Combination of C-Reactive Protein and Procalcitonin in Distinguishing Fungal from Bacterial Infections Early in Immunocompromised Children
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Subjects and Methods
2.2. Testing Methods
2.3. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Olivier-Gougenheim, L.; Rama, N.; Dupont, D.; Saultier, P.; Leverger, G.; AbouChahla, W.; Paillard, C.; Gandemer, V.; Theron, A.; Freycon, C.; et al. Invasive Fungal Infections in Immunocompromised Children: Novel Insight Following a National Study. J. Pediatrics 2021, 236, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Kaneda, M.; Sato, T.; Ichikawa, M.; Suzuki, D.; Ariga, T. The clinical feature of invasive fungal infection in pediatric patients with hematologic and malignant diseases: A 10-year analysis at a single institution at Japan. J. Pediatric Hematol./Oncol. 2008, 30, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Mor, M.; Gilad, G.; Kornreich, L.; Fisher, S.; Yaniv, I.; Levy, I. Invasive fungal infections in pediatric oncology. Pediatric Blood Cancer 2011, 56, 1092–1097. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.M.; Caniza, M.; Fynn, A.; Vescina, C.; Ruiz, C.D.; Iglesias, D.; Sosa, F.; Sung, L. Fungal infections in hematopoietic stem cell transplantation in children at a pediatric children’s hospital in Argentina. Transpl. Infect. Dis. 2018, 20, e12913. [Google Scholar] [CrossRef]
- Cesaro, S.; Tridello, G.; Castagnola, E.; Calore, E.; Carraro, F.; Mariotti, I.; Colombini, A.; Perruccio, K.; Decembrino, N.; Russo, G.; et al. Retrospective study on the incidence and outcome of proven and probable invasive fungal infections in high-risk pediatric onco-hematological patients. Eur. J. Haematol. 2017, 99, 240–248. [Google Scholar] [CrossRef]
- Sahbudak Bal, Z.; Yilmaz Karapinar, D.; Karadas, N.; Sen, S.; Onder Sivis, Z.; Akinci, A.B.; Balkan, C.; Kavakli, K.; Vardar, F.; Aydinok, Y. Proven and probable invasive fungal infections in children with acute lymphoblastic leukaemia: Results from an university hospital, 2005–2013. Mycoses 2015, 58, 225–232. [Google Scholar] [CrossRef]
- O’Connor, D.; Bate, J.; Wade, R.; Clack, R.; Dhir, S.; Hough, R.; Vora, A.; Goulden, N.; Samarasinghe, S. Infection-related mortality in children with acute lymphoblastic leukemia: An analysis of infectious deaths on UKALL2003. Blood 2014, 124, 1056–1061. [Google Scholar] [CrossRef]
