A Review: Antimicrobial Therapy for Human Pythiosis
Abstract
1. Introduction
2. Principles of Antimicrobial Therapy
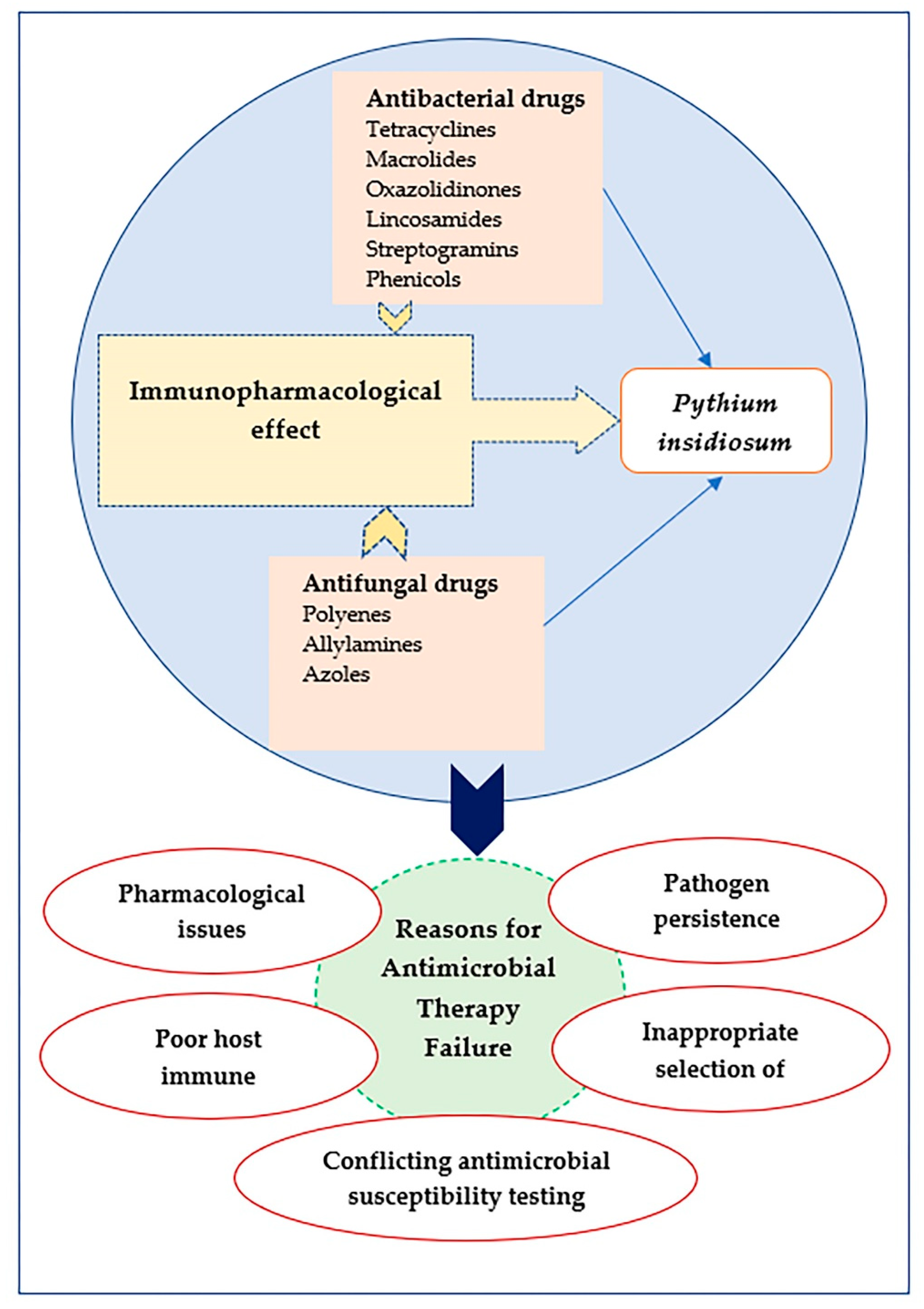
3. Why Do Antimicrobial Treatments Fail?
4. Immune Response and Antimicrobial Therapy
5. Antibacterial Drugs against P. insidiosum
5.1. Tetracyclines
5.2. Macrolides
5.3. Oxazolidinones
5.4. Lincosamides, Streptogramins, and Phenicols
5.5. Aminoglycosides
5.6. Miscellaneous Antibacterial Drugs
6. Antifungal Drugs against P. insidiosum
6.1. Polyenes
6.2. Allylamines and Azoles
6.3. Echinocandins
6.4. Miscellaneous Antifungal Drugs
7. Repurposing Antimicrobials against P. insidiosum
8. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Permpalung, N.; Worasilchai, N.; Chindamporn, A. Human Pythiosis: Emergence of Fungal-Like Organism. Mycopathologia 2020, 185, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Gaastra, W.; Lipman, L.J.; De Cock, A.W.; Exel, T.K.; Pegge, R.B.; Scheurwater, J.; Vilela, R.; Mendoza, L. Pythium insidiosum: An overview. Vet. Microbiol. 2010, 146, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Mar Htun, Z.; Laikul, A.; Pathomsakulwong, W.; Yurayart, C.; Lohnoo, T.; Yingyong, W.; Kumsang, Y.; Payattikul, P.; Sae-Chew, P.; Rujirawat, T.; et al. Identification and Biotyping of Pythium insidiosum Isolated from Urban and Rural Areas of Thailand by Multiplex PCR, DNA Barcode, and Proteomic Analyses. J. Fungi 2021, 7, 242. [Google Scholar] [CrossRef] [PubMed]
- Chitasombat, M.N.; Jongkhajornpong, P.; Lekhanont, K.; Krajaejun, T. Recent update in diagnosis and treatment of human pythiosis. PeerJ 2020, 8, e8555. [Google Scholar] [CrossRef]
- Krajaejun, T.; Imkhieo, S.; Intaramat, A.; Ratanabanangkoon, K. Development of an immunochromatographic test for rapid serodiagnosis of human pythiosis. Clin. Vaccine Immunol. 2009, 16, 506–509. [Google Scholar] [CrossRef]
- Zanette, R.A.; Bitencourt, P.E.R.; Alves, S.H.; Fighera, R.A.; Flores, M.M.; Wolkmer, P.; Hecktheuer, P.A.; Thomas, L.R.; Pereira, P.L.; Loreto, E.S.; et al. Insights into the pathophysiology of iron metabolism in Pythium insidiosum infections. Vet. Microbiol. 2013, 162, 826–830. [Google Scholar] [CrossRef]
- Chitasombat, M.N.; Larbcharoensub, N.; Chindamporn, A.; Krajaejun, T. Clinicopathological features and outcomes of pythiosis. Int. J. Infect. Dis. 2018, 71, 33–41. [Google Scholar] [CrossRef]
- Mendoza, L.; Ajello, L.; McGinnis, M.R. Infections Caused by the Oomycetous Pathogen Pythium insidiosum. J. Med. Mycol. 1996, 6, 151–164. [Google Scholar]
- Yolanda, H.; Krajaejun, T. Review of methods and antimicrobial agents for susceptibility testing against Pythium insidiosum. Heliyon 2020, 6, e03737. [Google Scholar] [CrossRef]
- Mendoza, L.; Newton, J.C. Immunology and immunotherapy of the infections caused by Pythium insidiosum. Med. Mycol. 2005, 43, 477–486. [Google Scholar] [CrossRef]
- Wanachiwanawin, W.; Mendoza, L.; Visuthisakchai, S.; Mutsikapan, P.; Sathapatayavongs, B.; Chaiprasert, A.; Suwanagool, P.; Manuskiatti, W.; Ruangsetakit, C.; Ajello, L. Efficacy of immunotherapy using antigens of Pythium insidiosum in the treatment of vascular pythiosis in humans. Vaccine 2004, 22, 3613–3621. [Google Scholar] [CrossRef] [PubMed]
- Yolanda, H.; Krajaejun, T. History and Perspective of Immunotherapy for Pythiosis. Vaccines 2021, 9, 1080. [Google Scholar] [CrossRef] [PubMed]
- Tondolo, J.S.M.; Ledur, P.C.; Loreto, E.S.; Verdi, C.M.; Bitencourt, P.E.R.; de Jesus, F.P.K.; Rocha, J.P.; Alves, S.H.; Sassaki, G.L.; Santurio, J.M. Extraction, characterization and biological activity of a (1,3)(1,6)-beta-d-glucan from the pathogenic oomycete Pythium insidiosum. Carbohydr. Polym. 2017, 157, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Permpalung, N.; Worasilchai, N.; Plongla, R.; Upala, S.; Sanguankeo, A.; Paitoonpong, L.; Mendoza, L.; Chindamporn, A. Treatment outcomes of surgery, antifungal therapy and immunotherapy in ocular and vascular human pythiosis: A retrospective study of 18 patients. J. Antimicrob. Chemother. 2015, 70, 1885–1892. [Google Scholar] [CrossRef]
- Wittayapipath, K.; Yenjai, C.; Prariyachatigul, C.; Hamal, P. Evaluation of antifungal effect and toxicity of xanthyletin and two bacterial metabolites against Thai isolates of Pythium insidiosum. Sci. Rep. 2020, 10, 4495. [Google Scholar] [CrossRef]
