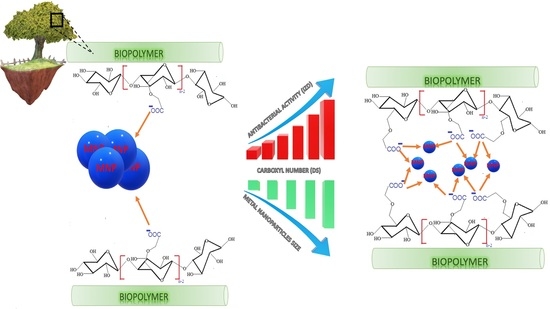
Synthesis of Metal-Loaded Carboxylated Biopolymers with Antibacterial Activity through Metal Subnanoparticle Incorporation
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Cation- and Metal-Loaded Materials
2.3. Material Characterization
2.4. Antibacterial Tests
3. Results and Discussion
3.1. Biopolymer and Composite Behavior in Aqueous Media
3.1.1. Effects of Biopolymer Structure and Clay Addition
3.1.2. Effect of Metal Incorporation
3.1.3. Zeta Potential–pH–Material Particle Size Interdependence
3.2. XPS Assessments of Induced Interactions
3.2.1. XPS Evidence of Cation-Matrix Interactions
3.2.2. Biopolymer Interactions with MNP and Montmorillonite
