Prophylactic Antibiofilm Activity of Antibiotic-Loaded Bone Cements against Gram-Negative Bacteria
Abstract
:1. Introduction
2. Methods
2.1. Bacterial Strains
2.2. Antibiotic-Loaded Bone Cements
2.3. Preparation of ALBC Elution Solutions
2.4. Determination of the Prophylactic Antibiofilm Effect of ALBCs Elution Solutions
2.5. Graphical Representation and Statistical Analysis
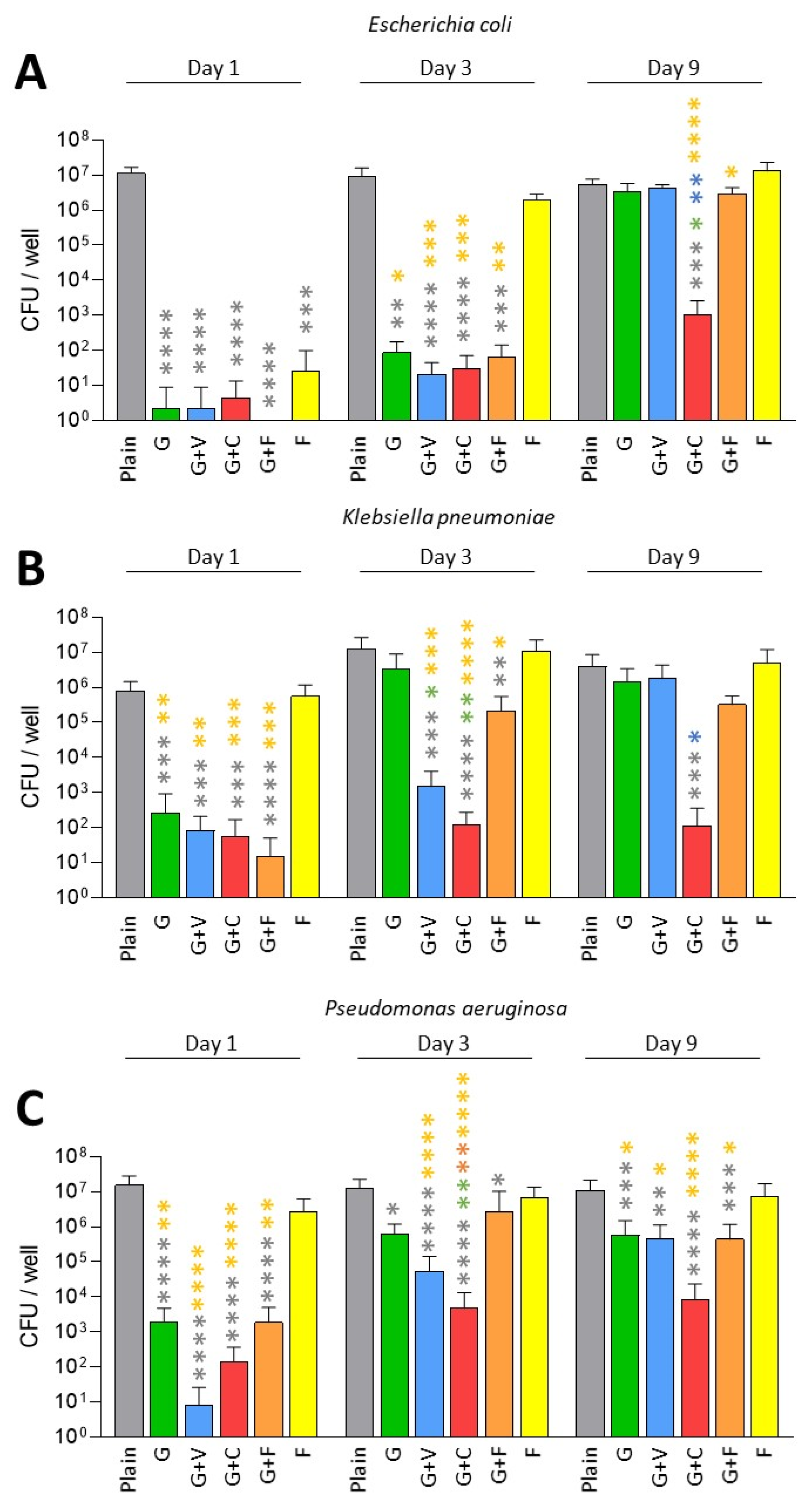
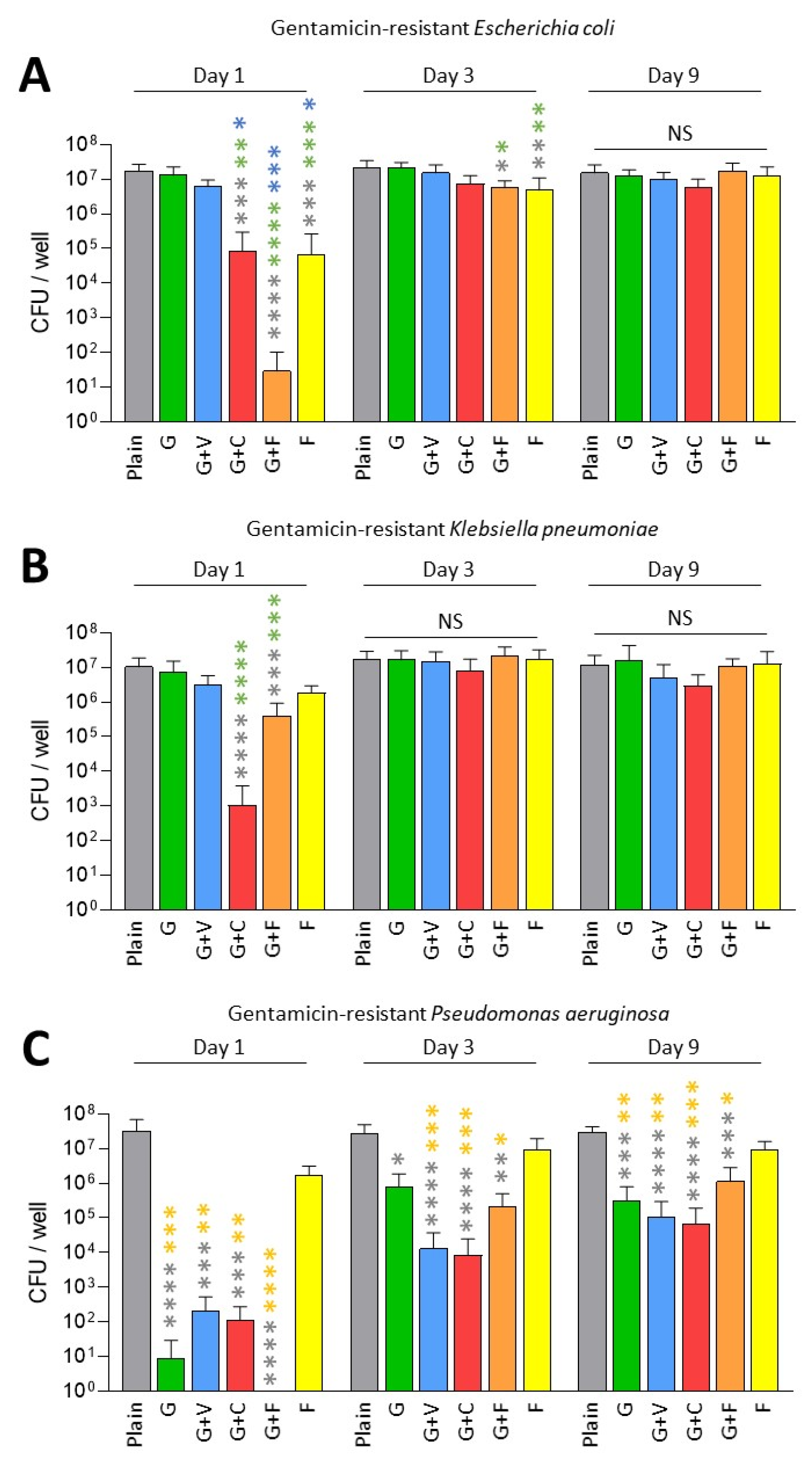
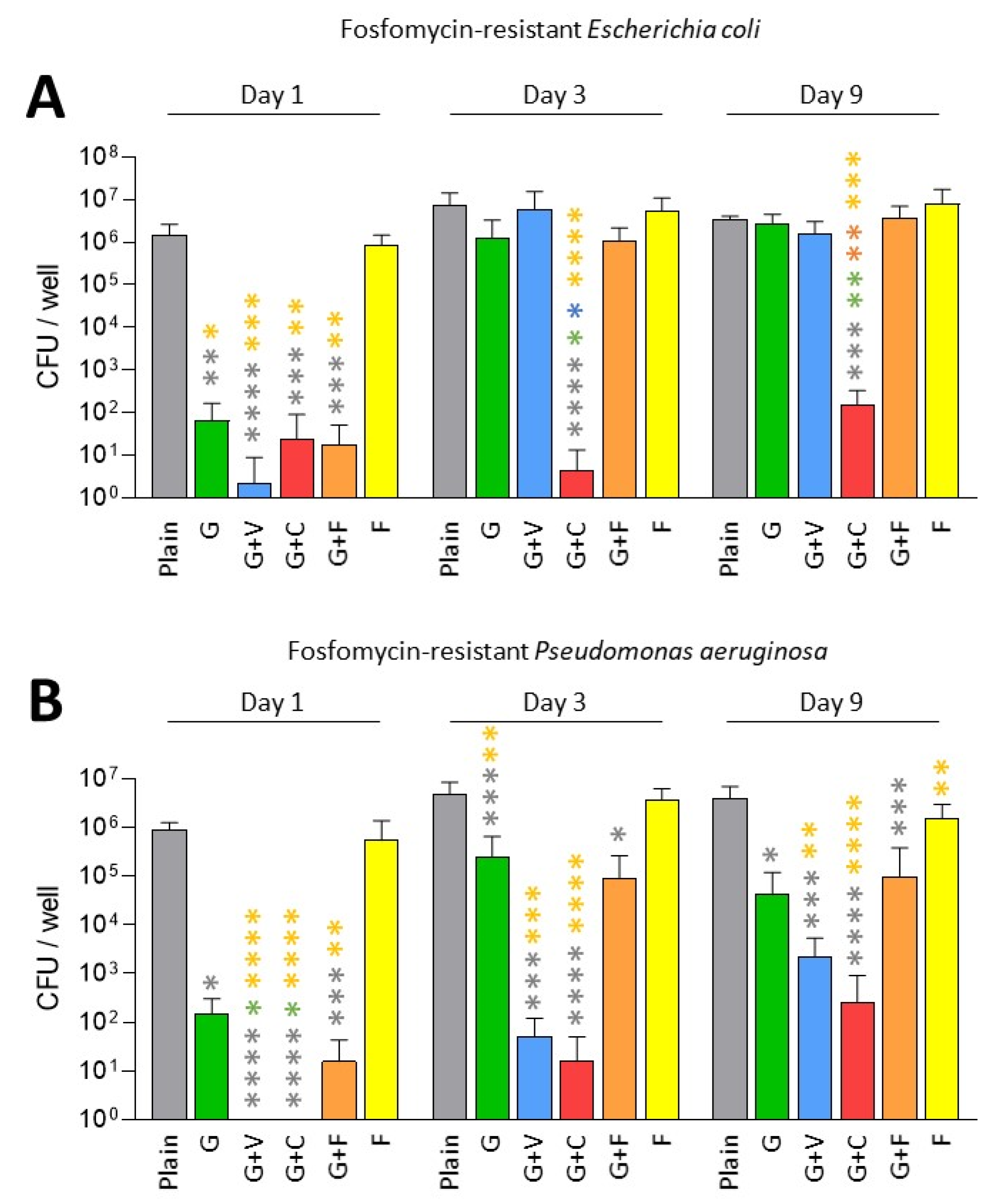
3. Results
3.1. Prevention of Biofilm Formation by ALBCs with Gentamicin- and Fosfomycin-Susceptible Strains
3.2. Prevention of Biofilm Formation by ALBCs with Gentamicin-Resistant Strains
3.3. Prevention of Biofilm Formation by ALBCs with Fosfomycin-Resistant Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buechel, F.F.; Femino, F.P.; D’Alessio, J. Primary Exchange Revision Arthroplasty for Infected Total Knee Replacement: A Long-Term Study. Am. J. Orthop. 2004, 33, 190–198; discussion 198. [Google Scholar]
- Gehrke, T.; Alijanipour, P.; Parvizi, J. The Management of an Infected Total Knee Arthroplasty. Bone Jt. J. 2015, 97-B, 20–29. [Google Scholar] [CrossRef]
- Tande, A.J.; Patel, R. Prosthetic Joint Infection. Clin. Microbiol. Rev. 2014, 27, 302–345. [Google Scholar] [CrossRef] [Green Version]
- Lemaignen, A.; Bernard, L.; Marmor, S.; Ferry, T.; Grammatico-Guillon, L.; Astagneau, P. Scientific Committee for Complex Bone and Joint Infections Reference Centers (CRIOAc), on behalf of the CRIOAc network Epidemiology of Complex Bone and Joint Infections in France Using a National Registry: The CRIOAc Network. J. Infect. 2021, 82, 199–206. [Google Scholar] [CrossRef]
- Rodríguez-Pardo, D.; Pigrau, C.; Lora-Tamayo, J.; Soriano, A.; del Toro, M.D.; Cobo, J.; Palomino, J.; Euba, G.; Riera, M.; Sánchez-Somolinos, M.; et al. Gram-negative Prosthetic Joint Infection: Outcome of a Debridement, Antibiotics and Implant Retention Approach. A Large Multicentre Study. Clin. Microbiol. Infect. 2014, 20, O911–O919. [Google Scholar] [CrossRef] [Green Version]
