Phytochemical Profile of the Ethanol Extract of Malvaviscus arboreus Red Flower and Investigation of the Antioxidant, Antimicrobial, and Cytotoxic Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the Extract
2.2. Phytochemical Examination
2.3. Determination of Total Phenolic Content (TPC) and Total Flavonoid Content (TFC)
2.4. Antioxidant Activities (ABTS+, FRAP, DPPH, Metal Chelating Property, and ORAC)
2.5. GC-MS Analysis
2.6. HPLC Determination of Phenolics and Flavonoids
2.7. 1HNMR Fingerprint Analysis
2.8. Antibacterial Activity
2.8.1. Test Microorganism
2.8.2. Bacterial Inactivation by ERF
2.8.3. Minimum Inhibitory Concentration (MIC)
2.8.4. Fungal Inactivation by ERF
2.8.5. Time-Kill Assay
2.8.6. Synergistic Activity
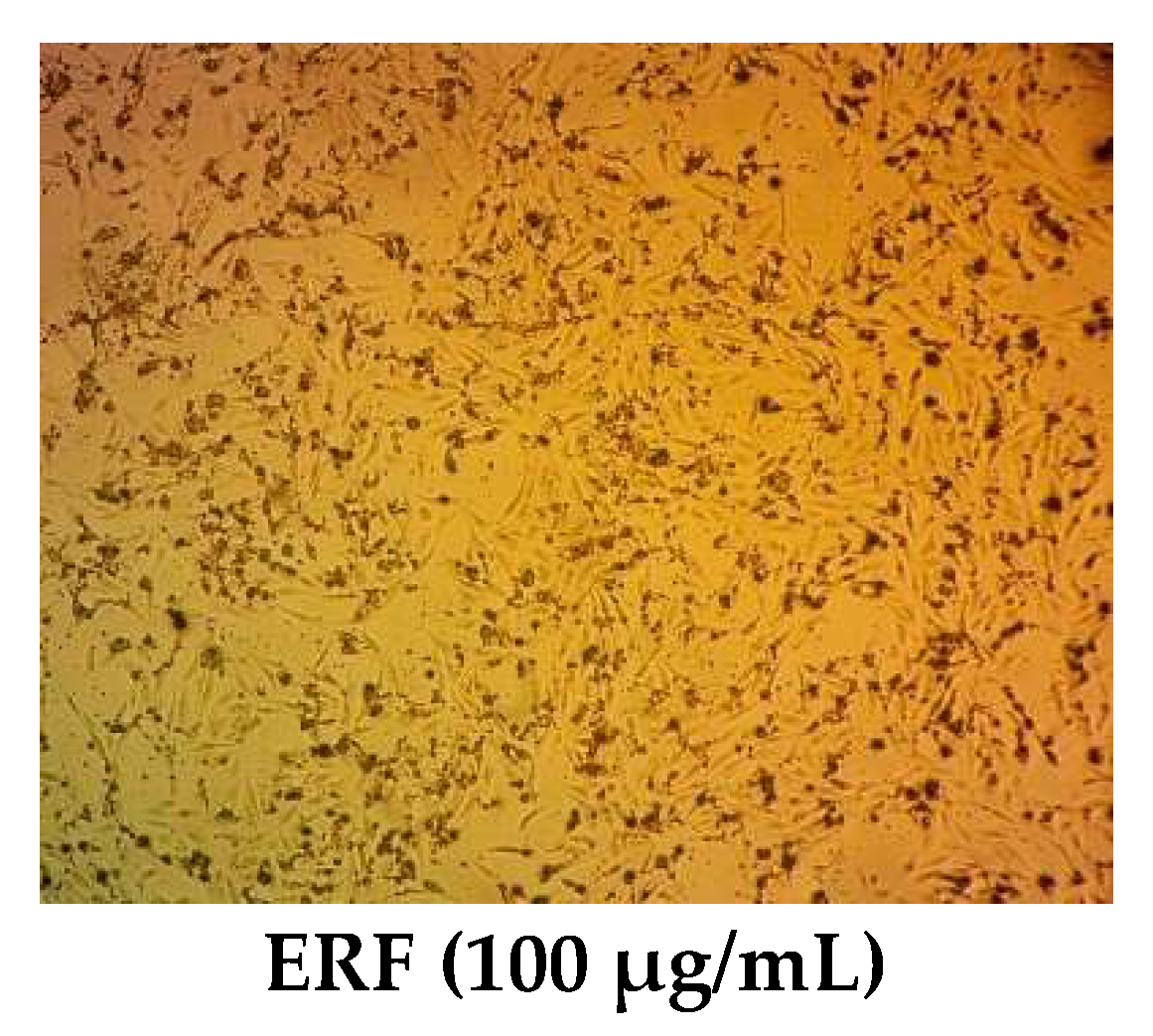
2.9. Cytotoxic Study
2.9.1. Cell Lines
2.9.2. Cytotoxicity Assay
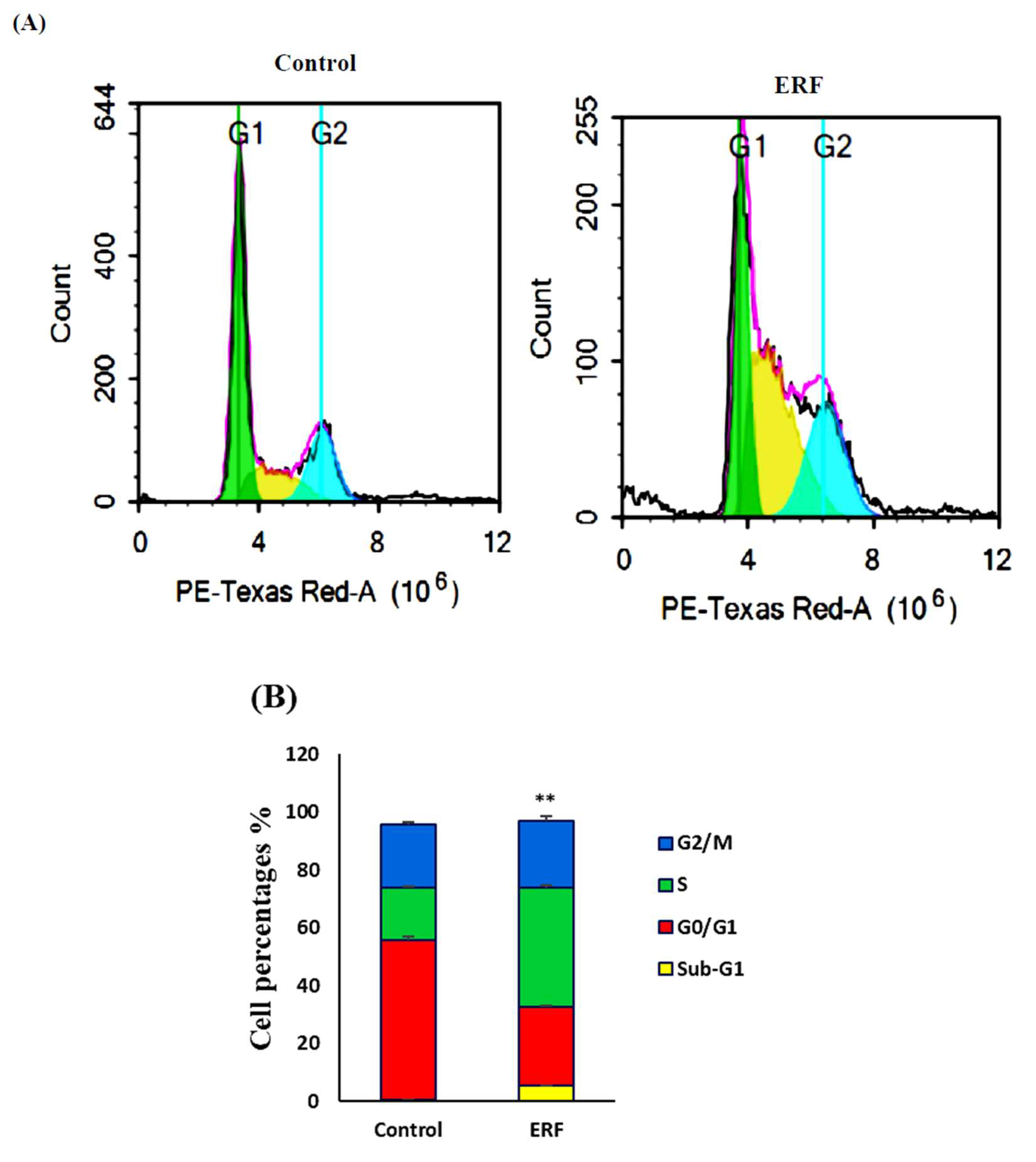
2.9.3. Analysis of Cell Cycle Distribution
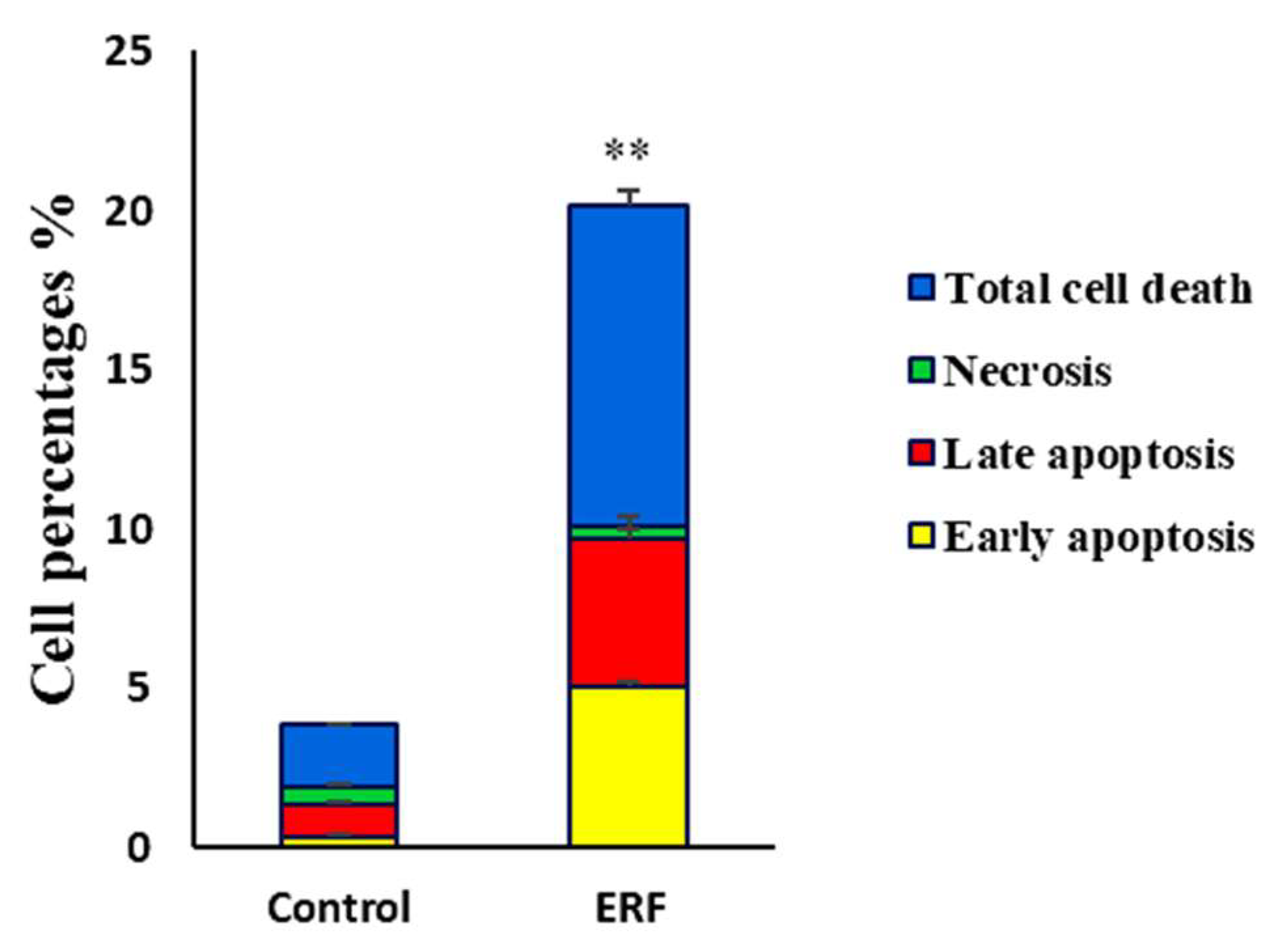
2.9.4. Apoptosis Assay
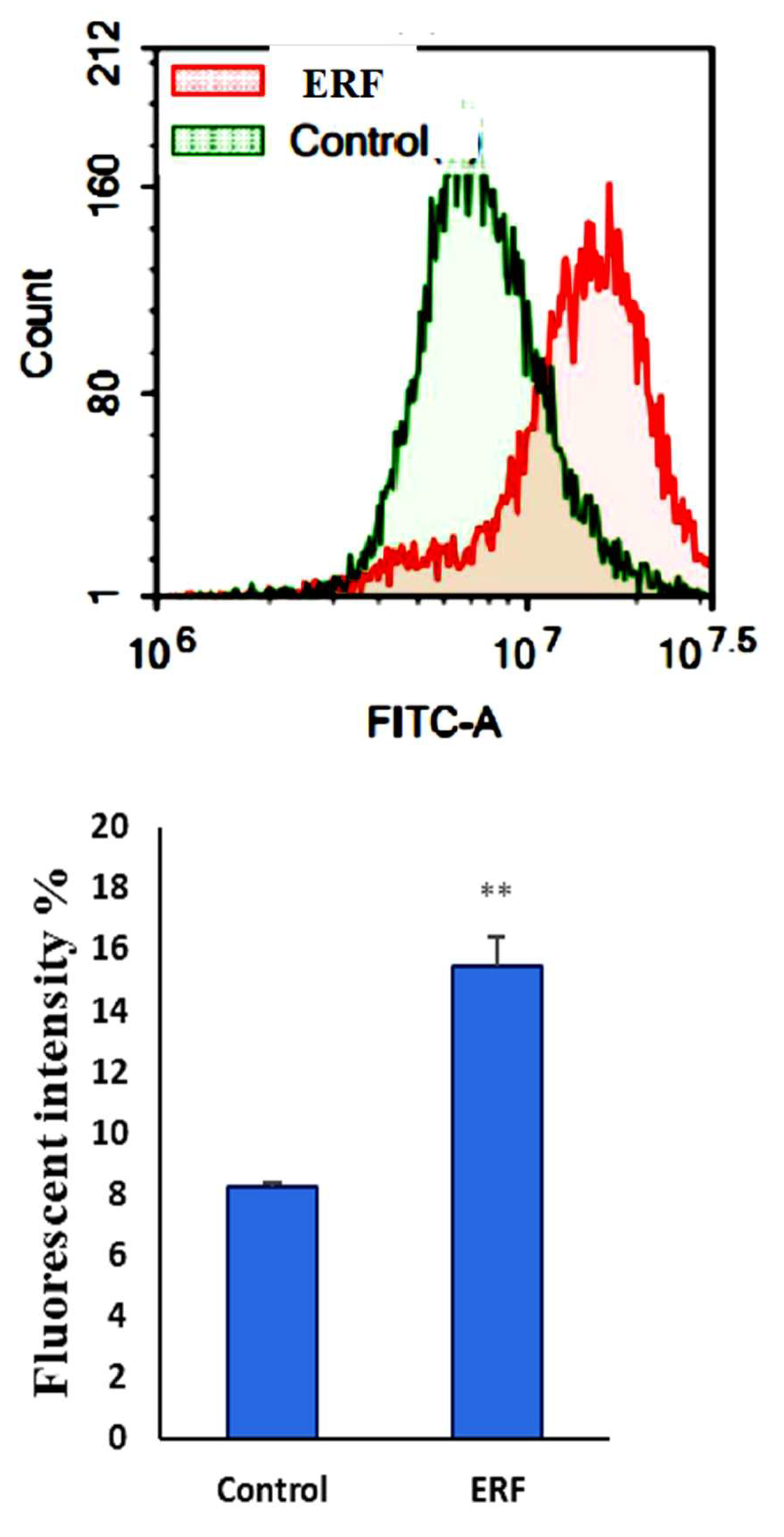
2.9.5. Autophagy Assay
2.9.6. Caspase-Glo 3/7 Activity
2.10. Statistical Analysis
3. Results and Discussion
3.1. Phytochemical Evaluation of ERF of M. arboreus
3.1.1. Phytochemical Screening
3.1.2. Total Flavonoid and Phenolic Contents
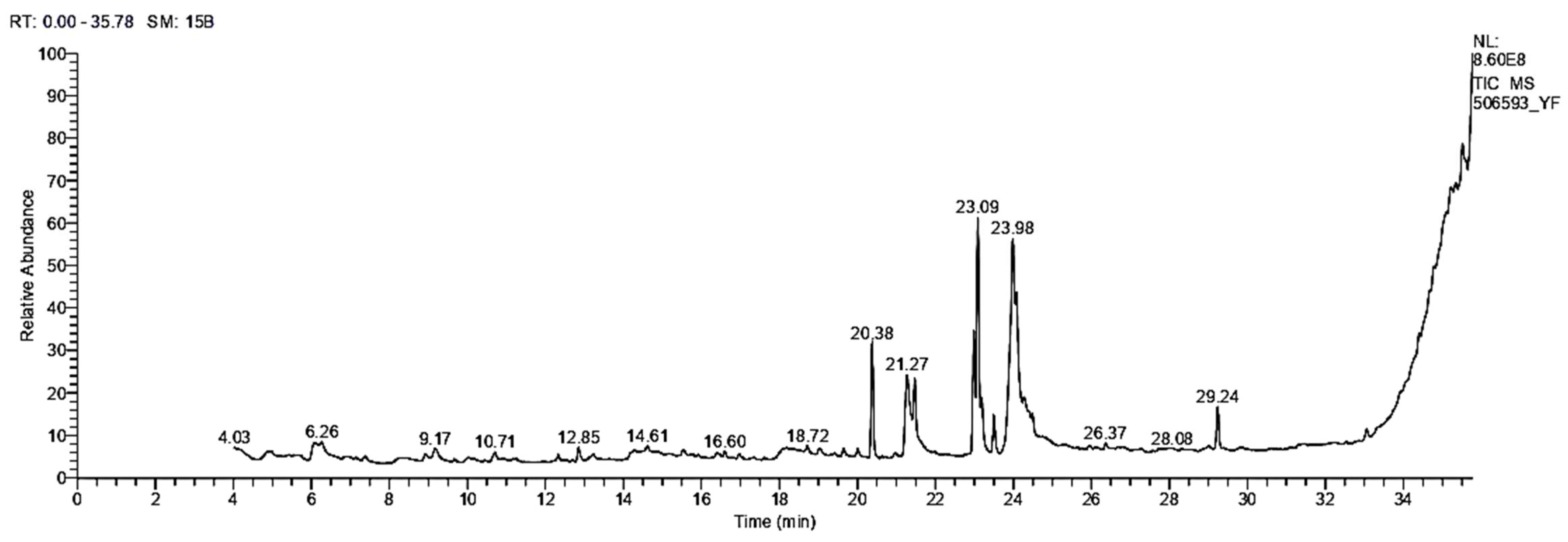
3.1.3. GC/MS Analysis
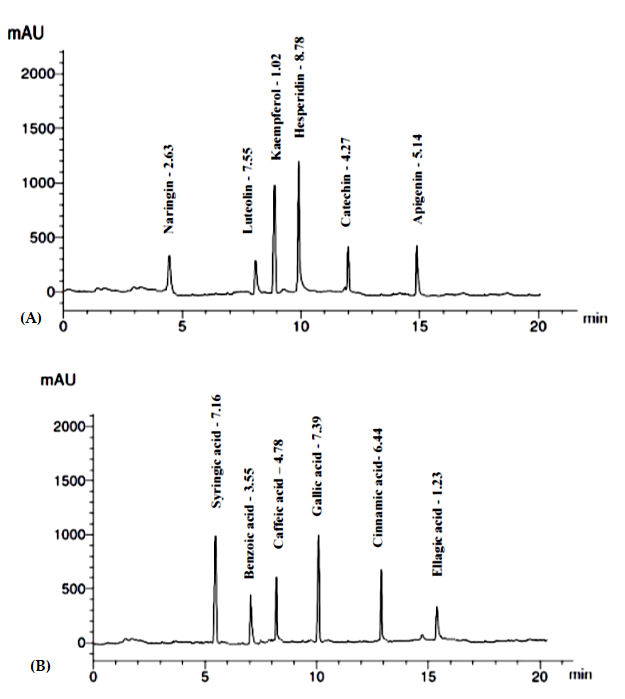
3.1.4. Identification and Quantification of Phenolics and Flavonoids
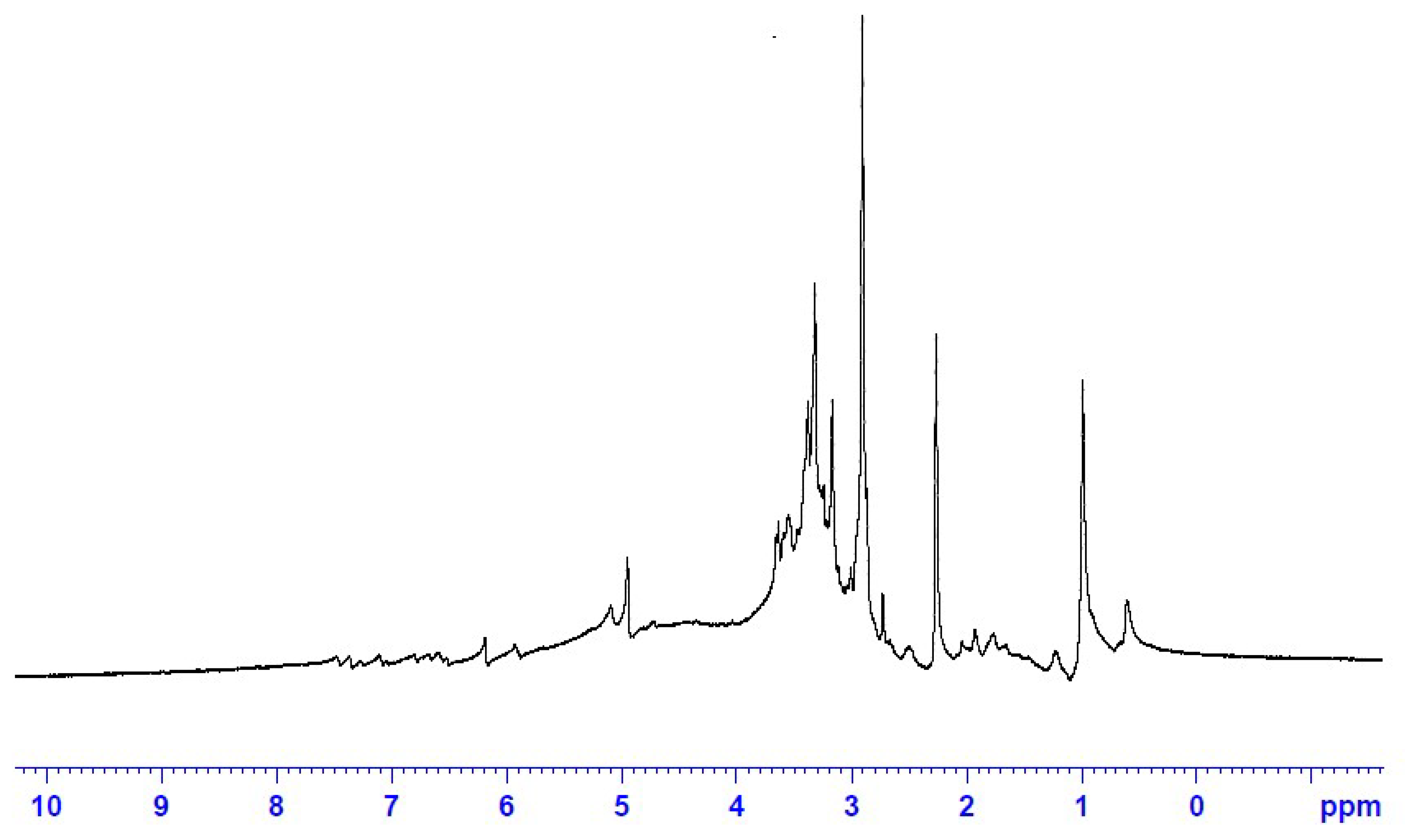
3.1.5. 1HNMR Fingerprint of the ERF
3.2. Antioxidant Capacities of ERF of M. arboreus
3.3. Antibacterial and Antifungal Activities of Extract
3.3.1. Bacterial Killing Kinetics Assay of ERF against Marine V. damsela
3.3.2. Analysis of the Synergistic Impact of ERF
3.4. Cytotoxic Activity
3.4.1. Cell Cycle Analysis
3.4.2. Assessing Cell Apoptosis with Annexin V-FITC
3.4.3. Assessment of Autophagy
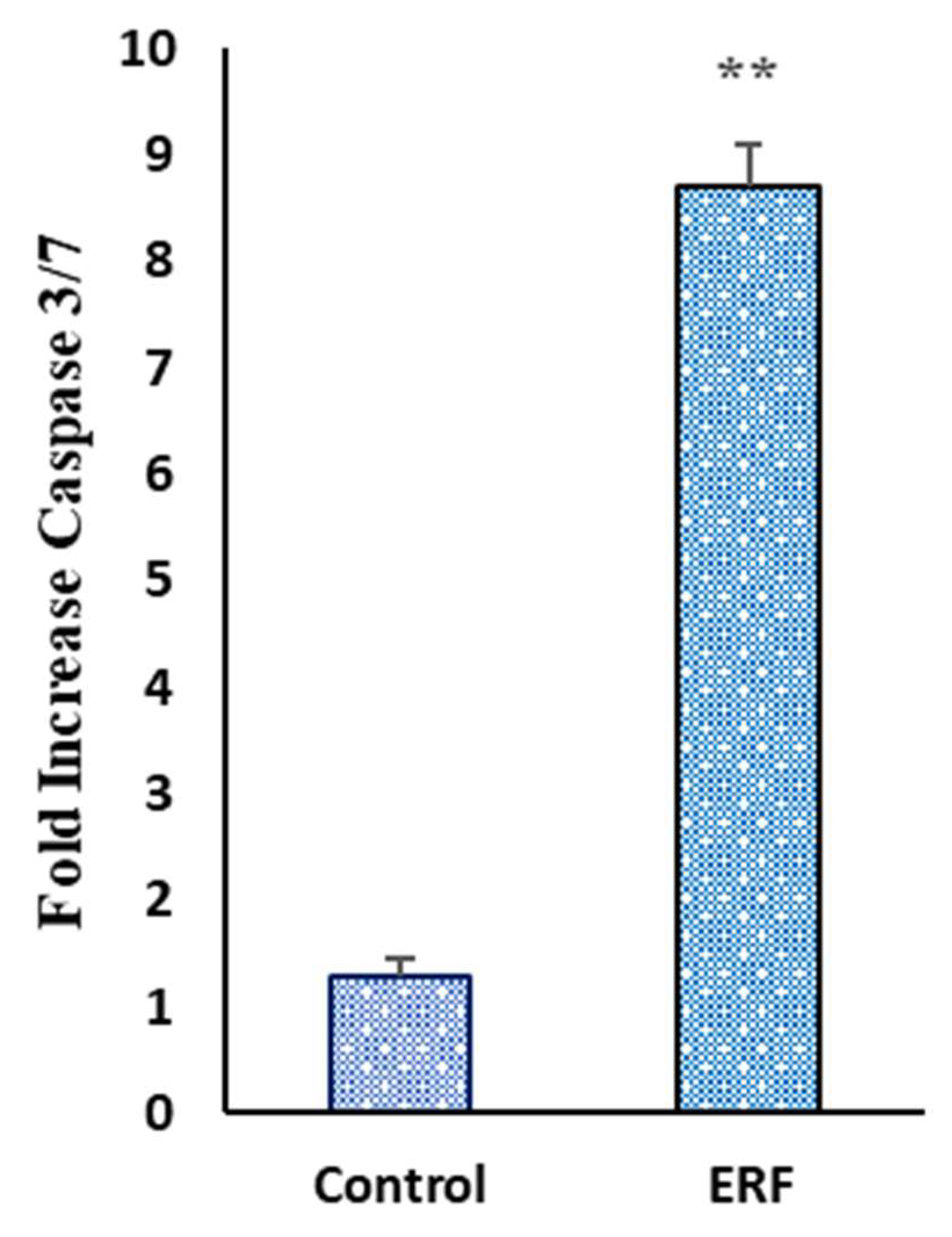
3.4.4. Effect of ERF on the Activity of Caspase 3/7
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, A. Hepatocellular carcinoma. N. Engl. J. Med. 2019, 380, 1450–1462. [Google Scholar] [CrossRef] [PubMed]
- Di Bisceglie, A.M.; Rustgi, V.K.; Hoofnagle, J.H.; Dusheiko, G.M.; Lotze, M.T. NIH conference. Hepatocellular carcinoma. Ann. Intern. Med. 1988, 108, 390–401. [Google Scholar] [CrossRef] [PubMed]