- Lerksuthirat, T.; Sangcakul, A.; Lohnoo, T.; Yingyong, W.; Rujirawat, T.; Krajaejun, T. Evolution of the Sterol Biosynthetic Pathway of Pythium insidiosum and Related Oomycetes Contributes to Antifungal Drug Resistance. Antimicrob. Agents Chemother. 2017, 61, e02352-16. [Google Scholar] [CrossRef]
- Thom, K.A.; Schweizer, M.L.; Osih, R.B.; McGregor, J.C.; Furuno, J.P.; Perencevich, E.N.; Harris, A.D. Impact of empiric antimicrobial therapy on outcomes in patients with Escherichia coli and Klebsiella pneumoniae bacteremia: A cohort study. BMC Infect. Dis. 2008, 8, 116. [Google Scholar] [CrossRef]
- Rawson, T.M.; Wilson, R.C.; O’Hare, D.; Herrero, P.; Kambugu, A.; Lamorde, M.; Ellington, M.; Georgiou, P.; Cass, A.; Hope, W.W.; et al. Optimizing antimicrobial use: Challenges, advances and opportunities. Nat. Rev. Microbiol. 2021, 19, 747–758. [Google Scholar] [CrossRef]
- Jarrell, A.S.; Kruer, R.M.; Johnson, D.; Lipsett, P.A. Antimicrobial Pharmacokinetics and Pharmacodynamics. Surg. Infect. (Larchmt) 2015, 16, 375–379. [Google Scholar] [CrossRef]
- Melida, H.; Sandoval-Sierra, J.V.; Dieguez-Uribeondo, J.; Bulone, V. Analyses of extracellular carbohydrates in oomycetes unveil the existence of three different cell wall types. Eukaryot. Cell 2013, 12, 194–203. [Google Scholar] [CrossRef]
- Pires, L.; Bosco Sde, M.; Baptista, M.S.; Kurachi, C. Photodynamic therapy in Pythium insidiosum—An in vitro study of the correlation of sensitizer localization and cell death. PLoS ONE 2014, 9, e85431. [Google Scholar] [CrossRef] [PubMed]
- Kammarnjassadakul, P.; Rangsipanuratn, W.; Sriprapun, M.; Ngamsakulrungruj, P.; Palaga, T.; Sritunyalucksana, K.; Chindamporn, A. Cytochrome Oxidase 2 (COX2), β-Tubulin (TUB) and Chitin Synthase Subunit 2 (CHS2) Expression in Pythium insidiosum Thai Strains. Walailak J. Sci. Technol. 2021, 18, 9433–9438. [Google Scholar] [CrossRef]
- Lerksuthirat, T.; Lohnoo, T.; Inkomlue, R.; Rujirawat, T.; Yingyong, W.; Khositnithikul, R.; Phaonakrop, N.; Roytrakul, S.; Sullivan, T.D.; Krajaejun, T. The elicitin-like glycoprotein, ELI025, is secreted by the pathogenic oomycete Pythium insidiosum and evades host antibody responses. PLoS ONE 2015, 10, e0118547. [Google Scholar] [CrossRef] [PubMed]
- Maeno, S.; Oie, Y.; Sunada, A.; Tanibuchi, H.; Hagiwara, S.; Makimura, K.; Nishida, K. Successful medical management of Pythium insidiosum keratitis using a combination of minocycline, linezolid, and chloramphenicol. Am. J. Ophthalmol. Case Rep. 2019, 15, 100498. [Google Scholar] [CrossRef]
- Gurnani, B.; Narayana, S.; Christy, J.; Rajkumar, P.; Kaur, K.; Gubert, J. Successful management of pediatric pythium insidiosum keratitis with cyanoacrylate glue, linezolid, and azithromycin: Rare case report. Eur. J. Ophthalmol. 2021, 11206721211006564. [Google Scholar] [CrossRef] [PubMed]
- Mercer, D.K.; Torres, M.D.T.; Duay, S.S.; Lovie, E.; Simpson, L.; von Kockritz-Blickwede, M.; de la Fuente-Nunez, C.; O’Neil, D.A.; Angeles-Boza, A.M. Antimicrobial Susceptibility Testing of Antimicrobial Peptides to Better Predict Efficacy. Front. Cell Infect. Microbiol. 2020, 10, 326. [Google Scholar] [CrossRef]
- Leekha, S.; Terrell, C.L.; Edson, R.S. General principles of antimicrobial therapy. Mayo. Clin. Proc. 2011, 86, 156–167. [Google Scholar] [CrossRef]
- Worasilchai, N.; Chindamporn, A.; Plongla, R.; Torvorapanit, P.; Manothummetha, K.; Chuleerarux, N.; Permpalung, N. In Vitro Susceptibility of Thai Pythium insidiosum Isolates to Antibacterial Agents. Antimicrob. Agents Chemother. 2020, 64, e02099-19. [Google Scholar] [CrossRef]
- Loreto, E.S.; Tondolo, J.S.; Pilotto, M.B.; Alves, S.H.; Santurio, J.M. New insights into the in vitro susceptibility of Pythium insidiosum. Antimicrob. Agents Chemother. 2014, 58, 7534–7537. [Google Scholar] [CrossRef]
- Loreto, E.S.; Tondolo, J.S.M.; Santurio, J.M.; Alves, S.H. Screening of antibacterial drugs for antimicrobial activity against Pythium insidiosum. Med. Mycol. 2019, 57, 523–525. [Google Scholar] [CrossRef]
- Mahl, D.L.; de Jesus, F.P.; Loreto, E.; Zanette, R.A.; Ferreiro, L.; Pilotto, M.B.; Alves, S.H.; Santurio, J.M. In vitro susceptibility of Pythium insidiosum isolates to aminoglycoside antibiotics and tigecycline. Antimicrob. Agents Chemother. 2012, 56, 4021–4023. [Google Scholar] [CrossRef] [PubMed]