- Hsieh, P.-H.; Lee, M.S.; Hsu, K.-Y.; Chang, Y.-H.; Shih, H.-N.; Ueng, S.W. Gram-negative Prosthetic Joint Infections: Risk Factors and Outcome of Treatment. Clin. Infect. Dis. 2009, 49, 1036–1043. [Google Scholar] [CrossRef]
- Zmistowski, B.; Fedorka, C.J.; Sheehan, E.; Deirmengian, G.; Austin, M.S.; Parvizi, J. Prosthetic Joint Infection Caused by Gram-negative Organisms. J. Arthroplast. 2011, 26, 104–108. [Google Scholar] [CrossRef]
- Crémet, L.; Corvec, S.; Bémer, P.; Bret, L.; Lebrun, C.; Lesimple, B.; Miegeville, A.-F.; Reynaud, A.; Lepelletier, D.; Caroff, N. Orthopaedic-Implant Infections by Escherichia Coli: Molecular and Phenotypic Analysis of the Causative Strains. J. Infect. 2012, 64, 169–175. [Google Scholar] [CrossRef]
- Malhotra, R.; Dhawan, B.; Garg, B.; Shankar, V.; Nag, T.C. A Comparison of Bacterial Adhesion and Biofilm Formation on Commonly Used Orthopaedic Metal Implant Materials: An In Vitro Study. Indian J. Orthop. 2019, 53, 148–153. [Google Scholar] [CrossRef]
- Josse, J.; Valour, F.; Maali, Y.; Diot, A.; Batailler, C.; Ferry, T.; Laurent, F. Interaction Between Staphylococcal Biofilm and Bone: How Does the Presence of Biofilm Promote Prosthesis Loosening? Front. Microbiol. 2019, 10, 1602. [Google Scholar] [CrossRef]
- Berberich, C.E.; Josse, J.; Laurent, F.; Ferry, T. Dual Antibiotic Loaded Bone Cement in Patients at High Infection Risks in Arthroplasty: Rationale of Use for Prophylaxis and Scientific Evidence. World J. Orthop. 2021, 12, 119–128. [Google Scholar] [CrossRef]
- Gandhi, R.; Backstein, D.; Zywiel, M.G. Antibiotic-Laden Bone Cement in Primary and Revision Hip and Knee Arthroplasty. J. Am. Acad. Orthop. Surg. 2018, 26, 727–734. [Google Scholar] [CrossRef]
