Prevalence and Characteristics of Streptococcus agalactiae from Freshwater Fish and Pork in Hong Kong Wet Markets
Abstract
:1. Introduction
2. Results
2.1. Prevalence of GBS in Food Animals and Its Climatic Association
2.2. Antibiotic Susceptibility and Biofilm Formation in Fish and Pig GBS
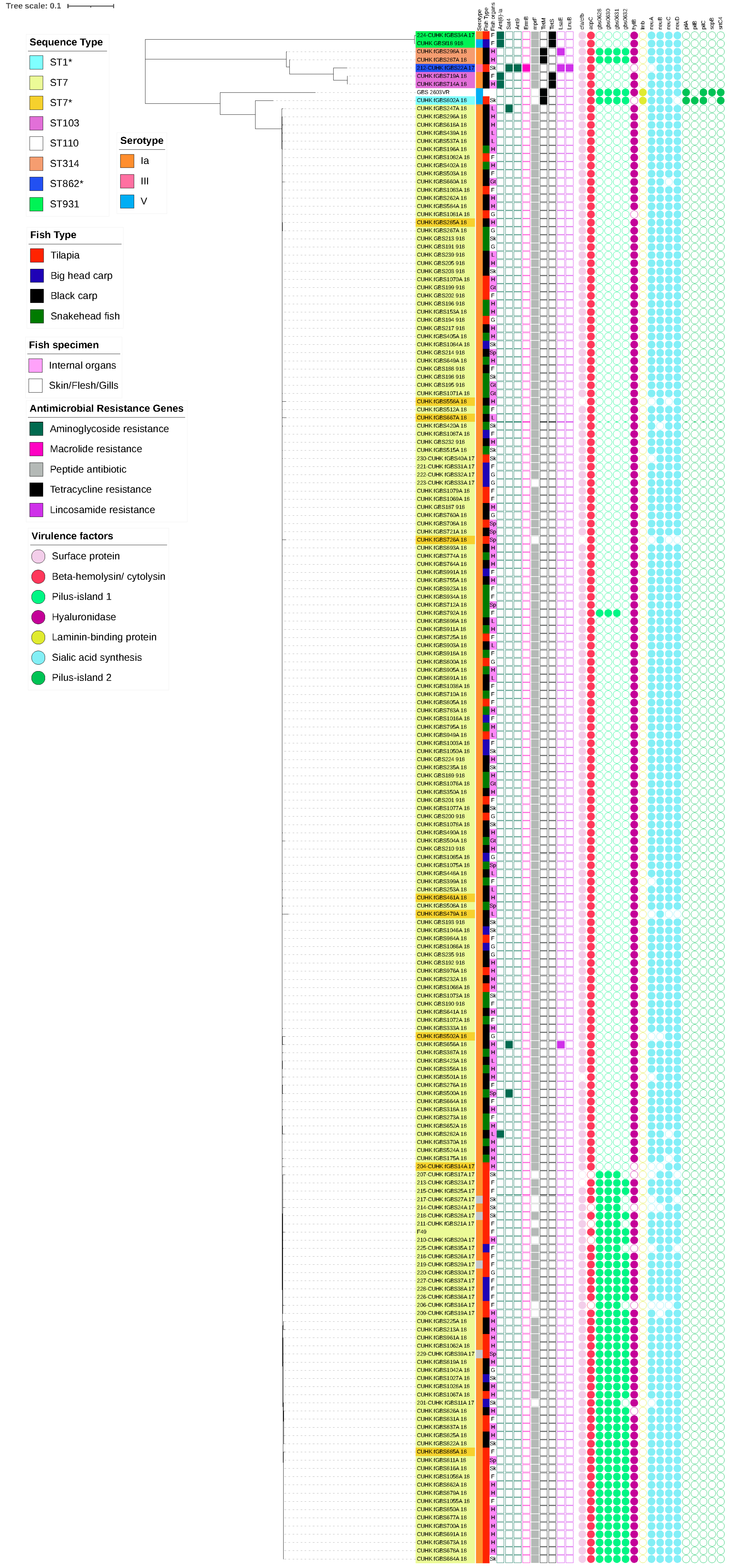
2.3. Molecular Characteristics of Capsular Serotypes among Freshwater Fish and Pigs
3. Discussion
4. Materials and Methods
4.1. Animal Sampling
4.2. Isolation of GBS
4.3. Antibiotic Susceptibility Testing
4.4. Biofilm Formation
4.5. Molecular Characterization of GBS Isolates and Whole-Genome Sequencing
5. Limitations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lancefield, R.C.; Hare, R. The serological differentiation of pathogenic and non-pathogenic strains of hemolytic streptococci from parturient women. J. Exp. Med. 1935, 28, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Paveenkittiporn, W.; Ungcharoen, R.; Kerdsin, A. Streptococcus agalactiae infections and clinical relevance in adults, Thailand. Diagn. Microbiol. Infect. Dis. 2020, 97, 115005. [Google Scholar] [CrossRef] [PubMed]
- Elliott, J.A.; Facklam, R.R.; Richter, C.B. Whole-cell protein patterns of nonhemolytic group B, type ib, streptococci isolated from humans, mice, cattle, frogs, and fish. J. Clin. Microbiol. 1990, 28, 628–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, A.; Nam, G.H.; Gim, J.A.; Lee, H.E.; Jo, A.; Kim, H.S. Current challenges of streptococcus infection and effective molecular, cellular, and environmental control methods in aquaculture. Mol. Cells 2018, 41, 495–505. [Google Scholar] [PubMed]
- Streptococcosis in Tilapia-Global Aquaculture Advocate. Global Aquaculture Alliance. Available online: https://www.aquaculturealliance.org/advocate/streptococcosis-in-tilapia/ (accessed on 9 April 2020).
