89. [Google Scholar] [CrossRef]
- Yaman, G.; Akyar, I.; Can, S. Evaluation of the MALDI TOF-MS method for identification of Candida strains isolated from blood cultures. Diagn. Microbiol. Infect. Dis. 2012, 73, 65–67. [Google Scholar] [CrossRef] [PubMed]
- Camp, I.; Spettel, K.; Willinger, B. Molecular methods for the diagnosis of invasive candidiasis. J Fungi 2020, 6, 101. [Google Scholar] [CrossRef] [PubMed]
- Wey, S.B.; Mori, M.; Pfaller, M.A.; Woolson, R.F.; Wenzel, R.P. Risk factors for hospital-acquired candidemia. A matched case-control study. Arch. Intern. Med. 1989, 149, 2349–2353. [Google Scholar] [CrossRef] [PubMed]
- Brillowska-Dabrowska, A.; Bergmann, O.; Jensen, I.M.; Jarløv, J.O.; Arendrup, M.C. Typing of Candida isolates from patients with invasive infection and concomitant colonization. Scand. J. Infect. Dis. 2010, 42, 109–113. [Google Scholar] [CrossRef]
- Jensen, R.H.; Johansen, H.K.; Søes, L.M.; Lemming, L.E.; Rosenvinge, F.S.; Nielsen, L.; Olesen, B.; Kristensen, L.; Dzajic, E.; Astvad, K.M.; et al. Posttreatment antifungal resistance among colonizing Candida isolates in candidemia patients: Results from a systematic multicenter study. Antimicrob. Agents Chemother. 2015, 60, 1500–1508. [Google Scholar] [CrossRef] [PubMed]
- Dupuis, C.; Le Bihan, C.; Maubon, D.; Calvet, L.; Ruckly, S.; Schwebel, C.; Bouadma, L.; Azoulay, E.; Cornet, M.; Timsit, J.F.; et al. Performance of repeated measures of (1-3)-β-D-Glucan, mannan antigen, and antimannan antibodies for the diagnosis of invasive candidiasis in ICU patients: A preplanned ancillary analysis of the EMPIRICUS randomized clinical trial. Open Forum Infect. Dis. 2021, 8, ofab080. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Yu, X.; Ye, L.; Zhou, G.; Wang, L.; Luo, Y. Clinical value of (1,3)-β-D-glucan, mannan, antimannan IgG and IgM antibodies in diagnosis of invasive candidiasis. Med. Mycol. 2019, 57, 976–986. [Google Scholar] [CrossRef]
- León, C.; Ruiz-Santana, S.; Saavedra, P.; Castro, C.; Loza, A.; Zakariya, I.; Úbeda, A.; Parra, M.; Macías, D.; Tomás, J.I.; et al. Contribution of Candida biomarkers and DNA detection for the diagnosis of invasive candidiasis in ICU patients with severe abdominal conditions. Crit. Care 2016, 20, 149. [Google Scholar] [CrossRef]
- Meng, Y.; Kang, M.; Li, D.; Wang, T.; Kuang, Z.; Ma, Y. Performance of a new Candida anti-mannan IgM and IgG assays in the diagnosis of candidemia. Rev. Inst. Med. Trop. Sao Paulo 2020, 62, e25. [Google Scholar] [CrossRef]
- Hanson, K.E.; Pfeiffer, C.D.; Lease, E.D.; Balch, A.H.; Zaas, A.K.; Perfect, J.R.; Alexander, B.D. β-D-glucan surveillance with preemptive anidulafungin for invasive candidiasis in intensive care unit patients: A randomized pilot study. PLoS ONE 2012, 7, e42282. [Google Scholar] [CrossRef]
- Tissot, F.; Lamoth, F.; Hauser, P.M.; Orasch, C.; Flückiger, U.; Siegemund, M.; Zimmerli, S.; Calandra, T.; Bille, J.; Eggimann, P.; et al. β-glucan antigenemia anticipates diagnosis of blood culture-negative intraabdominal candidiasis. Am. J. Respir. Crit. Care Med. 2013, 188, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Hartl, B.; Zeller, I.; Manhart, A.; Selitsch, B.; Lass-Flörl, C.; Willinger, B. A Retrospective assessment of four antigen assays for the detection of invasive candidiasis among high-risk hospitalized patients. Mycopathologia 2018, 183, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Mohr, J.F.; Sims, C.; Paetznick, V.; Rodriguez, J.; Finkelman, M.A.; Rex, J.H.; Ostrosky-Zeichner, L. Prospective survey of (1→3)-beta-D-glucan and its relationship to invasive candidiasis in the surgical intensive care unit setting. J. Clin. Microbiol. 