- Alhaqbani, A.H.; Almusalam, A.; Alnadhari, A.; AlSalem, A.; Alrasheed, M.; Alalwan, A.; Aldamkh, B. The prevalence and associated factors of viral hepatitis and cryptogenic related hepatocellular carcinoma at King Abdulaziz Medical city-Riyadh. Int. J. Community Med. Public Health 2019, 6, 561. [Google Scholar] [CrossRef]
- Wang, Z.; Li, Z.; Ye, Y.; Xie, L.; Li, W. Oxidative Stress and Liver Cancer: Etiology and Therapeutic Targets. Oxidative Med. Cell. Longev. 2016, 2016, 7891574. [Google Scholar] [CrossRef]
- McLoughlin, M.R.; Orlicky, D.J.; Prigge, J.R.; Krishna, P.; Talago, E.A.; Cavigli, I.R.; Eriksson, S.; Miller, C.G.; Kundert, J.A.; Sayin, V.I.; et al. TrxR1, Gsr, and oxidative stress determine hepatocellular carcinoma malignancy. Proc. Natl. Acad. Sci. USA 2019, 116, 11408–11417. [Google Scholar] [CrossRef]
- Arteel, G.E. Oxidants and antioxidants in alcohol-induced liver disease. Gastroenterology 2003, 124, 778–790. [Google Scholar] [CrossRef]
- Gordaliza, M. Natural products as leads to anticancer drugs. Clin. Transl. Oncol. 2007, 9, 767–776. [Google Scholar] [CrossRef]
- Abdulaev, F.I. Plant-derived agents against cancer. In Pharmacology and Therapeutics in the New Millennium; Gupta, S.K., Ed.; Narosa Publishing House: New Delhi, India, 2001; p. 345. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef]
- Puckhaber, L.S.; Stipanovic, R.D.; Bost, G.A. Analyses for flavonoid aglycones in fresh and preserved Hibiscus flowers. In Trends in New Crops and New Uses; ASHS Press: Alexandria, Egypt, 2002; pp. 556–563. [Google Scholar]
- Manian, R.; Anusuya, N.; Siddhuraju, P.; Manian, S. The antioxidant activity and free radical scavenging potential of two different solvent extracts of Camellia sinensis (L.) O. Kuntz, Ficus bengalensis L. and Ficus racemosa L. Food Chem. 2008, 107, 1000–1007. [Google Scholar] [CrossRef]
- Laulloo, S.J.; Bhowon, M.G.; Soyfoo, S.; Chua, L.S. Nutritional and Biological Evaluation of Leaves of Mangifera indica from Mauritius. J. Chem. 2018, 2018, 6869294. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Adedapo, A.A.; Jimoh, F.A.; Koduru, S.; Afolayan, A.J.; Masika, P.J. Antibacterial and antioxidant properties of the methanol extracts of the leaves and stems of Calpurnia aurea. BMC Complement. Altern. Med. 2008, 8, 53. [Google Scholar] [CrossRef]
- Gülçin, I.; Küfrevioǧlu, Ö.İ.; Oktay, M.; Büyükokuroǧlu, M.E. Antioxidant, antimicrobial, antiulcer and analgesic activities of nettle (Urtica dioica L.). J. Ethnopharmacol. 2004, 90, 205–215. [Google Scholar] [CrossRef]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and Validation of an Improved Oxygen Radical Absorbance Capacity Assay Using Fluorescein as the Fluorescent Probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef]
