Abstract
This systematic review aims to evaluate the antimicrobial activity of α-mangostin derived from Garcinia mangostana against different microbes. A literature search was performed using PubMed and Science Direct until March 2022. The research question was developed based on a PICO (Population, Intervention, Control and Outcomes) model. In this study, the population of interest was microbes, α-mangostin extracted from Garcinia mangostana was used as exposure while antibiotics were used as control, followed by the outcome which is determined by the antimicrobial activity of α-mangostin against studied microbes. Two reviewers independently performed the comprehensive literature search following the predetermined inclusion and exclusion criteria. A methodological quality assessment was carried out using a scoring protocol and the risk of bias in the studies was analyzed. Reward screening was performed among the selected articles to perform a meta-analysis based on the pre-determined criteria. Case groups where α-mangostin extracted from Garcinia mangostana was incorporated were compared to groups using different antibiotics or antiseptic agents (control) to evaluate their effectiveness. A total of 30 studies were included; they were heterogeneous in their study design and the risk of bias was moderate. The results showed a reduction in microbial counts after the incorporation of α-mangostin, which resulted in better disinfection and effectiveness against multiple microbes. Additionally, the meta-analysis result revealed no significant difference (p > 0.05) in their effectiveness when α-mangostin was compared to commercially available antibiotics. α-mangostin worked effectively against the tested microbes and was shown to have inhibitory effects on microbes with antibiotic resistance.
1. Introduction
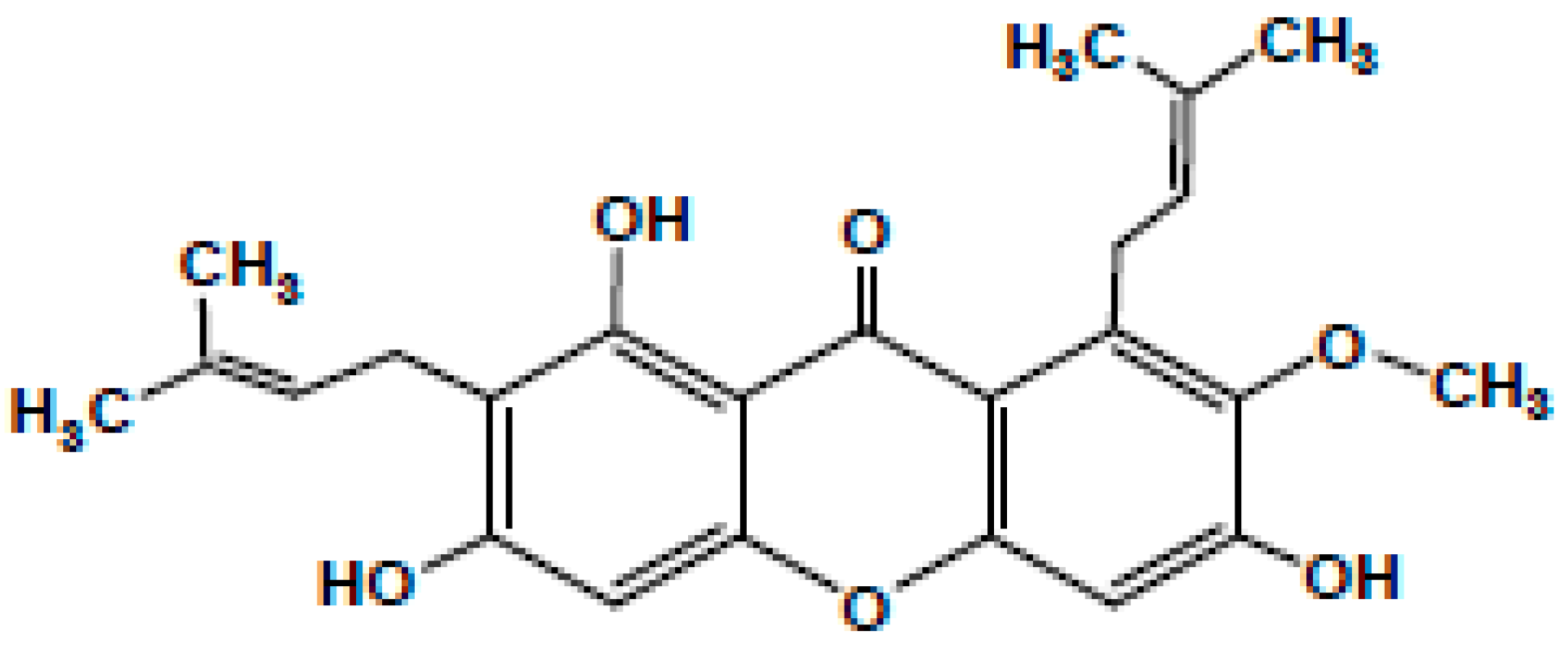
Garcinia mangostana Linn. (commonly known as mangostin), family Guttiferae a delicious and aromatic fruit native to China, India, Indonesia, Malaysia, Myanmar, the Philippines, Thailand, and other parts of Southeast Asia, is synonymous with good health and has been labelled as a ‘super fruit’ [1,2]. The fruit is intense with a slightly acidic taste [3]. It is well established to have medicinal properties that make it an intrinsic part of traditional Chinese, Thai, and Ayurvedic medicines [4,5] These useful properties occur due to the bioactive compounds such as xanthones, particularly the α, β, γ-mangostins found in Garcinia fruits [6]. Besides these, tannins, terpenes, anthocyanins, benzophenones, depsidones, phloroglucinols, polyphenols, and flavonoids are also present in Garcinia fruits [3,4] Along with the fruits, the pericarp, hull, rind, peel, roots, and bark are also rich in mangostins [5]. These xanthones have a tricyclic aromatic structure, occur naturally in plants, and are antibacterial [7]. Of these, the most prominent is α-mangostin, which has been chemically identified as 3,6,8-trihydroxy-2-methoxy-1,7-bis (3-methyl but-2-enyl) xanthan-9-one as shown in Figure 1, with a molecular weight of 410.45 g/mol [8]. The only difference between α-mangostin and its β version is the presence of a methyl group instead of a hydroxyl group [9,10,11]. The pharmacological aspects of α-mangostin were reviewed and it has been reported that its bioactivities vary from acting as an antiseptic to being an analgesic, antipyretic, antimicrobial, anti-parasitic, anti-malarial, anti-leishmanial, anti-hypertensive, anti-obesity, anti-proliferative, antioxidant, anti-inflammatory, anti-aggregation of β-amyloid, anti-tumour, anti-allergic, anti-mutagen, anti-cancer, cholinesterase inhibition, anti-HIV, anti-cariogenic, pro-apoptotic, anti-ageing, and neuroprotective agent against Alzheimer’s and Parkinson’s disorders [12]. Thus, α-mangostin has been employed for its antimicrobial properties, especially for dental treatments [3,4,9,13,14,15,16]. This can be critical for dentists, as the primary recognized reason for failure in any endodontic treatment has been connected to the presence of microbes in the apical part of the root canal [17]. Recently, Kasemwattananaroj et al. (2019) reported its immunomodulatory properties for the lymphocyte lineage and cytokine generation in human peripheral blood mononuclear cells (PBMCs) [18].
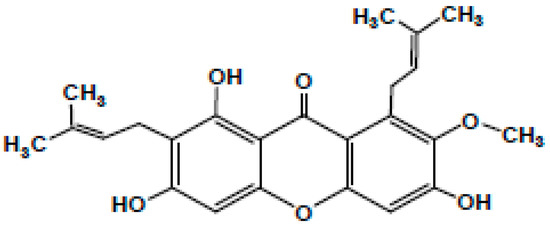
Figure 1.
Chemical structure of a-mangostin.
The extent of the antimicrobial action of α-mangostin has been explored by many researchers and it has been observed that it is not just effective against bacteria, but also against other microbes such as fungi and mycobacteria. However, the level of antibacterial activity varies from species to species. The major species of bacteria that have been studied include facultative anaerobic Gram-positive species such as Streptococcus, Enterococcus [9,19], Staphylococcus aureus [9,14,19], Propionibacterium acnes [1,20,21], Staphylococcus epidermidis and Salmonella [1,21,22,23]. In addition to this, the germicidal action of α-mangostin was also successfully reported against Gram-negative bacteria such as Pseudomonas aeruginosa, Klebsiella pneumoniae [24], and Escherichia coli [8,14]; fungi such as Candida albicans [14] and Aspergillus niger [8]; mycobacteria such as Mycobacterium tuberculosis; and viruses such as dengue [25]. In addition to this, Charernsriwilaiwat et al. (2013) successfully loaded an electrospun chitosan nanofiber mat with α-mangostin and used it to help in wound healing processes [26]. The mode of action of the mat starts with the penetration and breakdown of the lipid membranes due to the strong hydrophobic bonds or CAMP-like molecules attached to the bacterial surface via electrostatic bonds [27,28]. In some cases, the isolated compound was modified to match the needs, as there was no observed activity with the Gram-negative bacteria [24].