- Simms-Waldrip, T.; Rosen, G.; Nielsen-Saines, K.; Ikeda, A.; Brown, B.; Moore, T. Invasive fungal infections in pediatric hematopoietic stem cell transplant patients. Infect. Dis. 2015, 47, 218–224. [Google Scholar] [CrossRef]
- Oz, Y.; Kiraz, N. Diagnostic methods for fungal infections in pediatric patients: Microbiological, serological and molecular methods. Expert Rev. Anti-Infect. Ther. 2011, 9, 289–298. [Google Scholar] [CrossRef]
- Grim, S.A.; Berger, K.; Teng, C.; Gupta, S.; Layden, J.E.; Janda, W.M.; Clark, N.M. Timing of susceptibility-based antifungal drug administration in patients with Candida bloodstream infection: Correlation with outcomes. J. Antimicrob. Chemother. 2012, 67, 707–714. [Google Scholar] [CrossRef]
- Roilides, E.; Pana, Z.D. Application of diagnostic markers to invasive aspergillosis in children. Ann. N. Y. Acad. Sci. 2012, 1272, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Donnelly, J.P.; Chen, S.C.; Kauffman, C.A.; Steinbach, W.J.; Baddley, J.W.; Verweij, P.E.; Clancy, C.J.; Wingard, J.R.; Lockhart, S.R.; Groll, A.H.; et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease From the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin. Infect. Dis. 2020, 71, 1367–1376. [Google Scholar] [CrossRef] [PubMed]
- Ostrosky-Zeichner, L. Invasive mycoses: Diagnostic challenges. Am. J. Med. 2012, 125 (Suppl. 1), S14–S24. [Google Scholar] [CrossRef] [PubMed]
- Groll, A.H.; Pana, D.; Lanternier, F.; Mesini, A.; Ammann, R.A.; Averbuch, D.; Castagnola, E.; Cesaro, S.; Engelhard, D.; Garcia-Vidal, C.; et al. 8th European Conference on Infections in Leukaemia: 2020 guidelines for the diagnosis, prevention, and treatment of invasive fungal diseases in paediatric patients with cancer or post-haematopoietic cell transplantation. Lancet. Oncol. 2021, 22, e254–e269. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Robinson, P.; Fisher, B.; Alexander, S.; Ammann, R.A.; Beauchemin, M.; Carlesse, F.; Groll, A.H.; Haeusler, G.M.; Santolaya, M.; et al. Guideline for the Management of Fever and Neutropenia in Children With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. J. Clin. Oncol. 2017, 35, 2082–2094. [Google Scholar] [CrossRef]
- Oguz, S.S.; Sipahi, E.; Dilmen, U. C-reactive protein and interleukin-6 responses for differentiating fungal and bacterial aetiology in late-onset neonatal sepsis. Mycoses 2011, 54, 212–216. [Google Scholar] [CrossRef]