- Abdel-Shafi, S.; Al-Mohammadi, A.-R.; Sitohy, M.; Mosa, B.; Ismaiel, A.; Enan, G.; Osman, A. Antimicrobial Activity and Chemical Constitution of the Crude, Phenolic-Rich Extracts of Hibiscus sabdariffa, Brassica oleracea and Beta vulgaris. Molecules 2019, 24, 4280. [Google Scholar] [CrossRef]
- Mizzi, L.; Chatzitzika, C.; Gatt, R.; Valdramidis, V. HPLC Analysis of Phenolic Compounds and Flavonoids with Overlapping Peaks. Food Technol. Biotechnol. 2020, 58, 12–19. [Google Scholar] [CrossRef]
- Abdelhakim, H.; El-Sayed, E.; Rashidi, F. Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. J. Appl. Microbiol. 2020, 128, 1634–1646. [Google Scholar] [CrossRef]
- Pia, S.P.; Mahmud, R.; Perumal, S. Determination of Antibacterial Activity of Essential Oil of Myristica fragrans Houtt. using Tetrazolium Microplate Assay and its Cytotoxic Activity Against Vero Cell Line. Int. J. Pharmacol. 2012, 8, 572–576.e6. [Google Scholar] [CrossRef]
- Pourali, P.; Badiee, S.H.; Manafi, S.; Noorani, T.; Rezaei, A.; Yahyaei, B. Biosynthesis of gold nanoparticles by two bacterial and fungal strains, Bacillus cereus and Fusarium oxysporum, and assessment and comparison of their nanotoxicity in vitro by direct and indirect assays. Electron. J. Biotechnol. 2017, 29, 86–93. [Google Scholar] [CrossRef]
- Shabih, S.; Hajdari, A.; Mustafa, B.; Quave, C.L. Medicinal plants in the Balkans with antimicrobial properties. Med. Plants Anti-Infect. 2022, 2022, 103–138. [Google Scholar] [CrossRef]
- Saeloh, D.; Visutthi, M. Efficacy of Thai Plant Extracts for Antibacterial and Anti-Biofilm Activities against Pathogenic Bacteria. Antibiotics 2021, 10, 1470. [Google Scholar] [CrossRef]
- Gupta, M.; Tomar, R.S.; Kaushik, S.; Mishra, R.K.; Sharma, D. Effective Antimicrobial Activity of Green ZnO Nano Particles of Catharanthus roseus. Front. Microbiol. 2018, 9, 2030. [Google Scholar] [CrossRef]
- Fekry, M.; Ezzat, S.M.; Salama, M.M.; AlShehri, O.Y.; Al-Abd, A.M. Bioactive glycoalkaloides isolated from Solanum melongena fruit peels with potential anticancer properties against hepatocellular carcinoma cells. Sci. Rep. 2019, 9, 1746. [Google Scholar] [CrossRef]
- Sreevidya, N.; Mehrotra, S. Spectrometric method for estimation of precipitable with Dragendor’s reagent in plant material. J. Aoac. Int. 2003, 36, 1124–1127. [Google Scholar] [CrossRef]