Alternate sources of α-mangostin from plant species other than G. mangostana and their bioactivities have also been reported by several researchers. Negi et al. (2008) described the antibacterial properties of the fruit rinds of G. cowa and G. pedunculata against Bacillus cereus, B. coagulans, Bacillus subtilis, Staphylococcus aureus, and E. coli [29]. Taher et al. (2012) studied the chemistry of the stembark of G. malaccensis and observed its activity against S. aureus and B. anthracis [30]. As alternatives to these plant products, certain chemicals such as chlorhexidine digluconate, [31] alexidine, chlorhexidine, cetrimide [32], and sodium hypochlorite [33] have been employed as topical antimicrobial agents. However, these have been known to cause harmful side effects such as allergic reactions and irritation [34]. There are hardly any reviews consolidating the action of mangostins in overcoming these microbial infections. Moreover, Chen et al. (2018) pointed out that the present knowledge does not give a clear picture of the mechanism of action of α-mangostin [10]. Therefore, an in-depth study of the advantages of α-mangostin as a potent antimicrobial agent is imperative for the development of new forms of microbial agents. This systematic review aims to fill this lacuna in the use of plant products as antimicrobial agents using data from experimental studies. This systematic review will extend our knowledge of the therapeutic utilization of α-mangostin concerning the development of antimicrobial agents and the extent of their interactions. α-mangostin may be beneficial as an agent against specific infections in human beings as well as in other applications such as the development of germ-free food products and cosmetics.
2. Materials and Methods
2.1. Study Design and Selection
This is a systematic review of experiments that aimed to measure the antimicrobial activities of α-mangostin extracted from Garcinia mangostana. This systematic review was carried out per the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [35].
2.2. PICO
The population or patient or problem, intervention or exposure, comparison, or control (microbes), intervention or exposure and outcome (PICO) principal strategy used for the structured review questions was as follows:
- Population/problem: microbes.
- Intervention or exposure: use of α-mangostin extracted from Garcinia mangostana.
- Comparison or control: antibiotics.
- Outcome: antimicrobial activity of α-mangostin against studied microbes.
Based on these, the following free form of the research question was constructed:
“Does α-mangostin extracted from Garcinia mangostana have effective antimicrobial properties?”
2.3. Search Strategy
Systematic searches were performed using standard electronic databases such as PubMed and ScienceDirect. These databases were selected based on the researchers’ belief that these repositories offered articles of the highest quality and were most relevant to the topic of the research. In the PubMed database, the diagnostic search terms utilized were “Garcinia mangostana”, “mangostin”, and “anti-bacterial” or “anti-fungal” or “antimicrobial” or “anti-infective agents”. Each database was searched from inception until March 2022. Different keywords in different combinations were used alongside these two terms to conduct the searches. Boolean search operators (AND, OR) were used to connect keywords where appropriate. Quotation marks were used to search for an exact phrase, and the truncation symbol (*) was used where appropriate to search for all words starting with a particular combination of letters, such as Garcinia mangostana, mangostin, alpha mangostin, anti-bacterial agents, anti-infective agents, antimicrobial, etc. For Science Direct, search words such as Garcinia mangostana, mangostin, alpha mangostin, α-mangostin, antimicrobial, antibacterial, and antifungal were used. The search was complemented by the manual screening of the shortlisted papers and individually checked for eligibility criteria. Duplicate references were removed as and when they were detected by the researcher. An example of the search strategy that was used for PubMed is illustrated in Table 1.

Table 1.
Keywords used for the search strategy in PubMed.
2.4. Eligibility Criteria for Inclusion of Studies
2.4.1. Inclusion Criteria
All the full-text articles were considered for assessment. Only studies where the source plant was Garcinia mangostana and the antimicrobial agent was in the chemical form of α-mangostin were included. Moreover, quantitative results were included, and the language was restricted to studies in English and full-text. The articles were screened according to their titles, plant sources, main constituents, and the name of the microbe to exclude irrelevant papers. However, no restrictions in terms of time were imposed for the inclusion of studies.
2.4.2. Exclusion Criteria
All plant sources other than Garcinia mangostana, such as G. cowa, G. malaccensis, G. smeathmanni, and Tetrigona melanoleuca were excluded from the study. Additionally, the studies were screened for the mention of α-mangostin specifically. Any studies related to β- or γ- mangostins or any other xanthones that did not mention the specific type of mangostin or plant species used were also excluded from this study. Additionally, all review papers, abstracts, and book chapters were eliminated from this study. Moreover, all papers which had studied α-mangostin from Garcinia mangostana, but which did not mention any microbes were also removed from this study.
2.5. Data Extraction and Analysis
The characteristics of the included studies were extracted independently using a standardized form. These included the country of the study; the antimicrobial agent used; the plant part used in preparing α-mangostin; the types of microbes involved, and antibacterial activity measured in terms of minimum inhibitory concentration (MIC), minimum bactericidal concentration (MBC), or minimum fungicidal concentration (MFC).
Outcome Measures
The primary outcomes measured were the antimicrobial activity of α-mangostin against studied microbes.
2.6. Quality Assessment [Risk of Bias]
A critical quality assessment of the included studies was conducted using the Joanna Briggs Institute (JBI) critical appraisal (JBI, 2017) [36]. The nine questions are shown in Appendix A. One point was assigned to every ‘yes’ answer, while answers of ‘no’ or which were unclear were given zero points. Data extraction was carried out independently by two reviewers based on a set of items considered key to the study outcome. Any disagreements between the reviewers were resolved by reaching a consensus, and, when necessary, differences were resolved by a third reviewer. A quality assessment was used to categorize the level of evidence provided by the included studies.
2.7. Meta-Analysis Scoring Criteria Assessment by Using Newcastle–Ottawa
The meta-analysis was carried out based on articles that evaluated the effectiveness of α-mangostin derived from Garcinia mangostana as an antimicrobial agent (case group) and compared them with different commercially available antibiotics (control group). A modified Newcastle–Ottawa scoring scale was used to determine the eligibility criteria for this meta-analysis. Please refer to Table 2 for the scoring scale. Among all the articles, only those articles were selected that received a score of 3 and others that scored <3 were excluded from this meta-analysis. The excluded articles along with their scores are recorded in Table 3.

Table 2.
Newcastle–Ottawa scoring criteria used for meta-analysis.

Table 3.
Articles excluded from this meta-analysis with their reasons for exclusion.
Data Extracted for Meta-Analysis
The meta-analysis result was synthesized using the statistical method of inverse variance of random effect with a 95% confidence interval. Cochrane Review Manager 5.4.1 was used to evaluate the heterogenicity with Tau2, chi2, p-value, and I2 and test for the overall effect with Z.
3. Results
3.1. Search Results
A total of 55 and 39 potentially relevant papers were identified using the shortlisted search words in the PubMed and ScienceDirect databases, respectively (Table 4). After reading the articles, manual screening was conducted, keeping the inclusion and exclusion criteria in mind. All the 94 articles included had been published as full texts. Of these, Samprasit et al., 2014, was the one paper not found as a full text in the library of the researcher [37]. The information required for screening was obtained from the abstract of that paper. Within the PubMed database, 6 out of 55 articles were excluded, as 2 of them were reviewed, 2 of them involved the use of G. cowa as the plant source, 1 of them involved G. malaccensis as the plant source, and 1 of them used Tetrigona melanoleuca as the plant source. G. mangostana was included in 49 articles. However, α-mangostin was used in only 33 articles. One of the articles mentioned γ-mangostin, and 15 of them failed to mention the type of mangostin or tested different derivatives/xanthones in their studies. Therefore, these 16 articles were excluded from our analysis. Three articles were later excluded as no microbes were involved. Therefore, 28 relevant articles on this subject were obtained from the PubMed database.

Table 4.
Summary of the screening process.