- Manning, S.D.; Springman, A.C.; Million, A.D.; Milton, N.R.; McNamara, S.E.; Somsel, P.A.; Bartlett, P.; Davies, H.D. Association of group B streptococcus colonization and bovine exposure: A prospective cross-sectional cohort study. PLoS ONE 2010, 5, e8795. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Lin, Y.; Foo, K.; Koh, H.F.; Tow, C.; Zhang, Y.; Ang, L.W.; Cui, L.; Badaruddin, H.; Ooi, P.L.; et al. Group B streptococcus serotype III sequence type 283 bacteremia associated with consumption of raw fish, Singapore. Emerg. Infect. Dis. 2016, 22, 1970–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barkham, T.; Zadoks, R.N.; Azmai, M.N.A.; Baker, S.; Bich, V.T.N.; Chalker, V.; Chau, M.L.; Dance, D.; Deepak, R.N.; van Doorn, H.R.; et al. One hypervirulent clone, sequence type 283, accounts for a large proportion of invasive streptococcus agalactiae isolated from humans and diseased tilapia in southeast Asia. PLoS Negl. Trop. Dis. 2019, 13, e0007421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainability in Action. Rome, Italy. 2020. Available online: http://www.fao.org/3/ca9229en/ca9229en.pdf (accessed on 14 March 2022).
- National Pork Board. Top 10 Pork-Producing Countries. Pork Checkoff. Available online: https://www.pork.org/facts/stats/u-s-pork-exports/top-10-pork-producing-countries/ (accessed on 14 March 2022).
- Food and Environmental Hygiene Department. Public Markets. Available online: https://www.fehd.gov.hk/english/pleasant_environment/tidy_market/overview.html (accessed on 11 June 2020).
- Food and Environmental Hygiene Department. Slaughterhouses and Meat Inspection. Available online: https://www.cfs.gov.hk/english/import/import_smi.html (accessed on 5 March 2020).
- The Government of the Hong Kong Special Administration Region. LCQ9: Live Poultry Trade. Available online: https://www.info.gov.hk/gia/general/202004/22/P2020042200277.htm (accessed on 22 April 2021).
