In Vitro Assessment of Antimicrobial Activity of Phytobiotics Composition towards of Avian Pathogenic Escherichia coli (APEC) and Other E. coli Strains Isolated from Broiler Chickens
Abstract
1. Introduction
2. Results
2.1. Anatomopathological Examination
2.2. Identification of Isolates
2.3. Somatic Antigen
2.4. Virulence Gene Detection
2.5. Prevalence of Multiple Drug Resistance
2.6. Antimicrobial Resistance Profile
2.7. Genotypic Resistance
2.8. Effectiveness of Phytoncides Composition
3. Discussion
4. Materials and Methods
4.1. Phytoncides Mixtures
4.2. Broiler Sampling
4.3. Escherichia coli Isolation and Identification
4.4. Somatic Antigen Identification
4.5. APEC Genes Detection
4.6. Antimicrobial Susceptibility of Escherichia coli Isolates
4.7. Detection of Antibiotic Resistance Genes
4.8. Phytoncides Mixture Test by Broth Microdilution Method
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FDA. Outbreaks of Foodborne Illness. Available online: https://www.fda.gov/food/recalls-outbreaks-emergencies/outbreaks-foodborne-illness (accessed on 8 November 2022).
- Parvin, M.S.; Talukder, S.; Ali, M.Y.; Chowdhury, E.H.; Rahman, M.T.; Islam, M.T. Antimicrobial Resistance Pattern of Escherichia coli Isolated from Frozen Chicken Meat in Bangladesh. Pathogens 2020, 9, 420. [Google Scholar] [CrossRef] [PubMed]
- Sengun, I.Y.; Kilic, G.; Ozturk, B. The effects of koruk products used as marination liquids against foodborne pathogens (Escherichia coli O157:H7, Listeria monocytogenes and Salmonella Typhimurium) inoculated on poultry meat. LWT 2020, 133, 110148. [Google Scholar] [CrossRef]
- Farhoumand, P.; Hassanzadazar, H.; Soltanpour, M.S.; Aminzare, M.; Abbasi, Z. Prevalence, genotyping and antibiotic resistance of Listeria monocytogenes and Escherichia coli in fresh beef and chicken meats marketed in Zanjan, Iran. Iran. J. Microbiol. 2020, 12, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Kaesbohrer, A.; Bakran-Lebl, K.; Irrgang, A.; Fischer, J.; Kämpf, P.; Schiffmann, A.; Werckenthin, C.; Busch, M.; Kreienbrock, L.; Hille, K. Diversity in prevalence and characteristics of ESBL/pAmpC producing E. coli in food in Germany. Vet. Microbiol. 2019, 233, 52–60. [Google Scholar] [CrossRef]