2011, 49, 58–61. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Jiménez, M.C.; Muñoz, P.; Valerio, M.; Vena, A.; Guinea, J.; Bouza, E. Combination of Candida biomarkers in patients receiving empirical antifungal therapy in a Spanish tertiary hospital: A potential role in reducing the duration of treatment. J. Antimicrob. Chemother. 2015, 70, 3107–3115. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Mikulska, M.; Tumbarello, M.; Furfaro, E.; Spadaro, M.; Losito, A.R.; Mesini, A.; De Pascale, G.; Marchese, A.; Bruzzone, M.; et al. Combined use of serum (1,3)-β-D-glucan and procalcitonin for the early differential diagnosis between candidaemia and bacteraemia in intensive care units. Crit. Care 2017, 21, 176. [Google Scholar] [CrossRef]
- Fortún, J.; Meije, Y.; Buitrago, M.J.; Gago, S.; Bernal-Martinez, L.; Pemán, J.; Pérez, M.; Gómez-G Pedrosa, E.; Madrid, N.; Pintado, V.; et al. Clinical validation of a multiplex real-time PCR assay for detection of invasive candidiasis in intensive care unit patients. J. Antimicrob. Chemother. 2014, 69, 3134–3141. [Google Scholar] [CrossRef]
- Parra-Sánchez, M.; Zakariya-Yousef Breval, I.; Castro Méndez, C.; García-Rey, S.; Loza Vazquez, A.; Úbeda Iglesias, A.; Macías Guerrero, D.; Romero Mejías, A.; León Gil, C.; Martín-Mazuelos, E.; et al. Candida albicans germ-tube antibody: Evaluation of a new automatic assay for diagnosing invasive candidiasis in ICU patients. Mycopathologia 2017, 182, 645–652. [Google Scholar] [CrossRef]
- Pfeiffer, C.D.; Samsa, G.P.; Schell, W.A.; Reller, L.B.; Perfect, J.R.; Alexander, B.D. Quantitation of Candida CFU in initial positive blood cultures. J. Clin. Microbiol. 2011, 49, 2879–2883. [Google Scholar] [CrossRef]
- Sautour, M.; Lemaître, J.-P.; Ranjard, L.; Truntzer, C.; Basmaciyan, L.; Depret, G.; Hartmann, A.; Dalle, F. Detection and survival of Candida albicans in soils. Environ. DNA 2021, 3, 1093–1101. [Google Scholar] [CrossRef]
- Chang, S.S.; Hsieh, W.H.; Liu, T.S.; Lee, S.H.; Wang, C.H.; Chou, H.C.; Yeo, Y.H.; Tseng, C.P.; Lee, C.C. Multiplex PCR system for rapid detection of pathogens in patients with presumed sepsis—A systemic review and meta-analysis. PLoS ONE 2013, 8, e62323. [Google Scholar] [CrossRef]
- Jordana-Lluch, E.; Giménez, M.; Quesada, M.D.; Rivaya, B.; Marcó, C.; Domínguez, M.J.; Arméstar, F.; Martró, E.; Ausina, V. Evaluation of the broad-range PCR/ESI-MS technology in blood specimens for the molecular diagnosis of bloodstream infections. PLoS ONE 2015, 10, e0140865. [Google Scholar] [CrossRef] [PubMed]
- Desmet, S.; Maertens, J.; Bueselinck, K.; Lagrou, K. Broad-Range PCR Coupled with electrospray ionization time of flight mass spectrometry for detection of bacteremia and fungemia in patients with neutropenic fever. J. Clin. Microbiol. 2016, 54, 2513–2520. [Google Scholar] [CrossRef] [PubMed]