- Wall, V.; Nguyen, T.-H.; Nguyen, N.; Tran, P.A. Controlling Antibiotic Release from Polymethylmethacrylate Bone Cement. Biomedicines 2021, 9, 26. [Google Scholar] [CrossRef]
- Sebastian, S.; Liu, Y.; Christensen, R.; Raina, D.B.; Tägil, M.; Lidgren, L. Antibiotic Containing Bone Cement in Prevention of Hip and Knee Prosthetic Joint Infections: A Systematic Review and Meta-Analysis. J. Orthop. Translat. 2020, 23, 53–60. [Google Scholar] [CrossRef]
- Farhan-Alanie, M.M.; Burnand, H.G.; Whitehouse, M.R. The Effect of Antibiotic-Loaded Bone Cement on Risk of Revision Following Hip and Knee Arthroplasty. Bone Jt. J. 2020, 103-B, 7–15. [Google Scholar] [CrossRef]
- Sanz-Ruiz, P.; Matas-Diez, J.A.; Sanchez-Somolinos, M.; Villanueva-Martinez, M.; Vaquero-Martín, J. Is the Commercial Antibiotic-Loaded Bone Cement Useful in Prophylaxis and Cost Saving After Knee and Hip Joint Arthroplasty? The Transatlantic Paradox. J. Arthroplast. 2017, 32, 1095–1099. [Google Scholar] [CrossRef]
- Jenny, J.-Y.; Hamon, M.; Klein, S.; Reiter-Schatz, A.; Rondé-Oustau, C.; Boéri, C.; Wisniewski, S.; Gaudias, J. Cement Loaded with High-Dose Gentamicin and Clindamycin Reduces the Risk of Subsequent Infection After One-Stage Hip or Knee Arthroplasty Exchange for Periprosthetic Infection: A Preliminary Study. J. Arthroplast. 2021, 36, 3973–3978. [Google Scholar] [CrossRef]
- Cara, A.; Ballet, M.; Hemery, C.; Ferry, T.; Laurent, F.; Josse, J. Antibiotics in Bone Cements Used for Prosthesis Fixation: An Efficient Way to Prevent Staphylococcus aureus and Staphylococcus epidermidis Prosthetic Joint Infection. Front. Med. 2020, 7, 576231. [Google Scholar] [CrossRef]
- Poelstra, K.A.; Busscher, H.J.; Schenk, W.; van Horn, J.R.; Van der Mei, H.C. Effect of Gentamicin Loaded PMMA Bone Cement on Staphylococcus aureus Biofilm Formation. Biofouling 1999, 14, 249–254. [Google Scholar] [CrossRef]