- Worasilchai, N.; Permpalung, N.; Chongsathidkiet, P.; Leelahavanichkul, A.; Mendoza, A.L.; Palaga, T.; Reantragoon, R.; Finkelman, M.; Sutcharitchan, P.; Chindamporn, A. Monitoring Anti-Pythium insidiosum IgG Antibodies and (1-->3)-beta-d-Glucan in Vascular Pythiosis. J. Clin. Microbiol. 2018, 56, e00610-18. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.; Fessler, A.T.; Bottner, A.; Koper, L.M.; Wallmann, J.; Schwarz, S. Reasons for antimicrobial treatment failures and predictive value of in-vitro susceptibility testing in veterinary practice: An overview. Vet. Microbiol. 2020, 245, 108694. [Google Scholar] [CrossRef] [PubMed]
- Bassetti, M.; Montero, J.G.; Paiva, J.A. When antibiotic treatment fails. Intens Care Med. 2018, 44, 73–75. [Google Scholar] [CrossRef]
- Cicchese, J.M.; Dartois, V.; Kirschner, D.E.; Linderman, J.J. Both Pharmacokinetic Variability and Granuloma Heterogeneity Impact the Ability of the First-Line Antibiotics to Sterilize Tuberculosis Granulomas. Front. Pharmacol. 2020, 11, 333. [Google Scholar] [CrossRef]
- Shah, S.; Barton, G.; Fischer, A. Pharmacokinetic considerations and dosing strategies of antibiotics in the critically ill patient. J. Intensive Care Soc. 2015, 16, 147–153. [Google Scholar] [CrossRef]
- Veiga, R.P.; Paiva, J.A. Pharmacokinetics-pharmacodynamics issues relevant for the clinical use of beta-lactam antibiotics in critically ill patients. Crit. Care 2018, 22, 233. [Google Scholar] [CrossRef]
- Alghamdi, W.A.; Al-Shaer, M.H.; Klinker, K.P.; Peloquin, C.A. Variable linezolid exposure and response and the role of therapeutic drug monitoring: Case series. Clin. Case Rep. 2020, 8, 1126–1129. [Google Scholar] [CrossRef]
- Fugi, M.A.; Gunasekera, K.; Ochsenreiter, T.; Guan, X.; Wenk, M.R.; Maser, P. Genome profiling of sterol synthesis shows convergent evolution in parasites and guides chemotherapeutic attack. J. Lipid Res. 2014, 55, 929–938. [Google Scholar] [CrossRef]
- Levison, M.E.; Levison, J.H. Pharmacokinetics and pharmacodynamics of antibacterial agents. Infect. Dis. Clin. N. Am. 2009, 23, 791–815. [Google Scholar] [CrossRef]
- Jacobs, M.R. Optimisation of antimicrobial therapy using pharmacokinetic and pharmacodynamic parameters. Clin. Microbiol. Infect. 2001, 7, 589–596. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Qin, X.; Kong, F.; Chen, P.; Pan, G. Improving cellular uptake of therapeutic entities through interaction with components of cell membrane. Drug Deliv. 2019, 26, 328–342. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.R.; Cho, I.H.; Jeong, B.C.; Lee, S.H. Strategies to minimize antibiotic resistance. Int. J. Environ. Res. Public. Health 2013, 10, 4274–4305. [Google Scholar] [CrossRef] [PubMed]
- Actor, J.K. Innate Immunity. In Elsevier’s Integrated Review Immunology and Microbiology, 2nd ed.; Elsevier/Mosby: Philadelphia, PA, USA, 2012; pp. 43–51. [Google Scholar]
- Aristizábal, B.; González, A. Innate immune system. In Autoimmunity: From Bench to Bedside; Anaya, J.M., Shoenfeld, Y., Rojas-Villarraga, A., Levy, R.A., Cervera, R., Eds.; El Rosario University Press: Bogota, Colombia, 2013. [Google Scholar]
- Bergman, P.; Raqib, R.; Rekha, R.S.; Agerberth, B.; Gudmundsson, G.H. Host Directed Therapy against Infection by Boosting Innate Immunity. Front. Immunol. 2020, 11, 1209. [Google Scholar] [CrossRef] [PubMed]
- Gjini, E.; Brito, P.H. Integrating Antimicrobial Therapy with Host Immunity to Fight Drug-Resistant Infections: Classical vs. Adaptive Treatment. PLoS Comput. Biol. 2016, 12, e1004857. [Google Scholar] [CrossRef]
- Zheng, W.J.; Xu, Q.; Zhang, Y.Y.; E, X.F.; Gao, W.; Zhang, M.G.; Zhai, W.J.; Rajkumar, R.S.; Liu, Z.J. Toll-like receptor-mediated innate immunity against herpesviridae infection: A current perspective on viral infection signaling pathways. Virol. J. 2020, 17, 192. [Google Scholar] [CrossRef] [PubMed]
- Wongprompitak, P.; Pleewan, N.; Tantibhedhyangkul, W.; Chaiprasert, A.; Prabhasawat, P.; Inthasin, N.; Ekpo, P. Involvement of Toll-like receptor 2 on human corneal epithelium during an infection of Pythium insidiosum. Asian Pac. J. Allergy 2020, 38, 129–138. [Google Scholar] [CrossRef]
