Biosynthesis and Chemical Synthesis of Albomycin Nucleoside Antibiotics
Abstract
1. Introduction
2. Discovery and Structure Elucidation
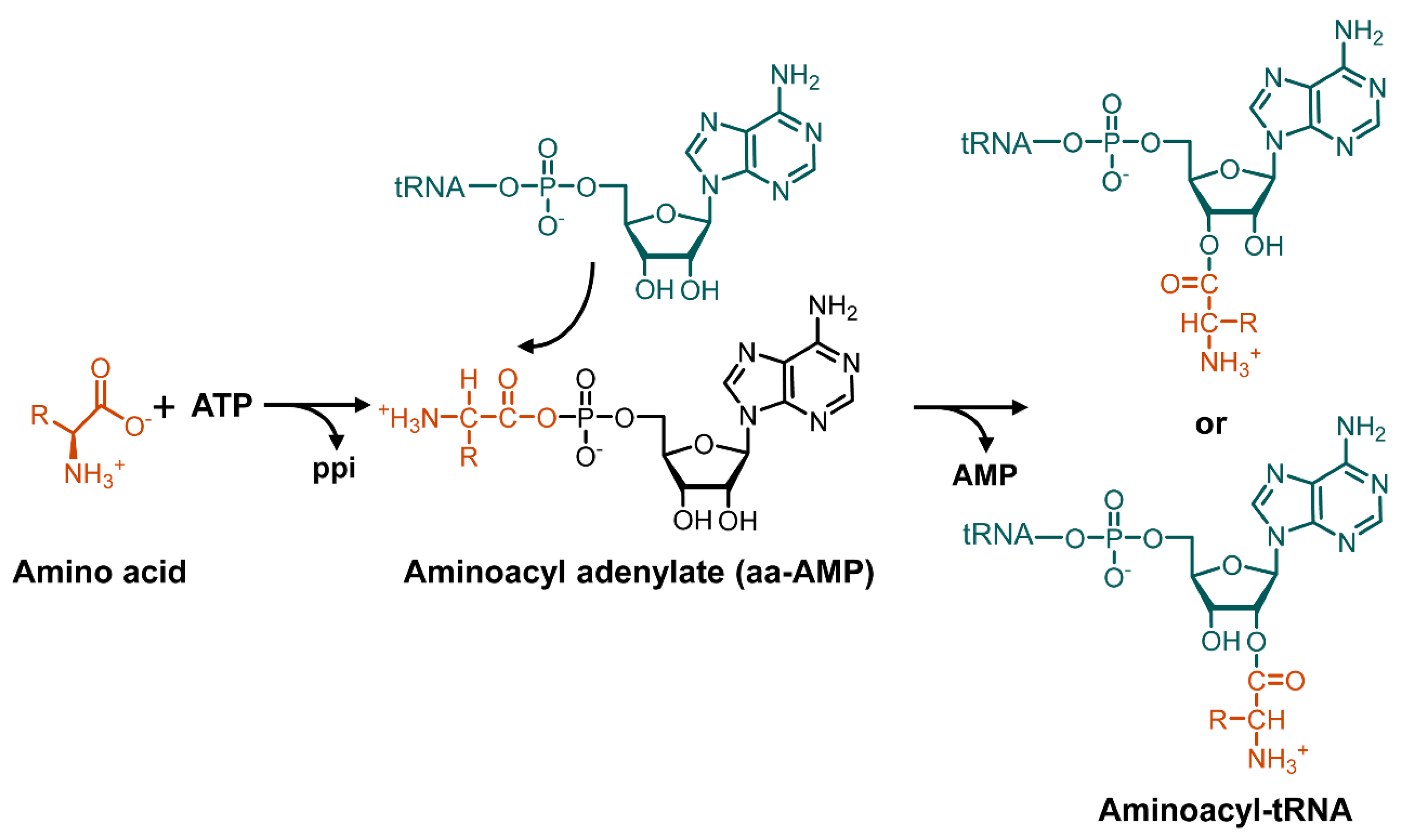
3. Mechanism of Action
4. Biosynthetic Pathway
5. Self-Resistance
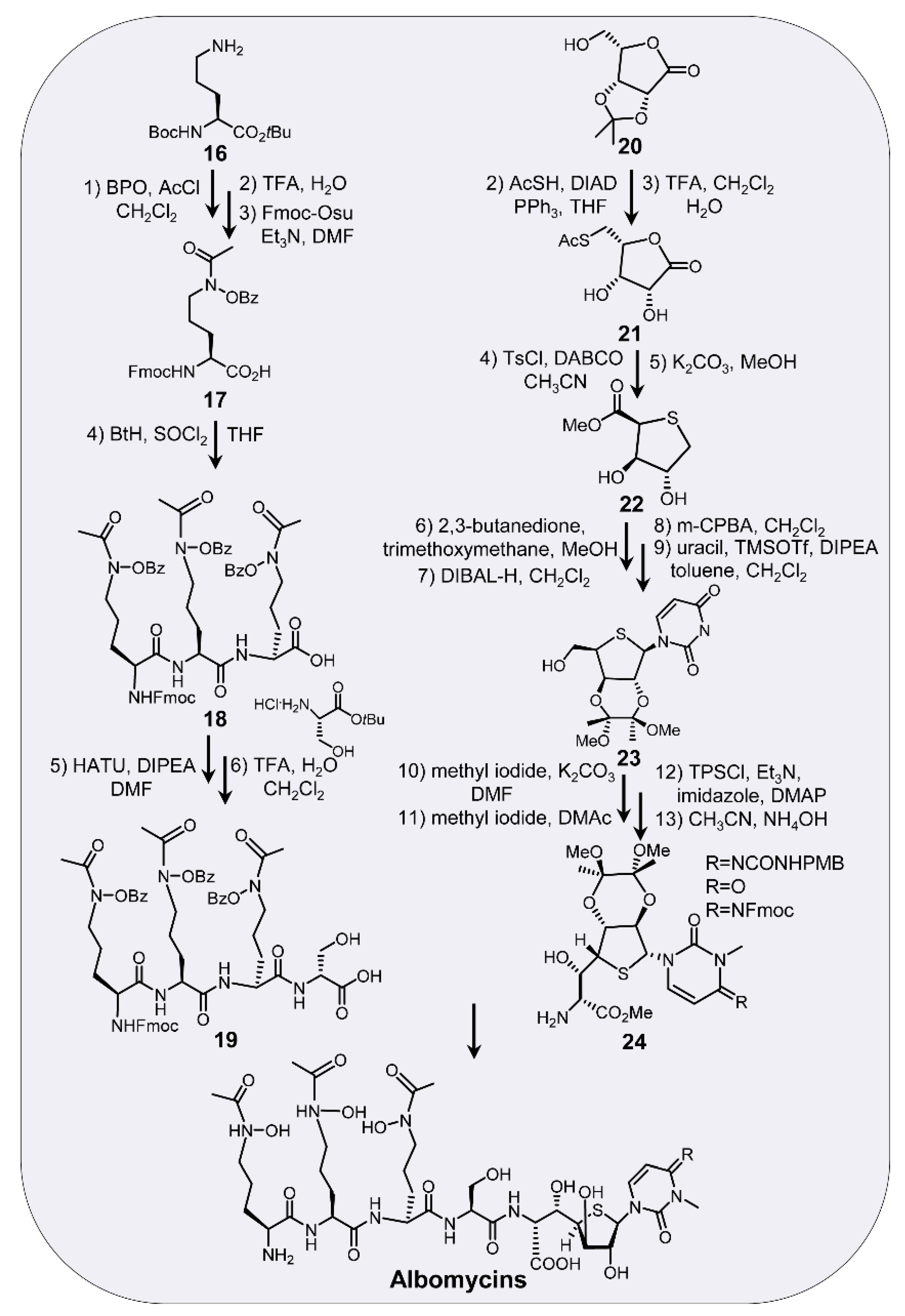
6. Chemical Synthesis
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Niu, G. Genomics-driven natural product discovery in actinomycetes. Trends Biotechnol. 2018, 36, 238–241. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Li, W. Next-generation drug discovery to combat antimicrobial resistance. Trends Biochem. Sci. 2019, 44, 961–972. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Shen, B. A new golden age of natural products drug discovery. Cell 2015, 163, 1297–1300. [Google Scholar] [CrossRef]
- Harvey, A.L.; Edrada-Ebel, R.; Quinn, R.J. The re-emergence of natural products for drug discovery in the genomics era. Nat. Rev. Drug Discov. 2015, 14, 111–129. [Google Scholar] [CrossRef]
- Pang, L.; Weeks, S.D.; Van Aerschot, A. Aminoacyl-tRNA synthetases as valuable targets for antimicrobial drug discovery. Int. J. Mol. Sci. 2021, 22, 1750. [Google Scholar] [CrossRef]
- Ibba, M.; Soll, D. Aminoacyl-tRNA synthesis. Annu. Rev. Biochem. 2000, 69, 617–650. [Google Scholar] [CrossRef]
- Gorska, A.; Sloderbach, A.; Marszall, M.P. Siderophore-drug complexes: Potential medicinal applications of the ‘Trojan horse’ strategy. Trends Pharmacol. Sci. 2014, 35, 442–449. [Google Scholar] [CrossRef]
- Braun, V.; Pramanik, A.; Gwinner, T.; Koberle, M.; Bohn, E. Sideromycins: Tools and antibiotics. Biometals 2009, 22, 3–13. [Google Scholar] [CrossRef]
- Travin, D.Y.; Severinov, K.; Dubiley, S. Natural Trojan horse inhibitors of aminoacyl-tRNA synthetases. RSC Chem. Biol. 2021, 2, 468–485. [Google Scholar] [CrossRef]
- Vondenhoff, G.H.; Van Aerschot, A. Microcin C: Biosynthesis, mode of action, and potential as a lead in antibiotics development. Nucleosides Nucleotides Nucleic. Acids 2011, 30, 465–474. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.G.; Park, B.K.; Kim, S.U.; Choi, D.; Nahm, B.H.; Moon, J.S.; Reader, J.S.; Farrand, S.K.; Hwang, I. Bases of biocontrol: Sequence predicts synthesis and mode of action of agrocin 84, the Trojan horse antibiotic that controls crown gall. Proc. Natl. Acad. Sci. USA 2006, 103, 8846–8851. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Palencia, A.; Virus, C.; Schulwitz, S.; Temple, B.R.; Cusack, S.; Reader, J. Structural characterization of antibiotic self-immunity tRNA synthetase in plant tumour biocontrol agent. Nat. Commun. 2016, 7, 12928. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, D.M.; Schatz, A.; Waksman, S.A. Grisein, a new antibiotic produced by a strain of Streptomyces griseus. Proc. Soc. Exp. Biol. Med. 1947, 64, 50–54. [Google Scholar] [CrossRef]
- Reynolds, D.M.; Waksman, S.A. Grisein, an antibiotic produced by certain strains of Streptomyces griseus. J. Bacteriol. 1948, 55, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Gause, G.F. Recent studies on albomycin, a new antibiotic. Br. Med. J. 1955, 2, 1177–1179. [Google Scholar] [CrossRef]
- Stapley, E.O.; Ormond, R.E. Similarity of albomycin and grisein. Science 1957, 125, 587–589. [Google Scholar] [CrossRef]
- Maehr, H.; Pitcher, R.G. Identity of albomycin δ2 and antibiotic Ro 5-2667. J. Antibiot. 1971, 24, 830–834. [Google Scholar] [CrossRef]
- Maehr, H. Antibiotics and other naturally occurring hydroxamic acids and hydroxamates. Pure Appl. Chem. 1971, 28, 603–636. [Google Scholar] [CrossRef]
- Benz, G.; Schröder, T.; Kurz, J.; Wünsche, C.; Karl, W.; Steffens, G.; Pfitzner, J.; Schmidt, D. Constitution of the deferriform of the albomycins δ1, δ2 and ε. Angew. Chem. Int. Ed. Engl. 1982, 21, 527–528. [Google Scholar] [CrossRef]
- Benz, G.; Schröder, T.; Kurz, J.; Wünsche, C.; Karl, W.; Steffens, G.; Pfitzner, J.; Schmidt, D. Konstitution der Desferriform der Albomycine δ1, δ2, ε. Angew. Chem. Int. Ed. Engl. 1982, 21, 1322–1335. [Google Scholar] [CrossRef]
- Haas, H. Fungal siderophore metabolism with a focus on Aspergillus fumigatus. Nat. Prod. Rep. 2014, 31, 1266–1276. [Google Scholar] [CrossRef] [PubMed]