- Bantawa, K.; Sah, S.N.; Subba Limbu, D.; Subba, P.; Ghimire, A. Antibiotic resistance patterns of Staphylococcus aureus, Escherichia coli, Salmonella, Shigella and Vibrio isolated from chicken, pork, buffalo and goat meat in eastern Nepal. BMC Res. Notes 2019, 12, 766. [Google Scholar] [CrossRef]
- Smith, B.A.; Meadows, S.; Meyers, R.; Parmley, E.J.; Fazil, A. Seasonality and zoonotic foodborne pathogens in Canada: Relationships between climate and Campylobacter, E. coli and Salmonella in meat products. Epidemiol. Infect. 2019, 147, e190. [Google Scholar] [CrossRef]
- Markland, S.M.; LeStrange, K.J.; Sharma, M.; Kniel, K.E. Old Friends in New Places: Exploring the Role of Extraintestinal E. coli in Intestinal Disease and Foodborne Illness. Zoonoses Public Health 2015, 62, 491–496. [Google Scholar] [CrossRef]
- Johnson, T.J.; Wannemuehler, Y.; Johnson, S.J.; Stell, A.L.; Doetkott, C.; Johnson, J.R.; Kim, K.S.; Spanjaard, L.; Nolan, L.K. Comparison of extraintestinal pathogenic Escherichia coli strains from human and avian sources reveals a mixed subset representing potential zoonotic pathogens. Appl. Environ. Microbiol. 2008, 74, 7043–7050. [Google Scholar] [CrossRef]
- Alonso, P.L.; Sacarlal, J.; Aponte, J.J.; Leach, A.; Macete, E.; Milman, J.; Mandomando, I.; Spiessens, B.; Guinovart, C.; Espasa, M.; et al. Efficacy of the RTS,S/AS02A vaccine against Plasmodium falciparum infection and disease in young African children: Randomised controlled trial. Lancet 2004, 364, 1411–1420. [Google Scholar] [CrossRef]
- Jakobsen, L.; Garneau, P.; Kurbasic, A.; Bruant, G.; Stegger, M.; Harel, J.; Jensen, K.S.; Brousseau, R.; Hammerum, A.M.; Frimodt-Møller, N. Microarray-based detection of extended virulence and antimicrobial resistance gene profiles in phylogroup B2 Escherichia coli of human, meat and animal origin. J. Med. Microbiol. 2011, 60, 1502–1511. [Google Scholar] [CrossRef] [PubMed]
- Tadesse, D.A.; Zhao, S.; Tong, E.; Ayers, S.; Singh, A.; Bartholomew, M.J.; McDermott, P.F. Antimicrobial drug resistance in Escherichia coli from humans and food animals, United States, 1950–2002. Emerg. Infect. Dis. 2012, 18, 741–749. [Google Scholar] [CrossRef] [PubMed]
- FDA. Summary Report on Antimicrobials Sold or Distributed for Use in Food-Producing Animals; Department of Health and Human Services: Washington, DC, USA, 2021. [Google Scholar]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef]