- Akin, H.; Akalin, H.; Budak, F.; Ener, B.; Ocakoğlu, G.; Gürcüoğlu, E.; Göral, G.; Oral, H.B. Alterations of serum cytokine levels and their relation with inflammatory markers in candidemia. Med. Mycol. 2015, 53, 258–268. [Google Scholar] [CrossRef][Green Version]
- Dou, Y.H.; Du, J.K.; Liu, H.L.; Shong, X.D. The role of procalcitonin in the identification of invasive fungal infection-a systemic review and meta-analysis. Diagn. Microbiol. Infect. Dis. 2013, 76, 464–469. [Google Scholar] [CrossRef]
- Dornbusch, H.J.; Strenger, V.; Kerbl, R.; Lackner, H.; Schwinger, W.; Sovinz, P.; Urban, C. Procalcitonin—A marker of invasive fungal infection? Supportive Care Cancer 2005, 13, 343–346. [Google Scholar] [CrossRef]
- Marková, M.; Brodská, H.; Malíčková, K.; Válková, V.; Cetkovský, P.; Kolář, M.; Haluzík, M. Substantially elevated C-reactive protein (CRP), together with low levels of procalcitonin (PCT), contributes to diagnosis of fungal infection in immunocompromised patients. Supportive Care Cancer 2013, 21, 2733–2742. [Google Scholar] [CrossRef]
- Stoma, I.; Karpov, I.; Uss, A.; Krivenko, S.; Iskrov, I.; Milanovich, N.; Vlasenkova, S.; Lendina, I.; Belyavskaya, K.; Cherniak, V. Combination of sepsis biomarkers may indicate an invasive fungal infection in haematological patients. Biomarkers 2019, 24, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Eklund, C.M. Proinflammatory cytokines in CRP baseline regulation. Adv. Clin. Chem. 2009, 48, 111–136. [Google Scholar] [CrossRef] [PubMed]
- Lehrnbecher, T.; Robinson, P.D.; Fisher, B.T.; Castagnola, E.; Groll, A.H.; Steinbach, W.J.; Zaoutis, T.E.; Negeri, Z.F.; Beyene, J.; Phillips, B.; et al. Galactomannan, β-D-Glucan, and Polymerase Chain Reaction-Based Assays for the Diagnosis of Invasive Fungal Disease in Pediatric Cancer and Hematopoietic Stem Cell Transplantation: A Systematic Review and Meta-Analysis. Clin. Infect. Dis. 2016, 63, 1340–1348. [Google Scholar] [CrossRef] [PubMed]

| Characteristic | Number (%) |
|---|---|
| Age (y), median (IQR) | 5 (3, 10) |
| Male | 77 (61.1) |
| Primary disease: | |
| Acute lymphoblastic leukemia | 95 (75.3) |
| Acute myeloid leukemia | 19 (15.1) |
| Mixed phenotype acute leukemia | 4 (3.2) |
| Lymphoma | 3 (2.4) |
| Solid tumor | 4 (3.2) |
| Aplastic anemia | 1 (0.8) |
| Pathogens: | |
| Bacterial: | 102 |
| Coagulase-negative Staph. | 21 (20.6) |
| K. pneumoniae | 20 (19.6) |
| S. aureus | 18 (17.6) |
| E. coli | 16 (15.7) |
| P. aeruginosa | 9 (8.8) |
| Streptococcus spp. | 7 (6.9) |