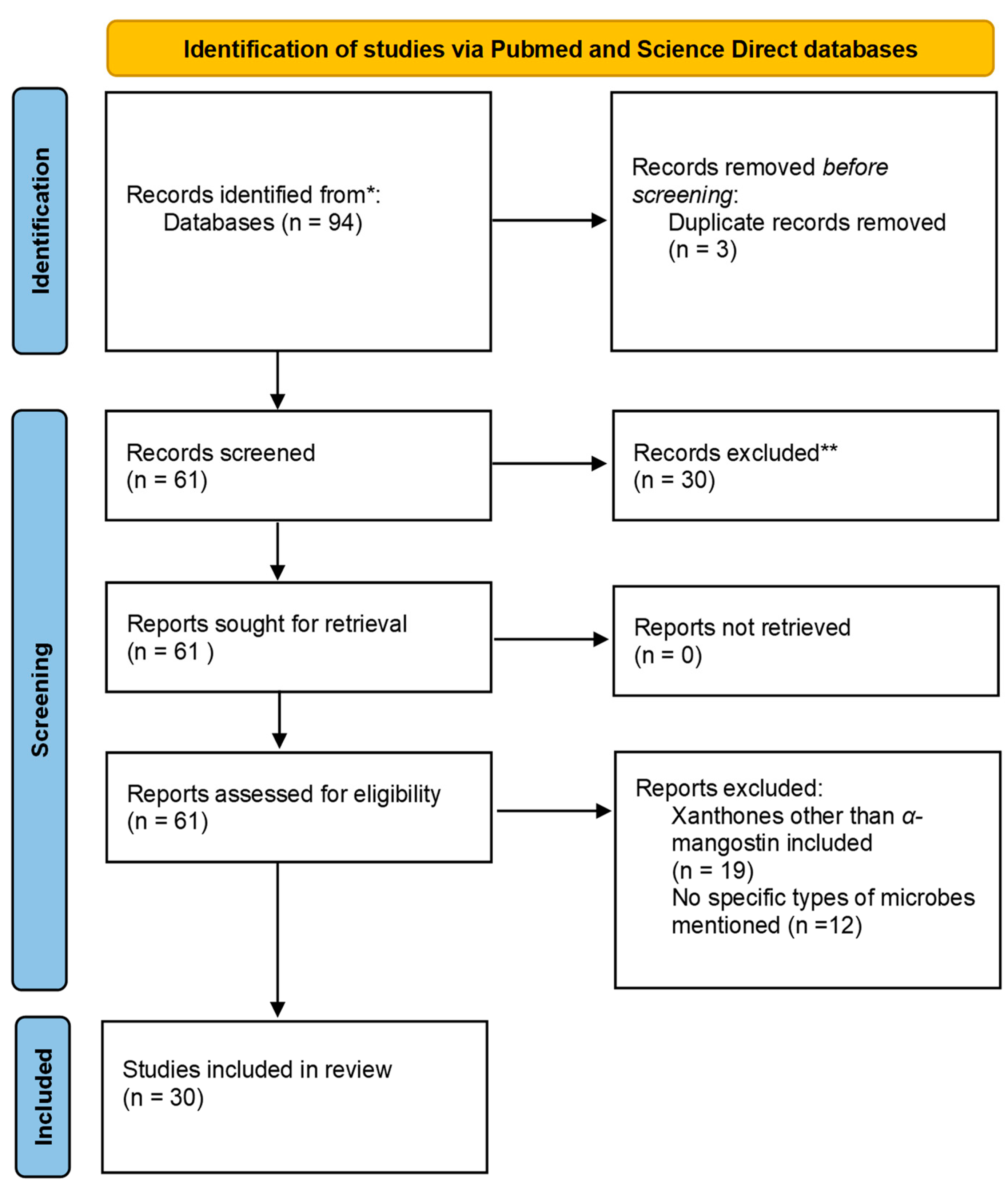
Similarly, 12 relevant articles were found from the list of articles obtained after screening the Science Direct database and removing the reviews. Of these, 9 involved studies on G. mangostana, and only 6 involved α-mangostin extracted from the fruit. Of these six articles, four were determined to be repeats from the PubMed search. Therefore, the Science Direct search gave us two unique articles to be included in this study. The PRISMA 2020 flowchart used is presented in Figure 2 [35].
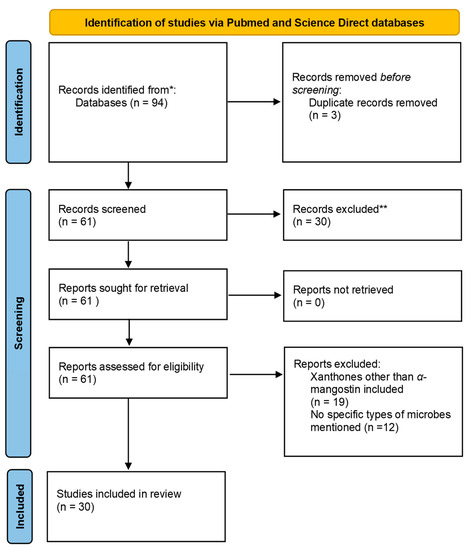
Figure 2.
Flowchart demonstrating the PRISMA chart. * Records Identified; ** Records Excluded.
3.2. Methodical Characterization of the Included Studies
Table 3 presents a summary of the 30 studies shortlisted for our review. These selected articles were published between the years 1996 and 2022. Many of them were reported from Thailand and Vietnam. However, there were a few instances from other countries such as Malaysia [38], Saudi Arabia [39], and Indonesia [7].
3.3. Risk of Bias in the Included Studies
All the included studies were evaluated for their risk of bias using the JBI appraisal tool (2017) as depicted in Table 5 [36]. The overall risk of bias in the included studies was moderate in all the cases. The differences in the studies were between the use of a control group and the use of some statistical tool for analysis. There was no control group in three studies [37,40,41], while statistical analysis was missing from 11 studies (Table 4). The quality of the score varied from five to six in total. The blinding of the participants was not possible due to the nature of the studies. Moreover, the allocation of the intervention groups was not concealed from the allocator in any of the studies. There were no multiple measurements of the outcome either before or after the intervention/exposure in any of them. Moreover, the reliability of the outcome was also unclear in all cases. The sixth question regarding the completion of the follow-up and any differences between groups in terms of whether their follow-up was adequately described and analyzed was not applicable for this study.

Table 5.
Risk of bias of included studies.
3.4. Meta-Analysis Result
Based on the predefined scoring criteria, eight articles were selected for this meta-analysis. These articles scored three and met all inclusion criteria. However, the rest of the articles that did not satisfy the criteria and scored <3, were excluded and are noted in Table 3.
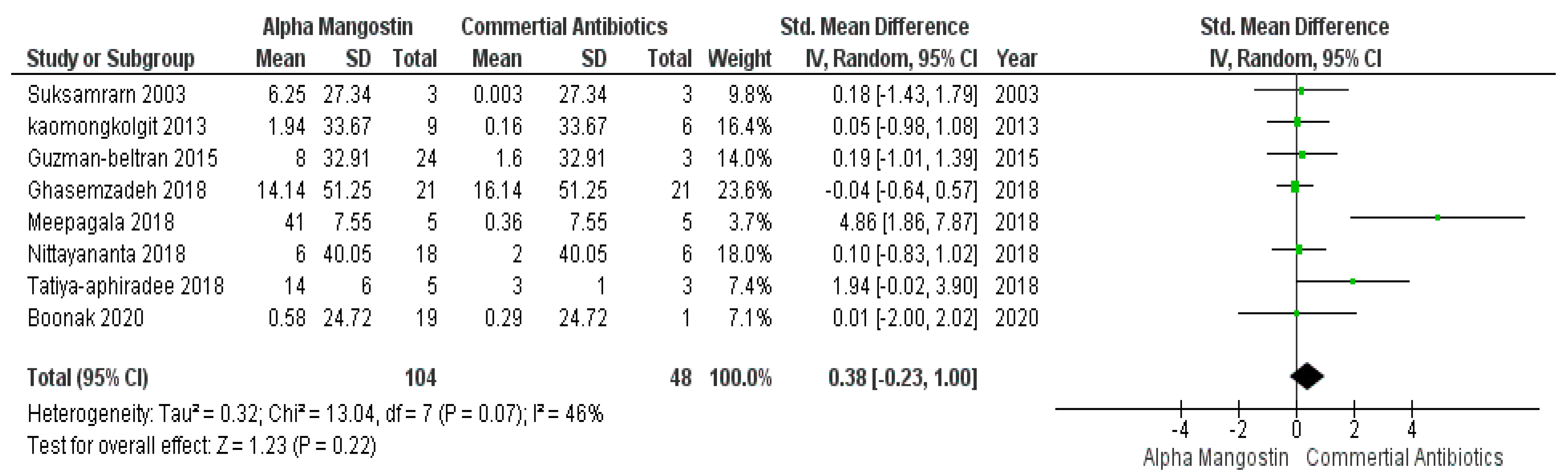
In Figure 3, studies those used commercially available antibiotics are plotted in control groups and those that incorporated α-mangostin are in the case group. This meta-analysis result evaluated that, based on the effectiveness, there were no significant differences (p > 0.05) between commercially available antibiotics and α-mangostin extracted from Garcinia mangostana, which means that both worked parallelly against different microbes. While the overall forest plot is showing no significant differences as the black diamond within Figure 3 exhibited inclination within the vertical line (i.e., the line of no effect), one study by Meepagala et al. [42] showed a significant difference hence the green square along with the horizontal line is situated away from the line of no effect that means individually the study showed an inclination towards the commercially available antibiotics over α-mangostin at a 95% confidence interval, I2 was 46% with heterogeneity Tau2 = 0.32, Chi2 = 13.04, df = 7 (p = 0.07), and test for overall effect, Z = 1.23 (p = 0.22).
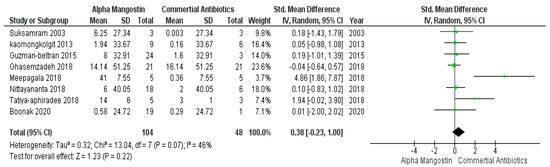
Figure 3.
Forest plot of studies on commercially available antibiotics and alpha mangostin.
4. Discussion
4.1. Standardization of the Garcinia Mangostana Extracts
The pericarp (hulls and peels) of G. mangostana was the most used plant part in the majority of studies included in this review; however, stem bark was used by Sakagami et al. (2005) [9]. The process of the extraction of xanthones and their isolation was initiated by the fractionation of the plant parts with hexane, benzene, acetone, and methanol; then, they were chromatographed on silica gel [7,9]. Methylene chloride was also used as a solvent and isolated β-mangostin along with α-mangostin, while Chomnawang et al. (2009) used chloroform and ethyl acetate in combination with other substances [43]. Pothitirat et al. (2009) extracted α-mangostin from G. mangostana using maceration and concentration techniques to avoid degradation and tested them for other bioactive compounds such as total phenolic compounds employing the Folin–Ciocalteu method, total flavonoids using the aluminum chloride colorimetric process, and total tannins using a protein precipitation assay [21]. The structural identification of the active isolates of α-mangostin was conducted using a spectrophotometer [7] or HPLC [1,21]. It was observed that the extracts from the younger fruits were richer in phenolics, flavonoids, and tannins compared to the older ones. To evaluate the actual effectiveness of α-mangostin against microbes, some studies had made comparisons between commercially available antibiotic agents and α-mangostin. These findings are in agreement with the current meta-analysis results which suggest no significant differences (p > 0.05) between the two groups.