- Garrido-Mesa, J.; Rodriguez-Nogales, A.; Algieri, F.; Vezza, T.; Hidalgo-Garcia, L.; Garrido-Barros, M.; Utrilla, M.P.; Garcia, F.; Chueca, N.; Rodriguez-Cabezas, M.E.; et al. Immunomodulatory tetracyclines shape the intestinal inflammatory response inducing mucosal healing and resolution. Br. J. Pharmacol. 2018, 175, 4353–4370. [Google Scholar] [CrossRef]
- Yamauchi, K.; Shibata, Y.; Kimura, T.; Abe, S.; Inoue, S.; Osaka, D.; Sato, M.; Igarashi, A.; Kubota, I. Azithromycin suppresses interleukin-12p40 expression in lipopolysaccharide and interferon-gamma stimulated macrophages. Int. J. Biol. Sci. 2009, 5, 667–678. [Google Scholar] [CrossRef]
- Takahashi, G.; Sato, N.; Yaegashi, Y.; Kojika, M.; Matsumoto, N.; Kikkawa, T.; Shozushima, T.; Akitomi, S.; Aoki, K.; Ito, N.; et al. Effect of linezolid on cytokine production capacity and plasma endotoxin levels in response to lipopolysaccharide stimulation of whole blood. J. Infect. Chemother. 2010, 16, 94–99. [Google Scholar] [CrossRef]
- Pichereau, S.; Moran, J.J.; Hayney, M.S.; Shukla, S.K.; Sakoulas, G.; Rose, W.E. Concentration-dependent effects of antimicrobials on Staphylococcus aureus toxin-mediated cytokine production from peripheral blood mononuclear cells. J. Antimicrob. Chemother. 2012, 67, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Lambers, C.; Burian, B.; Binder, P.; Ankersmit, H.J.; Wagner, C.; Muller, M.; Zeitlinger, M. Early immunomodulatory effects of linezolid in a human whole blood endotoxin model. Int. J. Clin. Pharmacol. Ther. 2010, 48, 419–424. [Google Scholar] [CrossRef]
- Hirata, N.; Hiramatsu, K.; Kishi, K.; Yamasaki, T.; Ichimiya, T.; Nasu, M. Pretreatment of mice with clindamycin improves survival of endotoxic shock by modulating the release of inflammatory cytokines. Antimicrob. Agents Chemother. 2001, 45, 2638–2642. [Google Scholar] [CrossRef] [PubMed]
- Veringa, E.M.; Lambe, D.W., Jr.; Ferguson, D.A., Jr.; Verhoef, J. Enhancement of opsonophagocytosis of Bacteroides spp. by clindamycin in subinhibitory concentrations. J. Antimicrob. Chemother. 1989, 23, 577–587. [Google Scholar] [CrossRef] [PubMed]
- Trostdorf, F.; Reinert, R.R.; Schmidt, H.; Nichterlein, T.; Stuertz, K.; Schmitz-Salue, M.; Sadowski, I.; Bruck, W.; Nau, R. Quinupristin/dalfopristin attenuates the inflammatory response and reduces the concentration of neuron-specific enolase in the cerebrospinal fluid of rabbits with experimental Streptococcus pneumoniae meningitis. J. Antimicrob. Chemother. 1999, 43, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, S.; Dutta, K.; Manna, S.K.; Basu, A.; Bishayi, B. Possible protective role of chloramphenicol in TSST-1 and coagulase-positive Staphylococcus aureus-induced septic arthritis with altered levels of inflammatory mediators. Inflammation 2011, 34, 269–282. [Google Scholar] [CrossRef]
- Suschek, C.V.; Bonmann, E.; Kapsokefalou, A.; Hemmrich, K.; Kleinert, H.; Forstermann, U.; Kroncke, K.D.; Mahotka, C.; Kolb-Bachofen, V. Revisiting an old antimicrobial drug: Amphotericin B induces interleukin-1-converting enzyme as the main factor for inducible nitric-oxide synthase expression in activated endothelia. Mol. Pharmacol. 2002, 62, 936–946. [Google Scholar] [CrossRef]
- Mizuno, K.; Fukami, T.; Toyoda, Y.; Nakajima, M.; Yokoi, T. Terbinafine stimulates the pro-inflammatory responses in human monocytic THP-1 cells through an ERK signaling pathway. Life Sci. 2010, 87, 537–544. [Google Scholar] [CrossRef]
- Vora, S.; Purimetla, N.; Brummer, E.; Stevens, D.A. Activity of voriconazole, a new triazole, combined with neutrophils or monocytes against Candida albicans: Effect of granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor. Antimicrob. Agents Chemother. 1998, 42, 907–910. [Google Scholar] [CrossRef][Green Version]
- Baltch, A.L.; Smith, R.P.; Franke, M.A.; Ritz, W.J.; Michelsen, P.B.; Bopp, L.H. Effects of cytokines and fluconazole on the activity of human monocytes against Candida albicans. Antimicrob. Agents Chemother. 2001, 45, 96–104. [Google Scholar] [CrossRef]
- Itaqui, S.R.; Verdi, C.M.; Tondolo, J.S.; da Luz, T.S.; Alves, S.H.; Santurio, J.M.; Loreto, E.S. In Vitro Synergism between Azithromycin or Terbinafine and Topical Antimicrobial Agents against Pythium insidiosum. Antimicrob. Agents Chemother. 2016, 60, 5023–5025. [Google Scholar] [CrossRef] [PubMed]