- Metzgar, D.; Frinder, M.W.; Rothman, R.E.; Peterson, S.; Carroll, K.C.; Zhang, S.X.; Avornu, G.D.; Rounds, M.A.; Carolan, H.E.; Toleno, D.M.; et al. The IRIDICA BAC BSI assay: Rapid, sensitive and culture-independent identification of bacteria and candida in blood. PLoS ONE 2016, 11, e0158186. [Google Scholar] [CrossRef]
- White, P.L.; Hibbitts, S.J.; Perry, M.D.; Green, J.; Stirling, E.; Woodford, L.; McNay, G.; Stevenson, R.; Barnes, R.A. Evaluation of a commercially developed semiautomated PCR-surface-enhanced raman scattering assay for diagnosis of invasive fungal disease. J. Clin. Microbiol. 2014, 52, 3536–3543. [Google Scholar] [CrossRef] [PubMed]
- Mylonakis, E.; Clancy, C.J.; Ostrosky-Zeichner, L.; Garey, K.W.; Alangaden, G.J.; Vazquez, J.A.; Groeger, J.S.; Judson, M.A.; Vinagre, Y.M.; Heard, S.O.; et al. T2 magnetic resonance assay for the rapid diagnosis of candidemia in whole blood: A clinical trial. Clin. Infect. Dis. 2015, 60, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Krifors, A.; Ullberg, M.; Castegren, M.; Petersson, J.; Sparrelid, E.; Hammarström, H.; Sjölin, J.; Özenci, V.; Blennow, O. T2Candida assay in the diagnosis of intraabdominal candidiasis: A prospective multicenter study. J. Fungi 2022, 8, 86. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Could Candida Overgrowth Be Involved in the Pathophysiology of Autism? J. Clin. Med. 2022, 11, 442. [Google Scholar] [CrossRef]
- Li, L.; Liao, Z.; Yang, Y.; Lv, L.; Cao, Y.; Zhu, Z. Metabolomic profiling for the identification of potential biomarkers involved in a laboratory azole resistance in Candida albicans. PLoS ONE 2018, 13, e0192328. [Google Scholar] [CrossRef]
- Liu, R.; Bao, Z.X.; Zhao, P.J.; Li, G.H. Advances in the study of metabolomics and metabolites in some species interactions. Molecules 2021, 26, 3311. [Google Scholar] [CrossRef]
- Smeekens, S.P.; van de Veerdonk, F.L.; Netea, M.G. An omics perspective on Candida infections: Toward next-generation diagnosis and therapy. Front. Microbiol. 2016, 7, 154. [Google Scholar] [CrossRef]
- Kumar, V.; Cheng, S.C.; Johnson, M.D.; Smeekens, S.P.; Wojtowicz, A.; Giamarellos-Bourboulis, E.; Karjalainen, J.; Franke, L.; Withoff, S.; Plantinga, T.S.; et al. Immunochip SNP array identifies novel genetic variants conferring susceptibility to candidaemia. Nat. Commun. 2014, 5, 4675. [Google Scholar] [CrossRef] [PubMed]
- CLSI Performance Standards for Antifungal Susceptibility Testing of Yeasts, 2nd ed.; CLSI supplement M60; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2020.
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 10.0. 2020. Available online: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ (accessed on 14 April 2022).
- Berkow, E.L.; Lockhart, S.R.; Ostrosky-Zeichner, L. Antifungal susceptibility testing: Current approaches. Clin. Microbiol. Rev. 2020, 33, e00069-19. [Google Scholar] [CrossRef] [PubMed]
- Nunnally, N.S.; Damm, T.; Lockhart, S.R.; Berkow, E.L. Categorizing susceptibility of clinical isolates of Candida auris to amphotericin B, caspofungin, and fluconazole by use of the CLSI M44-A2 disk diffusion method. J. Clin. Microbiol. 2021, 59, e02355-20. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antifungal Susceptibility Testing and Interpretation. Available online: https://www.cdc.gov/fungal/candida-auris/c-auris-antifungal.html (accessed on 14 April 2022).