4.2. The Efficacy of α-Mangostin on Microbes
From the data in Table 6, it can be summarised that xanthones—in particular, α-mangostin—are naturally-occurring antimicrobial agents. Iinuma et al., as early as 1996, reported the intense antibacterial properties of extracts obtained from G. mangostana and G. dioica against methicillin-resistant Staphylococcus aureus [7]. Similarly, Suksamrarn et al. (2003) has also reported that a-mangostin exhibited a strong inhibitory effect against Mycobacterium Tuberculosis when evaluated against standard drugs Rifampicin, Isoniazid, and Kanamycin [44]. Along with methicillin-resistant S. aureus, Sakagami et al. (2005) explored the possibility of employing antibiotics such as ampicillin, gentamicin, minocycline, and vancomycin hydrochloride in combination with α-mangostin against vancomycin-resistant Enterococci (VRE) with success; however, α-mangostin was found to be ineffective against E. coli, Proteus vulgaris, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Serratia marcescens [9]. Chomnawang et al. (2009) evaluated extracts from 17 different medicinal plants, including Garcinia, against S. aureus. Moreover, in addition to G. mangostana, inhibition was observed for other extracts as well. However, maximum inhibition was observed for G. mangostana, followed by Eupatorium odoratum [43]. Pothitirat et al. (2009) observed that the extracts from the younger fruits were richer in phenolics, flavonoids, and tannins compared to those from older ones. However, the reverse was true for antibacterial properties, where the rinds of matured fruit showed an increased amount of α-mangostin, indicating their higher levels of bacterial inhibition [21]. Therefore, it can be suggested that the age of the fruit has an impact on the expected application of α-mangostin. For cosmetic purposes, younger fruits are better; however, for antimicrobial properties, matured ones will function better.
Pothitirat et al. (2010) tested the antimicrobial properties of α-mangostin extracted by different means against Propionibacterium acnes and Staphylococcus epidermidis. Among all five methods of extraction—maceration, percolation, magnetic stirring, ultrasonic apparatus, and the Soxhlet process—the more efficient antimicrobial properties were observed in extracts obtained through the Soxhlet method [1]. In another study by Nguyen and Marquis (2011), cariogenic oral bacteria such as Streptococcus mutans were tested against α-mangostin, and it was observed that the glycolysis, membrane-bound enzymes, glycolytic enzymes, alkali production, and respiration of the bacterial cells were inhibited by α-mangostin. Moreover, it had a killer effect on the bacteria at high concentrations [45]. Arunrattiyakorn et al. (2011) carried out the first microbial transformation of α-mangostin into four modified novel xanthones and tested them against endophytic fungi such as Colletotrichum gloeosporioides, Neosartorya spathulate, and Mycobacterium tuberculosis. Only two of the five derivatives were found to be effective [46]. Kaomongkolgit et al. (2013) explored the effect of α-mangostin on Enterococcus faecalis, a bacterium commonly found in root canal irrigations, and observed it to have anti-bactericidal properties [47]. Additionally, the current study also supports the claim because within the forest plot there are no significant differences when a comparison was made between α-mangostin and commercially available antibiotics. Moreover, α-mangostin had a higher rate of killing the microbe than chlorhexidine (CHX), which is commonly used as an irrigating solution in root canal procedures. Koh et al. (2013) investigated the antimicrobial properties of α-mangostin against the methicillin-resistant Staphylococcus aureus and reported it to be the most potent anti-infective agent. The mode of action by which α-mangostin controls the bacteria was also elaborated. It was reported that α-mangostin targets the membrane of bacteria by depolarizing it and inducing approximately 37% leakage due to vesicle lysis within a very short period (about 5 to 10 min) [28]. Based on pharmaceutical nanotechnology, alternate forms of healing using nanofiber mats as effective wound dressings were developed and G. mangostana was integrated into these through the ultra-spinning method [26]. The mats created were then tested against S. aureus and E. coli and found to be highly bactericidal and nontoxic and to possess high tensile strength. The synergistic effects of plant products and antibiotics on enhancing the ‘antimicrobial spectrum’ were evaluated by Seesom et al. (2013) [48]. Al-Massaarani et al. (2013) investigated the antimicrobial properties of α-mangostin against multiple microbes such as Plasmodium falciparum, Leishmania infantum, Trypanosoma cruzi, T. brucei, Candida albicans, Escherichia coli, Pseudomonas aeruginosa, Bacillus subtilis, Staphylococcus aureus, Mycobacterium smegmatis, M. cheleneoi, M. xenopi, and M. intracellulare and reported a moderate amount of bacterial selectivity, leading to inconclusive results regarding its antimicrobial action [39]. Nguyen et al. (2014) reported that the virulent aspects of S. mutans and its biofilms were curtailed by α-mangostin [40]. Similarly, Asasutjarit et al. (2014) also reported on the anti-bacterial properties of α-mangostin against Propionibacterium acnes, starting from amounts of 1 to 32 μg/mL of α-mangostin [49].
Mohamed et al. (2014) pointed out that α-mangostin had a quorum-sensing inhibitory action against Chromobacterium violaceum, a weak inhibitory activity against E. coli and S. aureus, and no action against Candida albicans [3]. In 2015, Samprasit et al. (2015) reported the use of G. mangostana-integrated mucoadhesive electrospun nanofiber mats against pathogens involved in dental caries. The antibacterial activity of these nanofiber mats was successfully tested against S. mutans and S. sanguinis. A nanofiber mat imbued with Garcinia extract showed the highest bactericidal activity and decreased both in vivo and in vitro oral bacteria [50]. The effect of α-mangostin in synergy with antibiotics such as oxacillin on oxacillin-resistant S. saprophyticus was studied by Phitaktim et al. (2016). It was observed that the growth of the bacterial strain was restricted by isolated α-mangostin in conjugation with the antibiotic. The 1,4-benopyrone structure of α-mangostin was responsible for restricting the bacteria. It was suggested that the mode of action for inhibiting the bacteria follows a three-step process. First, the cytoplasmic membrane is disrupted, causing increased permeability. This is followed by the restriction of the β-lactamase activity and, lastly, the impairment of the peptidoglycan [13]. In vivo antibacterial activity against methicillin-resistant Staphylococcus aureus was also reported by Tatiya-aphiradee et al. (2016) in the pericarp of G. mangostana using a superficial skin infection following the tape-stripping mouse model. This was the first study that evaluated the efficacy of the use of Garcinia extracts in infections in mice. It was also suggested that α-mangostin extract is an optimal candidate for the development of an alternate topical formulation to overcome infections [16]. The sensitivity of α-mangostin toward Mycobacterium tuberculosis was evaluated and compared against commercially available antibiotics by Guzmán-Beltrán et al. (2016). It was observed that α-mangostin was able to inhibit the infection at both high and low concentrations in human macrophages which means the study is in agreement with current study findings [51]. Narasimhan et al. (2017) assessed the use of α-mangostin and its synthetic derivative byproducts as antimicrobial agents against microbes contaminating food packaging, such as E. coli, Bacillus subtilis, S. aureus, and P. aeruginosa, and fungi such as Candida albicans and Aspergillus niger. The zone of inhibition was measured for each bacterium, and all 13 derivatives displayed antimicrobial properties against all the bacteria and fungi investigated [8]. Phuong et al. (2017) isolated α-mangostin from the peel of mangosteen fruit and examined its antibacterial activity against three strains of biofilms of S. aureus in Vietnam. It was proven that α-mangostin was the key antimicrobial compound. However, it was speculated that the α-mangostin also interacted with the extracellular matrix proteins along with the membrane proteins [23]. Ghasemzadeh et al. (2018) optimized the extraction process of α-mangostin from the plant source to improve the quality of the α-mangostin extracted and investigated it against various bacterial strains such as Listeria ivanovii, Staphylococcus aureus, Mycobacterium smegmatis, Steptococcus uberis, Vibrio parahaemolyticus, Enterobacter cloacae, and E. coli. There was a significant difference in the antimicrobial activity between the optimized and non-optimized extracts. The inhibition zone was the largest in the extracts optimized against S. aureus. This was reported to be the first study focusing on the antimicrobial activities of optimized extracts against microbes, apart from S. aureus and E. coli [38]. Nittayananta et al. (2018) extended the use of natural plant products such as α-mangostin, which is a common antimicrobial agent used against oral pathogens in oral sprays. α-mangostin was evaluated against Candida albicans, Streptococcus mutans, and Porphyromonas gingivalis, and it was observed that α-mangostin restricted the growth of these microbes without causing cytotoxicity. Therefore, it was suggested as a complementary mode of treatment along with conventional dental treatments [52]. Meepagala et al. (2018) discovered that α-mangostin was found to be 10-fold less active than γ-mangostin against catfish pathogens, Flavobacterium columnare [42]. In contrast to a study carried out by Tatiya-aphiradee et al. (2016) [16], it was revealed that MRSA infected wounds treated with G. mangostana Pericarp Extract (GME) were completely healed on the 10th day as compared to incomplete healing when treated with α-mangostin. Tatiya-aphiradee et al. (2019) suggested that the greater anti-inflammatory and anti-bacterial activity of GME is attributed to the presence of other constituents in the GM pericarp [53]. Recently, Chokpaisarn et al. (2019) reported the use of Ya-Samarn-Phlae, a type of traditional Thai medicine containing G. mangostana, along with Curcuma longa, Oryza sativa, and Areca catechu against Pseudomonas aeruginosa. It showed a high level of inhibition of the bacteria [54]. Larsuporm et al. (2019) performed the first in vitro study against Staphylococcus pseudintermedius isolated from cases of canine pyoderma, which are commonly seen in dogs and cats. The results revealed that α-mangostin in mangostin crude extract measured by HPLC was effective against two strains of Staphylococcus pseudintermedius, MRSP and MSSP with no significant difference reported between both. It was suggested by the authors that mangosteen crude extract might be a good substitute against chlorhexidine due to reports of chlorhexidine resistance in MRSA and increasing MICs among humans [41]. Five xanthones were isolated from C. cochinchinense and G. mangostana and tested for antibacterial activity against MRSA and P. aeruginosa as described by Boonak et al. (2020). One of the isolates, α-mangostin, exhibited the highest antibacterial activity; however, it possessed poor pharmacokinetic properties rendering it unsuitable to be used in the in-vivo model due to hepatoxicity and mutagenicity problems. Hence, α-mangostin analogues were produced by partially modifying the xanthone under the acidic condition which was then proven to show high anti-MRSA and P. aerugionsa activity along with better pharmacokinetic properties as tested by ADMET software. It is also noted that one of the analogues exhibited a synergistic effect against MRSA and P. aeruginosa when coupled with vancomycin [55]. Thus, our study was able to consolidate the bactericidal tendencies of α-mangostin against various forms of microbes, which may be beneficial for controlling infection in general. An antimicrobial study performed in Malaysia concluded that, without any combination with the other antimicrobial agent, α-mangostin cannot play the role of an effective antimicrobial agent whereas commercial antibiotics alone could show effective results in fighting against microbes [56]. These findings are partially contrary to the findings of the current study but are supported by Meepagala et al., who suggested the commercially available antibiotics show a better antimicrobial effect over α-mangostin [42].