- Li, C.; Sapugahawatte, D.N.; Yang, Y.; Wong, K.T.; Lo, N.W.S.; Ip, M. Multidrug-resistant Streptococcus agalactiae strains found in human and fish with high penicillin and cefotaxime non-susceptibilities. Microorganisms 2020, 8, 1055. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 27th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2019. [Google Scholar]
- Kayansamruaj, P.; Pirarat, N.; Kondo, H.; Hirono, I.; Rodkhum, C. Genomic Comparison between Pathogenic Streptococcus Agalactiae Isolated from Nile Tilapia in Thailand and Fish-Derived ST7 Strains. Infect. Genet. Evol. 2015, 36, 307–314. Available online: http://www.sciencedirect.com/science/article/pii/S1567134815300095 (accessed on 14 March 2022). [CrossRef] [PubMed]
- Bowater, R.O.; Forbes-Faulkner, J.; Anderson, I.G.; Condon, K.; Robinson, B.; Kong, F.; Gilbert, G.L.; Reynolds, A.; Hyland, S.; McPherson, G.; et al. Natural outbreak of streptococcus agalactiae (GBS) infection in wild giant Queensland grouper, Epinephelus lanceolatus (Bloch), and other wild fish in northern Queensland, Australia. J. Fish Dis. 2012, 35, 173–186. [Google Scholar] [CrossRef]
- Evans, J.J.; Bohnsack, J.F.; Klesius, P.H.; Whiting, A.A.; Garcia, J.C.; Shoemaker, C.A.; Takahashi, S. Phylogenetic relationships among Streptococcus agalactiae isolated from piscine, dolphin, bovine and human sources: A dolphin and piscine lineage associated with a fish epidemic in Kuwait is also associated with human neonatal infections in Japan. J. Med. Microbiol. 2008, 57 Pt 11, 1369–1376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, N.; Bohnsack, J.F.; Takahashi, S.; Oliver, K.A.; Chan, M.S.; Kunst, F.; Glaser, P.; Rusniok, C.; Crook, D.W.; Harding, R.M.; et al. Multilocus sequence typing system for group B streptococcus. J. Clin. Microbiol. 2003, 41, 2530–2536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wibawan, I.W.; Lämmler, C.; Smola, J. Properties and type antigen patterns of group B streptococcal isolates from pigs and nutrias. J. Clin. Microbiol. 1993, 31, 762–764. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, Z.; Qu, P.; Ke, P.; Yang, X.; Zhou, Q.; Lan, K.; He, M.; Cao, N.; Qin, S.; Huang, X. Antibiotic resistance and molecular epidemiological characteristics of Streptococcus agalactiae isolated from pregnant women in Guangzhou, South China. Can. J. Infect. Dis. Med. Microbiol. 2020, 2020, 1368942. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, J.; Zheng, X.; Chen, L.; He, M.; Ma, S.; Lin, Y.; Lin, X.; Chen, C. Identification of group B streptococcus serotypes and genotypes in late pregnant women and neonates that are associated with neonatal early-onset infection in a south china population. Front. Paediatr. 2020, 8, 265. Available online: https://www.frontiersin.org/article/10.3389/fped.2020.00265 (accessed on 14 March 2022).
- Chu, C.; Huang, P.Y.; Chen, H.M.; Wang, Y.-H.; Tsai, I.-A.; Lu, C.-C.; Chen, C.-C. Genetic and pathogenic difference between Streptococcus agalactiae serotype Ia fish and human isolates. BMC Microbiol. 2016, 16, 175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agriculture, Fisheries and Conservation Department. Agriculture and Fisheries. 2018. Available online: https://www.afcd.gov.hk/english/quarantine/qua_live/qua_live_amr/introAMR.html (accessed on 5 April 2020).
- Laws Compilation and Publication Unit. Cap 139N. Public Health (Animals and Birds) (Chemical Residues) Regulation; Department of Justice: Hong Kong, China, 2006.
- Ip, M.; Ang, I.; Fung, K.; Liyanapathirana, V.; Luo, M.J.; Lai, R. Hypervirulent clone of group B streptococcus serotype III sequence type 283, hong kong, 1993–2012. Emerg. Infect. Dis. 2016, 22, 1800–1803. [Google Scholar] [CrossRef] [Green Version]
- Anonymous. Isolating Group B Streptococcus from a Food (Fish) Sample. Available online: https://10minus6cosm.tumblr.com/post/135763399281/isolating-group-b-streptococcus-from-a-food-sample (accessed on 25 December 2020).