- Aigner, M.; Erbeznik, T.; Gschwentner, M.; Lass-Flörl, C. Etest and Sensititre YeastOne susceptibility testing of echinocandins against Candida species from a single center in Austria. Antimicrob. Agents Chemother. 2017, 61, e00512-17. [Google Scholar] [CrossRef]
- Altinbaş, R.; Barış, A.; Şen, S.; Öztürk, R.; Kiraz, N. Comparison of the Sensititre YeastOne antifungal method with the CLSI M27-A3 reference method to determine the activity of antifungal agents against clinical isolates of Candida spp. Turk. J. Med. Sci. 2020, 50, 2024–2031. [Google Scholar] [CrossRef]
- Córdoba, S.; Abiega, C.; Agorio, I.; Amigot, S.; Ardizzoli, K.; Giusiano, G.; Guelfand, L.; López Moral, L.; Maldonado, I.; Pineda, G.; et al. Utilidad del panel Sensititre YeastOne® para detectar especies de Candida resistentes a los antifúngicos [Usefulness of the Sensititre YeastOne® panel to detect Candida species resistant to antifungal drugs]. Rev. Argent. Microbiol. 2022, 54, 9–14. [Google Scholar] [CrossRef]
- Siqueira, R.A.; Doi, A.M.; de Petrus Crossara, P.P.; Koga, P.C.M.; Marques, A.G.; Nunes, F.G.; Pasternak, J.; Martino, M.D.V. Evaluation of two commercial methods for the susceptibility testing of Candida species: Vitek 2® and Sensititre YeastOne®. Rev. Iberoam. Micol. 2018, 35, 83–87. [Google Scholar] [CrossRef]
- Arendrup, M.C.; Prakash, A.; Meletiadis, J.; Sharma, C.; Chowdhary, A. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob. Agents Chemother. 2017, 61, e00485-17. [Google Scholar] [CrossRef]
- Magobo, R.E.; Corcoran, C.; Seetharam, S.; Govender, N.P. Candida auris-associated candidemia, South Africa. Emerg. Infect. Dis. 2014, 20, 1250–1251. [Google Scholar] [CrossRef]
- Kathuria, S.; Singh, P.K.; Sharma, C.; Prakash, A.; Masih, A.; Kumar, A.; Meis, J.F.; Chowdhary, A. Multidrug-resistant Candida auris misidentified as Candida haemulonii: Characterization by Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry and DNA sequencing and its antifungal susceptibility profile variability by Vitek 2, CLSI broth microdilution, and Etest method. J. Clin. Microbiol. 2015, 53, 1823–1830. [Google Scholar] [CrossRef]
- Kritikos, A.; Neofytos, D.; Khanna, N.; Schreiber, P.W.; Boggian, K.; Bille, J.; Schrenzel, J.; Mühlethaler, K.; Zbinden, R.; Bruderer, T.; et al. Accuracy of Sensititre YeastOne echinocandins epidemiological cut-off values for identification of FKS mutant Candida albicans and Candida glabrata: A ten year national survey of the Fungal Infection Network of Switzerland (FUNGINOS). Clin. Microbiol. Infect. 2018, 24, 1214. [Google Scholar] [CrossRef] [PubMed]
- Dalyan Cilo, B.; Ener, B. Comparison of Clinical Laboratory Standards Institute (CLSI) Microdilution method and VITEK 2 automated antifungal susceptibility system for the determination of antifungal susceptibility of Candida species. Cureus 2021, 13, e20220. [Google Scholar] [CrossRef] [PubMed]
- Calandra, T.; Roberts, J.A.; Antonelli, M.; Bassetti, M.; Vincent, J.L. Diagnosis and management of invasive candidiasis in the ICU: An updated approach to an old enemy. Crit. Care 2016, 20, 125. [Google Scholar] [CrossRef] [PubMed]
- Jung, P.; Mischo, C.E.; Gunaratnam, G.; Spengler, C.; Becker, S.L.; Hube, B.; Jacobs, K.; Bischoff, M. Candida albicans adhesion to central venous catheters: Impact of blood plasma-driven germ tube formation and pathogen-derived adhesins. Virulence 2020, 11, 1453–1465. [Google Scholar] [CrossRef]
- Kollef, M.; Micek, S.; Hampton, N.; Doherty, J.A.; Kumar, A. Septic shock attributed to Candida infection: Importance of empiric therapy and source control. Clin. Infect. Dis. 2012, 54, 1739–1746. [Google Scholar] [CrossRef] [PubMed]
