Therapeutic Drug Monitoring of Amphotericin-B in Plasma and Peritoneal Fluid of Pediatric Patients after Liver Transplantation: A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patients’ Characteristics
2.2. Determination of Amphotericin-B Levels by LC-MS/MS
2.3. Statistical Analysis
3. Results
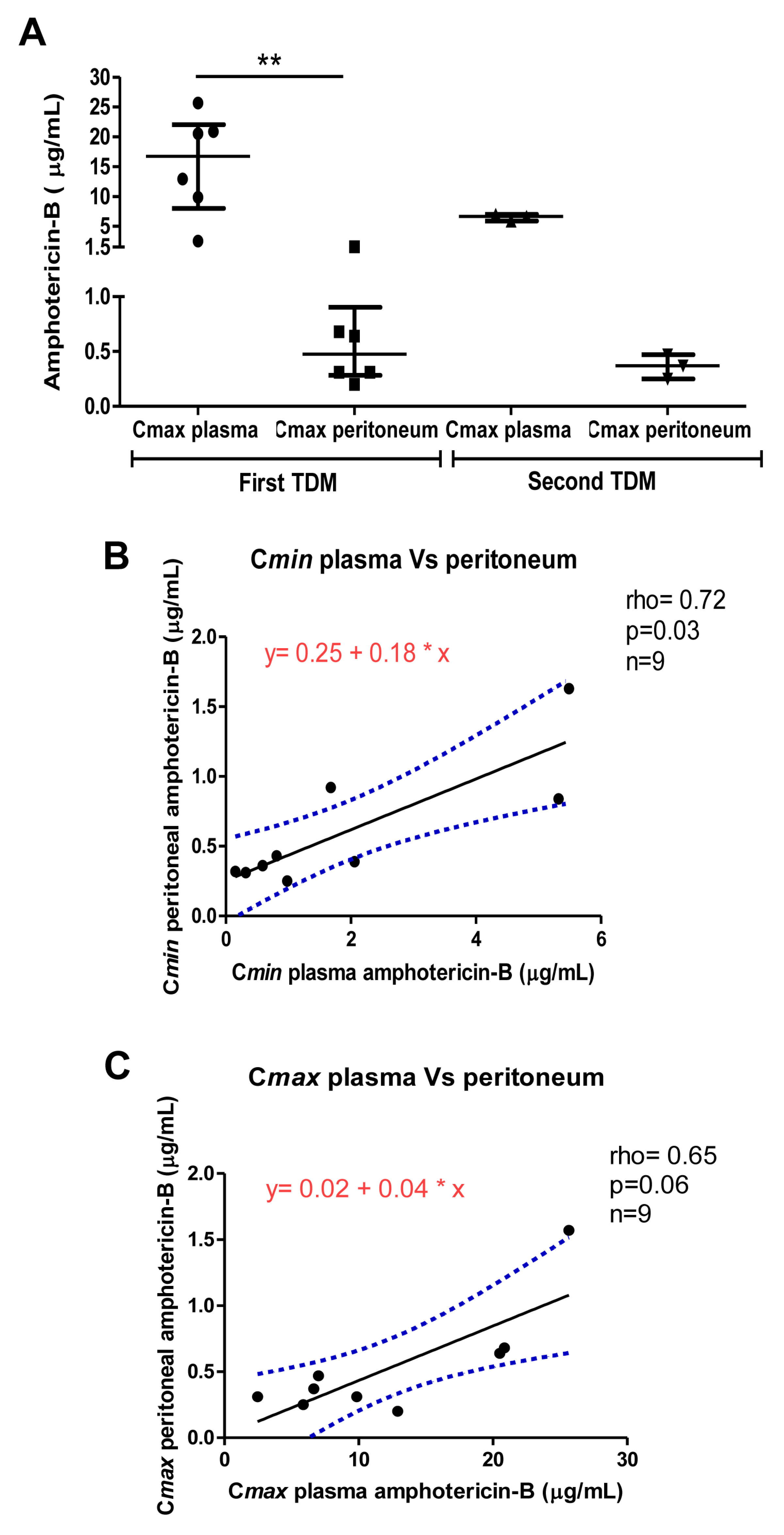
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grimaldi, C.; di Francesco, F.; Chiusolo, F.; Angelico, R.; Monti, L.; Muiesan, P.; de Ville de Goyet, J. Aggressive prevention and preemptive management of vascular complications after pediatric liver transplantation: A major impact on graft survival and long-term outcome. Pediatric Transplant. 2018, 22, e13288. [Google Scholar] [CrossRef] [PubMed]
- Albillos, A.; Lario, M.; Álvarez-Mon, M. Cirrhosis-associated immune dysfunction: Distinctive features and clinical relevance. J. Hepatol. 2014, 61, 1385–1396. [Google Scholar] [CrossRef]
- Ferrarese, A.; Cattelan, A.; Cillo, U.; Gringeri, E.; Russo, F.P.; Germani, G.; Gambato, M.; Burra, P.; Senzolo, M. Invasive fungal infection before and after liver transplantation. World J. Gastroenterol. 2020, 26, 7485–7496. [Google Scholar] [CrossRef]
- John, J.; Loo, A.; Mazur, S.; Walsh, T.J. Therapeutic drug monitoring of systemic antifungal agents: A pragmatic approach for adult and pediatric patients. Expert Opin. Drug Metab. Toxicol. 2019, 15, 881–895. [Google Scholar] [CrossRef] [PubMed]
- Lepak, A.J.; Andes, D.R. Antifungal pharmacokinetics and pharmacodynamics. Cold Spring Harb. Perspect. Med. 2014, 5, a019653. [Google Scholar] [CrossRef] [PubMed]
- Abbo, L.M.; Grossi, P.A. Surgical site infections: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13589. [Google Scholar] [CrossRef] [PubMed]
- Rex, J.H.; Walsh, T.J.; Sobel, J.D.; Filler, S.G.; Pappas, P.G.; Dismukes, W.E.; Edwards, J.E. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin. Infect. Dis. 2000, 30, 662–678. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Neupane, R.; Anjum, H.; Surani, S. Fungal infections following liver transplantation. World J. Hepatol. 2021, 13, 1653–1662. [Google Scholar] [CrossRef]
- Haidar, G.; Green, M. Intra-abdominal infections in solid organ transplant recipients: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13595. [Google Scholar] [CrossRef] [PubMed]
- Ikura, M.; Nakamura, T.; Uno, T.; Nakagita, K.; Takenaka, H.; Matsuda, S.; Oda, R.; Wada, K.; Hattori, Y.; Seguchi, O.; et al. Discontinuation of oral amphotericin B therapy does not influence the pharmacokinetics of tacrolimus in heart transplant patients. Int. J. Clin. Pharmacol. Ther. 2021, 59, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Gòmez-Lòpez, A. Antifungal therapeutic drug monitoring: Focus on drugs without a clear recommendation. Clin. Microbiol. Infect. 2020, 26, 1481–1487. [Google Scholar] [CrossRef]