- Bachir, R.G.; Benali, M. Antibacterial activity of the essential oils from the leaves of Eucalyptus globulus against Escherichia coli and Staphylococcus aureus. Asian Pac. J. Trop. Biomed. 2012, 2, 739–742. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Gupta, R.P.; Lather, D.; Bagri, P. Ameliorating effect of Withania somnifera root extract in Escherichia coli–infected broilers. Poult. Sci. 2020, 99, 1875–1887. [Google Scholar] [CrossRef] [PubMed]
- Happy, A.; Soumya, M.; Venkat Kumar, S.; Rajeshkumar, S.; Sheba, R.D.; Lakshmi, T.; Deepak Nallaswamy, V. Phyto-assisted synthesis of zinc oxide nanoparticles using Cassia alata and its antibacterial activity against Escherichia coli. Biochem. Biophys. Rep. 2019, 17, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Pourahmad Jaktaji, R.; Mohammadi, P. Effect of total alkaloid extract of local Sophora alopecuroides on minimum inhibitory concentration and intracellular accumulation of ciprofloxacin, and acrA expression in highly resistant Escherichia coli clones. J. Glob. Antimicrob. Resist. 2018, 12, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bazargani, M.M.; Rohloff, J. Antibiofilm activity of essential oils and plant extracts against Staphylococcus aureus and Escherichia coli biofilms. Food Control 2016, 61, 156–164. [Google Scholar] [CrossRef]
- Gambi, L.; Crippa, C.; Lucchi, A.; De Cesare, A.; Parisi, A.; Manfreda, G.; Pasquali, F. Research note: The resistome of commensal Escherichia coli isolated from broiler carcasses “produced without the use of antibiotics”a. Poult. Sci. 2022, 101, 101770. [Google Scholar] [CrossRef]
- Miladi, H.; Zmantar, T.; Kouidhi, B.; Al Qurashi, Y.M.A.; Bakhrouf, A.; Chaabouni, Y.; Mahdouani, K.; Chaieb, K. Synergistic effect of eugenol, carvacrol, thymol, p-cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb. Pathog. 2017, 112, 156–163. [Google Scholar] [CrossRef]
- Si, H.; Hu, J.; Liu, Z.; Zeng, Z.L. Antibacterial effect of oregano essential oil alone and in combination with antibiotics against extended-spectrum beta-lactamase-producing Escherichia coli. FEMS Immunol. Med. Microbiol. 2008, 53, 190–194. [Google Scholar] [CrossRef]
- Rosato, A.; Piarulli, M.; Corbo, F.; Muraglia, M.; Carone, A.; Vitali, E.M.; Vitali, C. In Vitro Synergistic Action of Certain Combinations of Gentamicin and Essential Oils. Curr. Med. Chem. 2010, 17, 3289–3295. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.; Li, H.; Zhou, H.; Wang, M.; Zhao, X.; Sun, X.; Li, C.; Zhang, X. Effects of Taraxacum and Astragalus extracts combined with probiotic Bacillus subtilis and Lactobacillus on Escherichia coli–infected broiler chickens. Poult. Sci. 2021, 100, 101007. [Google Scholar] [CrossRef] [PubMed]