- Vergidis, P.; Clancy, C.J.; Shields, R.K.; Park, S.Y.; Wildfeuer, B.N.; Simmons, R.L.; Nguyen, M.H. Intra-abdominal candidiasis: The importance of early source control and antifungal treatment. PLoS ONE 2016, 11, e0153247. [Google Scholar] [CrossRef]
- Andes, D.R.; Safdar, N.; Baddley, J.W.; Playford, G.; Reboli, A.C.; Rex, J.H.; Sobel, J.D.; Pappas, P.G.; Kullberg, B.J.; Mycoses Study Group. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: A patient-level quantitative review of randomized trials. Clin. Infect. Dis. 2012, 54, 1110–1122. [Google Scholar] [CrossRef]
- Cornely, O.A.; Bassetti, M.; Calandra, T.; Garbino, J.; Kullberg, B.J.; Lortholary, O.; Meersseman, W.; Akova, M.; Arendrup, M.C.; Arikan-Akdagli, S.; et al. ESCMID* guideline for the diagnosis and management of Candida diseases 2012: Non-neutropenic adult patients. Clin. Microbiol. Infect. 2012, 18, 19–37. [Google Scholar] [CrossRef] [PubMed]
- Cavassin, F.B.; Baú-Carneiro, J.L.; Vilas-Boas, R.R.; Queiroz-Telles, F. Sixty years of amphotericin B: An overview of the main antifungal agent used to treat invasive fungal infections. Infect. Dis. Ther. 2021, 10, 115–147. [Google Scholar] [CrossRef]
- Anderson, T.M.; Clay, M.C.; Cioffi, A.G.; Diaz, K.A.; Hisao, G.S.; Tuttle, M.D.; Nieuwkoop, A.J.; Comellas, G.; Maryum, N.; Wang, S.; et al. Amphotericin forms an extramembranous and fungicidal sterol sponge. Nat. Chem. Biol. 2014, 10, 400–406. [Google Scholar] [CrossRef]
- Robbins, N.; Caplan, T.; Cowen, L.E. Molecular evolution of antifungal drug resistance. Annu. Rev. Microbiol. 2017, 71, 753–775. [Google Scholar] [CrossRef] [PubMed]
- Apsemidou, A.; Füller, M.A.; Idelevich, E.A.; Kurzai, O.; Tragiannidis, A.; Groll, A.H. Candida lusitaniae breakthrough fungemia in an immuno-compromised adolescent: Case report and review of the literature. J. Fungi 2020, 6, 380. [Google Scholar] [CrossRef] [PubMed]
- Faustino, C.; Pinheiro, L. Lipid systems for the delivery of amphotericin B in antifungal therapy. Pharmaceutics 2020, 12, 29. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hafez, Y.; Siaj, H.; Janajri, M.; Abu-Baker, Y.; Nazzal, Z.; Hamdan, Z.; Adwan, R.; Aiesh, B.M.; Anaya, A.I. Tolerability and epidemiology of nephrotoxicity associated with conventional amphotericin B therapy: A retrospective study in tertiary care centers in Palestine. BMC Nephrol. 2022, 23, 132. [Google Scholar] [CrossRef]
- Caputo, R.; Asprea, M.; Giovannetti, L.; Messori, A. Nephrotoxicity of three formulations of amphotericin B: Trial sequential analysis. Arch. Med. Sci. 2020, 16, 1493–1495. [Google Scholar] [CrossRef]
- Zhang, H.; Qu, W.; Nazzal, M.; Ortiz, J. Burn patients with history of kidney transplant experience increased incidence of wound infection. Burns 2020, 46, 609–615. [Google Scholar] [CrossRef]
- Peyclit, L.; Yousfi, H.; Rolain, J.-M.; Bittar, F. Drug repurposing in medical mycology: Identification of compounds as potential antifungals to overcome the emergence of multidrug-resistant fungi. Pharmaceuticals 2021, 14, 488. [Google Scholar] [CrossRef]
- Delma, F.Z.; Al-Hatmi, A.M.S.; Brüggemann, R.J.M.; Melchers, W.J.G.; de Hoog, S.; Verweij, P.E.; Buil, J.B. Molecular mechanisms of 5-Fluorocytosine resistance in yeasts and filamentous fungi. J. Fungi 2021, 7, 909. [Google Scholar] [CrossRef]
- Botero-Calderon, L.; Benjamin, D.K., Jr.; Cohen-Wolkowiez, M. Advances in the treatment of invasive neonatal candidiasis. Expert Opin. Pharmacother. 2015, 16, 1035–1048. [Google Scholar] [CrossRef]
- Testoni, D.; Smith, P.B.; Benjamin, D.K., Jr. The use of antifungal therapy in neonatal intensive care. Clin. Perinatol. 2012, 39, 83–98. [Google Scholar] [CrossRef]