Another study proved that α-mangostin is not effective in the inhibition of gram-negative bacteria. However, α-mangostin alone or in a mixture with gentamicin against vancomycin-resistant Enterococci, and α-mangostin in a mixture with vancomycin hydrochloride against methicillin-resistant S. aureus, could be more effective in infection control measures. α-mangostin could thus play a role, used alone or in combination, in the inhibition of certain bacteria, as is partially supported by our study results. However, it is suggested that α-mangosteen should use in a combination with any commercial antibiotic agent to maximize its antimicrobial property [9]. It was also discovered from our study that certain microbes, such as the bacteria Enterococcus faecalis and Staphylococcus epidermidis and fungi Candida albicans, cause periodontic infections that have rarely been studied by researchers. This research can form the basis for future studies in many disciplines of medicine and dentistry.
4.3. Strengths and Limitations of This Study
The major strengths of this review involve the lack of any time limitations and the inclusion of all studies in which the fruits of Garcinia mangostana were used as a direct plant source. Moreover, this review compiles information from the major studies on this topic conducted across the world and gives a brief list of all the microbes that have been tested against α-mangostin. Our study also consolidates the amount of inhibition caused by these substances along with the antibiotic-resistant species. However, no study is without its limitations. Our study was subject to all limitations experienced by systematic reviews. Moreover, this review was limited to only two database searches and was confined to experimental studies concerning α-mangostin extracted from the plant parts of Garcinia mangostana. Other forms of mangostins or other plant sources, despite having microbiocidal properties, were not considered. No strict guidelines regarding the comparison with the control were followed. The reports were mostly confined to Southeast Asia. The assessment of study quality was limited due to the experimental nature of the studies. Moreover, the methodologies used by the reported studies were not of a standardized nature.
5. Conclusions
From the results of our systematic review and meta-analysis, it can be concluded that α-mangostin is effective against multiple microbes, including ones with antibiotic resistance. Additionally, α-mangostin, though plant-based, produced similar antimicrobial activity in comparison with commercially available antibiotics. To the best of our knowledge, this is the first systematic review on the potential of α-mangostin as an antimicrobial agent. Our study results suggested that both α-mangostin and commercial antibiotics showed similar antimicrobial effects in the inhibition of reported microorganisms such as M. tuberculosis, E. faecalis, L. ivanovii, S. aureus, M. smegmatis, S. uberis, V. parahaemolyticus, E. cloacae, E. coli, F. columnare, methicillin-resistant S. aureus, C. albicans, S. mutans, P. gingivalis, S. typhi, S. sonei and P. aeruginosa.
Implications for Future Studies
The lack of natural healing agents in today’s world necessitates the investigation of many pharmacological aspects of natural antimicrobial agents such as α-mangostin. The biggest advantage of α-mangostin lies in its non-toxic and safe nature. Moreover, the use of antibiotics as antimicrobial agents must also consider any toxic side effects. Thus, this study was undertaken in an attempt to understand the functioning of α-mangostin from Garcinia mangostana as an effective antimicrobial agent. Compounds such as this are the backbone of the drug industry and have wide pharmaceutical applications in the field of medical science. It was found that α-mangostin plays an active role in wound healing and is also useful in cosmetology and the food packaging and processing industry. Our study’s scope includes the usefulness of this substance to researchers from microbiology, botany, and ethnopharmacology; drug therapists; and all kinds of doctors dealing with the control of infection in various body parts. Based on our study, it can be suggested that α-mangostin is an ideal candidate for the development of a new category of antimicrobial compounds to overcome antibiotic resistance; these compounds have potential applications in the area of future pharmacology and healthcare and may also be used in the treatment of immune diseases. Apart from this, these compounds can be advantageous in the treatment of dental issues and for wound-healing purposes. The range of uses of α-mangostin can be vast, extending from the field of biomedicine to biomaterials, biomedical devices, food preservation, and many others.

Table 6.
Characteristics of the studies and their outcomes and results.
Table 6.
Characteristics of the studies and their outcomes and results.