- Asai, Y.; Hiratsuka, T.; Ueda, M.; Kawamura, Y.; Asamizu, S.; Onaka, H.; Arioka, M.; Nishimura, S.; Yoshida, M. Differential biosynthesis and roles of two ferrichrome-type siderophores, ASP2397/AS2488053 and ferricrocin, in Acremonium persicinum. ACS Chem. Biol. 2022, 17, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Niu, G.; Tan, H. Nucleoside antibiotics: Biosynthesis, regulation, and biotechnology. Trends Microbiol. 2015, 23, 110–119. [Google Scholar] [CrossRef]
- Shiraishi, T.; Kuzuyama, T. Recent advances in the biosynthesis of nucleoside antibiotics. J. Antibiot. 2019, 72, 913–923. [Google Scholar] [CrossRef]
- Pramanik, A.; Stroeher, U.H.; Krejci, J.; Standish, A.J.; Bohn, E.; Paton, J.C.; Autenrieth, I.B.; Braun, V. Albomycin is an effective antibiotic, as exemplified with Yersinia enterocolitica and Streptococcus pneumoniae. Int. J. Med. Microbiol. 2007, 297, 459–469. [Google Scholar] [CrossRef]
- Pramanik, A.; Braun, V. Albomycin uptake via a ferric hydroxamate transport system of Streptococcus pneumoniae R6. J. Bacteriol. 2006, 188, 3878–3886. [Google Scholar] [CrossRef]
- Al Shaer, D.; Al Musaimi, O.; de la Torre, B.G.; Albericio, F. Hydroxamate siderophores: Natural occurrence, chemical synthesis, iron binding affinity and use as Trojan horses against pathogens. Eur. J. Med. Chem. 2020, 208, 112791. [Google Scholar] [CrossRef]
- Neilands, J.B. Microbial envelope proteins related to iron. Annu. Rev. Microbiol. 1982, 36, 285–309. [Google Scholar] [CrossRef]
- Braun, V. Active transport of siderophore-mimicking antibacterials across the outer membrane. Drug Resist. Update 1999, 2, 363–369. [Google Scholar] [CrossRef]
- Ferguson, A.D.; Braun, V.; Fiedler, H.P.; Coulton, J.W.; Diederichs, K.; Welte, W. Crystal structure of the antibiotic albomycin in complex with the outer membrane transporter FhuA. Protein Sci. 2000, 9, 956–963. [Google Scholar] [CrossRef] [PubMed]
- Killmann, H.; Herrmann, C.; Torun, A.; Jung, G.; Braun, V. TonB of Escherichia coli activates FhuA through interaction with the β-barrel. Microbiology 2002, 148, 3497–3509. [Google Scholar] [CrossRef] [PubMed]