- Yassien, E.E.; Hamed, M.M.; Abdelmohsen, U.R.; Hassan, H.M.; Gazwi, H.S.S. In vitro antioxidant, antibacterial, and antihyperlipidemic potential of ethanolic Avicennia marina leaves extract supported by metabolic profiling. Environ. Sci. Pollut. Res. 2021, 28, 27207–27217. [Google Scholar] [CrossRef]
- Knothe, G.; Kenar, J.A. Determination of the fatty acid profile by 1H-NMR spectroscopy. Eur. J. Lipid Sci. Technol. 2004, 106, 88–96. [Google Scholar] [CrossRef]
- Chua, L.S.; Hidayathulla, S. Phytochemical profile of fresh and senescent leaves due to storage for Ficus deltoidea. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2015, 151, 74–83. [Google Scholar] [CrossRef]
- Kasote, D.M.; Katyare, S.S.; Hegde, M.V.; Bae, H. Significance of Antioxidant Potential of Plants and its Relevance to Therapeutic Applications. Int. J. Biol. Sci. 2015, 11, 982–991. [Google Scholar] [CrossRef]
- Mahady, G.B.; Huang, Y.; Doyle, B.J.; Locklear, T. Natural Products as Antibacterial Agents. Stud. Nat. Prod. Chem. 2008, 35, 423–444. [Google Scholar] [CrossRef]
- Chen, B.-J.; Jamaludin, N.S.; Khoo, C.-H.; See, T.-H.; Sim, J.-H.; Cheah, Y.-K.; Halim, S.N.A.; Seng, H.-L.; Tiekink, E.R. In vitro antibacterial and time-kill evaluation of phosphanegold (I) dithiocarbamates, R3PAu [S2CN (iPr) CH2CH2OH] for R = Ph, Cy and Et, against a broad range of Gram-positive and Gram-negative bacteria. Gold Bull. 2014, 47, 225–236. [Google Scholar]
- Schinor, E.C.; Salvador, M.J.; Ito, I.Y.; Dias, D.A. Evaluation of the antimicrobial activity of crude extracts and isolated constituents from Chresta scapigera. Braz. J. Microbiol. 2007, 38, 145–149. [Google Scholar] [CrossRef]
- Wu, H.-S.; Raza, W.; Fan, J.-Q.; Sun, Y.-G.; Bao, W.; Shen, Q.-R. Cinnamic Acid Inhibits Growth but Stimulates Production of Pathogenesis Factors by in Vitro Cultures of Fusarium oxysporum f.sp. niveum. J. Agric. Food Chem. 2008, 56, 1316–1321. [Google Scholar] [CrossRef] [PubMed]
- Korošec, B.; Sova, M.; Turk, S.; Kraševec, N.; Novak, M.; Lah, L.; Stojan, J.; Podobnik, B.; Berne, S.; Zupanec, N.; et al. Antifungal activity of cinnamic acid derivatives involves inhibition of benzoate 4-hydroxylase (CYP53). J. Appl. Microbiol. 2014, 116, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Johannes, E.; Litaay, M.; Syahribulan. The bioactivity of hexadecanoic acid compound isolated from hydroid Aglaophenia cupressina lamoureoux as antibacterial agent against Salmonella typhi. Int. J. Biol. Med. Res. 2016, 7, 5469–5472. [Google Scholar]