- Imperi, M.; Pataracchia, M.; Alfarone, G.; Baldassarri, L.; Orefici, G.; Creti, R. A multiplex PCR assay for the direct identification of the capsular type (Ia to IX) of streptococcus agalactiae. J. Microbiol. Methods 2010, 80, 212–214. [Google Scholar] [CrossRef]
- Abdul-Lateef, L.A.; Gatea, A.K.; Jwad, T.S. Biofilm formation by Streptococcus agalactiae is affected by pH changes in vitro. J. Pharm. Sci. Res. 2018, 10, 3216. Available online: http://easyaccess.lib.cuhk.edu.hk/login?url=https://www.proquest.com/scholarly-journals/biofilm-formation-streptococcus-agalactiae-is/docview/2164972220/se-2?accountid=10371 (accessed on 14 March 2022).
- Sapugahawatte, D.N.; Li, C.; Zhu, C.; Dharmaratne, P.; Wong, K.T.; Lo, N.; Ip, M. Prevalence and characteristics ofextended-spectrum-β-lactamase-producing and carbapenemase-producing Enterobacteriaceae from freshwater fish and pork in wet markets of Hong Kong. mSphere 2020, 5, e00107-20. [Google Scholar] [CrossRef] [Green Version]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zankari, E.; Hasman, H.; Cosentino, S.; Vestergaard, M.; Rasmussen, S.; Lund, O.; Aarestrup, F.M.; Larsen, M.V. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 2012, 67, 2640–2644. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jolley, K.A.; Maiden, M.C.J. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinform. 2010, 11, 595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treangen, T.J.; Ondov, B.D.; Koren, S.; Phillippy, A.M. The harvest suite for rapid core-genome alignment and visualization of thousands of intraspecific microbial genomes. Genome Biol. 2014, 15, 524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. Interactive tree of life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Fish Type/Food Source (n) * | GBS Prevalence %, (n) | |
|---|---|---|
| Freshwater fish (n = 191/992, 19.3%) | Tilapia (n = 182) | 34.1% (62) |
| Big Head Carp (n = 187) | 10.1% (19) | |
| Snakehead Fish (n = 314) | 13.6% (43) | |
| Black Carp (n = 309) | 22.3% (67) | |
| Pigs (n = 61/361, 16.9%) | Tongue (n = 193) | 24.8% (48) |
| Small intestine (n = 92) | 3.2% (3) | |
| Large intestine (n = 76) | 13% (10) |
| Class of Antibiotic | Antibiotic | Concentration Range (μg/mL) | Freshwater Fish GBS | Pig GBS | ||||
|---|---|---|---|---|---|---|---|---|
| MIC (mg/L) a | % (No./191) Resistance | MIC (mg/L) a | % (No./61) Resistance | |||||
| MIC50 | MIC90 | MIC50 | MIC90 | |||||
| Penicillins | Penicillin | 2–0.0625 | 0.03 | 0.06 | 0.5 (1) | 0.015 | 0.03 | 0 (0) |
| Glycopeptides | Vancomycin | 8–0.25 | 0.25 | 0.5 | 0 (0) | 0.12 | 0.25 | 0 (0) |
| Tetracyclines | Doxycycline | 32–0.12 | 0.25 | 2 | 37.1 (71) | 16 | 16 | 90.1 (55) |
| Minocycline | 32–0.12 | ≤0.12 | 1 | 5.7 (11) | 16 | 16 | 85.2 (52) | |
| Tetracycline | 32–0.12 | ≤0.12 | 1 | 5.7 (11) | 16 | 16 | 90.1 (55) | |