- Jesus, F.P.; Ferreiro, L.; Bizzi, K.S.; Loreto, E.S.; Pilotto, M.B.; Ludwig, A.; Alves, S.H.; Zanette, R.A.; Santurio, J.M. In vitro activity of carvacrol and thymol combined with antifungals or antibacterials against Pythium insidiosum. J. Mycol. Med. 2015, 25, e89–e93. [Google Scholar] [CrossRef] [PubMed]
- Jesus, F.P.; Ferreiro, L.; Loreto, E.S.; Pilotto, M.B.; Ludwig, A.; Bizzi, K.; Tondolo, J.S.; Zanette, R.A.; Alves, S.H.; Santurio, J.M. In vitro synergism observed with azithromycin, clarithromycin, minocycline, or tigecycline in association with antifungal agents against Pythium insidiosum. Antimicrob. Agents Chemother. 2014, 58, 5621–5625. [Google Scholar] [CrossRef] [PubMed]
- Chopra, I.; Roberts, M. Tetracycline antibiotics: Mode of action, applications, molecular biology, and epidemiology of bacterial resistance. Microbiol. Mol. Biol. Rev. 2001, 65, 232–260. [Google Scholar] [CrossRef]
- Nakao, C.; Angel, M.; Mateo, S.D.; Komesu, M.C. Effects of Topical Tetracycline in Wound Healing on Experimental Diabetes in Rats. Open Diabetes J. 2009, 2, 53–59. [Google Scholar] [CrossRef]
- Zimmermann, C.E.P.; Jesus, F.P.K.; Schlemmer, K.B.; Loreto, E.S.; Tondolo, J.S.M.; Driemeier, D.; Alves, S.H.; Ferreiro, L.; Santurio, J.M. In vivo effect of minocycline alone and in combination with immunotherapy against pythium insidiosum. Vet. Microbiol. 2020, 243, 108616. [Google Scholar] [CrossRef]
- Patel, P.H.; Hashmi, M.F. Macrolides; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Cramer, C.L.; Patterson, A.; Alchakaki, A.; Soubani, A.O. Immunomodulatory indications of azithromycin in respiratory disease: A concise review for the clinician. Postgrad. Med. 2017, 129, 493–499. [Google Scholar] [CrossRef]
- Allen, J.E.; Wynn, T.A. Evolution of Th2 immunity: A rapid repair response to tissue destructive pathogens. PLoS Pathog. 2011, 7, e1002003. [Google Scholar] [CrossRef]
- Chen, F.; Liu, Z.; Wu, W.; Rozo, C.; Bowdridge, S.; Millman, A.; Van Rooijen, N.; Urban, J.F., Jr.; Wynn, T.A.; Gause, W.C. An essential role for TH2-type responses in limiting acute tissue damage during experimental helminth infection. Nat. Med. 2012, 18, 260–266. [Google Scholar] [CrossRef]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef]
- Jesus, F.P.; Loreto, E.S.; Ferreiro, L.; Alves, S.H.; Driemeier, D.; Souza, S.O.; Franca, R.T.; Lopes, S.T.; Pilotto, M.B.; Ludwig, A.; et al. In Vitro and In Vivo Antimicrobial Activities of Minocycline in Combination with Azithromycin, Clarithromycin, or Tigecycline against Pythium insidiosum. Antimicrob. Agents Chemother. 2016, 60, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Bozdogan, B.; Appelbaum, P.C. Oxazolidinones: Activity, mode of action, and mechanism of resistance. Int. J. Antimicrob. Agents 2004, 23, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Xia, L.; Wang, R.; Cai, Y. Linezolid and Its Immunomodulatory Effect: In Vitro and In Vivo Evidence. Front. Pharmacol. 2019, 10, 1389. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, L.K.; Kalra, P.; Sharma, S.; Mohamed, A.; Mittal, R.; Das, S.; Bagga, B. Linezolid shows high safety and efficacy in the treatment of Pythium insidiosum keratitis in a rabbit model. Exp. Eye Res. 2021, 202, 108345. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, S.; Shen, J.; Kadlec, K.; Wang, Y.; Brenner Michael, G.; Fessler, A.T.; Vester, B. Lincosamides, Streptogramins, Phenicols, and Pleuromutilins: Mode of Action and Mechanisms of Resistance. Cold Spring Harb. Perspect. Med. 2016, 6, a027037. [Google Scholar] [CrossRef]
- Krause, K.M.; Serio, A.W.; Kane, T.R.; Connolly, L.E. Aminoglycosides: An Overview. Cold Spring Harb. Perspect. Med. 2016, 6, a027029. [Google Scholar] [CrossRef]
- McMeekin, D.; Mendoza, L. In vitro effect of streptomycin on clinical isolates of Pythium insidiosum. Mycologia 2000, 92, 371–373. [Google Scholar] [CrossRef]
- McOsker, C.C.; Fitzpatrick, P.M. Nitrofurantoin: Mechanism of action and implications for resistance development in common uropathogens. J. Antimicrob. Chemother. 1994, 33, 23–30. [Google Scholar] [CrossRef]