- Belt, H.V.D.; Neut, D.; Schenk, W.; Horn, J.R.V.; Mei, H.C.V.D.; Busscher, H.J. Gentamicin Release from Polymethylmethacrylate Bone Cements and Staphylococcus aureus Biofilm Formation. Acta Orthop. Scand. 2000, 71, 625–629. [Google Scholar] [CrossRef]
- Stefánsdóttir, A.; Johansson, D.; Knutson, K.; Lidgren, L.; Robertsson, O. Microbiology of the Infected Knee Arthroplasty: Report from the Swedish Knee Arthroplasty Register on 426 Surgically Revised Cases. Scand. J. Infect. Dis. 2009, 41, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Tai, C.-L.; Hsieh, P.-H.; Ueng, S.W.N. Gentamicin in Bone Cement. Bone Jt. Res. 2013, 2, 220–226. [Google Scholar] [CrossRef]
- Kauffman, C.A.; Ramundo, N.C.; Williams, S.G.; Dey, C.R.; Phair, J.P.; Watanakunakorn, C. Surveillance of Gentamicin-Resistant Gram-negative Bacilli in a General Hospital. Antimicrob. Agents Chemother. 1978, 13, 918–923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harbarth, S.; Rohner, P.; Safran, E.; Garbino, J.; Auckenthaler, R.; Pittet, D. Resistance to Amikacin and Gentamicin among Gram-negative Bloodstream Isolates in a University Hospital between 1989 and 1994. Clin. Microbiol. Infect. 1998, 4, 199–204. [Google Scholar] [CrossRef]
- Wang, L.; Di Luca, M.; Tkhilaishvili, T.; Trampuz, A.; Gonzalez Moreno, M. Synergistic Activity of Fosfomycin, Ciprofloxacin, and Gentamicin Against Escherichia coli and Pseudomonas aeruginosa Biofilms. Front. Microbiol. 2019, 10, 2522. [Google Scholar] [CrossRef] [Green Version]
- Stepanovic, S.; Vukovic, D.; Dakic, I.; Savic, B.; Svabic-Vlahovic, M. A Modified Microtiter-Plate Test for Quantification of Staphylococcal Biofilm Formation. J. Microbiol. Methods 2000, 40, 175–179. [Google Scholar] [CrossRef]
- Tasse, J.; Cara, A.; Saglio, M.; Villet, R.; Laurent, F. A Steam-Based Method to Investigate Biofilm. Sci. Rep. 2018, 8, 13040. [Google Scholar] [CrossRef]