| Oxazolidinones | Linezolid | 64–0.06 | 2 | 2 | 0 (0) | 2 | 2 | 0 (0) |
| Macrolides | Erythromycin | 4–0.12 | ≤0.12 | ≤0.12 | 3.1 (6) | >16 | >16 | 88.5 (54) |
| Lincosamides | Clindamycin | 4–0.12 | ≤0.12 | ≤0.12 | 1.5 (3) | >16 | >16 | 98.3 (60) |
| Fluoroquinolones | Ciprofloxacin ^ | 32–0.12 | 0.5 | 1 | 0.5 (1) | 0.5 | 0.5 | 0 (0) |
| Levofloxacin | 32–0.12 | 0.25 | 0.5 | 0 (0) | 0.5 | 0.5 | 0 (0) | |
| Food Type | No. a | Serotypes n (%) | ||||
|---|---|---|---|---|---|---|
| Ia | III-2 | III-NT | V | NT | ||
| Tilapia | 62 # | 56 | - | 1 | 1 | 4 |
| (90.3) | (1.6) | (1.6) | (6.3) | |||
| Big Head cap | 19 | 18 | - | - | 1 | - |
| (94.7) | (5.3) | |||||
| Snakehead | 43 | 43 | - | - | - | - |
| (100) | ||||||
| Black carp | 67 | 67 | - | - | - | - |
| (100) | ||||||
| Pig’s tongue | 48 | - | 2 | 45 | - | 1 |
| (4.2) | (93.8) | (2.1) | ||||
| Pig’s small intestine | 3 | - | - | 3 | - | - |
| (100) | ||||||
| Pig’s large intestine | 10 | - | - | 8 | - | 2 |
| (80) | (20) | |||||
| Total GBS strains collected | 252 | 184 | 2 | 57 | 2 | 7 |
| (73) | (0.8) | (22.6) | (0.8) | (2.8) | ||
| Source | Number of GBS Strains | Sequence Types (STs) n (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 7 | SLV7 | 103 | 314 | 651 | SLV651 | 862 | 931 | Unknown # | ||
| Fish | 191 | 1 (0.4) | 175 (69.4) | 9 (3.6) | 2 (0.8) | 2 (0.8) | - | - | - | 2 (0.8) | - |
| Pig | 61 | 1 (0.4) | - | - | - | - | 47 (18.7) | 6 (2.4) | 6 (2.4) | - | 1 (0.4) |
| Total | 252 | 2 (0.8) | 175 (69.4) | 9 (3.6) | 2 (0.8) | 2 (0.8) | 47 (18.7) | 6 (2.4) | 6 (2.4) | 2 (0.8) | 1 (0.4) |
| Antibiotic Group | Resistant Gene | Fish | Pig | ||
|---|---|---|---|---|---|
| No. of Strains Carrying the Gene (n = 191) | Percentage (%) | No. of Strains Carrying the Gene (n = 61) | Percentage (%) | ||
| Aminoglycoside | ant(6)-Ia | 5 | 2.6 | 10 | 16.4 |
| (AGly)aph(3′)-II | - | - | 37 | 60.7 | |
| sat4 | 3 | 1.6 | 42 | 68.9 | |
| (Agly)spw | - | - | 48 | 78.7 | |
| Peptide antibiotic | mprF | 179 | 93.7 | - | - |
| Tetracyclines | tetL | - | - | 43 | 70.5 |
| tetM | 4 | 2.1 | 2 | 3.3 | |
| tetS | 4 | 2.1 | 8 | 13.1 | |
| Lincosamide | lsaE | 2 | 1.0 | - | - |
| Macrolides | ermB | - | - | 50 | 82.0 |
| ermT | - | - | 3 | 4.9 | |
| ermB + ermT | - | - | 43 | 70.5 | |
| lnuB | - | - | 49 | 80.3 | |
| lnuC | - | - | 1 | 1.6 | |
| inuB + lnuC | - | - | 1 | 1.6 | |
| MREA | - | - | 55 | 90.2 | |
| Oxacillin | optrA | - | - | 43 | 70.5 |
| Phenicol | catA8 | - | - | 19 | 31.1 |
| fexA | - | - | 4 | 6.6 | |
| Trimethoprim | dfrG | - | - | 3 | 4.9 |
| MPRF | - | - | 55 | 90.2 | |
| Category | Virulence Gene | Total No. of Isolates with Virulence Genes (%) | |
|---|---|---|---|
| Fish GBS (n = 191) | Pig GBS (n = 61) | ||
| Adhesion | Lmb | 2 (1.0) | 2 (3.3) |
| gbs0628 a | 53 (27.7) | 2 (3.3) | |