| Study ID | Author | Country of Study | Main Antimicrobial Agent | Plant Part Used | Control | Microbes | Method | MIC * of Test | MIC * of Control | MBC **/MFC *** | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inhibitory (mm) | Count (µg/mL) | Inhibitory (mm) | Count (µg/mL) | Test Count (µg/mL) | Control Count (µg/mL) | ||||||||
| 1. | Chokpaisam et al. (2019) [54] | Thailand | Ya-Samarn-Phlae (YSP) | Pericarp of Garcinia, seeds of Areca catechu and Oryza sativa and rhizome of Cucurma longa | PBS | P. aeruginosa | Crystal violet assay | 12.29 μm | - | 18 | - | ||
| 2. | Ghasemzadeh et al. (2018) [38] | Malaysia | α-mangostin | Pericarp | Ciprofloxacin | L. ivanovii, S. aureus, Mycobacterium smegmatis, Streptococcus uberis, Vibrio parahaemolyticus, Enterobacter cloacae, E. coli | DPPH assay, FRAP assay, | 17, 18, 16, 14, 12, 12, 10 | 18, 16, 17, 18, 14, 12, 12, 10 | ||||
| 3. | Narasimhan et al. (2017) [8] | - | α-mangostin and synthetic derivatives | Dried fruits | Ciprofloxacin | E. coli, Bacillus subtilis, S. aureus, and P. aeruginosa, | Muller Hilton agar plates | 4 | 50 | 11 | 50 | ||
| Ketoconazole | Candida albicans, Aspergillus niger | Disc diffusion method | 13 | 100 | |||||||||
| 4. | Phuong et al. (2017) [23] | Vietnam | α-Mangostin | Peels | PBS | Staphylococcus aureus | Membrane activity assay | 4.58–9.15 μmol/L | 2 folds higher | ||||
| 5. | Phitaktim et al. (2016) [13] | Thailand | α-mangostin alone and combination with oxacillin and nisin | Matured dried fruit hulls | S. aureus | S. saprophyticus | MTT assays | 8 | 4 | ||||
| 6. | Tatiya-Aphiradee et al. (2016) [16] | Thailand | α- and γ-mangostin | Crude dried pericarp | Gentamicin, Erythromycin | Methicillin-resistant Staphylococcus aureus | Agar well diffusion assay | 10 | 0 | 6.25 | >10,000 | 100 | >10,000 |
| 7. | Samprasit et al. (2015) [50] | Thailand | α-mangostin | Pericarp | 0.2% w/v chlorhexidine | S. mutans and S. sanguinis | MTT assay | 0.1 mg/mL | 1 mg/mL | 0.2 mg/mL | |||
| 8. | Nguyen et al. (2014) [40] | Vietnam | α-mangostin | Peels | S. mutans | F-ATPase and phosphotransferase system (PTS) assays | |||||||
| 9. | Mohamed et al. (2014) [3] | Vietnam | Mangostanaxanthones I and II, 9-hydroxycalabaxanthone, parvifolixanthone C, α-mangostin and rubraxanthone | Air-dried pericarps | Ampicillin | Staphylococcus aureus, Bacillus cereus, Escherichia coli, and C. violaceum | Agar plate diffusion and dilution | 2 | 250 | 24 | - | ||
| Fluconazole | C. albicans and A. fumigatus | 0 | 20 | NA | NA | ||||||||
| 10. | Asasutjarit et al. (2014) [49] | Thailand | α-mangostin | Air-dried rind | Amoxicillin | Propionibacterium acnes | Microdilution assay | 0.5 | 0 | ||||
| 11. | Al-Massarani et al. (2013) [39] | Saudi Arabia | α-Mangostin | Pericarp | Amoxifen for MRC-5, chloroquine for P. falciparum, miltefosine for L. infantum, benznidazole for T. cruzi and suramin for T. brucei. | C. albicans, Plasmodium falciparum, Leishmania infantum and Trypanosoma cruzi and T. brucei, Escherichia coli, Pseudomonas aeruginosa, Bacillius subtilis, Staphylococcus aureus, Mycobacterium smegmatis, M. cheleneoi, M. xenopi and M. intracellulare. | Broth microdilution | NA | >200 µg/mL | NA | NA | NA | NA |
| 12. | Seesom et al. (2013) [48] | Thailand | α-, ϒ-Mangostin | Pericarp | Penicillin | Leptospira biflexa | Broth microdilution method | 200 to >800 | 0.39 to 6.25 | ||||
| 13. | Charernsriwilaiwat et al. (2013) [26] | - | α-Mangostin | Fruit hull | Penicillin | Staphylococcus aureus and Escherichia coli | Metal ion chelating assay | 0.5 | 0.5 | ||||
| 14. | Koh et al. (2013) [28] | - | 1,5,8-trihydroxy-3-methoxy-2-(3- methyl-2-butenyl) xanthone, γ-mangostin, garcinia E, α-mangostin and mangostenoneD | Fruit hull | Vancomycin | Methicillin-resistant Staphylococcus aureus (MRSA) | SYTOX green assay | 3.125 | 0.78–1.56 | ||||
| 15. | Arunrattiyakorn et al. (2011) [46] | - | a-Mangostin (1), mangostin 3-sulfate (2), mangosteen 6-sulfate (3), 17,18-dihydroxymangostanin 6-sulfate (4) and isomangostanin 3-sulfate (5). | Fruit hull | Rifampicin, streptomycin, isoniazid, and ofloxacin | Colletotrichum gloeosporioides and Neosartorya spathulata | Green fluorescent protein microplate assay (GFPMA) | 15.24 and 6.75 μm for 1 and 2, respectively, 3–5 showed no activity (MIC > 50 lg/mL). | 0.36 × 10−2, 1.46 × 10−2, 0.29–0.54, 0.17–0.34, and 1.08–2.16 μm for rifampicin, streptomycin, isoniazid, and ofloxacin, respectively. | ||||
| 16. | Nguyen et al. (2011) [45] | Vietnam | α-Mangostin | Peel | α-Mangostin with 25% ethanol | Streptococcus mutans | F-ATPase and phosphotransferase system (PTS) assays | 70% | |||||
| 17. | Pothitirat et al. (2010) [1] | Thailand | α-mangostin | Rind | other extracts (Hex, EtOH, H2O) | S. epidermidis, P. acne | Microdilution assay | 15.63, 7.8–15.63 | 3.91 µg/mL | NA | 7.81–500 µg/mL | 15.63 µg/mL | 31.25–(>500) µg/mL |
| 18. | Pothitirat et al. (2009) [21] | Thailand | α-mangostin | Matured rinds | Pure α-mangostin | S. epidermidis, P. acnes | Microdilution assay | NA | 15.63 for both | NA | 1.95 (P. acnes), 3.91 (S. epidermidis) | 15.63 (P.acnes), 31.25 (S. epidermidis) | 1.95 (P. acnes), 3.91 (S. epidermidis) |
| Young rinds | Pure α-mangostin | S. epidermidis, P. acnes | Microdilution assay | NA | 15.63 (P.acnes), 31.25 (S. epidermidis) | NA | 31.25 (P.acnes), 62.50 (S. epidermidis) | ||||||
| 19. | Chomnawang et al. (2009) [43] | Thailand | α-mangostin | Not mentioned | 16 other medicinal plants | Methicillin-resistant Staphylococcus aureus, S. epidermidis | Disc diffusion and microdilution assay | 11.3 ± 0.60 (S. aureus), 10.50 ± 0.70 (S. epidermidis) mm | 0.039 mg/mL for all | Not detected to 19.70 ± 0.60 mm | 0.625–(>5) mg/mL | 0.156 for both (mg/mL) | ≥5 mg/mL |
| 20. | Sakagami et al. (2005) [9] | - | α-, β- Mangostin | Stem bark | Gentamicin | Vancomycin-resistant Enterococci (VRE) | Agar Dilution | NA | 3.13 (α-Mangostin), 25 (β- Mangostin | NA | >100 | NA | NA |
| Methicillin-resistant Staphylococcus aureus (MRSA) | Agar Dilution | NA | 6.25 (α-Mangostin), >100 (β- Mangostin | NA | >100 | NA | NA | ||||||
| 21. | Iinuma et al. (1996) [7] | Indonesia | α-Mangostin | Dried and ground pericarp | Vancomycin, Gentamycin | Staphylococcus aureus | 1.57–>12.5 | 0.8 (Vancomycin) and 1.57 (Gentamicin) | |||||
| E. coli | Bioassay | 25 | >25 (Vancomycin) and 25 (Gentamicin) | ||||||||||
| 22. | Guzmán-Beltrán et al. (2015) [51] | - | α-Mangostin, NDGA | - | Rifampicin at 0.4 μg/mL | Mycobacterium tuberculosis | Colourimetric assay | 250 (NDGA), 62.5 (α-Mangostin) | |||||
| 23. | Kaomongkolgit et al. (2013) [47] | Thailand | α-Mangostin | Dried pericarps | NaOCl and CHX | Enterococcus faecalis | MTT assay | 1.97 | 0.15% (NaOCl), 2.5 (CHX) | 3.94 | 0.31% (NaOCl), 5 (CHX) | ||
| 24. | Nittayananta et al. (2018) [52] | Thailand | α-Mangostin and/or lawsone methyl ether (2-methoxy-1,4-naphthoquinone) (LME) | Pericarp | Gentamicin | Candida albicans | Microdilution assay | 625 mg/mL | 0.625 mg/mL | ||||
| Streptococcus mutans | Microdilution assay | 0.3125 mg/mL | >2.5 mg/mL | ||||||||||
| Porphyromonas gingivalis | Microdilution assay | 2.5 mg/mL | 2.5 mg/mL | ||||||||||
| 25 | Meepagala et al. (2018) [42] | USA | α-mangostinγ-Mangostin(-)-Epicatechin | Pericarp of Garcinia mangostana | Florfenicol | Flavobacterium columnare | MTT assay ALM-00-173 | - | 41.0 | - | 0.36 | - | - |
| 26 | Larsuprom et al. (2019) [41] | Thailand | a-mangostin | Pericarp of Garcinia mangostana | Methicillin-susceptible S. pseudintermedius (MSSP) methicillin-resistant S. pseudintermedius (MRSP) | Broth Microdilution Method | - | 0.53 ± 0.35 µg/mL, 0.47 ± 0.27 µg/mL | - | - | - | - | |
| 27 | Boonnak et al. (2020) [55] | Thailand | a-mangostin and derivatives norathyriol γ-Mangostin and deririatives dulxisxantone β-mangostin | CH2Cl2 extracts of the C. cochichinense resin and G. mangostana hulls | Vancomycin | MRSA B. subtilis E. faecalis VRE S. typhi S. sonei P. aeruginosa | Not stated | - | 2.34 2.34 150 150 18.75 150 2.34 | 2.34 | - | - | |
| 28. | Suksamsarn et al. (2003) [44] | Thailand | (1)a-mangostin | Fruit hulls and the edible arils and seeds of Garcinia mangostana | Rifam picin Isoniazid Kanamycin | Mycobacterium tuberculosis | Microplate Alamar Blue Assay | - | 6.25 | - | 0.003–0.0047, 0.025–0.05 1.25–2.5 | - | - |
| 29. | Tatiya-aphiradee et al. (2019) [53] | Thailand | a-mangostin | Pericarp extract of Garcinia mangostana | Oxacillin Erythromycin | MSSA ATCC 9144 MSSA ATCC 23235 MRSA DMST4738 MRSA DMST20651 MRSA DMST20654 | Micro-dilution method. | - | 3.625 7.250 12.50 6.25 6.25 | - | 0.800 0.800 O—25.00 E—>400 >400 O—200 E > 400 | - | - |
| 30. | Samprasit et al. (2014) [37] | - | α-Mangostin | - | Oral microbes | Time kill assay | Only abstract available to the researcher | ||||||
* MIC = minimum inhibitory concentration; ** MBC = minimum bactericidal concentration; *** MFC = minimum fungal concentration.