- Karaglani, M.; Tzitzikou, E.; Tottas, S.; Kougioumtzis, I.; Arvanitidis, K.; Kolios, G.; Chatzaki, E.; Drosos, G.I. Gentamycin Elution from Polymethylmethacrylate and Bone Graft Substitute: Comparison between Commercially Available and Home-Made Preparations. J. Orthop. 2020, 19, 9–13. [Google Scholar] [CrossRef]
- Ensing, G.T.; van Horn, J.R.; van der Mei, H.C.; Busscher, H.J.; Neut, D. Copal Bone Cement Is More Effective in Preventing Biofilm Formation than Palacos R-G. Clin. Orthop. Relat. Res. 2008, 466, 1492–1498. [Google Scholar] [CrossRef] [Green Version]
- González, M.J.; Da Cunda, P.; Notejane, M.; Zunino, P.; Scavone, P.; Robino, L. Fosfomycin Tromethamine Activity on Biofilm and Intracellular Bacterial Communities Produced by Uropathogenic Escherichia coli Isolated from Patients with Urinary Tract Infection. Pathog. Dis. 2019, 77, ftz022. [Google Scholar] [CrossRef]
- Robu, A.; Antoniac, A.; Grosu, E.; Vasile, E.; Raiciu, A.D.; Iordache, F.; Antoniac, V.I.; Rau, J.V.; Yankova, V.G.; Ditu, L.M.; et al. Additives Imparting Antimicrobial Properties to Acrylic Bone Cements. Materials 2021, 14, 7031. [Google Scholar] [CrossRef] [PubMed]
- Jackson, J.; Lo, J.; Hsu, E.; Burt, H.M.; Shademani, A.; Lange, D. The Combined Use of Gentamicin and Silver Nitrate in Bone Cement for a Synergistic and Extended Antibiotic Action against Gram-Positive and Gram-negative Bacteria. Materials 2021, 14, 3413. [Google Scholar] [CrossRef]
- Chen, I.-C.; Su, C.-Y.; Nien, W.-H.; Huang, T.-T.; Huang, C.-H.; Lu, Y.-C.; Chen, Y.-J.; Huang, G.-C.; Fang, H.-W. Influence of Antibiotic-Loaded Acrylic Bone Cement Composition on Drug Release Behavior and Mechanism. Polymers 2021, 13, 2240. [Google Scholar] [CrossRef] [PubMed]