3.3. Zero-Valent Metal Dispersion
3.4. Antibacterial Activity
3.4.1. Effect of Biopolymer Structure and Metal Incorporation
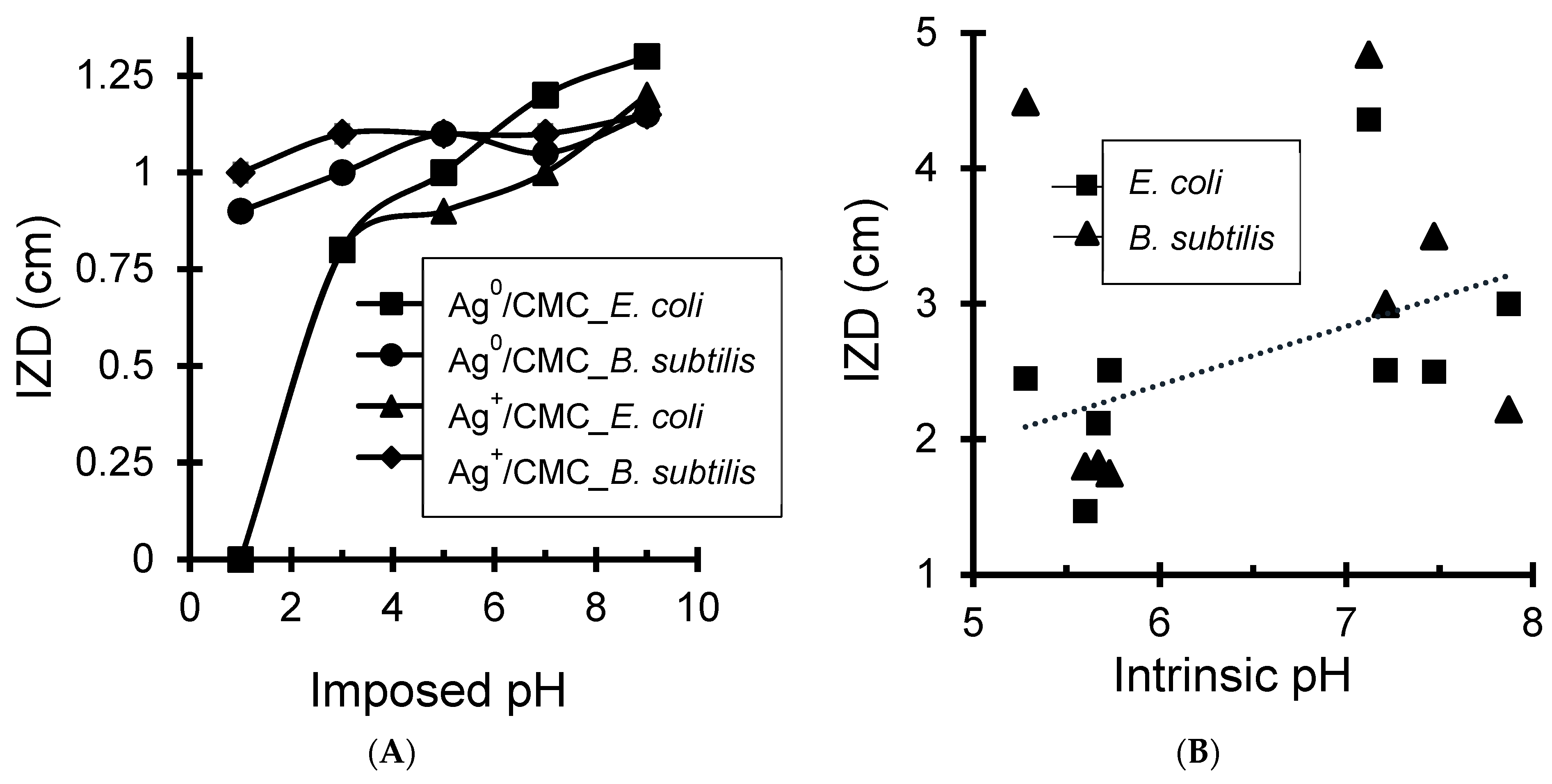
3.4.2. Effects of Cation Amount and pH
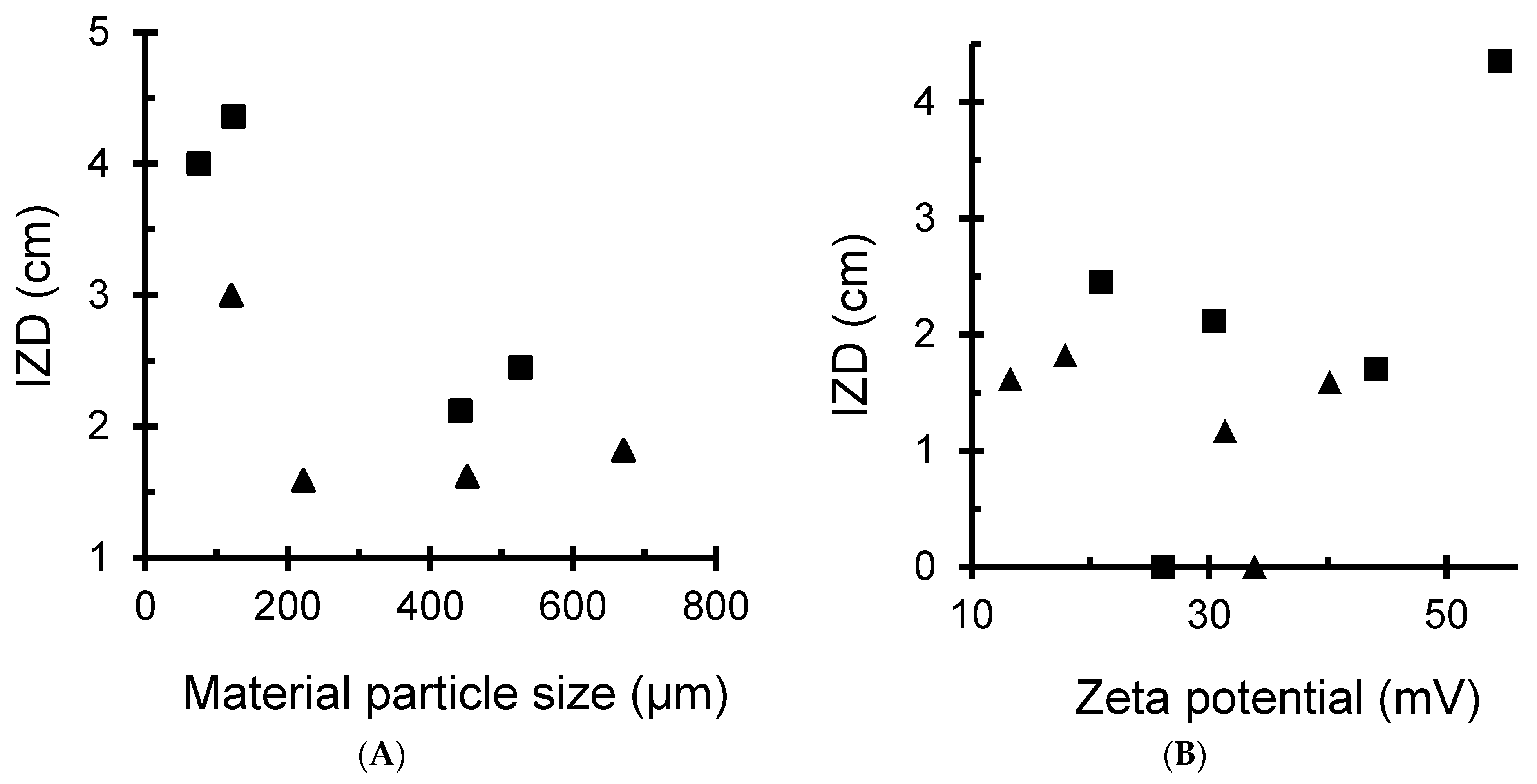
3.4.3. Effects of Material and Metal Dispersions
4. Conclusions
5. Highlights
- Carboxymethyl-functionalized biopolymers act as host-matrices for metal particles.
- Carboxymethyl group density influences the metal dispersion and particle size.
- Metal dispersion and stabilization involve interactions with matrices oxygen atoms.
- Metal type, dispersion, valence and amount are key factors in the antibacterial activity.