- Radiati, L.E. Mechanism of Virulence Inhibition of Enterophatogenic Bacteria by Ginger (Zingiber officinale Roscoe) Rhizome Extract. Ph.D. Dissertation, Postgraduate Program, Bogor Agricultural University, Bogor, Indonesia, 2002. [Google Scholar]
- Donato, M.; Tolosa, L.; Gómez-Lechón, M.J. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol. Biol. 2015, 1250, 77–93. [Google Scholar]
- Tirkey, N.; Pilkhwal, S.; Kuhad, A.; Chopra, K. Hesperidin, a citrus bioflavonoid, decreases the oxidative stress produced by carbon tetrachloride in rat liver and kidney. BMC Pharmacol. 2005, 5, 2. [Google Scholar] [CrossRef]
- Lee, C.J.; Wilson, L.; Jordan, M.A.; Nguyen, V.; Tang, J.; Smiyun, G. Hesperidin suppressed proliferations of both Human breast cancer and androgen-dependent prostate cancer cells. Phytother. Res. 2009, 24, S15–S19. [Google Scholar] [CrossRef]
- Abd El-Azim, M.H.M.; Abdelgawad, A.A.M.; El-Gerby, M.; Ali, S.; El-Mesallamy, A.M.D. Phenolic compounds and cytotoxic activities of methanol extract of basil (Ocimum basilicumL L.). J. Microb. Biochem. Technol. 2015, 7, 182–185. [Google Scholar] [CrossRef]
- Tanaka, M.; Kuei, C.W.; Nagashima, Y.; Taguchi, T. Application of antioxidative millard reaction products from histidine and glucose to sardine products. Nippon. Suisan Gakkaishil. 1998, 54, 1409–1414. [Google Scholar] [CrossRef]
- Dickson, M.A.; Schwartz, G.K. Development of Cell-Cycle Inhibitors for Cancer Therapy. Curr. Oncol. 2009, 16, 36–43. [Google Scholar] [CrossRef]
- Riccardi, C.; Nicoletti, I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat. Protoc. 2006, 1, 1458–1461. [Google Scholar] [CrossRef]
- Fadok, V.A.; Voelker, D.R.; Campbell, P.A.; Cohen, J.J.; Bratton, D.L.; Henson, P.M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immunol. 1992, 148, 2207–2216. [Google Scholar]
- Demchenko, A.P. Beyond annexin V: Fluorescence response of cellular membranes to apoptosis. Cytotechnology 2012, 65, 157–172. [Google Scholar] [CrossRef]
- Towers, C.G.; Torburn, A. Terapeutic targeting of autophagy. EBioMedicine 2016, 14, 15–23. [Google Scholar] [CrossRef]
- Hippert, M.M.; O'Toole, P.S.; Thorburn, A. Autophagy in Cancer: Good, Bad, or Both? Cancer Res. 2006, 66, 9349–9351. [Google Scholar] [CrossRef]