| gbs0630 a | 53 (27.7) | 2 (3.3) | |
| gbs0631 a | 53 (27.7) | 2 (3.3) | |
| gbs0632 a | 45 (23.6) | 2 (3.3) | |
| pilA | 2 (1.0) | 2 (3.3) | |
| pilB | 1 (0.5) | 1 (1.6) | |
| pilC | 2 (1.0) | 2 (3.3) | |
| scpB | 1 (0.5) | 0 (0.0) | |
| srC4 | 2 (1.0) | 2 (3.3) | |
| Invasion | cylA | 183 (95.8) | 59 (96.7) |
| cylB | 184 (96.3) | 59 (96.7) | |
| cylD | 179 (93.7) | 59 (96.7) | |
| cylF | 185 (96.9) | 58 (95.1) | |
| cylG | 188 (98.4) | 59 (96.7) | |
| cylI | 184 (96.3) | 57 (93.4) | |
| cylJ | 182 (95.3) | 58 (95.1) | |
| cylK | 181 (94.8) | 58 (95.1) | |
| cylL | 0 (0.0) | 0 (0.0) | |
| cylR1 | 0 (0.0) | 0 (0.0) | |
| cylR2 | 0 (0.0) | 0 (0.0) | |
| cylS | 0 (0.0) | 0 (0.0) | |
| cylX | 187 (97.9) | 59 (96.7) | |
| cylZ | 190 (99.5) | 59 (96.7) | |
| acpC | 186 (97.4) | 58 (95.1) | |
| hylB | 183 (95.8) | 58 (95.1) | |
| Immune evasion | cpsA | 173 (90.6) | 58 (95.1) |
| cpsB | 183 (95.8) | 59 (96.7) | |
| cpsC | 180 (94.2) | 58 (95.1) | |
| cpsD | 0 (0.0) | 59 (96.7) | |
| cpsE | 180 (94.2) | 56 (91.8) | |
| cpsF | 183 (95.8) | 59 (96.7) | |
| cpsG | 2 (1.0) | 2 (3.3) | |
| cpsH | 3 (1.6) | 2 (3.3) | |
| cpsI | 0 (0.0) | 0 (0.0) | |
| cpsJ | 3 (1.6) | 2 (3.3) | |
| cpsK | 166 (86.9) | 58 (95.1) | |
| cpsL | 178 (93.2) | 58 (95.1) | |
| cpsM | 3 (1.6) | 2 (3.3) | |
| cpsN | 3 (1.6) | 0 (0.0) | |
| cpsO | 3 (1.6) | 2 (3.3) | |
| neuA | 177 (92.7) | 58 (95.1) | |
| neuB | 184 (96.3) | 59 (96.7) | |
| neuC | 184 (96.3) | 57 (93.4) | |
| neuD | 186 (97.4) | 58 (95.1) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sapugahawatte, D.N.; Li, C.; Dharmaratne, P.; Zhu, C.; Yeoh, Y.K.; Yang, J.; Lo, N.W.S.; Wong, K.T.; Ip, M. Prevalence and Characteristics of Streptococcus agalactiae from Freshwater Fish and Pork in Hong Kong Wet Markets. Antibiotics 2022, 11, 397. https://doi.org/10.3390/antibiotics11030397
Sapugahawatte DN, Li C, Dharmaratne P, Zhu C, Yeoh YK, Yang J, Lo NWS, Wong KT, Ip M. Prevalence and Characteristics of Streptococcus agalactiae from Freshwater Fish and Pork in Hong Kong Wet Markets. Antibiotics. 2022; 11(3):397. https://doi.org/10.3390/antibiotics11030397
Chicago/Turabian StyleSapugahawatte, Dulmini Nanayakkara, Carmen Li, Priyanga Dharmaratne, Chendi Zhu, Yun Kit Yeoh, Jun Yang, Norman Wai Sing Lo, Kam Tak Wong, and Margaret Ip. 2022. "Prevalence and Characteristics of Streptococcus agalactiae from Freshwater Fish and Pork in Hong Kong Wet Markets" Antibiotics 11, no. 3: 397. https://doi.org/10.3390/antibiotics11030397
APA StyleSapugahawatte, D. N., Li, C., Dharmaratne, P., Zhu, C., Yeoh, Y. K., Yang, J., Lo, N. W. S., Wong, K. T., & Ip, M. (2022). Prevalence and Characteristics of Streptococcus agalactiae from Freshwater Fish and Pork in Hong Kong Wet Markets. Antibiotics, 11(3), 397. https://doi.org/10.3390/antibiotics11030397