- Parenti, M.A.; Hatfield, S.M.; Leyden, J.J. Mupirocin: A topical antibiotic with a unique structure and mechanism of action. Clin. Pharm. 1987, 6, 761–770. [Google Scholar]
- Noor, A.; Preuss, C.V. Amphotericin B; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Triscott, J.A.; Weedon, D.; Cabana, E. Human subcutaneous pythiosis. J. Cutan. Pathol. 1993, 20, 267–271. [Google Scholar] [CrossRef]
- Krajaejun, T.; Sathapatayavongs, B.; Pracharktam, R.; Nitiyanant, P.; Leelachaikul, P.; Wanachiwanawin, W.; Chaiprasert, A.; Assanasen, P.; Saipetch, M.; Mootsikapun, P.; et al. Clinical and epidemiological analyses of human pythiosis in Thailand. Clin. Infect. Dis. 2006, 43, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Sekhon, A.S.; Padhye, A.A.; Garg, A.K. In vitro sensitivity of Penicillium marneffei and Pythium insidiosum to various antifungal agents. Eur. J. Epidemiol. 1992, 8, 427–432. [Google Scholar] [CrossRef] [PubMed]
- Permpalung, N.; Worasilchai, N.; Manothummetha, K.; Torvorapanit, P.; Ratanawongphaibul, K.; Chuleerarux, N.; Plongla, R.; Chindamporn, A. Clinical outcomes in ocular pythiosis patients treated with a combination therapy protocol in Thailand: A prospective study. Med. Mycol. 2019, 57, 923–928. [Google Scholar] [CrossRef] [PubMed]
- Mesa-Arango, A.C.; Scorzoni, L.; Zaragoza, O. It only takes one to do many jobs: Amphotericin B as antifungal and immunomodulatory drug. Front. Microbiol. 2012, 3, 286. [Google Scholar] [CrossRef]
- Malone-Povolny, M.J.; Maloney, S.E.; Schoenfisch, M.H. Nitric Oxide Therapy for Diabetic Wound Healing. Adv. Healthc Mater. 2019, 8, e1801210. [Google Scholar] [CrossRef]
- Luo, J.D.; Chen, A.F. Nitric oxide: A newly discovered function on wound healing. Acta Pharmacol. Sin. 2005, 26, 259–264. [Google Scholar] [CrossRef]
- Vriens, K.; Kumar, P.T.; Struyfs, C.; Cools, T.L.; Spincemaille, P.; Kokalj, T.; Sampaio-Marques, B.; Ludovico, P.; Lammertyn, J.; Cammue, B.P.A.; et al. Increasing the Fungicidal Action of Amphotericin B by Inhibiting the Nitric Oxide-Dependent Tolerance Pathway. Oxid. Med. Cell Longev. 2017, 2017, 4064628. [Google Scholar] [CrossRef]
- Mesa-Arango, A.C.; Trevijano-Contador, N.; Roman, E.; Sanchez-Fresneda, R.; Casas, C.; Herrero, E.; Arguelles, J.C.; Pla, J.; Cuenca-Estrella, M.; Zaragoza, O. The production of reactive oxygen species is a universal action mechanism of Amphotericin B against pathogenic yeasts and contributes to the fungicidal effect of this drug. Antimicrob. Agents Chemother. 2014, 58, 6627–6638. [Google Scholar] [CrossRef]
- Ryder, N.S. Terbinafine: Mode of action and properties of the squalene epoxidase inhibition. Br. J. Dermatol. 1992, 126, 2–7. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Perfect, J.R. Antimicrobial resistance: Resistance to antifungal agents: Mechanisms and clinical impact. Clin. Infect. Dis. 2008, 46, 120–128. [Google Scholar] [CrossRef]
- Ghannoum, M.A.; Rice, L.B. Antifungal agents: Mode of action, mechanisms of resistance, and correlation of these mechanisms with bacterial resistance. Clin. Microbiol. Rev. 1999, 12, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Susaengrat, N.; Torvorapanit, P.; Plongla, R.; Chuleerarux, N.; Manothummetha, K.; Tuangsirisup, J.; Worasilchai, N.; Chindamporn, A.; Permpalung, N. Adjunctive antibacterial agents as a salvage therapy in relapsed vascular pythiosis patients. Int. J. Infect. Dis. 2019, 88, 27–30. [Google Scholar] [CrossRef] [PubMed]
- Cavalheiro, A.S.; Maboni, G.; de Azevedo, M.I.; Argenta, J.S.; Pereira, D.I.; Spader, T.B.; Alves, S.H.; Santurio, J.M. In Vitro activity of terbinafine combined with caspofungin and azoles against Pythium insidiosum. Antimicrob. Agents Chemother. 2009, 53, 2136–2138. [Google Scholar] [CrossRef] [PubMed]
- Shenep, J.L.; English, B.K.; Kaufman, L.; Pearson, T.A.; Thompson, J.W.; Kaufman, R.A.; Frisch, G.; Rinaldi, M.G. Successful medical therapy for deeply invasive facial infection due to Pythium insidiosum in a child. Clin. Infect. Dis. 1998, 27, 1388–1393. [Google Scholar] [CrossRef]
- Sucher, A.J.; Chahine, E.B.; Balcer, H.E. Echinocandins: The newest class of antifungals. Ann. Pharmacother. 2009, 43, 1647–1657. [Google Scholar] [CrossRef]