- Soung, Y.H.; Jeong, E.G.; Ahn, C.H.; Kim, S.S.; Song, S.Y.; Yoo, N.J.; Lee, S.H. Mutational analysis of caspase 1, 4, and 5 genes in common human cancers. Hum. Pathol. 2008, 39, 895–900. [Google Scholar] [CrossRef]
- Tee, T.T.; Cheah, Y.H.; Hawariah, L.P.A. F16, a fraction from Eurycoma longifolia jack extract, induces apoptosis via a caspase-9-independent manner in MCF-7 cells. Anticancer Res. 2007, 27, 3425–3430. [Google Scholar]
- Shao, W.; Yeretssian, G.; Doiron, K.; Hussain, S.N.; Saleh, M. The caspase-1 digestome identifies the glycolysis pathway as a target during infection and septic shock. J. Biol. Chem. 2007, 282, 36321–36329. [Google Scholar] [CrossRef]
- Lim, S.-W.; Loh, H.-S.; Ting, K.N.; Bradshaw, T.D.; Zeenathul, N.A. Antiproliferation and induction of caspase-8-dependent mitochondria-mediated apoptosis by β-tocotrienol in human lung and brain cancer cell lines. Biomed. Pharmacother. 2014, 68, 1105–1115. [Google Scholar] [CrossRef]




| Tests | Result |
|---|---|
| Flavonoids | + |
| Tannins | + |
| Coumarins | + |
| Saponins | ± |
| Steroids | ± |
| Glycosides | + |
| Phenols | + |
| Terpenoids | + |
| Emodins | + |
| Anthocyanins | + |
| Alkaloids | ± |
| Parameters | Result |
|---|---|
| TPC (mg GAE/g extract) | 46.25 ± 2.1 |
| TFC (mg QE/g extract) | 23.83 ± 2.9 |
| RT | Name of the Compound | MF | MW | Peak Area (%) | Biological Activity ** | |
|---|---|---|---|---|---|---|
| 1 | 6.07 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl | C6H8O4 | 144 | 2.85 | Antimicrobial, anti-inflammatory |
| 2 | 6.26 | Octadecanoic acid, ethyl ester | C20H40O2 | 312 | 1.46 | Anti-microbial |
| 3 | 9.17 | 3,5-Heptadienal, 2-ethylidene-6-methyl- | C10H14O | 150 | 1.14 | Anti-inflammatory, antitumor, antiviral |
| 4 | 10.71 | 4-(3,3-dimethyl-1-butynyl)-4-hydroxy-2,6,6-trimethyl-2-cyclohexen-1-one | C15H22O2 | 234 | 1.09 | Antioxidant, anti-inflammatory |
| 5 | 12.33 | 10,13-Octadecadiynoic acid, methyl ester | C19H30O2 | 290 | 0.60 | No activity reported |
| 6 | 12.84 | 1-(3-Methoxy-5-methylphenyl)-N-methylpropan-2-amine | C12H19NO | 193 | 1.20 | No activity reported |
| 7 | 18.72 | 9,12,15-Octadecatrienoic acid, 2,3-bis [(trimethylsilyl)oxy] propyl ester, (z, z, z)- | C27H52O4Si2 | 496 | 0.66 | Anticancer, hepatoprotective |
| 8 | 19.64 | Cis-13-Eicosenoic acid | C20H38O2 | 310 | 0.81 | Anti-inflammatory activity |
| 9 | 20.01 | 9-octadecenoic acid, (2-phenyl-1,3-dioxolan-4-yl) methyl ester, cis | C28H44O4 | 444 | 0.72 | Antimicrobial, anti-inflammatory |