- Vermes, A.; van Der Sijs, H.; Guchelaar, H.J. Flucytosine: Correlation between toxicity and pharmacokinetic parameters. Chemotherapy 2000, 46, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Vitale, R.G. Role of antifungal combinations in difficult to treat Candida infections. J. Fungi 2021, 7, 731. [Google Scholar] [CrossRef] [PubMed]
- Hassanmoghadam, F.; Shokohi, T.; Hedayati, M.T.; Aslani, N.; Haghani, I.; Nabili, M.; Lotfali, E.; Davari, A.; Moazeni, M. High prevalence of itraconazole resistance among Candida parapsilosis isolated from Iran. Curr. Med. Mycol. 2019, 5, 43–46. [Google Scholar] [CrossRef] [PubMed]
- Galia, L.; Pezzani, M.D.; Compri, M.; Callegari, A.; Rajendran, N.B.; Carrara, E.; Tacconelli, E.; The COMBACTE MAGNET EPI-Net Network. Surveillance of Antifungal Resistance in Candidemia Fails to Inform Antifungal Stewardship in European Countries. J. Fungi 2022, 8, 249. [Google Scholar] [CrossRef]
- Berkow, E.L.; Lockhart, S.R. Fluconazole resistance in Candida species: A current perspective. Infect. Drug Resist. 2017, 10, 237–245. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole antifungal resistance in Candida albicans and emerging non-albicans Candida species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef]
- Ham, Y.Y.; Lewis, J.S.; Thompson, G.R. Rezafungin: A novel antifungal for the treatment of invasive candidiasis. Future Microbiol. 2021, 16, 27–36. [Google Scholar] [CrossRef]
- Miesel, L.; Lin, K.Y.; Ong, V. Rezafungin treatment in mouse models of invasive candidiasis and aspergillosis: Insights on the PK/PD pharmacometrics of rezafungin efficacy. Pharmacol. Res. Perspect. 2019, 7, e00546. [Google Scholar] [CrossRef]
- Miesel, L.; Cushion, M.T.; Ashbaugh, A.; Lopez, S.R.; Ong, V. Efficacy of rezafungin in prophylactic mouse models of invasive Candidiasis, Aspergillosis, and Pneumocystis pneumonia. Antimicrob. Agents Chemother. 2021, 65, e01992-20. [Google Scholar] [CrossRef]
- Lepak, A.J.; Zhao, M.; Andes, D.R. Determination of pharmacodynamic target exposures for rezafungin against Candida tropicalis and Candida dubliniensis in the neutropenic mouse disseminated candidiasis model. Antimicrob. Agents Chemother. 2019, 63, e01556-19. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Carvalhaes, C.; Messer, S.A.; Rhomberg, P.R.; Castanheira, M. Activity of a long-acting echinocandin, rezafungin, and comparator antifungal agents tested against contemporary unvasive fungal isolates (SENTRY program, 2016 to 2018). Antimicrob. Agents Chemother. 2020, 64, e00099-20. [Google Scholar] [CrossRef]
- Farhadi, Z.; Farhadi, T.; Hashemian, S.M. Virtual screening for potential inhibitors of β(1,3)-D-glucan synthase as drug candidates against fungal cell wall. J. Drug Assess. 2020, 9, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Szymański, M.; Chmielewska, S.; Czyżewska, U.; Malinowska, M.; Tylicki, A. Echinocandins—Structure, mechanism of action and use in antifungal therapy. J. Enzym. Inhib. Med. Chem. 2022, 37, 876–894. [Google Scholar] [CrossRef] [PubMed]
- Hautala, N.; Köykkä, H.; Siiskonen, M.; Saari, J.; Kauranen, J.; Hautala, T. Effect of first-line antifungal treatment on ocular complication risk in Candida or yeast blood stream infection. BMJ Open Ophthalmol. 2021, 6, e000837. [Google Scholar] [CrossRef] [PubMed]
- Taur, Y.; Cohen, N.; Dubnow, S.; Paskovaty, A.; Seo, S.K. Effect of antifungal therapy timing on mortality in cancer patients with candidemia. Antimicrob Agents Chemother. 2010, 54, 184–190. [Google Scholar] [CrossRef] [PubMed]
- Mroczyńska, M.; Brillowska-Dąbrowska, A. Review on current status of echinocandins use. Antibiotic 2020, 9, 227. [Google Scholar] [CrossRef]