- Lamaris, G.A.; Lewis, R.E.; Chamilos, G.; May, G.S.; Safdar, A.; Walsh, T.J.; Raad, I.I.; Kontoyiannis, D.P. Caspofungin-mediated beta-glucan unmasking and enhancement of human polymorphonuclear neutrophil activity against Aspergillus and non-Aspergillus hyphae. J. Infect. Dis. 2008, 198, 186–192. [Google Scholar] [CrossRef]
- Katragkou, A.; Roilides, E.; Walsh, T.J. Role of Echinocandins in Fungal Biofilm-Related Disease: Vascular Catheter-Related Infections, Immunomodulation, and Mucosal Surfaces. Clin. Infect. Dis. 2015, 61 (Suppl. 6), S622–S629. [Google Scholar] [CrossRef]
- Zanette, R.A.; Jesus, F.P.; Pilotto, M.B.; Weiblen, C.; Potter, L.; Ferreiro, L.; Alves, S.H.; Santurio, J.M. Micafungin alone and in combination therapy with deferasirox against Pythium insidiosum. J. Mycol. Med. 2015, 25, 91–94. [Google Scholar] [CrossRef]
- Pereira, D.I.; Santurio, J.M.; Alves, S.H.; Argenta, J.S.; Potter, L.; Spanamberg, A.; Ferreiro, L. Caspofungin in vitro and in vivo activity against Brazilian Pythium insidiosum strains isolated from animals. J. Antimicrob. Chemother. 2007, 60, 1168–1171. [Google Scholar] [CrossRef]
- Polak, A.M. Preclinical data and mode of action of amorolfine. Clin. Exp. Dermatol. 1992, 17 (Suppl. 1), 8–12. [Google Scholar] [CrossRef]
- Ianiski, L.B.; Stibbe, P.C.; Denardi, L.B.; Weiblen, C.; Soares, M.P.; Valente, J.S.S.; Sangioni, L.A.; Pereira, D.I.B.; Santurio, J.M.; Botton, S.A. In vitro anti-Pythium insidiosum activity of amorolfine hydrochloride and azithromycin, alone and in combination. Med. Mycol. 2021, 59, 67–73. [Google Scholar] [CrossRef]
- Sahoo, B.M.; Ravi Kumar, B.V.V.; Sruti, J.; Mahapatra, M.K.; Banik, B.K.; Borah, P. Drug Repurposing Strategy (DRS): Emerging Approach to Identify Potential Therapeutics for Treatment of Novel Coronavirus Infection. Front. Mol. Biosci. 2021, 8, 628144. [Google Scholar] [CrossRef] [PubMed]
- Yacouba, A.; Olowo-Okere, A.; Yunusa, I. Repurposing of antibiotics for clinical management of COVID-19: A narrative review. Ann. Clin. Microbiol. Antimicrob. 2021, 20, 37. [Google Scholar] [CrossRef] [PubMed]
- Kaul, G.; Shukla, M.; Dasgupta, A.; Chopra, S. Update on drug-repurposing: Is it useful for tackling antimicrobial resistance? Future Microbiol. 2019, 14, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Stokes, M.; Abdijadid, S. Disulfiram; StatPearls: Treasure Island, FL, USA, 2021. [Google Scholar]
- Krajaejun, T.; Lohnoo, T.; Yingyong, W.; Rujirawat, T.; Kumsang, Y.; Jongkhajornpong, P.; Theerawatanasirikul, S.; Kittichotirat, W.; Reamtong, O.; Yolanda, H. The Repurposed Drug Disulfiram Inhibits Urease and Aldehyde Dehydrogenase and Prevents In Vitro Growth of the Oomycete Pythium insidiosum. Antimicrob. Agents Chemother. 2019, 63, e00609-19. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar] [CrossRef]
- Li, J.; Zheng, S.; Chen, B.; Butte, A.J.; Swamidass, S.J.; Lu, Z. A survey of current trends in computational drug repositioning. Brief Bioinform. 2016, 17, 2–12. [Google Scholar] [CrossRef]
- Ko, Y. Computational Drug Repositioning: Current Progress and Challenges. Appl. Sci. 2020, 10, 5076. [Google Scholar] [CrossRef]
- Rudrapal, M.; Khairnar, S.; Jadhav, A. Drug Repurposing (DR): An Emerging Approach in Drug Discovery. In Drug—Hypothesis, Molecular Aspects and Therapeutic Applications; BoD—Books on Demand: London, UK, 2020. [Google Scholar]
- Liu, Y.; Tong, Z.; Shi, J.; Li, R.; Upton, M.; Wang, Z. Drug repurposing for next-generation combination therapies against multidrug-resistant bacteria. Theranostics 2021, 11, 4910–4928. [Google Scholar] [CrossRef]
- Cheng, Y.S.; Williamson, P.R.; Zheng, W. Improving therapy of severe infections through drug repurposing of synergistic combinations. Curr. Opin. Pharmacol. 2019, 48, 92–98. [Google Scholar] [CrossRef]
- Nguyen, T.K.; Selvanayagam, R.; Ho, K.K.K.; Chen, R.; Kutty, S.K.; Rice, S.A.; Kumar, N.; Barraud, N.; Duong, H.T.T.; Boyer, C. Co-delivery of nitric oxide and antibiotic using polymeric nanoparticles. Chem. Sci. 2016, 7, 1016–1027. [Google Scholar] [CrossRef]
- Rouillard, K.R.; Novak, O.P.; Pistiolis, A.M.; Yang, L.; Ahonen, M.J.R.; McDonald, R.A.; Schoenfisch, M.H. Exogenous Nitric Oxide Improves Antibiotic Susceptibility in Resistant Bacteria. ACS Infect. Dis. 2021, 7, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.sanfordguide.com/ (accessed on 13 January 2022).