- Rohrbach, M.R.; Braun, V.; Koster, W. Ferrichrome transport in Escherichia coli K-12: Altered substrate specificity of mutated periplasmic FhuD and interaction of FhuD with the integral membrane protein FhuB. J. Bacteriol. 1995, 177, 7186–7193. [Google Scholar] [CrossRef] [PubMed]
- Schultz-Hauser, G.; Koster, W.; Schwarz, H.; Braun, V. Iron (III) hydroxamate transport in Escherichia coli K-12: FhuB-mediated membrane association of the FhuC protein and negative complementation of fhuC mutants. J. Bacteriol. 1992, 174, 2305–2311. [Google Scholar] [CrossRef][Green Version]
- Kadner, R.J.; Heller, K.; Coulton, J.W.; Braun, V. Genetic control of hydroxamate-mediated iron uptake in Escherichia coli. J. Bacteriol. 1980, 143, 256–264. [Google Scholar] [CrossRef]
- Braun, V.; Gunthner, K.; Hantke, K.; Zimmermann, L. Intracellular activation of albomycin in Escherichia coli and Salmonella typhimurium. J. Bacteriol. 1983, 156, 308–315. [Google Scholar] [CrossRef]
- Zeng, Y.; Kulkarni, A.; Yang, Z.; Patil, P.B.; Zhou, W.; Chi, X.; Van Lanen, S.; Chen, S. Biosynthesis of albomycin δ2 provides a template for assembling siderophore and aminoacyl-tRNA synthetase inhibitor conjugates. ACS Chem. Biol. 2012, 7, 1565–1575. [Google Scholar] [CrossRef]
- Ushimaru, R.; Liu, H.W. Biosynthetic origin of the atypical stereochemistry in the thioheptose core of albomycin nucleoside antibiotics. J. Am. Chem. Soc. 2019, 141, 2211–2214. [Google Scholar] [CrossRef]
- Ushimaru, R.; Chen, Z.; Zhao, H.; Fan, P.H.; Liu, H.W. Identification of the enzymes mediating the maturation of the Seryl-tRNA synthetase inhibitor SB-217452 during the biosynthesis of albomycins. Angew. Chem. Int. Ed. Engl. 2020, 59, 3558–3562. [Google Scholar] [CrossRef]
- Garg, R.P.; Gonzalez, J.M.; Parry, R.J. Biochemical characterization of VlmL, a Seryl-tRNA synthetase encoded by the valanimycin biosynthetic gene cluster. J. Biol. Chem. 2006, 281, 26785–26791. [Google Scholar] [CrossRef]
- Garg, R.P.; Qian, X.L.; Alemany, L.B.; Moran, S.; Parry, R.J. Investigations of valanimycin biosynthesis: Elucidation of the role of seryl-tRNA. Proc. Natl. Acad. Sci. USA 2008, 105, 6543–6547. [Google Scholar] [CrossRef] [PubMed]