- Lüdemann, M.; Jakuscheit, A.; Ewald, A.; Frühmann, L.; Hölscher-Doht, S.; Rudert, M.; von Hertzberg-Boelch, S.P. Influence of Tranexamic Acid on Elution Characteristics and Compressive Strength of Antibiotic-Loaded PMMA-Bone Cement with Gentamicin. Materials 2021, 14, 5639. [Google Scholar] [CrossRef] [PubMed]
| Gentamicin | Fosfomycin | |
|---|---|---|
| Escherichia coli | S | S |
| GentaR E. coli | R | S |
| FosfoR E. coli | S | R |
| Klebsiella pneumoniae | S | S |
| GentaR K. pneumoniae | R | S |
| Pseudomonas aeruginosa | S | S |
| GentaR P. aeruginosa | R | S |
| FosfoR P. aeruginosa | S | R |
| Cement | Antibiotic and Quantity | Commercial Name | |||
|---|---|---|---|---|---|
| Plain | - | - | - | - | PALACOS R |
| G | gentamicin | 0.5 g | - | - | PALACOS R+G |
| G+V | gentamicin | 0.5 g | Vancomycin | 2 g | COPAL G+V |
| G+C | gentamicin | 1 g | Clindamycin | 1 g | COPAL G+C |
| G+F | gentamicin | 0.5 g | Fosfomycin | 1.5 g | Not commercialized |
| F | - | - | Fosfomycin | 1.5 g | Not commercialized |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cara, A.; Ferry, T.; Laurent, F.; Josse, J. Prophylactic Antibiofilm Activity of Antibiotic-Loaded Bone Cements against Gram-Negative Bacteria. Antibiotics 2022, 11, 137. https://doi.org/10.3390/antibiotics11020137
Cara A, Ferry T, Laurent F, Josse J. Prophylactic Antibiofilm Activity of Antibiotic-Loaded Bone Cements against Gram-Negative Bacteria. Antibiotics. 2022; 11(2):137. https://doi.org/10.3390/antibiotics11020137
Chicago/Turabian StyleCara, Andréa, Tristan Ferry, Frédéric Laurent, and Jérôme Josse. 2022. "Prophylactic Antibiofilm Activity of Antibiotic-Loaded Bone Cements against Gram-Negative Bacteria" Antibiotics 11, no. 2: 137. https://doi.org/10.3390/antibiotics11020137
APA StyleCara, A., Ferry, T., Laurent, F., & Josse, J. (2022). Prophylactic Antibiofilm Activity of Antibiotic-Loaded Bone Cements against Gram-Negative Bacteria. Antibiotics, 11(2), 137. https://doi.org/10.3390/antibiotics11020137