Author Contributions
Conceptualization, O.S.S.; methodology, O.S.S. and H.K.K.; software, S.Z.E.; validation, S.Z.E., F.R. and N.B.J.; formal analysis, N.B.J. and A.F.D.; investigation, O.S.S.; resources, Y.Y.T. and J.A.S.; data curation, Y.Y.T. and J.A.S.; writing—original draft preparation, O.S.S.; writing—review and editing, O.S.S. and S.P.K.; visualization, A.F.D. and M.K.A.; supervision, M.K.A.; project administration, A.F.D.; funding acquisition, O.S.S. All authors have read and agreed to the published version of the manuscript.
Funding
The study was funded by internal research from International Medical University IMU PMHS I-2018 (01). The funders had no role in study design, data collection and analysis, decision, or preparation of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data supporting this systematic review and meta-analysis are sourced from previously reported studies and datasets that have been cited. The processed data are available from the corresponding author upon request.
Conflicts of Interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Appendix A

Reviewer ______________________________________ Date_______________________________
Author_______________________________________ Year_________ Record Number_________
| Yes | No | Unclear | Not applicable | |
| □ | □ | □ | □ |
| □ | □ | □ | □ |
| □ | □ | □ | □ |
| □ | □ | □ | □ |
| □ | □ | □ | □ |
| □ | □ | □ | □ |
| □ | □ | □ | □ |
| □ | □ | □ | □ |
| □ | □ | □ | □ |
Overall appraisal: Include □ Exclude □ Seek further info □
Comments (Including reason for exclusion)
______________________________________________________________________________________________
______________________________________________________________________________________________
____________________________________________________________________
References
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Free radical scavenging and anti-acne activities of mangosteen fruit rind extracts prepared by different extraction methods. Pharm. Biol. 2010, 48, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Janardhan, S.; Mahendra, J.; Girija, A.S.S.; Mahendra, L.; Priyadharsini, V. Antimicrobial effects of Garcinia mangostana on cariogenic microorganisms. J. Clin. Diagn. Res. 2017, 11, ZC19–ZC22. [Google Scholar]
- Mohamed, G.A.; Ibrahim, S.R.M.; Shaaban, M.I.A.; Ross, S.A. Mangostanaxanthones I and II, new xanthones from the pericarp of Garcinia mangostana. Fitoterapia 2014, 98, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Pedraza-Chaverri, J.; Cárdenas-Rodríguez, N.; Orozco-Ibarra, M.; Pérez-Rojas, J.M. Medicinal properties of mangosteen (Garcinia mangostana). Food Chem. Toxicol. 2008, 46, 3227–3239. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.A.; Al-Abd, A.M.; El-halawany, A.M.; Abdallah, H.M.; Ibrahim, S.R.M. New xanthones and cytotoxic constituents from Garcinia mangostana fruit hulls against human hepatocellular, breast, and colorectal cancer cell lines. J. Ethnopharmacol. 2017, 198, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Shibata, M.A.; Matoba, Y.; Tosa, H.; Iinuma, M. Effects of mangosteen pericarp extracts against mammary cancer. Altern. Integr. Med. 2013, 2, 1000139. [Google Scholar]
- Iinuma, M.; Tosa, H.; Tanaka, T.; Asai, F.; Kobayashi, Y.; Shimano, R.; Miyauchi, K.I. Antibacterial activity of xanthones from guttiferaeous plants against methicillin-resistant Staphylococcus aureus. J. Pharm. Pharmacol. 1996, 48, 861–865. [Google Scholar] [CrossRef]
- Narasimhan, S.; Maheshwaran, S.; Abu-Yousef, I.A.; Majdalawieh, A.F.; Rethavathi, J.; Das, P.E.; Poltronieri, P. Anti-bacterial and anti-fungal activity of xanthones obtained via semi-synthetic modification of α-mangostin from Garcinia mangostana. Molecules 2017, 22, 275. [Google Scholar] [CrossRef] [Green Version]
- Sakagami, Y.; Iinuma, M.; Piyasena, K.G.N.P.; Dharmaratne, H.R.W. Antibacterial activity of α-mangostin against vancomycin resistant Enterococci (VRE) and synergism with antibiotics. Phytomedicine 2005, 12, 203–208. [Google Scholar] [CrossRef]
- Chen, G.; Li, Y.; Wang, W.; Deng, L. Bioactivity and pharmacological properties of α-mangostin from the mangosteen fruit: A review. Expert Opin. Ther. Pat. 2018, 28, 415–427. [Google Scholar] [CrossRef]
- Wang, M.H.; Zhang, K.J.; Gu, Q.L.; Bi, X.L.; Wang, J.X. Pharmacology of mangostins and their derivatives: A comprehensive review. Chin. J. Nat. Med. 2017, 15, 81–93. [Google Scholar] [CrossRef]
- Steinmetz, K.A.; Potter, J.D. Vegetables, fruit, and cancer prevention: A review. J. Am. Diet. Assoc. 1996, 96, 1027–1039. [Google Scholar] [CrossRef]
- Phitaktim, S.; Chomnawang, M.; Sirichaiwetchakoon, K.; Dunkhunthod, B.; Hobbs, G.; Eumkeb, G. Synergism and the mechanism of action of the combination of α-mangostin isolated from Garcinia mangostana L. and oxacillin against an oxacillin-resistant Staphylococcus saprophyticus. BMC Microbiol. 2016, 16, 195. [Google Scholar] [CrossRef]
- Ragasa, C.Y.; Crisostomo, C.J.J.; Garcia, K.; Shen, C. Antimicrobial xanthones from Garcinia mangostana L. Philipp. Scient. 2010, 47, 63–75. [Google Scholar]
- Alsultan, Q.M.N.; Sijam, K.; Rashid, T.S.; Ahmad, K.B. GC-MS Analysis and antibacterial activity of mangosteen leaf extracts against plant pathogenic bacteria. Am. J. Plant Sci. 2016, 7, 1013. [Google Scholar] [CrossRef] [Green Version]
- Tatiya-aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. In vivo antibacterial activity of Garcinia mangostana pericarp extract against methicillin-resistant Staphylococcus aureus in a mouse superficial skin infection model. Pharm. Biol. 2016, 54, 2606–2615. [Google Scholar] [CrossRef] [Green Version]
- Murad, C.F.; Sassone, L.M.; Faveri, M.; Hirata, R.; Figueiredo, L.; Feres, M. Microbial diversity in persistent root canal infections investigated by checkerboard DNA-DNA hybridization. J. Endod. 2014, 40, 899–906. [Google Scholar] [CrossRef]
- Kasemwattanaroj, P.; Moongkarndi, P.; Pattanapanyasat, K.; Mangmool, S.; Rodpai, E.; Samer, J.; Sukapirom, K. Immunomodulatory Activities of α-Mangostin on Peripheral Blood Mononuclear Cells. Nat. Prod. Commun. 2019, 8, 1934578X1300800. [Google Scholar] [CrossRef] [Green Version]
- Dharmaratne, H.R.W.; Sakagami, Y.; Piyasena, K.G.P.; Thevanesam, V. Antibacterial activity of xanthones from Garcinia mangostana (L.) and their structure-activity relationship studies. Nat. Prod. Res. 2013, 27, 938–941. [Google Scholar] [CrossRef]
- Chomnawang, M.T.; Surassmo, S.; Nukoolkarn, V.S.; Gritsanapan, W. Antimicrobial effects of Thai medicinal plants against acne-inducing bacteria. J. Ethnopharmacol. 2005, 101, 330–333. [Google Scholar] [CrossRef]
- Pothitirat, W.; Chomnawang, M.T.; Supabphol, R.; Gritsanapan, W. Comparison of bioactive compounds content, free radical scavenging and anti-acne inducing bacteria activities of extracts from the mangosteen fruit rind at two stages of maturity. Fitoterapia 2009, 80, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Sivaranjani, M.; Prakash, M.; Gowrishankar, S.; Rathna, J.; Pandian, S.K.; Ravi, A.V. In vitro activity of alpha-mangostin in killing and eradicating Staphylococcus epidermidis RP62A biofilms. Appl. Microbiol. Biotechnol. 2017, 101, 3349–3359. [Google Scholar] [CrossRef] [PubMed]
- Phuong, N.T.M.; Van Quang, N.; Mai, T.T.; Anh, N.V.; Kuhakarn, C.; Reutrakul, V.; Bolhuis, A. Antibiofilm activity of α-mangostin extracted from Garcinia mangostana L. against Staphylococcus aureus. Asian Pac. J. Trop. Med. 2017, 10, 1154–1160. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Koh, J.J.; Li, J.; Qiu, S.; Aung, T.; Lin, H.; Beuerman, R. Design and synthesis of amphiphilic xanthone-based, membrane-targeting antimicrobials with improved membrane selectivity. J. Med. Chem. 2013, 56, 2359–2373. [Google Scholar] [CrossRef] [PubMed]
- Tarasuk, M.; Songprakhon, P.; Chimma, P.; Sratongno, P.; Na-Bangchang, K.; Yenchitsomanus, T.P. Alpha-mangostin inhibits both dengue virus production and cytokine/chemokine expression. Virus Res. 2017, 240, 180–189. [Google Scholar] [CrossRef]