- Bretagne, S.; Desnos-Ollivier, M.; Sitbon, K.; Lortholary, O.; Che, D.; Dromer, F.; Participants of the YEASTS. No impact of fluconazole to echinocandins replacement as first-line therapy on the epidemiology of yeast fungemia (Hospital-Driven Active Surveillance, 2004–2017, Paris, France). Front. Med. 2021, 8, 641965. [Google Scholar] [CrossRef]
- Bienvenu, A.L.; Pradat, P.; Guerin, C.; Aubrun, F.; Fellahi, J.L.; Friggeri, A.; Guichon, C.; Hernu, R.; Menotti, J.; Monard, C.; et al. Evaluation of first-line therapies for the treatment of candidemia in ICU patients: A propensity score analysis. Int. J. Infect. Dis. 2020, 93, 15–21. [Google Scholar] [CrossRef]
- Reboli, A.C.; Shorr, A.F.; Rotstein, C.; Pappas, P.G.; Kett, D.H.; Schlamm, H.T.; Reisman, A.L.; Biswas, P.; Walsh, T.J. Anidulafungin compared with fluconazole for treatment of candidemia and other forms of invasive candidiasis caused by Candida albicans: A multivariate analysis of factors associated with improved outcome. BMC Infect. Dis. 2011, 11, 261. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Sobel, J.D.; Ruhnke, M.; Pappas, P.G.; Viscoli, C.; Rex, J.H.; Cleary, J.D.; Rubinstein, E.; Church, L.W.; Brown, J.M.; et al. Voriconazole versus a regimen of amphotericin B followed by fluconazole for candidaemia in non-neutropenic patients: A randomised non-inferiority trial. Lancet 2005, 366, 1435–1442. [Google Scholar] [CrossRef]
- Vazquez, J.; Reboli, A.C.; Pappas, P.G.; Patterson, T.F.; Reinhardt, J.; Chin-Hong, P.; Tobin, E.; Kett, D.H.; Biswas, P.; Swanson, R. Evaluation of an early step-down strategy from intravenous anidulafungin to oral azole therapy for the treatment of candidemia and other forms of invasive candidiasis: Results from an open-label trial. BMC Infect. Dis. 2014, 14, 97. [Google Scholar] [CrossRef] [PubMed]
- Pappas, P.G.; Rotstein, C.M.; Betts, R.F.; Nucci, M.; Talwar, D.; De Waele, J.J.; Vazquez, J.A.; Dupont, B.F.; Horn, D.L.; Ostrosky-Zeichner, L.; et al. Micafungin versus caspofungin for treatment of candidemia and other forms of invasive candidiasis. Clin. Infect. Dis. 2007, 45, 883–893. [Google Scholar] [CrossRef] [PubMed]
- Kullberg, B.J.; Viscoli, C.; Pappas, P.G.; Vazquez, J.; Ostrosky-Zeichner, L.; Rotstein, C.; Sobel, J.D.; Herbrecht, R.; Rahav, G.; Jaruratanasirikul, S.; et al. Isavuconazole versus caspofungin in the treatment of candidemia and other invasive candida infections: The ACTIVE trial. Clin. Infect. Dis. 2019, 68, 1981–1989. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, M.; Aguado, J.M.; Almirante, B.; Lora-Pablos, D.; Padilla, B.; Puig-Asensio, M.; Montejo, M.; García-Rodríguez, J.; Pemán, J.; Ruiz Pérez de Pipaón, M.; et al. Initial use of echinocandins does not negatively influence outcome in Candida parapsilosis bloodstream infection: A propensity score analysis. Clin. Infect. Dis. 2014, 58, 1413–1421. [Google Scholar] [CrossRef]
- Kontoyiannis, D.P.; Bassetti, M.; Nucci, M.; Capparella, M.R.; Yan, J.L.; Aram, J.; Hogan, P.A. Anidulafungin for the treatment of candidaemia caused by Candida parapsilosis: Analysis of pooled data from six prospective clinical studies. Mycoses 2017, 60, 663–667. [Google Scholar] [CrossRef]
- Chiotos, K.; Vendetti, N.; Zaoutis, T.E.; Baddley, J.; Ostrosky-Zeichner, L.; Pappas, P.; Fisher, B.T. Comparative effectiveness of echinocandins versus fluconazole therapy for the treatment of adult candidaemia due to Candida parapsilosis: A retrospective observational cohort study of the Mycoses Study Group (MSG-12). J. Antimicrob. Chemother. 2016, 71, 3536–3539. [Google Scholar] [CrossRef]