- Highly dispersed copper and silver subnanoparticles show enhanced antibacterial activity.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roca, I.; Akova, M.; Baquero, F.; Carlet, J.; Cavaleri, M.; Coenen, S.; Cohen, J.; Findlay, D.; Gyssens, I.; Heure, O.E.; et al. The global threat of antimicrobial resistance: Science for intervention. New Microbes. New Infect. 2015, 6, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Jansen, W.; Van Der Bruggen, J.; Verhoef, J.; Fluit, A. Bacterial resistance: A sensitive issue: Complexity of the challenge and containment strategy in Europe. Drug Resist. Updat. 2006, 9, 123–133. [Google Scholar] [CrossRef]
- Penesyan, A.; Gillings, M.; Paulsen, I.T. Antibiotic Discovery: Combatting Bacterial Resistance in Cells and in Biofilm Communities. Molecules 2015, 20, 5286–5298. [Google Scholar] [CrossRef] [PubMed]
- Capita, R.; Alonso-Calleja, C. Antibiotic-Resistant Bacteria: A Challenge for the Food Industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Noori, F.; Neree, A.T.; Megoura, M.; Mateescu, M.A.; Azzouz, A. Insights into the metal retention role in the antibacterial behavior of montmorillonite and cellulose tissue-supported copper and silver nanoparticles. RSC Adv. 2021, 11, 24156–24171. [Google Scholar] [CrossRef]
- Keshari, A.K.; Srivastava, R.; Singh, P.; Yadav, V.B.; Nath, G. Antioxidant and antibacterial activity of silver nanoparticles synthesized by Cestrum nocturnum. J. Ayurveda Integr. Med. 2020, 11, 37–44. [Google Scholar] [CrossRef]
- Kim, S.-H.; Lee, H.-S.; Ryu, D.-S.; Choi, S.-J.; Lee, D.-S. Antibacterial activity of silver-nanoparticles against Staphylococcus aureus and Escherichia coli. Microbiol. Biotechnol. Lett. 2011, 39, 77–85. [Google Scholar]
- Bondarenko, O.; Ivask, A.; Käkinen, A.; Kurvet, I.; Kahru, A. Particle-Cell Contact Enhances Antibacterial Activity of Silver Nanoparticles. PLoS ONE 2013, 8, e64060. [Google Scholar] [CrossRef]
- Harikumar, P.; Aravind, A. Antibacterial activity of copper nanoparticles and copper nanocomposites against Escherichia coli bacteria. Int. J. Sci. 2016, 5, 83–90. [Google Scholar]
- Ramyadevi, J.; Jeyasubramanian, K.; Marikani, A.; Rajakumar, G.; Rahuman, A.A. Synthesis and antimicrobial activity of copper nanoparticles. Mater. Lett. 2012, 71, 114–116. [Google Scholar] [CrossRef]
- Faedmaleki, F.; Farshad, H.S.; Salarian, A.A.; Ahmadi Ashtiani, H.; Rastegar, H. Toxicity effect of silver nanoparticles on mice liver primary cell culture and hepg2 cell line. Iran J. Pharm. Res. 2014, 13, 235–242. [Google Scholar] [PubMed]