- Keeratijarut, A.; Lohnoo, T.; Rujirawat, T.; Yingyong, W.; Kalambaheti, T.; Miller, S.; Phuntumart, V.; Krajaejun, T. The Immunoreactive Exo-1,3-beta-Glucanase from the Pathogenic Oomycete Pythium insidiosum Is Temperature Regulated and Exhibits Glycoside Hydrolase Activity. PLoS ONE 2015, 10, e0135239. [Google Scholar] [CrossRef] [PubMed]
- Wittayapipath, K.; Laolit, S.; Yenjai, C.; Chio-Srichan, S.; Pakarasang, M.; Tavichakorntrakool, R.; Prariyachatigul, C. Analysis of xanthyletin and secondary metabolites from Pseudomonas stutzeri ST1302 and Klebsiella pneumoniae ST2501 against Pythium insidiosum. BMC Microbiol. 2019, 19, 78. [Google Scholar] [CrossRef] [PubMed]
- Subramaniam, S.; Joyce, P.; Thomas, N.; Prestidge, C.A. Bioinspired drug delivery strategies for repurposing conventional antibiotics against intracellular infections. Adv. Drug Deliv. Rev. 2021, 177, 113948. [Google Scholar] [CrossRef]
- Stocco, G.; Lucafo, M.; Decorti, G. Pharmacogenomics of Antibiotics. Int. J. Mol. Sci. 2020, 21, 5975. [Google Scholar] [CrossRef]


| Antimicrobial Class | Drug | MIC Determination Method(s) | Reference(s) |
|---|---|---|---|
| Tetracyclines | Tetracycline | Broth microdilution | [28] |
| Tigecycline | Broth microdilution, disk diffusion, and Etest | [28,29,31] | |
| Minocycline | Broth microdilution, disk diffusion, and Etest | [28,29] | |
| Macrolides | Azithromycin | Broth microdilution, disk diffusion, and Etest | [28,29] |
| Clarithromycin | Broth microdilution, disk diffusion, and Etest | [28,29] | |
| Oxazolidinones | Linezolid | Broth microdilution, disk diffusion, and Etest | [29] |
| Lincosamides | Clindamycin | Broth dilution | [30] |
| Streptogramins | Quinupristin and dalfopristin | Broth dilution | [30] |
| Phenicols | Chloramphenicol | Broth dilution | [30] |
| Aminoglycosides | Gentamicin | Broth microdilution | [31] |
| Neomycin | Broth microdilution | [31] | |
| Paromomycin | Broth microdilution | [31] | |
| Streptomycin | Broth microdilution | [31] | |
| Nitrofurantoin | Broth dilution | [30] | |
| Mupirocin | Broth microdilution, disk diffusion, and Etest | [29] | |
| Polyenes | Amphotericin B | Etest | [29] |
| Allylamines | Terbinafine | Broth dilution and radial growth | [9] |
| Azoles | Miconazole | Broth microdilution | [9] |
| Ketoconazole | Broth microdilution | [9] | |
| Fluconazole | Broth microdilution and agar diffusion | [9] | |
| Itraconazole | Broth microdilution, radial growth, and agar diffusion | [9] | |
| Posaconazole | Broth microdilution and agar diffusion | [9] | |
| Voriconazole | Broth microdilution, radial growth, and agar diffusion | [9] | |
| Echinocandins | Caspofungin | Broth dilution | [32] |
| Anidulafungin | Broth dilution | [32] |
| Antimicrobial Class | Drug | Immunopharmacological Effect | Reference(s) |
|---|---|---|---|
| Tetracyclines | Tigecycline, minocycline | Potentiate the innate immune response and augment resolution of inflammation | [50] |
| Macrolides | Azithromycin | Reduce the production of IL-12, resulting in enhanced Th2 response | [51] |
| Oxazolidinones | Linezolid | Suppress synthesis of proinflammatory cytokines, such as interleukin-1β (IL-1β), IL-6, IL-8, interferon-γ (IFN-γ), and tumor necrosis factor-α (TNF-α) | [52,53,54] |
| Lincosamides | Clindamycin | Suppress the release of inflammatory cytokines such as TNF-α and IL-1β and enhance the phagocytosis of microorganisms by host cells | [55,56] |
| Streptogramins | Quinupristin-dalfopristin | Decrease the concentration of pro-inflammatory cell wall components (lipoteichoic acid and teichoic acid) and the activity of TNF | [57] |
| Phenicols | Chloramphenicol | Elevate the anti-inflammatory IL-10 levels | [58] |
| Polyenes | Amphotericin B | Activate the host’s innate immunity and augment the IL-1β-induced inducible nitric-oxide synthase (iNOS) expression and the production of nitric oxide (NO) | [59] |
| Allylamines | Terbinafine | Stimulate proinflammatory cytokines | [60] |
| Azoles | Fluconazole, voriconazole | Enhance microbicidal activity of monocytes, macrophages, and neutrophils | [61,62] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Medhasi, S.; Chindamporn, A.; Worasilchai, N. A Review: Antimicrobial Therapy for Human Pythiosis. Antibiotics 2022, 11, 450. https://doi.org/10.3390/antibiotics11040450
Medhasi S, Chindamporn A, Worasilchai N. A Review: Antimicrobial Therapy for Human Pythiosis. Antibiotics. 2022; 11(4):450. https://doi.org/10.3390/antibiotics11040450
Chicago/Turabian StyleMedhasi, Sadeep, Ariya Chindamporn, and Navaporn Worasilchai. 2022. "A Review: Antimicrobial Therapy for Human Pythiosis" Antibiotics 11, no. 4: 450. https://doi.org/10.3390/antibiotics11040450
APA StyleMedhasi, S., Chindamporn, A., & Worasilchai, N. (2022). A Review: Antimicrobial Therapy for Human Pythiosis. Antibiotics, 11(4), 450. https://doi.org/10.3390/antibiotics11040450