- Peterson, L.R.; Kelty, R.H.; Hall, W.H.; Votava, H.J. Therapy of Candida peritonitis: Penetration of amphotericin B into peritoneal fluid. Postgrad. Med. J. 1978, 54, 340–342. [Google Scholar] [CrossRef][Green Version]
- Muther, R.S.; Bennett, W.M. Peritoneal clearance of amphotericin B and 5-fluorocytosine. West. J. Med. 1980, 133, 157–160. [Google Scholar]
- Felton, T.; Troke, P.F.; Hope, W.W. Tissue penetration of antifungal agents. Clin. Microbiol. Rev. 2014, 27, 68–88. [Google Scholar] [CrossRef]
- Cleary, J.D.; Hayman, J.; Sherwood, J.; Lasala, G.P.; Piazza-Hepp, T. Amphotericin B overdose in pediatric patients with associated cardiac arrest. Ann. Pharmacother. 1993, 27, 715–719. [Google Scholar] [CrossRef]
- Mohr, J.F.; Hall, A.C.; Ericsson, C.D.; Ostrosky-Zeichner, L. Fatal amphotericin B overdose due to administration of nonlipid formulation instead of lipid formulation. Pharmacotherapy 2005, 25, 426–428. [Google Scholar] [CrossRef]
- Van der Voort, P.H.J.; Boerma, E.C.; Yska, J.P. Serum and intraperitoneal levels of amphotericin B and flucytosine during intravenous treatment of critically ill patients with Candida peritonitis. J. Antimicrob. Chemother. 2007, 59, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, H.; Inoue, S.; Matsuhisa, A.; Nishiguchi, S. Diagnosis of spontaneous bacterial peritonitis and an in situ hybridization approach to detect an “unidentified” pathogen. Int. J. Hepatol. 2014, 2014, 634617. [Google Scholar] [CrossRef] [PubMed]
- Ross, J.T.; Matthay, M.A.; Harris, H.W. Secondary peritonitis: Principles of diagnosis and intervention. Br. Med. J. 2018, 36, k1407. [Google Scholar] [CrossRef] [PubMed]

| Patients | Age 1 | Weight 2 | Primary Disease | Cirrhosis | Candida Albicans in Peritoneum | Candida Albicans in Blood | Bacteria Peritoneum | Antimicrobial Therapy |
|---|---|---|---|---|---|---|---|---|
| Patient 1 | 242 | 50 | Biliary cirrhosis | yes | yes | yes | Enterococcus Faecalis Enterobacter Cloacae Klebsiella Pneumonie | vancomycin meropenem tigecycline |
| Patient 2 | 5 | 6 | Biliary atresia | yes | no | no | Enterococcus Faecalis Klebsiella Pneumoniae | teicoplanin meropenem amikacin |
| Patient 3 | 18 | 11 | Biliary atresia | yes | yes | no | Enterococcus Faecium Pseudomonas Aeruginosa | vancomycin meropenem |
| Patient 4 | 5 | 6 | Biliary atresia | yes | no | no | Enterococcus Faecalis | meropenem teicoplanin levofloxacin |
| Patient 5 | 92 | 14 | Alagille syndrome | yes | yes | no | Enterococcus Faecium Pseudomonas Aeruginosa | vancomycin meropenem amikacin |
| Patient 6 | 236 | 50 | Biliary cirrhosis | yes | no | no | Enterococcus Faecium | teicoplanin |
| Patient | WC Blood cell/µL | WC/PMN Peritoneum mm3 | CRP mg/dL | PCT ng/mL |
|---|---|---|---|---|
| Patient 1 1st TDM | 31,190 | 15/7 | 8.8 | 2.94 |