- Allen, S.L.; Sharma, J.N.; Zamborini, F.P. Aggregation-Dependent Oxidation of Metal Nanoparticles. J. Am. Chem. Soc. 2017, 139, 12895–12898. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Wang, H.; Zhou, D.; Lenihan, H.S.; Cherr, G.; Cardinale, B.J.; Miller, R.; Ji, Z. Stability and Aggregation of Metal Oxide Nanoparticles in Natural Aqueous Matrices. Environ. Sci. Technol. 2010, 44, 1962–1967. [Google Scholar] [CrossRef]
- Kalsin, A.M.; Pinchuk, A.O.; Smoukov, S.K.; Paszewski, M.; Schatz, G.C.; Grzybowski, B.A. Electrostatic Aggregation and Formation of Core−Shell Suprastructures in Binary Mixtures of Charged Metal Nanoparticles. Nano Lett. 2006, 6, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- De Souza, T.A.J.; Souza, L.R.R.; Franchi, L.P. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol. Environ. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef]
- Lin, C.; Fugetsu, B.; Su, Y.; Watari, F. Studies on toxicity of multi-walled carbon nanotubes on Arabidopsis T87 suspension cells. J. Hazard. Mater. 2009, 170, 578–583. [Google Scholar] [CrossRef] [PubMed]
- Le Ouay, B.; Stellacci, F. Antibacterial activity of silver nanoparticles: A surface science insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- Xiu, Z.-M.; Zhang, Q.-B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible Particle-Specific Antibacterial Activity of Silver Nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Iqbal, T.; Irfan, M.; Ramay, S.M.; Mahmood, A.; Saleem, M.; Siddiqi, S.A. ZnO—PVA Polymer Matrix with Transition Metals Oxide Nano-fillers for High Dielectric Mediums. J. Polym. Environ. 2020, 28, 2422–2432. [Google Scholar] [CrossRef]
- Pathak, T.K.; Kroon, R.; Craciun, V.; Popa, M.; Chifiriuc, M.C.; Swart, H. Influence of Ag, Au and Pd noble metals doping on structural, optical and antimicrobial properties of zinc oxide and titanium dioxide nanomaterials. Heliyon 2019, 5, e01333. [Google Scholar] [CrossRef] [PubMed]
- Diaz, C.; Barrientos, L.; Carrillo, D.; Valdebenito, J.; Valenzuela, M.L.; Allende, P.; Geaney, H.; O’Dwyer, C. Solvent-less method for efficient photocatalytic α-Fe2O3 nanoparticles using macromolecular polymeric precursors. New J. Chem. 2016, 40, 6768–6776. [Google Scholar] [CrossRef]
- Kosakowska, K.A.; Casey, B.K.; Albert, J.N.L.; Wang, Y.; Ashbaugh, H.S.; Grayson, S.M. Synthesis and Self-Assembly of Amphiphilic Star/Linear–Dendritic Polymers: Effect of Core versus Peripheral Branching on Reverse Micelle Aggregation. Biomacromolecules 2018, 19, 3177–3189. [Google Scholar] [CrossRef] [PubMed]