- Kullberg, B.J.; Vasquez, J.; Mootsikapun, P.; Nucci, M.; Paiva, J.A.; Garbino, J.; Yan, J.L.; Aram, J.; Capparella, M.R.; Conte, U.; et al. Efficacy of anidulafungin in 539 patients with invasive candidiasis: A patient-level pooled analysis of six clinical trials. J. Antimicrob. Chemother. 2017, 72, 2368–2377. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.A.; Diekema, D.J.; Gibbs, D.L.; Newell, V.A.; Nagy, E.; Dobiasova, S.; Rinaldi, M.; Barton, R.; Veselov, A.; Global Antifungal Surveillance Group. Candida krusei, a multidrug-resistant opportunistic fungal pathogen: Geographic and temporal trends from the ARTEMIS DISK Antifungal Surveillance Program, 2001 to 2005. J. Clin. Microbiol. 2008, 46, 515–521. [Google Scholar] [CrossRef]
- Ademe, M.; Girma, F. Candida auris: From multidrug resistance to pan-resistant strains. Infect. Drug Resist. 2020, 13, 1287–1294. [Google Scholar] [CrossRef]
- McCarty, T.P.; Pappas, P.G. Antifungal Pipeline. Front. Cell. Infect. Microbiol. 2021, 11, 732223. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Cai, X.; Li, R.; Zheng, B.; Yang, E.; Liang, T.; Yang, X.; Wan, Z.; Liu, W. Two sequential clinical isolates of Candida glabrata with multidrug-resistance to posaconazole and echinocandins. Antibiotics 2021, 10, 1217. [Google Scholar] [CrossRef]
- Bandara, N.; Samaranayake, L. Emerging and future strategies in the management of recalcitrant Candida auris. Med. Mycol. 2022, 60, myac008. [Google Scholar] [CrossRef] [PubMed]
- Centre for Disease Prevention and Control. Candida auris Outbreak in Healthcare in Northern Italy, 2019–2021; ECDC: Stockholm, Sweden, 2022.
- Černáková, L.; Roudbary, M.; Brás, S.; Tafaj, S.; Rodrigues, C.F. Candida auris: A quick review on identification, current treatments, and challenges. Int. J. Mol. Sci. 2021, 22, 4470. [Google Scholar] [CrossRef] [PubMed]
- Denning, D.W. Antifungal drug resistance: An update. Eur. J. Hosp. Pharm. 2022, 29, 109–112. [Google Scholar] [CrossRef] [PubMed]
- Jamiu, A.T.; Albertyn, J.; Sebolai, O.M.; Pohl, C.H. Update on Candida krusei, a potential multidrug-resistant pathogen. Med. Mycol. 2021, 59, 14–30. [Google Scholar] [CrossRef]
| Test | Turnaround Time | Diagnostic Value | Sensitivity | Specificity | Notes |
|---|---|---|---|---|---|
| Culture | 2–4 days | Positive | 21–71% | N/A | Allows susceptibility testing |
| T2Candida | 3–5 h | Positive | 91% | 99% | Approved for the detection of C. albicans, C. krusei, C. tropicalis, C. parapsilosis, and C. glabrata in whole blood. |
| β-D-glucan (Fungitell) | 1 h | ≥80 ng/L | 92% | 81% | Can be positive in other fungal infections |
| β-D-glucan + procalcitonin | 1 h | ≥80 ng/L <0.2 ng/mL | 96% | 98% | Can be positive in other fungal infections |
| Etiologic Agent of Invasive Candidiasis Therapy | C. albicans, C. parapsilosis, C. tropicalis | C. krusei, C. glabrata | C. auris |
|---|---|---|---|
| First-line therapy * | Echinocandin | Echinocandin | Echinocandin |
| Alternative first-line therapy | Fluconazole | Amphotericin B lipid formulations | Amphotericin B lipid formulations |
| Step-down therapy ** | Fluconazole | Voriconazole | Susceptibility data required |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barantsevich, N.; Barantsevich, E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics 2022, 11, 718. https://doi.org/10.3390/antibiotics11060718
Barantsevich N, Barantsevich E. Diagnosis and Treatment of Invasive Candidiasis. Antibiotics. 2022; 11(6):718. https://doi.org/10.3390/antibiotics11060718
Chicago/Turabian StyleBarantsevich, Natalia, and Elena Barantsevich. 2022. "Diagnosis and Treatment of Invasive Candidiasis" Antibiotics 11, no. 6: 718. https://doi.org/10.3390/antibiotics11060718
APA StyleBarantsevich, N., & Barantsevich, E. (2022). Diagnosis and Treatment of Invasive Candidiasis. Antibiotics, 11(6), 718. https://doi.org/10.3390/antibiotics11060718