- Lekeshmanaswamy, M.; Anusiyadevi, K. Biosynthesis of silver nanoparticles using Pergularia daemia (Hamilton, 1822) leaf extract and its enhanced antibacterial activity against gram negative bacteria (Escherichia coli). Mater. Today Proc. 2022, 48, 201–206. [Google Scholar] [CrossRef]
- Swelum, A.A.; Elbestawy, A.R.; El-Saadony, M.T.; Hussein, E.O.S.; Alhotan, R.; Suliman, G.M.; Taha, A.E.; Ba-Awadh, H.; El-Tarabily, K.A.; Abd El-Hack, M.E. Ways to minimize bacterial infections, with special reference to Escherichia coli, to cope with the first-week mortality in chicks: An updated overview. Poult. Sci. 2021, 100, 101039. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.-s.; Zhou, F.; Ji, B.-p.; Xu, J. Evaluation of Combined Antibacterial Effects of Eugenol, Cinnamaldehyde, Thymol, and Carvacrol against E. coli with an Improved Method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef] [PubMed]
- Irawan, A.; Hidayat, C.; Jayanegara, A.; Ratriyanto, A. Essential oils as growth-promoting additives on performance, nutrient digestibility, cecal microbes, and serum metabolites of broiler chickens: A meta-analysis. Anim Biosci 2021, 34, 1499–1513. [Google Scholar] [CrossRef]
- Iwiński, H.; Wódz, K.; Chodkowska, K.; Nowak, T.; Różański, H. In Vitro Evaluation of Antimicrobial Effect of Phytobiotics Mixture on Salmonella spp. Isolated from Chicken Broiler. Antibiotics 2022, 11, 868. [Google Scholar] [CrossRef]
- Balta, I.; Linton, M.; Pinkerton, L.; Kelly, C.; Stef, L.; Pet, I.; Stef, D.; Criste, A.; Gundogdu, O.; Corcionivoschi, N. The effect of natural antimicrobials against Campylobacter spp. and its similarities to Salmonella spp, Listeria spp., Escherichia coli, Vibrio spp., Clostridium spp. and Staphylococcus spp. Food Control 2021, 121, 107745. [Google Scholar] [CrossRef]
- da Silva, A.P.; Nascimento da Silva, L.C.; Martins da Fonseca, C.S.; de Araújo, J.M.; Correia, M.T.; Cavalcanti Mda, S.; Lima, V.L. Antimicrobial Activity and Phytochemical Analysis of Organic Extracts from Cleome spinosa Jaqc. Front. Microbiol. 2016, 7, 963. [Google Scholar] [CrossRef]