- Mullowney, M.W.; McClure, R.A.; Robey, M.T.; Kelleher, N.L.; Thomson, R.J. Natural products from thioester reductase containing biosynthetic pathways. Nat. Prod. Rep. 2018, 35, 847–878. [Google Scholar] [CrossRef] [PubMed]
- Močibob, M.; Rokov-Plavec, J.; Godinic-Mikulcic, V.; Gruic-Sovulj, I. Seryl-tRNA synthetases in translation and beyond. Croat. Chem. Acta 2016, 89, 261–276. [Google Scholar] [CrossRef]
- Zeng, Y.; Roy, H.; Patil, P.B.; Ibba, M.; Chen, S. Characterization of two seryl-tRNA synthetases in albomycin-producing Streptomyces sp. strain ATCC 700974. Antimicrob. Agents Chemother. 2009, 53, 4619–4627. [Google Scholar] [CrossRef]
- Yanagisawa, T.; Kawakami, M. How does Pseudomonas fluorescens avoid suicide from its antibiotic pseudomonic acid?: Evidence for two evolutionarily distinct isoleucyl-tRNA synthetases conferring self-defense. J. Biol. Chem. 2003, 278, 25887–25894. [Google Scholar] [CrossRef]
- Olano, C.; Wilkinson, B.; Sanchez, C.; Moss, S.J.; Sheridan, R.; Math, V.; Weston, A.J.; Brana, A.F.; Martin, C.J.; Oliynyk, M.; et al. Biosynthesis of the angiogenesis inhibitor borrelidin by Streptomyces parvulus Tu4055: Cluster analysis and assignment of functions. Chem. Biol. 2004, 11, 87–97. [Google Scholar]
- Vecchione, J.J.; Sello, J.K. Characterization of an inducible, antibiotic-resistant aminoacyl-tRNA synthetase gene in Streptomyces coelicolor. J. Bacteriol. 2008, 190, 6253–6257. [Google Scholar] [CrossRef]
- Kitabatake, M.; Ali, K.; Demain, A.; Sakamoto, K.; Yokoyama, S.; Soll, D. Indolmycin resistance of Streptomyces coelicolor A3(2) by induced expression of one of its two tryptophanyl-tRNA synthetases. J. Biol. Chem. 2002, 277, 23882–23887. [Google Scholar] [CrossRef]
- Yan, Y.; Liu, N.; Tang, Y. Recent developments in self-resistance gene directed natural product discovery. Nat. Prod. Rep. 2020, 37, 879–892. [Google Scholar] [CrossRef]
- Flemming, A. Antibacterials: Resistance-guided discovery of new antibiotics. Nat. Rev. Drug Discov. 2013, 12, 826. [Google Scholar] [CrossRef]
- Benz, G.; Schmidt, D. Albomycine, IV. Isolierung und totalsynthese von (N5-acetyl-N5-hydroxy-L-ornithyl)-(N5-acetyl-N5-hydroxy-L-ornithyl)-N5-acetyl-N5-hydroxy-L-ornithin. Liebigs Ann. Chem. 1984, 1984, 1434–1440. [Google Scholar] [CrossRef]