- Ujihara, M.; Imae, T. Hierarchical structures of dendritic polymers. Polym. Int. 2010, 59, 137–144. [Google Scholar] [CrossRef]
- Furukawa, Y.; Watkins, J.L.; Kim, J.; Curry, K.J.; Bennett, R.H. Aggregation of montmorillonite and organic matter in aqueous media containing artificial seawater. Geochem. Trans. 2009, 10, 2. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Pooresmaeil, M.; Namazi, H. Green one-pot synthesis of carboxymethylcellulose/Zn-based metal-organic framework/graphene oxide bio-nanocomposite as a nanocarrier for drug delivery system. Carbohydr. Polym. 2019, 208, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, S.; Pooresmaeil, M.; Hashemi, H.; Namazi, H. Carboxymethylcellulose capsulated cu-based metal-organic framework-drug nanohybrid as a ph-sensitive nanocomposite for ibuprofen oral delivery. Int. J. Biol. Macromol. 2018, 119, 588–596. [Google Scholar] [PubMed]
- Xu, L.; Chen, G.; Peng, C.; Qiao, H.; Ke, F.; Hou, R.; Li, D.; Cai, H.; Wan, X. Adsorptive removal of fluoride from drinking water using porous starch loaded with common metal ions. Carbohydr. Polym. 2017, 160, 82–89. [Google Scholar] [CrossRef]
- Wang, J.; Ji, B.; Shu, Y.; Chen, W.; Zhu, L.; Chen, F. Cr (VI) Removal from Aqueous Solution Using Starch and Sodium Carboxymethyl Cellulose-Coated Fe and Fe /Ni Nanoparticles. Pol. J. Environ. Stud. 2018, 27, 2785–2792. [Google Scholar] [CrossRef]
- Ounkaew, A.; Kasemsiri, P.; Jetsrisuparb, K.; Uyama, H.; Hsu, Y.-I.; Boonmars, T.; Artchayasawat, A.; Knijnenburg, J.T.; Chindaprasirt, P. Synthesis of nanocomposite hydrogel based carboxymethyl starch/polyvinyl alcohol/nanosilver for biomedical materials. Carbohydr. Polym. 2020, 248, 116767. [Google Scholar] [CrossRef] [PubMed]
- Labelle, M.; Ispas-Szabo, P.; Mateescu, M.A. Structure-Functions Relationship of Modified Starches for Pharmaceutical and Biomedical Applications. Starch-Stärke 2020, 72, 2000002. [Google Scholar] [CrossRef]
- Ogushi, Y.; Sakai, S.; Kawakami, K. Synthesis of enzymatically-gellable carboxymethylcellulose for biomedical applications. J. Biosci. Bioeng. 2007, 104, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Pal, K.; Banthia, A.K.; Majumdar, D.K. Development of carboxymethyl cellulose acrylate for various biomedical applications. Biomed. Mater. 2006, 1, 85–91. [Google Scholar] [CrossRef]
- Farrokhpay, S. A review of polymeric dispersant stabilisation of titania pigment. Adv. Colloid Interface Sci. 2009, 151, 24–32. [Google Scholar] [CrossRef]