- Wang, Y.; Yam, K.L. Inhibitory effect of thymol via different modes of delivery on growth of Escherichia coli DH5α. Food Packag. Shelf Life 2018, 16, 92–96. [Google Scholar] [CrossRef]
- Bassolé, I.H.N.; Juliani, H.R. Essential Oils in Combination and Their Antimicrobial Properties. Molecules 2012, 17, 3989–4006. [Google Scholar] [CrossRef] [PubMed]
- Soulaimani, B.; Hidar, N.E.; Ben El Fakir, S.; Mezrioui, N.; Hassani, L.; Abbad, A. Combined antibacterial activity of essential oils extracted from Lavandula maroccana (Murb.), Thymus pallidus Batt. and Rosmarinus officinalis L. against antibiotic-resistant Gram-negative bacteria. Eur. J. Integr. Med. 2021, 43, 101312. [Google Scholar] [CrossRef]
- Aumeeruddy-Elalfi, Z.; Gurib-Fakim, A.; Mahomoodally, M.F. Chapter 13 Antimicrobial and Antibiotic Potentiating Activity of Essential Oils From Tropical Medicinal Herbs and Spices. In Antibiotic Resistance; Academic Press, Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 271–289. [Google Scholar]
- Cox, S.D.; Mann, C.M.; Markham, J.L. Interactions between components of the essential oil of Melaleuca alternifolia. J. Appl. Microbiol. 2001, 91, 492–497. [Google Scholar] [CrossRef] [PubMed]
- Połeć, K.; Wyżga, B.; Olechowska, K.; Hąc-Wydro, K. On the synergy/antagonism of selected terpenes in the effect on lipid membranes studied in model systems. J. Mol. Liq. 2022, 349, 118473. [Google Scholar] [CrossRef]
- Luo, B.; Kastrat, E.; Morcol, T.; Cheng, H.; Kennelly, E.; Long, C. Gaultheria longibracteolata, an alternative source of wintergreen oil. Food Chem. 2021, 342, 128244. [Google Scholar] [CrossRef]
- Nikolić, M.; Marković, T.; Mojović, M.; Pejin, B.; Savić, A.; Perić, T.; Marković, D.; Stević, T.; Soković, M. Chemical composition and biological activity of Gaultheria procumbens L. essential oil. Ind. Crops Prod. 2013, 49, 561–567. [Google Scholar] [CrossRef]
- Burt, S.A.; Vlielander, R.; Haagsman, H.P.; Veldhuizen, E.J. Increase in activity of essential oil components carvacrol and thymol against Escherichia coli O157:H7 by addition of food stabilizers. J. Food Prot. 2005, 68, 919–926. [Google Scholar] [CrossRef]
- Price, C.T.D.; Lee, I.R.; Gustafson, J.E. The effects of salicylate on bacteria. Int. J. Biochem. Cell Biol. 2000, 32, 1029–1043. [Google Scholar] [CrossRef]