- Benz, G. Albomycins, III. Synthesis of N5-acetyl-N5-hydroxy-L-ornithine from L-glutamic acid. Liebigs Ann. Chem. 1984, 8, 1424–1433. [Google Scholar] [CrossRef]
- Lin, Y.-M.; Miller, M.J. Practical synthesis of hydroxamate-derived siderophore components by an indirect oxidation method and syntheses of a DIG−siderophore conjugate and a biotin−siderophore conjugate. J. Org. Chem. 1999, 64, 7451–7458. [Google Scholar] [CrossRef]
- Dolence, E.K.; Lin, C.E.; Miller, M.J.; Payne, S.M. Synthesis and siderophore activity of albomycin-like peptides derived from N5-acetyl-N5-hydroxy-L-ornithine. J. Med. Chem. 1991, 34, 956–968. [Google Scholar] [CrossRef]
- Dolence, E.K.; Minnick, A.A.; Miller, M.J. N5-acetyl-N5-hydroxy-L-ornithine-derived siderophore-carbacephalosporin beta-lactam conjugates: Iron transport mediated drug delivery. J. Med. Chem. 1990, 33, 461–464. [Google Scholar] [CrossRef]
- Bredenkamp, A.D.; Martin, W.; Holzapfel, C.W.; Swanepoel, A.D. Synthesis of the thionucleoside moiety of albomycin δ1. S. Afr. Chem. 1991, 44, 31–33. [Google Scholar]
- Gadakh, B.; Vondenhoff, G.; Pang, L.; Nautiyal, M.; De Graef, S.; Strelkov, S.V.; Weeks, S.D.; Van Aerschot, A. Synthesis and structural insights into the binding mode of the albomycin δ1 core and its analogues in complex with their target aminoacyl-tRNA synthetase. Bioorg. Med. Chem. 2020, 28, 115645. [Google Scholar] [CrossRef]
- Paulsen, H.; Brieden, M.; Benz, G. Verzweigte und kettenverlängerte Zucker, XXXI. Synthese des Sauerstoffanalogons der Desferriform von δ1-Albomycin. Liebigs Ann. Chem. 1987, 1987, 565–575. [Google Scholar] [CrossRef]
- Lin, Z.; Xu, X.; Zhao, S.; Yang, X.; Guo, J.; Zhang, Q.; Jing, C.; Chen, S.; He, Y. Total synthesis and antimicrobial evaluation of natural albomycins against clinical pathogens. Nat. Commun. 2018, 9, 3445. [Google Scholar] [CrossRef]
- Vondenhoff, G.H.; Gadakh, B.; Severinov, K.; Van Aerschot, A. Microcin C and albomycin analogues with aryl-tetrazole substituents as nucleobase isosters are selective inhibitors of bacterial aminoacyl tRNA synthetases but lack efficient uptake. Chembiochem 2012, 13, 1959–1969. [Google Scholar] [CrossRef]