- Arus, A.V.; Tahir, M.N.; Sennour, R.; Shiao, T.C.; Sallam, L.M.; Nistor, I.D.; Roy, R.; Azzouz, A. Cu0and Pd0loaded Organo-Bentonites as Sponge-like Matrices for Hydrogen Reversible Capture at Ambient Conditions. ChemistrySelect 2016, 1, 1452–1461. [Google Scholar] [CrossRef]
- Bouazizi, N.; Barrimo, D.; Nousir, S.; Ben Slama, R.; Shiao, T.; Roy, R.; Azzouz, A. Metal-loaded polyol-montmorillonite with improved affinity towards hydrogen. J. Energy Inst. 2016, 91, 110–119. [Google Scholar] [CrossRef]
- Sennour, R.; Shiao, T.C.; Arus, V.A.; Tahir, M.N.; Bouazizi, N.; Roy, R.; Azzouz, A. Cu0-Loaded organo-montmorillonite with improved affinity towards hydrogen: An insight into matrix–metal and non-contact hydrogen–metal interactions. Phys. Chem. Chem. Phys. 2017, 19, 29333–29343. [Google Scholar] [CrossRef]
- Tahir, M.N.; Sennour, R.; Arus, V.A.; Sallam, L.M.; Roy, R.; Azzouz, A. Metal organoclays with compacted structure for truly physical capture of hydrogen. Appl. Surf. Sci. 2017, 398, 116–124. [Google Scholar] [CrossRef]
- Alothman, M.; Ispas-Szabo, P.; Mateescu, M.A. Design of Catalase Monolithic Tablets for Intestinal Targeted Delivery. Pharmaceutics 2021, 13, 69. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, M.; Gosselin, P.; Mateescu, M.A. Carboxymethyl high amylose starch as excipient for controlled drug release: Mechanistic study and the influence of degree of substitution. Int. J. Pharm. 2009, 382, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, Ž.; Jeremić, K.; Jovanović, S.; Lechner, M.D. A Comparison of Some Methods for the Determination of the Degree of Substitution of Carboxymethyl Starch. Starch-Stärke 2005, 57, 79–83. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Potera, C. Understanding the Germicidal Effects of Silver Nanoparticles. Environ. Heal. Perspect. 2012, 120, a386. [Google Scholar] [CrossRef][Green Version]
- Qing, Y.; Cheng, L.; Li, R.; Liu, G.; Zhang, Y.; Tang, X.; Wang, J.; Liu, H.; Qin, Y. Potential antibacterial mechanism of silver nanoparticles and the optimization of orthopedic implants by advanced modification technologies. Int. J. Nanomed. 2018, 13, 3311–3327. [Google Scholar] [CrossRef]
- Gordon, O.; Slenters, T.V.; Brunetto, P.S.; Villaruz, A.E.; Sturdevant, D.E.; Otto, M.; Landmann, R.; Fromm, K.M. Silver Coordination Polymers for Prevention of Implant Infection: Thiol Interaction, Impact on Respiratory Chain Enzymes, and Hydroxyl Radical Induction. Antimicrob. Agents Chemother. 2010, 54, 4208–4218. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Li, L.; Liu, L.; Cai, J.; Zhang, Y.; Zhou, J.; Zhang, L. Construction of Cellulose Based ZnO Nanocomposite Films with Antibacterial Properties through One-Step Coagulation. ACS Appl. Mater. Interfaces 2015, 7, 2597–2606. [Google Scholar] [CrossRef] [PubMed]