- Akinola, S.A.; Mwanza, M.; Ateba, C.N. Occurrence, Genetic Diversities And Antibiotic Resistance Profiles Of Salmonella Serovars Isolated From Chickens. Infect. Drug Resist. 2019, 12, 3327–3342. [Google Scholar] [CrossRef]
- Kozak, G.K.; Boerlin, P.; Janecko, N.; Reid-Smith, R.J.; Jardine, C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl. Environ. Microbiol. 2009, 75, 559–566. [Google Scholar] [CrossRef]
- Pławińska-Czarnak, J.; Wódz, K.; Kizerwetter-Świda, M.; Bogdan, J.; Kwieciński, P.; Nowak, T.; Strzałkowska, Z.; Anusz, K. Multi-Drug Resistance to Salmonella spp. When Isolated from Raw Meat Products. Antibiotics 2022, 11, 876. [Google Scholar] [CrossRef] [PubMed]
- Chodkowska, K.A.; Abramowicz-Pindor, P.A.; Tuśnio, A.; Gawin, K.; Taciak, M.; Barszcz, M. Effect of Phytobiotic Composition on Production Parameters, Oxidative Stress Markers and Myokine Levels in Blood and Pectoral Muscle of Broiler Chickens. Animals 2022, 12, 2625. [Google Scholar] [CrossRef] [PubMed]

| E. coli Strain | ||
|---|---|---|
| Genes | Negative n (%) | Positive n (%) |
| astA | 19 (20.65) | 73 (79.35) |
| iss | 8 (8.69) | 84 (91.31) |
| irp2 | 26 (28.26) | 66 (71.74) |
| papC | 63 (68.48) | 29 (31.52) |
| cvi/cva | 47 (58.09) | 45 (48.91) |
| iucD | 23 (25.00) | 69 (75.00) |
| tsh | 58 (63.04) | 34 (36.96) |
| vat | 53 (57.61) | 39 (42.39) |
| iutA | 59 (64.13) | 33 (35.87) |
| ompT | 73 (73.35) | 19 (20.65) |
| Type of Poultry | astA n (%) | Iss n (%) | irp2 n (%) | papC n (%) | cvi/cva n (%) | iucD n (%) | tsh n (%) | Vat n (%) | iutA n (%) | ompT n (%) |
|---|---|---|---|---|---|---|---|---|---|---|
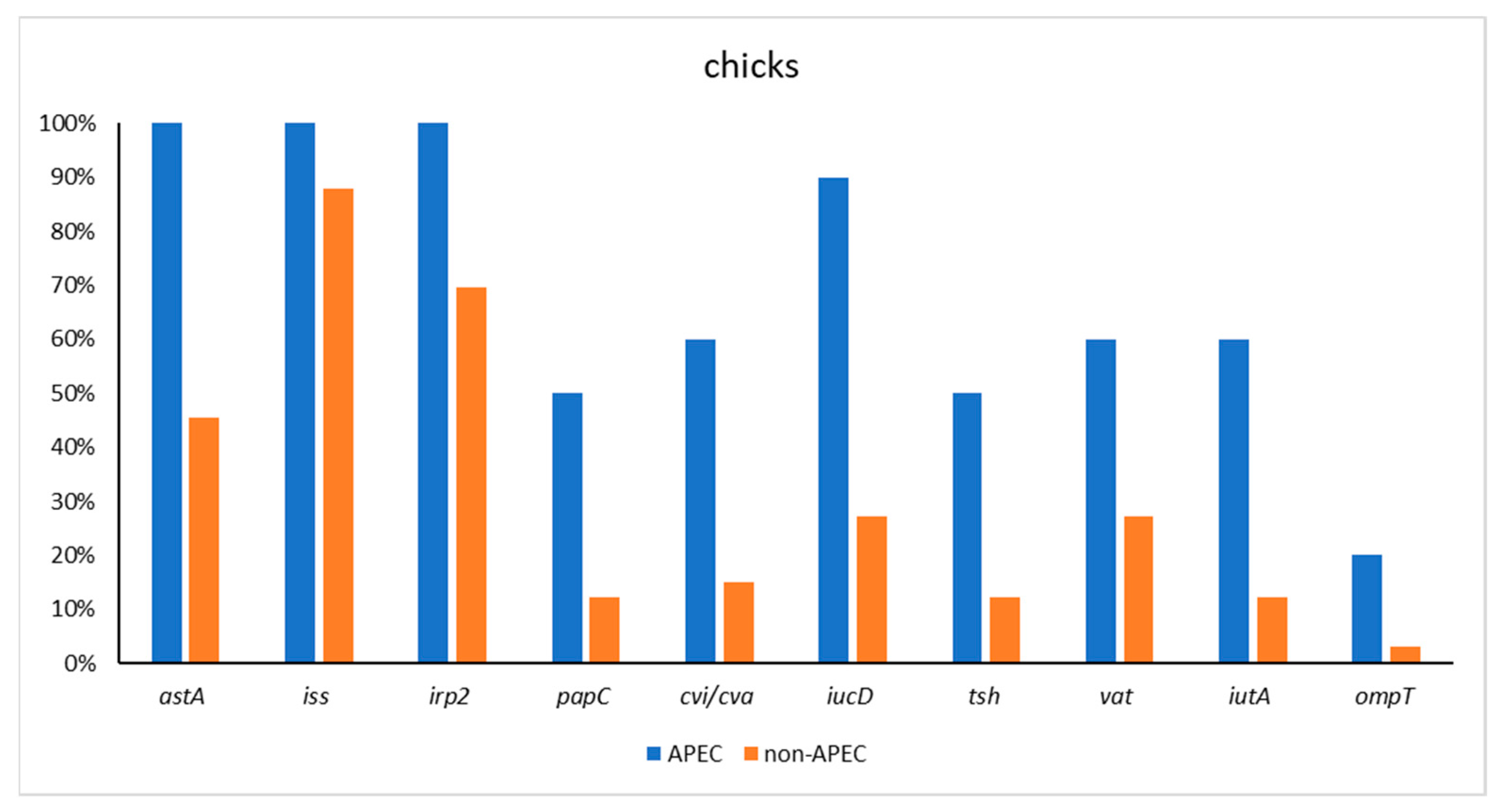
| DOCs APEC (n = 10) | 10 (100) | 10 (100) | 10 (100) | 5 (50) | 6 (60) | 9 (90) | 5 (50) | 6 (60) | 6 (60) | 2 (20) |
| DOCs non-APEC (n = 23) | 15 (45.45) | 29 (87.88) | 23 (69.7) | 4 (12.12) | 5 (15.15) | 9 (27.27) | 4 (12.12) | 9 (27.27) | 4 (12.12) | 1 (3.03) |
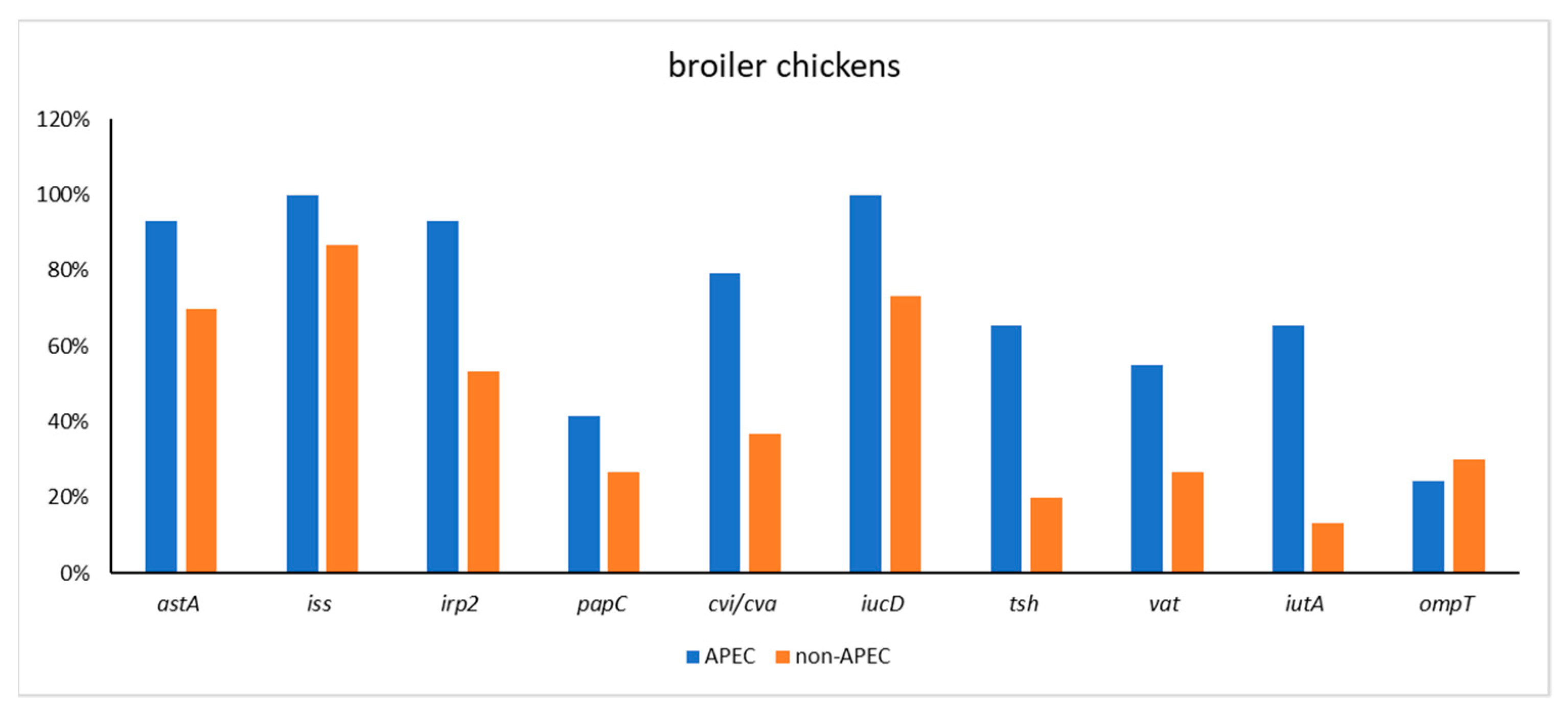
| broiler chicken APEC (n = 29) | 27 (93.10) | 29 (100) | 27 (93.10) | 12 (41.38) | 23 (79.31) | 29 (100) | 19 (65.52) | 16 (55.17) | 19 (65.52) | 7 (24.14) |
| broiler chicken non-APEC (n = 30) | 21 (70) | 26 (86.67) | 16 (53.33) | 8 (26.67) | 11 (36.67) | 22 (73.33) | 6 (20) | 8 (26.67) | 4 (13.33) | 9 (30) |
| Escherichia coli Strains | Dilution H1 | |||||||||||
| 1:2 | 1:4 | 1:16 | 1:32 | 1:64 | 1:118 | 1:256 | 1:512 | 1:1024 | 1:2048 | 1:4096 | 1:8192 | |