- Hill, J.M.; Yu, G.; Shue, Y.K.; Zydowsky, T.M.; Rebek, J. Aminoacyl Adenylate Mimics as Novel Antimicrobial and Antiparasitic Agents. U.S. Patent 5,726,195, 10 March 1998. [Google Scholar]
- Khoshnood, S.; Heidary, M.; Asadi, A.; Soleimani, S.; Motahar, M.; Savari, M.; Saki, M.; Abdi, M. A review on mechanism of action, resistance, synergism, and clinical implications of mupirocin against Staphylococcus aureus. Biomed. Pharmacother. 2019, 109, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Tucaliuc, A.; Blaga, A.C.; Galaction, A.I.; Cascaval, D. Mupirocin: Applications and production. Biotechnol. Lett. 2019, 41, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Barak, O.; Loo, D.S. AN-2690, a novel antifungal for the topical treatment of onychomycosis. Curr. Opin. Investig. Drugs 2007, 8, 662–668. [Google Scholar] [PubMed]
- Coghi, P.S.; Zhu, Y.; Xie, H.; Hosmane, N.S.; Zhang, Y. Organoboron compounds: Effective antibacterial and antiparasitic agents. Molecules 2021, 26, 3309. [Google Scholar] [CrossRef] [PubMed]
- Pines, M.; Snyder, D.; Yarkoni, S.; Nagler, A. Halofuginone to treat fibrosis in chronic graft-versus-host disease and scleroderma. Biol. Blood Marrow Transplant. 2003, 9, 417–425. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, M.; Zhang, Y.; Lv, L.; Kong, D.; Niu, G. Biosynthesis and Chemical Synthesis of Albomycin Nucleoside Antibiotics. Antibiotics 2022, 11, 438. https://doi.org/10.3390/antibiotics11040438
Wang M, Zhang Y, Lv L, Kong D, Niu G. Biosynthesis and Chemical Synthesis of Albomycin Nucleoside Antibiotics. Antibiotics. 2022; 11(4):438. https://doi.org/10.3390/antibiotics11040438
Chicago/Turabian StyleWang, Meiyan, Yuxin Zhang, Lanxin Lv, Dekun Kong, and Guoqing Niu. 2022. "Biosynthesis and Chemical Synthesis of Albomycin Nucleoside Antibiotics" Antibiotics 11, no. 4: 438. https://doi.org/10.3390/antibiotics11040438
APA StyleWang, M., Zhang, Y., Lv, L., Kong, D., & Niu, G. (2022). Biosynthesis and Chemical Synthesis of Albomycin Nucleoside Antibiotics. Antibiotics, 11(4), 438. https://doi.org/10.3390/antibiotics11040438