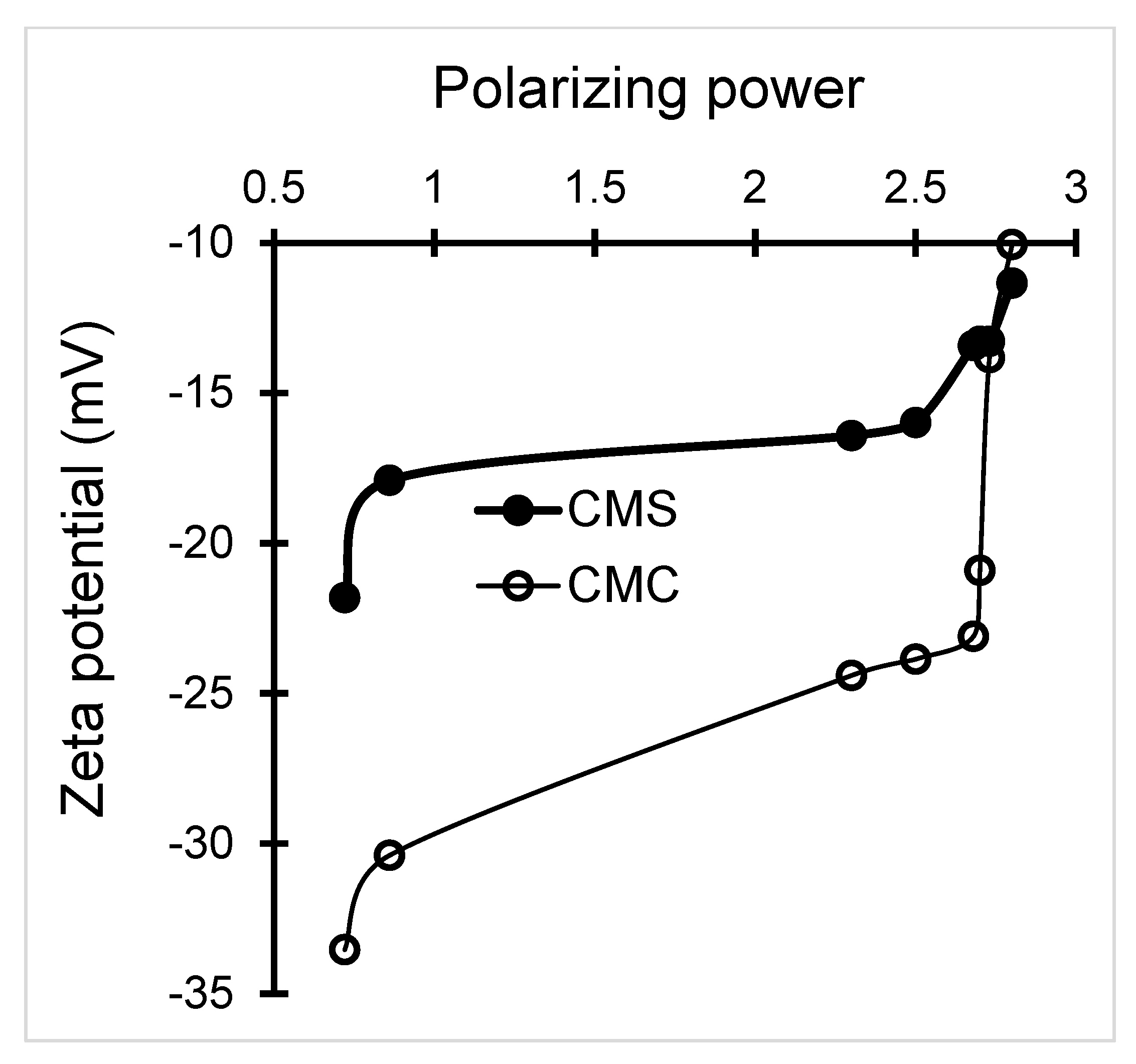

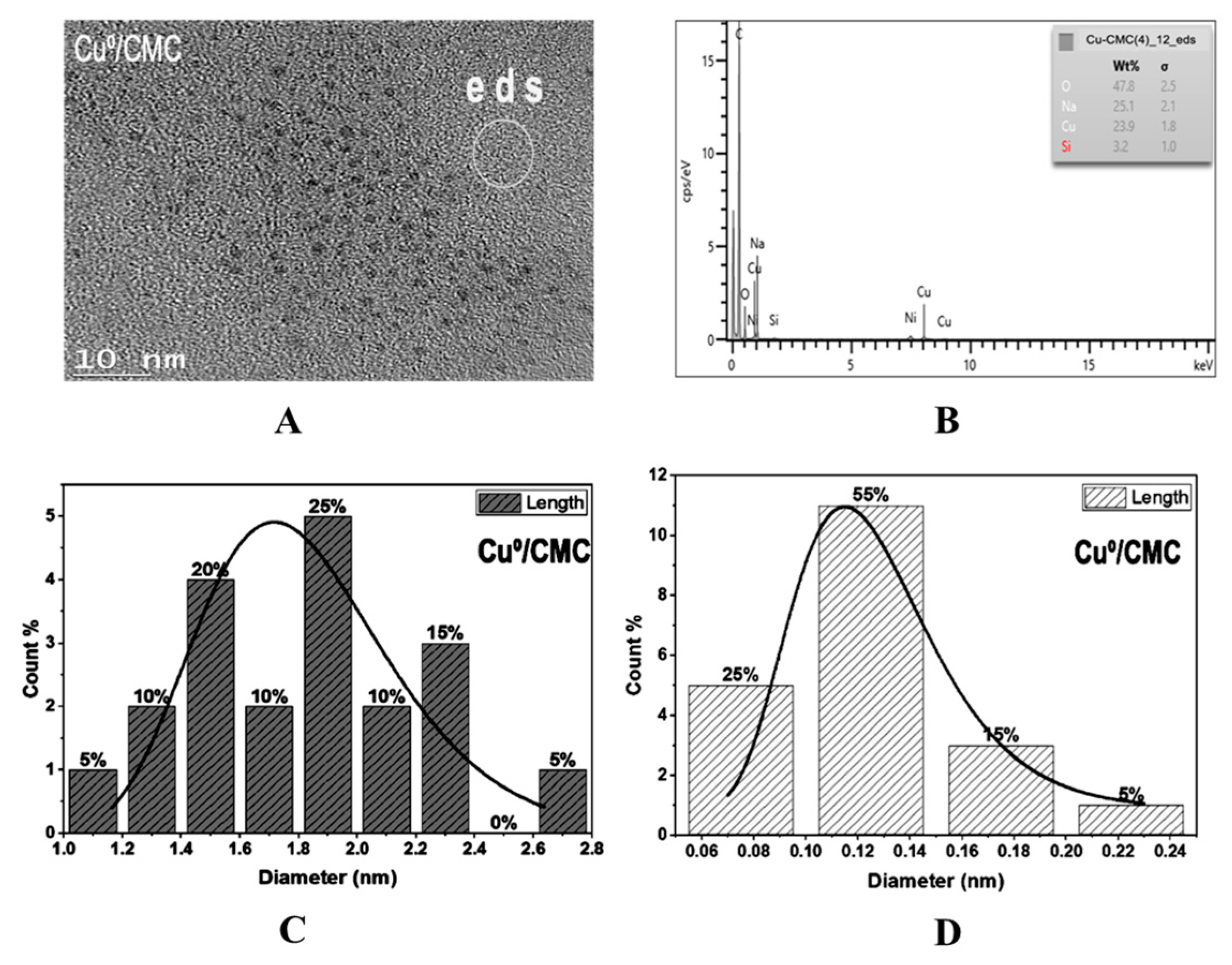

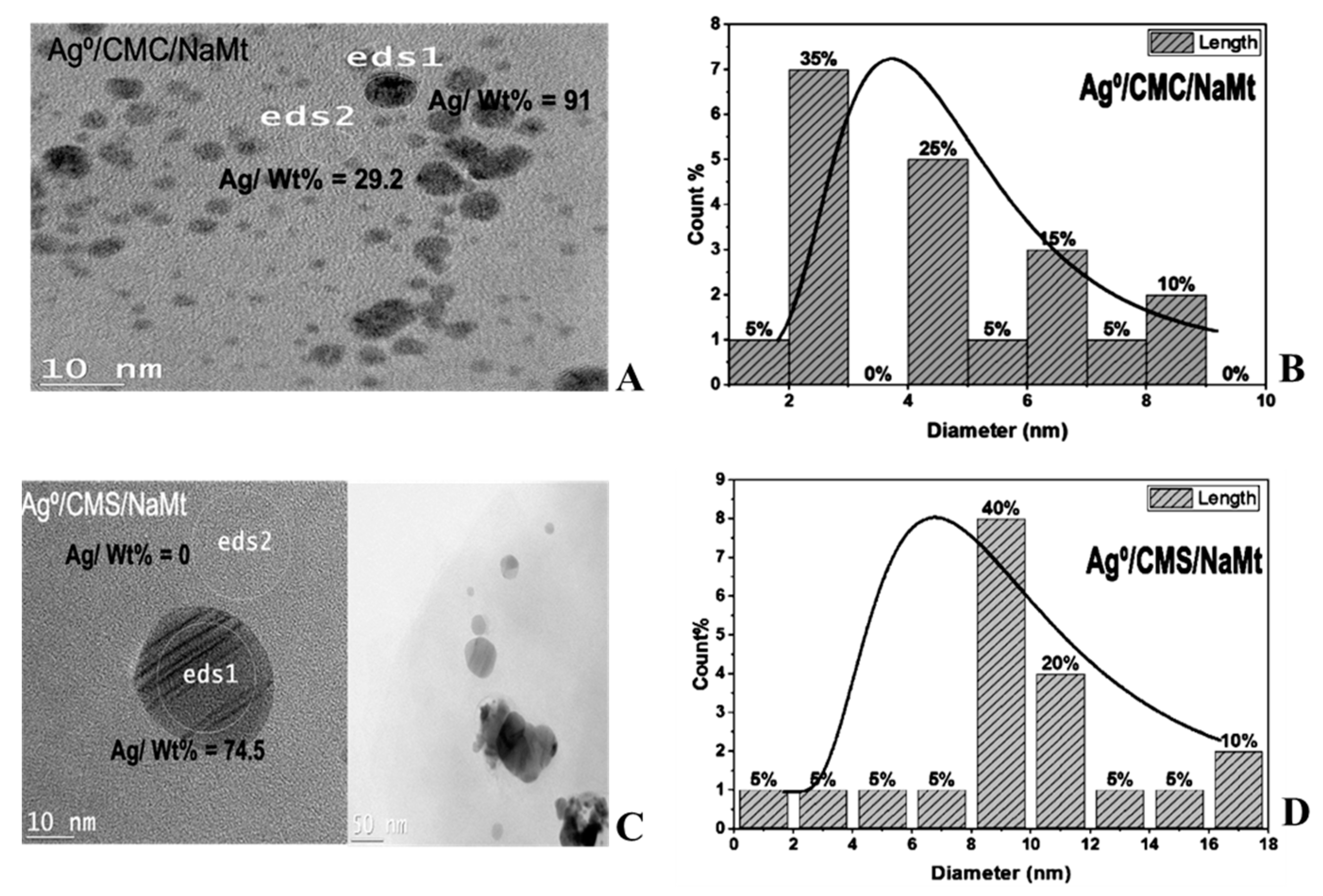


| Biopolymer | Sample | Zeta Potential * | Particle Size * | Degree of Substitution ** | pH * | % Error | ||
|---|---|---|---|---|---|---|---|---|
| mV | % Error | nm | % Error | |||||
| Carboxymethyl cellulose | CMC | −33.83 | 5.80 | 289.30 | 1.40 | 0.92 + 0.01 | 6.37 | 2.10 |
| Cu2+/CMC | −13.82 | 1.04 | 526.20 | 3.82 | 5.60 | 0.63 | ||
| Ag+/CMC | −30.39 | 3.51 | 441.70 | 3.30 | 5.28 | 3.21 | ||
| Cu0/CMC | −44.05 | 4.40 | 123.10 | 2.40 | 6.37 | 4.32 | ||
| Ag0/CMC | −54.53 | 4.50 | 75.42 | 5.03 | 6.00 | 3.10 | ||
| CMC/NaMt | −48.36 | 3.80 | 266.60 | 6.04 | - | 6.32 | 2.22 | |
| Cu2+/CMC-NaMt | −23.74 | 1.62 | 605.50 | 0.47 | 5.66 | 2.32 | ||
| Ag+/CMC-NaMt | −39.21 | 3.62 | 450.20 | 2.30 | 5.90 | 3.21 | ||
| Cu0/CMC-NaMt | −48.36 | 2.36 | 206.25 | 1.45 | 6.90 | 1.26 | ||
| Ag0/CMC-NaMt | −47.35 | 3.19 | 128.10 | 5.51 | 6.00 | 1.22 | ||
| Carboxymethyl Starch | CMS | −26.11 | 3.50 | 350.20 | 1.50 | 0.51 + 0.01 | 6.56 | 3.40 |
| Cu2+/CMS | −13.28 | 2.21 | 670.60 | 2.45 | 5.67 | 4.32 | ||
| Ag+/CMS | −17.90 | 1.02 | 451.40 | 2.19 | 5.73 | 4.02 | ||
| Cu0/CMS | −31.36 | 1.20 | 221.73 | 1.64 | 6.47 | 2.04 | ||
| Ag0/CMS | −40.16 | 1.58 | 120.65 | 0.28 | 6.21 | 0.89 | ||
| CMS/NaMt | −33.42 | 6.80 | 299.00 | 2.32 | - | 6.41 | 2.11 | |
| Cu2+/CMS-NaMt | −17.89 | 1.54 | 596.30 | 2.45 | 5.90 | 6.32 | ||
| Ag+/CMS-NaMt | −32.34 | 1.13 | 537.10 | 1.93 | 5.83 | 3.21 | ||
| Cu0/CMS-NaMt | −34.12 | 2.25 | 213.10 | 6.76 | 6.37 | 4.23 | ||
| Ag0/CMS-NaMt | −33.54 | 2.10 | 165.15 | 1.81 | 6.00 | 0.41 | ||
| XPS Signal | Binding Energy (eV) * | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CMC-Based Samples | CMS-Based Samples | ||||||||||
| Matrix | Alone | +Cu2+ | +Cu0 | +Ag+ | +Ag0 | Alone | + Cu2+ | +Cu0 | +Ag+ | +Ag0 | |
| O1S | O=C | 530.68 | 530.28 | 529.89 | 530.18 | 529.38 | 530.78 | 530.08 | 529.88 | 530.28 | 529.98 |
| H-O-C | 532.58 | 531.28 | 530.89 | 532.28 | 531.08 | 532.58 | 531.58 | 531.18 | 532.28 | 532.11 | |
| C1S | C-O-C | 286.18 | 286.28 | 287.08 | 286.38 | 286.22 | 286.18 | 286.28 | 285.98 | 286.29 | 286.37 |
| C-C | 284.58 | 284.78 | 284.90 | 284.68 | 284.78 | 284.58 | 284.78 | 284.48 | 284.78 | 284.68 | |