| DOCs APEC | − | − | − | − | − | − | − | + | + | + | + | + |
| DOCs non-APEC | − | − | − | − | − | − | − | + | + | + | + | + |
| broiler chicken APEC | − | − | − | − | − | − | − | − | + | + | + | + |
| broiler chicken non-APEC | − | − | − | − | − | − | − | + | + | + | + | + |
| Dilution H2 | ||||||||||||
| 1:2 | 1:4 | 1:16 | 1:32 | 1:64 | 1:118 | 1:256 | 1:512 | 1:1024 | 1:2048 | 1:4096 | 1:8192 | |
| DOCs APEC | − | − | − | − | − | − | − | − | + | + | + | + |
| DOCs non-APEC | − | − | − | − | − | − | − | + | + | + | + | + |
| broiler chicken APEC | − | − | − | − | − | − | − | − | + | + | + | + |
| broiler chicken non-APEC | − | − | − | − | − | − | − | + | + | + | + | + |
| No. of Band in Positive Control | Toxin Gene | Expected PCR Product Size |
|---|---|---|
| 1 | vat | 978 bp |
| 2 | tsh | 824 bp |
| 3 | iucD | 693 bp |
| 4 | cvi/cva | 598 bp |
| 5 | papC | 501bp |
| 6 | irp2 | 413 bp |
| 7 | iss | 309 bp |
| 8 | astA | 111 bp |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chodkowska, K.A.; Iwiński, H.; Wódz, K.; Nowak, T.; Różański, H. In Vitro Assessment of Antimicrobial Activity of Phytobiotics Composition towards of Avian Pathogenic Escherichia coli (APEC) and Other E. coli Strains Isolated from Broiler Chickens. Antibiotics 2022, 11, 1818. https://doi.org/10.3390/antibiotics11121818
Chodkowska KA, Iwiński H, Wódz K, Nowak T, Różański H. In Vitro Assessment of Antimicrobial Activity of Phytobiotics Composition towards of Avian Pathogenic Escherichia coli (APEC) and Other E. coli Strains Isolated from Broiler Chickens. Antibiotics. 2022; 11(12):1818. https://doi.org/10.3390/antibiotics11121818
Chicago/Turabian StyleChodkowska, Karolina A., Hubert Iwiński, Karolina Wódz, Tomasz Nowak, and Henryk Różański. 2022. "In Vitro Assessment of Antimicrobial Activity of Phytobiotics Composition towards of Avian Pathogenic Escherichia coli (APEC) and Other E. coli Strains Isolated from Broiler Chickens" Antibiotics 11, no. 12: 1818. https://doi.org/10.3390/antibiotics11121818
APA StyleChodkowska, K. A., Iwiński, H., Wódz, K., Nowak, T., & Różański, H. (2022). In Vitro Assessment of Antimicrobial Activity of Phytobiotics Composition towards of Avian Pathogenic Escherichia coli (APEC) and Other E. coli Strains Isolated from Broiler Chickens. Antibiotics, 11(12), 1818. https://doi.org/10.3390/antibiotics11121818