| Patient 1 2nd TDM | 19,890 | 6867/4666 | 12.59 | 2.97 |
| Patient 2 1st TDM | 8690 | 1793/987 | 5.44 | 0.29 |
| Patient 3 1st TDM | 10,050 | 996/628 | 9.88 | 2.72 |
| Patient 3 2nd TDM | 6650 | 764/542 | 4.33 | 0.14 |
| Patient 4 1st TDM | 8780 | 82/36 | 5.26 | 0.51 |
| Patient 4 2nd TDM | 11,370 | 23/16 | 6.28 | 0.91 |
| Patient 5 1st TDM | 6480 | 450/387 | 3.89 | 0.18 |
| Patient 6 1st TDM | 13,111 | 809/679 | 0.57 | 1.06 |
| Patient 6 2nd TDM | 26,840 | 671/553 | 4.6 | 0.6 |
| First TDM | Second TDM | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Dosage mg/kg | Days after Amphotericin Start | Cmin Plasma | Cmin Peritoneum | Cmax Plasma | Cmax Peritoneum | Dosage mg/kg | Days after Amphotericin Start | Cmin Plasma | Cmin Peritoneum | Cmax Plasma | Cmax Peritoneum |
| 1 | 3 | 34 | 5.32 | 0.84 | 20.51 | 0.64 | 5 | 41 | 4.55 | 0.13 | --º | 0.21 |
| 2 | 3 | 9 | 0.59 | 0.36 | 2.47 | 0.31 | 3 | --º | --º | --º | --º | --º |
| 3 | 3 | 6 | 0.98 | 0.25 | 9.86 | 0.31 | 3 | 10 | 0.81 | 0.43 | 7.01 | 0.47 |
| 4 | 3 | 6 | 0.32 | 0.31 | 12.91 | 0.2 | 3 | 10 | 0.15 | 0.32 | 5.88 | 0.25 |
| 5 | 3 | 4 | 1.68 | 0.92 | 20.85 | 0.68 | 3 | --º | --º | --º | --º | --º |
| 6 | 3 | 5 | 5.49 | 1.63 | 25.66 | 1.57 | 3 | 10 | 2.06 | 0.39 | 6.65 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tortora, F.; Dei Giudici, L.; Simeoli, R.; Chiusolo, F.; Cairoli, S.; Bernaschi, P.; Bianchi, R.; Picardo, S.G.; Dionisi Vici, C.; Goffredo, B.M. Therapeutic Drug Monitoring of Amphotericin-B in Plasma and Peritoneal Fluid of Pediatric Patients after Liver Transplantation: A Case Series. Antibiotics 2022, 11, 640. https://doi.org/10.3390/antibiotics11050640
Tortora F, Dei Giudici L, Simeoli R, Chiusolo F, Cairoli S, Bernaschi P, Bianchi R, Picardo SG, Dionisi Vici C, Goffredo BM. Therapeutic Drug Monitoring of Amphotericin-B in Plasma and Peritoneal Fluid of Pediatric Patients after Liver Transplantation: A Case Series. Antibiotics. 2022; 11(5):640. https://doi.org/10.3390/antibiotics11050640
Chicago/Turabian StyleTortora, Francesca, Luigi Dei Giudici, Raffaele Simeoli, Fabrizio Chiusolo, Sara Cairoli, Paola Bernaschi, Roberto Bianchi, Sergio Giuseppe Picardo, Carlo Dionisi Vici, and Bianca Maria Goffredo. 2022. "Therapeutic Drug Monitoring of Amphotericin-B in Plasma and Peritoneal Fluid of Pediatric Patients after Liver Transplantation: A Case Series" Antibiotics 11, no. 5: 640. https://doi.org/10.3390/antibiotics11050640
APA StyleTortora, F., Dei Giudici, L., Simeoli, R., Chiusolo, F., Cairoli, S., Bernaschi, P., Bianchi, R., Picardo, S. G., Dionisi Vici, C., & Goffredo, B. M. (2022). Therapeutic Drug Monitoring of Amphotericin-B in Plasma and Peritoneal Fluid of Pediatric Patients after Liver Transplantation: A Case Series. Antibiotics, 11(5), 640. https://doi.org/10.3390/antibiotics11050640