- Charernsriwilaiwat, N.; Rojanarata, T.; Ngawhirunpat, T.; Sukma, M.; Opanasopit, P. Electrospun chitosan-based nanofiber mats loaded with Garcinia mangostana extracts. Int. J. Pharm. 2013, 452, 333–343. [Google Scholar] [CrossRef]
- Wimley, W.C. Describing the mechanism of antimicrobial peptide action with the interfacial activity model. ACS Chem. Biol. 2010, 5, 905–917. [Google Scholar] [CrossRef] [Green Version]
- Koh, J.J.; Qiu, S.; Zou, H.; Lakshminarayanan, R.; Li, J.; Zhou, X.; Beuerman, R.W. Rapid bactericidal action of alpha-mangostin against MRSA as an outcome of membrane targeting. Biochim. Biophys. Acta-Biomembr. 2013, 1828, 834–844. [Google Scholar] [CrossRef] [Green Version]
- Negi, P.S.; Jayaprakasha, G.K.; Jena, B.S. Antibacterial activity of the extracts from the fruit rinds of Garcinia cowa and Garcinia pedunculata against food borne pathogens and spoilage bacteria. LWT-Food Sci. Technol. 2008, 41, 1857–1861. [Google Scholar] [CrossRef]
- Taher, M.; Susanti, D.; Rezali, M.F.; Zohri, F.S.A.; Ichwan, S.J.A.; Alkhamaiseh, S.I.; Ahmad, F. Apoptosis, antimicrobial and antioxidant activities of phytochemicals from Garcinia malaccensis Hk.f. Asian Pac. J. Trop. Med. 2012, 5, 136–141. [Google Scholar] [CrossRef]
- McBain, A.J.; Bartolo, R.G.; Catrenich, C.E.; Charbonneau, D.; Ledder, R.G.; Gilbert, P. Effects of a chlorhexidine gluconate-containing mouthwash on the vitality and antimicrobial susceptibility of in vitro oral bacterial ecosystems. Appl. Environ. Microbiol. 2003, 69, 4770–4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Linares, M.; Ferrer-Luque, C.M.; Arias-Moliz, T.; de Castro, P.; Aguado, B.; Baca, P. Antimicrobial activity of alexidine, chlorhexidine and cetrimide against Streptococcus mutans biofilm. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vianna, M.E.; Gomes, B.P.; Berber, V.B.; Zaia, A.A.; Ferraz, C.C.R.; de Souza-Filho, F.J. In vitro evaluation of the antimicrobial activity of chlorhexidine and sodium hypochlorite. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2004, 97, 79–84. [Google Scholar] [CrossRef]
- Sodata, P.; Juntavee, A.; Juntavee, N.; Peerapattana, J. Optimization of Adhesive Pastes for Dental Caries Prevention. AAPS PharmSciTech 2017, 18, 3087–3096. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- JBI Checklist for Quasi-Experimental Studies (Non-Randomized Experimental Studies). 2017. Available online: http://joannabriggs.org/research/critical-appraisal-tools.html (accessed on 11 April 2022).
- Samprasit, W.; Opanasopit, P.; Sukma, M.; Kaomongkolgit, R. Antibacterial activity of Garcinia mangostana extracts on oral pathogens. Minerva Stomatol. 2014, 63, 249–257. [Google Scholar]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Baghdadi, A.; Tayebi-Meigooni, A. Alpha-mangostin-rich extracts from mangosteen pericarp: Optimization of green extraction protocol and evaluation of the biological activity. Molecules 2018, 23, 1852. [Google Scholar] [CrossRef] [Green Version]
- Al-Massarani, S.M.; El Gamal, A.A.; Al-Musayeib, N.M.; Mothana, R.A.; Basudan, O.A.; Al-Rehaily, A.J.; Maes, L. Phytochemical, antimicrobial and antiprotozoal evaluation of Garcinia mangostana pericarp and α-mangostin, its major xanthone derivative. Molecules 2013, 18, 10599–10608. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, P.T.M.; Falsetta, M.L.; Hwang, G.; Gonzalez-Begne, M.; Koo, H. α-mangostin disrupts the development of Streptococcus mutans biofilms and facilitates its mechanical removal. PLoS ONE 2014, 9, e111312. [Google Scholar] [CrossRef] [Green Version]
- Larsuprom, L.; Rungroj, N.; Lekcharoensuk, C.; Pruksakorn, C.; Kongkiatpaiboon, S.; Chen, C.; Sukatta, U. In vitro antibacterial activity of mangosteen (Garcinia mangostana Linn.) crude extract against Staphylococcus pseudintermedius isolates from canine pyoderma. Vet. Dermatol. 2019, 30, 487. [Google Scholar] [CrossRef]
- Meepagala, K.; Schrader, K. Antibacterial Activity of Constituents from Mangosteen Garcinia mangostana Fruit Pericarp against Several Channel Catfish Pathogens. J. Aquat. Anim. Health 2018, 30, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Chomnawang, M.T.; Surassmo, S.; Wongsariya, K.; Bunyapraphatsara, N. Antibacterial Activity of Thai Medicinal Plants against Methicillin-resistant Staphylococcus aureus. Fitoterapia 2009, 80, 102–104. [Google Scholar] [CrossRef] [PubMed]
- Suksamrarn, S.; Suwannapoch, N.; Phakhodee, W.; Thanuhiranlert, J.; Ratananukul, P.; Chimnoi, N.; Suksamrarn, A. Antimycobacterial Activity of Prenylated Xanthones from the Fruits of Garcinia mangostana. Chem. Pharm. Bull. 2003, 51, 857–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, P.T.M.; Marquis, R.E. Antimicrobial actions of α-mangostin against oral streptococci. Can. J. Microbiol. 2011, 57, 217–225. [Google Scholar] [CrossRef] [PubMed]
- Arunrattiyakorn, P.; Suksamrarn, S.; Suwannasai, N.; Kanzaki, H. Microbial metabolism of α-mangostin isolated from Garcinia mangostana L. Phytochemistry 2011, 72, 730–734. [Google Scholar] [CrossRef] [PubMed]
- Kaomongkolgit, R.; Jamdee, K.; Pumklin, J.; Pavasant, P. Laboratory evaluation of the antibacterial and cytotoxic effect of alpha-mangostin when used as a root canal irrigant. Indian J. Dent. 2013, 4, 12–17. [Google Scholar] [CrossRef]
- Seesom, W.; Jaratrungtawee, A.; Suksamrarn, S.; Mekseepralard, C.; Ratananukul, P.; Sukhumsirichart, W. Antileptospiral activity of xanthones from Garcinia mangostana and synergy of gamma-mangostin with penicillin G. BMC Complementary Altern. Med. 2013, 13, 182. [Google Scholar] [CrossRef] [Green Version]
- Asasutjarit, R.; Larpmahawong, P.; Fuongfuchat, A.; Sareedenchai, V.; Veeranondha, S. Physicochemical Properties and Anti-Propionibacterium acnes activity of film-forming solutions containing alpha-mangostin-rich extract. AAPS PharmSciTech 2014, 15, 306–316. [Google Scholar] [CrossRef] [Green Version]
- Samprasit, W.; Kaomongkolgit, R.; Sukma, M.; Rojanarata, T.; Ngawhirunpat, T.; Opanasopit, P. Mucoadhesive electrospun chitosan-based nanofibre mats for dental caries prevention. Carbohydr. Polym. 2015, 117, 933–940. [Google Scholar] [CrossRef]
- Guzmán-Beltrán, S.; Rubio-Badillo, M.Á.; Juárez, E.; Hernández-Sánchez, F.; Torres, M. Nordihydroguaiaretic acid (NDGA) and α-mangostin inhibit the growth of Mycobacterium tuberculosis by inducing autophagy. Int. Immunopharmacol. 2016, 31, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Nittayananta, W.; Limsuwan, S.; Srichana, T.; Sae-Wong, C.; Amnuaikit, T. Oral spray containing plant-derived compounds is effective against common oral pathogens. Arch. Oral Biol. 2018, 90, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Tatiya-aphiradee, N.; Chatuphonprasert, W.; Jarukamjorn, K. Anti-inflammatory effect of Garcinia mangostana Linn. pericarp extract in methicillin-resistant Staphylococcus aureus-induced superficial skin infection in mice. Biomed. Pharmacother. 2019, 111, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Chokpaisam, J.; Yincharoen, K.; Sanpinit, S.; Karutha Pandian, S.T.; Nandhini, J.R.; Gowrishankar, S.; Chusri, S. Effects of a traditional Thai polyherbal medicine ‘Ya-Samarn-Phlae’ as a natural anti-biofilm agent against Pseudomonas aeruginosa. Microb. Pathog. 2019, 128, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Boonnak, N.; Chantrapromma, S.; Sathirakul, K.; Kaewpiboon, C. Modified tetra-oxygenated xanthones analogues as anti-MRSA and P. aeruginosa agent and their synergism with vancomycin. Bioorganic Med. Chem. Lett. 2020, 30, 127494. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.H.; Taher, M.; Susanti, D.; Amiroudine, M.Z.; Ahmed, Q.U. Direct and indirect antimicrobial effects of α-mangostin on the pathogenic microorganism. J. Coast. Life Med. 2014, 2, 70–75. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).