| 10 | 20.38 | Hexadecanoic acid, methyl ester | C17H34O2 | 270 | 8.88 | Antioxidant, antimicrobial, antihypercholesterolemic property |
| 11 | 21.27 | Hexadecanoic acid | C16H32O2 | 256 | 8.52 | Anti-inflammatory, antioxidant, antihypercholesterolemic |
| 12 | 21.47 | Hexadecanoic acid, ethyl ester | C18H36O2 | 284 | 3.66 | Antioxidant, antihypercholesterolemic antiandrogenic |
| 13 | 22.99 | 9,12-Octadecadienoic acid (Z, Z)-, methyl ester | C19H34O2 | 294 | 9.46 | Hepatoprotective, antihistamine, hypocholesterolemia, anti-eczema |
| 14 | 23.09 | 11-Octadecenoic acid, methyl ester | C19H36O2 | 19.49 | Antioxidant, antimicrobial | |
| 15 | 23.19 | 16-Octadecenoic acid, methyl ester | C19H36O2 | 296 | 3.86 | Selectively inhibit eukaryotic DNA polymerase activities in vitro |
| 16 | 23.50 | Octadecanoic acid, methyl ester | C19H38O2 | 298 | 3.03 | Antimicrobial |
| 17 | 23.98 | 9,12-Octadecadienoic acid (Z, Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C21H38O4 | 354 | 18.41 | Antiarthritic, hepatoprotective, antiandrogenic, anticoronary, antieczemic, anticancer |
| 18 | 24.08 | Ethyl oleate | C18H34O2 | 282 | 7.73 | Antibacterial, anticancer |
| 19 | 24.28 | Oleic acid | C20H38O2 | 310 | 1.25 | It is used as a vehicle for intramuscular drug delivery, progesterone |
| 20 | 24.41 | Linoleic acid ethyl ester | C20H36O2 | 308 | 0.71 | Anti-arthritic, anti-acne, hepatoprotective, anti-histaminic, anti-coronary |
| 21 | 24.49 | Octadecanoic acid, 2,3-dihydroxypropyl ester | C21H42O4 | 358 | 1.00 | Anticancer, antimicrobial |
| Components | RT (min) | Conc. (µg/mg) |
|---|---|---|
| Flavonoid compounds | ||
| Naringin | 4.6 | 2.63 |
| Hesperidin | 10.0 | 8.78 |
| Kaempferol | 8.1 | 1.02 |
| Luteolin | 9.0 | 7.55 |
| Apigenin | 15.0 | 5.14 |
| Catechin | 12.0 | 4.27 |
| Phenolic compounds | ||
| Caffeic acid | 8.1 | 4.78 |
| Cinnamic acid | 13.0 | 6.44 |
| Gallic acid | 10.0 | 7.39 |
| Syringic acid | 5.2 | 7.16 |
| Benzoic acid | 7.0 | 3.55 |
| Ellagic acid | 15. 6 | 1.23 |
| Parameters | Result |
|---|---|
| DPPH (IC50 μg/mL) | 115.6 ± 16.9 |
| ABTS (µM TE/mg extract) | 716.45 ± 16.12 |
| FRAP (μM TE/mg extract) | 99.15 ± 4.96 |
| ORAC (µM TE/mg extract) | 1138.11 ± 79.65 |
| Metal chelating property (µM EDTA eq/mg extract) | 57.58 ± 3.5 |
| Pathogens | Inhibition Zone (mm) | Amoxicillin/Clavulanic (Positive Control) | DMSO (Negative Control) |
|---|---|---|---|
| B. subtillus | 0.0 | 14.0 ± 0.5 | 0.0 |