| O-C=O | 287.58 | 287.88 | 287.98 | 287.68 | 287.78 | 287.68 | 288.18 | 288.10 | 287.98 | 287.78 | |
| Biopolymer | Incorporated Species | Samples | Inhibition Zone Diameter (IZD) (cm) * | |||
|---|---|---|---|---|---|---|
| E. coli DH5α | % Error | B. subtilis S168 | % Error | |||
| CMC | None | CMC | 0.00 | 0.00 | 0.00 | 0.00 |
| Metal cation | Cu2+/CMC | 2.12 | 2.10 | 3.81 | 3.23 | |
| Ag+/CMC | 4.36 | 3.43 | 5.40 | 3.67 | ||
| Zero-valent metal | Cu0/CMC | 1.70 | 5.33 | 4.06 | 1.83 | |
| Ag0/CMC | 3.81 | 1.11 | 4.70 | 4.34 | ||
| Montmorillonite | CMC/NaMt | 0.00 | 2.32 | 0.00 | 4.23 | |
| Metal cation | Cu2+/CMC/NaMt | 1.50 | 4.32 | 3.90 | 2.36 | |
| Ag+/CMC/NaMt | 2.29 | 6.12 | 1.70 | 3.21 | ||
| Zero-valent metal | Cu0/CMC/NaMt | 1.27 | 3.12 | 3.81 | 5.23 | |
| Ag0/CMC/NaMt | 2.11 | 2.10 | 1.27 | 4.21 | ||
| CMS | None | CMS | 0.00 | 2.98 | 0.00 | 3.12 |
| Metal cation | Cu2+/CMS | 1.82 | 3.29 | 3.80 | 3.54 | |
| Ag+/CMS | 3.06 | 4.55 | 5.00 | 6.14 | ||
| Zero-valent metal | Cu0/CMS | 3.12 | 6.01 | 4.06 | 4.53 | |
| Ag0/CMS | 2.54 | 1.21 | 3.81 | 2.12 | ||
| Montmorillonite | CMS/NaMt | 0.00 | 2.04 | 0.00 | 3.24 | |
| Metal cation | Cu2+/CMS/NaMt | 1.00 | 2.32 | 4.50 | 6.55 | |
| Ag+/CMS/NaMt | 2.17 | 2.43 | 1.70 | 3.45 | ||
| Zero-valent metal | Cu0/CMS/NaMt | 1.52 | 5.32 | 3.30 | 2.17 | |
| Ag0/CMS/NaMt | 1.20 | 1.00 | 0.20 | 2.91 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noori, F.; Megoura, M.; Labelle, M.-A.; Mateescu, M.A.; Azzouz, A. Synthesis of Metal-Loaded Carboxylated Biopolymers with Antibacterial Activity through Metal Subnanoparticle Incorporation. Antibiotics 2022, 11, 439. https://doi.org/10.3390/antibiotics11040439
Noori F, Megoura M, Labelle M-A, Mateescu MA, Azzouz A. Synthesis of Metal-Loaded Carboxylated Biopolymers with Antibacterial Activity through Metal Subnanoparticle Incorporation. Antibiotics. 2022; 11(4):439. https://doi.org/10.3390/antibiotics11040439
Chicago/Turabian StyleNoori, Farzaneh, Meriem Megoura, Marc-André Labelle, Mircea Alexandru Mateescu, and Abdelkrim Azzouz. 2022. "Synthesis of Metal-Loaded Carboxylated Biopolymers with Antibacterial Activity through Metal Subnanoparticle Incorporation" Antibiotics 11, no. 4: 439. https://doi.org/10.3390/antibiotics11040439
APA StyleNoori, F., Megoura, M., Labelle, M.-A., Mateescu, M. A., & Azzouz, A. (2022). Synthesis of Metal-Loaded Carboxylated Biopolymers with Antibacterial Activity through Metal Subnanoparticle Incorporation. Antibiotics, 11(4), 439. https://doi.org/10.3390/antibiotics11040439