| Enterococcus faecium | 1 (1.08) |
| Gemella Berger | 1 (1.08) |
| Roseomonas | 1 (1.08) |
| Abiotrophia defectiva | 1 (1.08) |
| Salmonella typhi | 1 (1.08) |
| M.luteus | 1 (1.08) |
| Citrobacter freundii | 1 (1.08) |
| Propionibacterium acnes | 1 (1.08) |
| Listeria monocytogenes | 1 (1.08) |
| Rothia | 1 (1.08) |
| Fungal: | 24 |
| Candida spp. | 9 (37.5) |
| Aspergillus spp. | 5 (20.8) |
| T.asahii | 4 (16.6) |
| Mucor | 3 (12.5) |
| Haematonectria haematococca | 1 (4.2) |
| Trichosporon inkin | 1 (4.2) |
| Pneumocystis jirovecii | 1 (4.2) |
| Variable | Coefficients | OR | 95% CI | p |
|---|---|---|---|---|
| CRP | 0.042 | 1.043 | 1.024–1.062 | <0.001 † |
| PCT | −1.029 | 0.357 | 0.140–0.914 | 0.032 † |
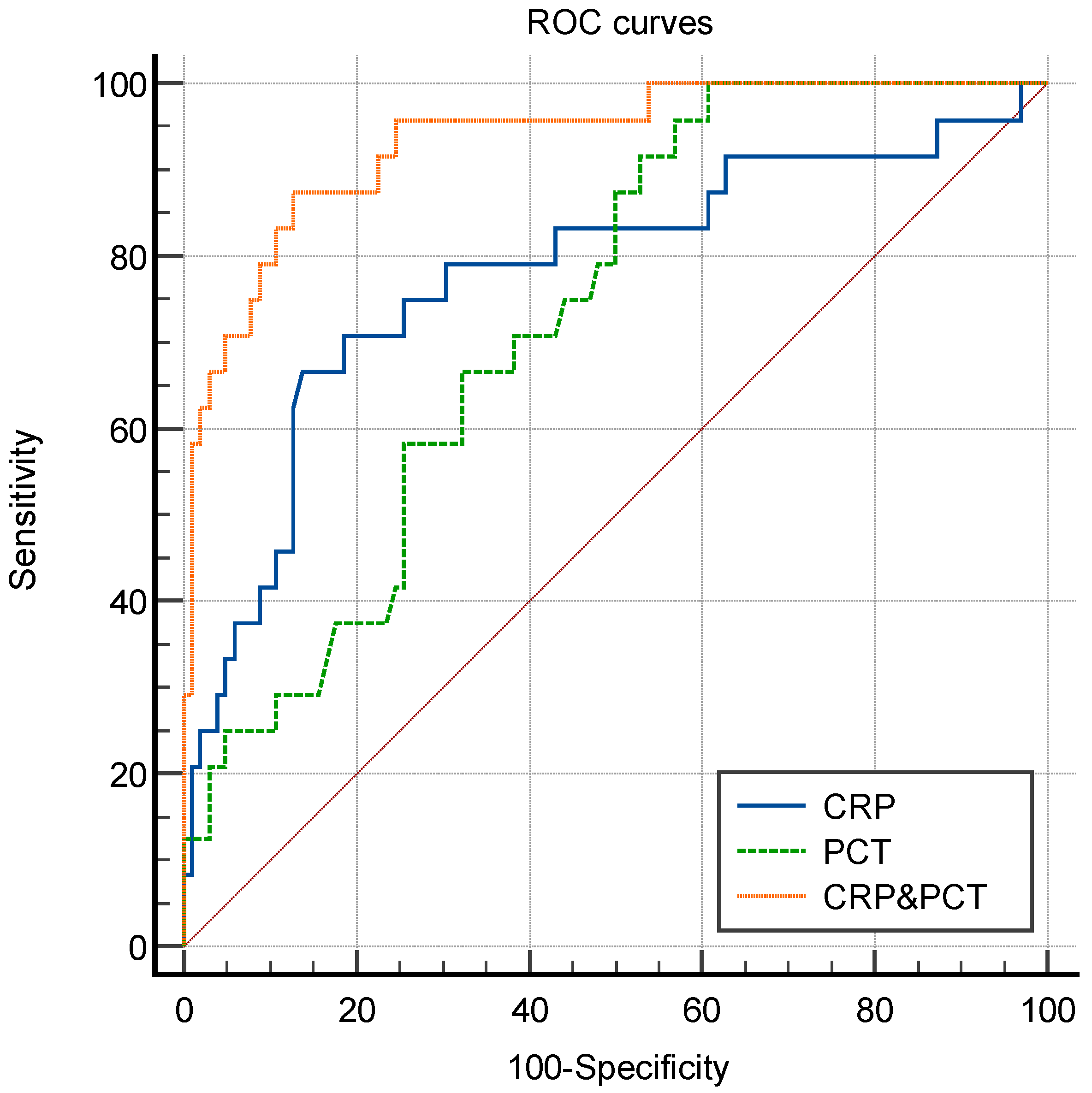
| Biomarker | AUC | Cut-Off * | Sensitivity (%) | Specificity (%) | PLR | NLR | PPV (%) | NPV (%) |
|---|---|---|---|---|---|---|---|---|
| CRP | 0.780 (0.664–0.896) | 94.93 mg/L | 66.7 (46.7–82.0) | 86.3 (78.3–91.6) | 4.86 | 0.39 | 53.3 | 91.7 |
| PCT | 0.731 (0.634–0.828) | 2.00 μg/L | 95.8 (79.8–99.8) | 43.1 (34.0–52.8) | 1.69 | 0.10 | 28.4 | 97.8 |
| CRP&PCT | 0.934 (0.881–0.987) | - | 87.5 (69.0–95.7) | 87.3 (79.4–92.4) | 6.87 | 0.14 | 88.2 | 91.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Zhang, X.; Yue, T.; Tang, Y.; Ke, Z.; Li, Y.; Luo, X.; Huang, L. Combination of C-Reactive Protein and Procalcitonin in Distinguishing Fungal from Bacterial Infections Early in Immunocompromised Children. Antibiotics 2022, 11, 730. https://doi.org/10.3390/antibiotics11060730
Liu Y, Zhang X, Yue T, Tang Y, Ke Z, Li Y, Luo X, Huang L. Combination of C-Reactive Protein and Procalcitonin in Distinguishing Fungal from Bacterial Infections Early in Immunocompromised Children. Antibiotics. 2022; 11(6):730. https://doi.org/10.3390/antibiotics11060730
Chicago/Turabian StyleLiu, Yingli, Xiaoli Zhang, Tianfang Yue, Yanlai Tang, Zhiyong Ke, Yu Li, Xuequn Luo, and Libin Huang. 2022. "Combination of C-Reactive Protein and Procalcitonin in Distinguishing Fungal from Bacterial Infections Early in Immunocompromised Children" Antibiotics 11, no. 6: 730. https://doi.org/10.3390/antibiotics11060730
APA StyleLiu, Y., Zhang, X., Yue, T., Tang, Y., Ke, Z., Li, Y., Luo, X., & Huang, L. (2022). Combination of C-Reactive Protein and Procalcitonin in Distinguishing Fungal from Bacterial Infections Early in Immunocompromised Children. Antibiotics, 11(6), 730. https://doi.org/10.3390/antibiotics11060730