| S. aureus | 12.0 ± 0.6 | 12.0. ± 0.2 | 0.0 |
| E. faecalis | 10.0 ± 0.1 | 18.0 ± 0.5 | 0.0 |
| P. aeruginosa | 14.0 ± 0.1 | 14.0 ± 0.2 | 0.0 |
| V. fluvialis | 16.0 ± 0.4 | 22.0 ± 0.6 | 0.0 |
| V. damsela | 20.0 ± 0.2 | 24.0 ± 0.2 | 0.0 |
| B. cereus | 0.0 | 10.0 ± 0.3 | 0.0 |
| S. typhimurium | 16.0 ± 0.2 | 20.0 ± 0.3 | 0.0 |
| Pathogens | MIC (mg/mL) |
|---|---|
| S. aureus | 10.0 ± 0.06 |
| E. faecalis | 12.5 ± 0.02 |
| P. aeruginosa | 10.0 ± 0.01 |
| V. fluvialis | 2.5 ± 0.05 |
| V. damsela | 1.5 ± 0.02 |
| S. typhimurium | 5.0 ± 0.01 |
| A. fumigatus | 1.0 ± 0.02 |
| A. niger | 1.75 ± 0.06 |
| A. flavus | 1.25 ± 0.01 |
| A. terreus | 0.75 ± 0.01 |
| Amoxicillin/Clavulanic & ERF | Inhibition Zone (mm) of V. damsela (10−6 CFU/mL) |
|---|---|
| Amoxicillin/clavulanic (20/10 mcg) | 24.0 ± 0.2 |
| ERF (250 µg/mL) | 20.0 ± 0.2 |
| ERF (500 µg/mL) + Amoxicillin/clavulanic (20/10 mcg) | 26.0 ± 0.2 |
| ERF (750 µg/mL) + Amoxicillin/clavulanic (20/10 mcg) | 26.9 ± 0.2 |
| ERF (1000 µg/mL) + Amoxicillin/clavulanic (20/10 mcg) | 28.0 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gazwi, H.S.S.; Shoeib, N.A.; Mahmoud, M.E.; Soltan, O.I.A.; Hamed, M.M.; Ragab, A.E. Phytochemical Profile of the Ethanol Extract of Malvaviscus arboreus Red Flower and Investigation of the Antioxidant, Antimicrobial, and Cytotoxic Activities. Antibiotics 2022, 11, 1652. https://doi.org/10.3390/antibiotics11111652
Gazwi HSS, Shoeib NA, Mahmoud ME, Soltan OIA, Hamed MM, Ragab AE. Phytochemical Profile of the Ethanol Extract of Malvaviscus arboreus Red Flower and Investigation of the Antioxidant, Antimicrobial, and Cytotoxic Activities. Antibiotics. 2022; 11(11):1652. https://doi.org/10.3390/antibiotics11111652
Chicago/Turabian StyleGazwi, Hanaa S. S., Nagwa A. Shoeib, Magda E. Mahmoud, Osama I. A. Soltan, Moaz M. Hamed, and Amany E. Ragab. 2022. "Phytochemical Profile of the Ethanol Extract of Malvaviscus arboreus Red Flower and Investigation of the Antioxidant, Antimicrobial, and Cytotoxic Activities" Antibiotics 11, no. 11: 1652. https://doi.org/10.3390/antibiotics11111652
APA StyleGazwi, H. S. S., Shoeib, N. A., Mahmoud, M. E., Soltan, O. I. A., Hamed, M. M., & Ragab, A. E. (2022). Phytochemical Profile of the Ethanol Extract of Malvaviscus arboreus Red Flower and Investigation of the Antioxidant, Antimicrobial, and Cytotoxic Activities. Antibiotics, 11(11), 1652. https://doi.org/10.3390/antibiotics11111652








