Prevalence of Multidrug-Resistant and Extended-Spectrum Beta-Lactamase-Producing Shigella Species in Asia: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Results
2.1. Study Selection and Characteristics of the Included Studies
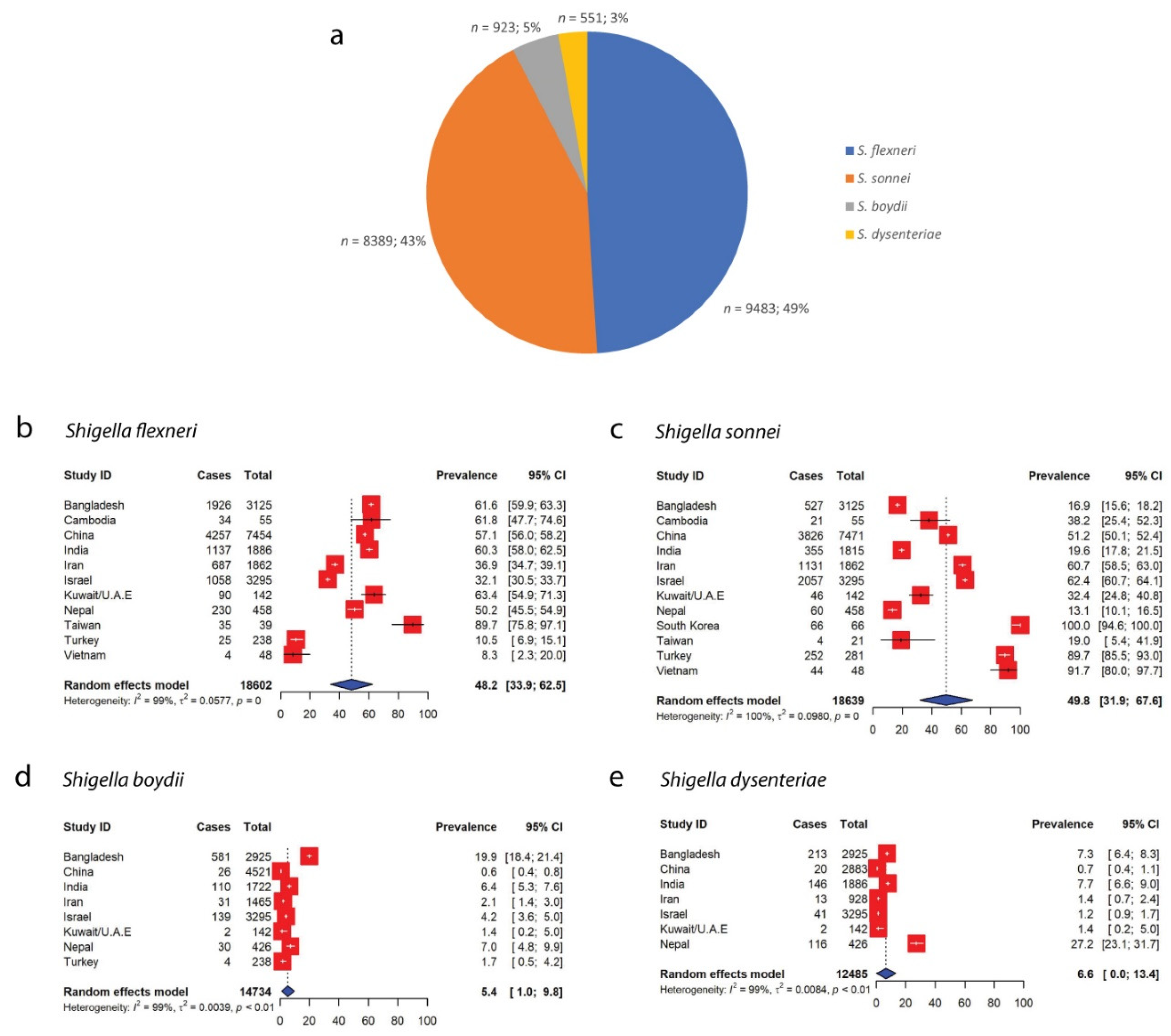
2.2. Prevalence of Shigella species in Asia
2.3. Antimicrobial Resistance Patterns of Shigella spp.
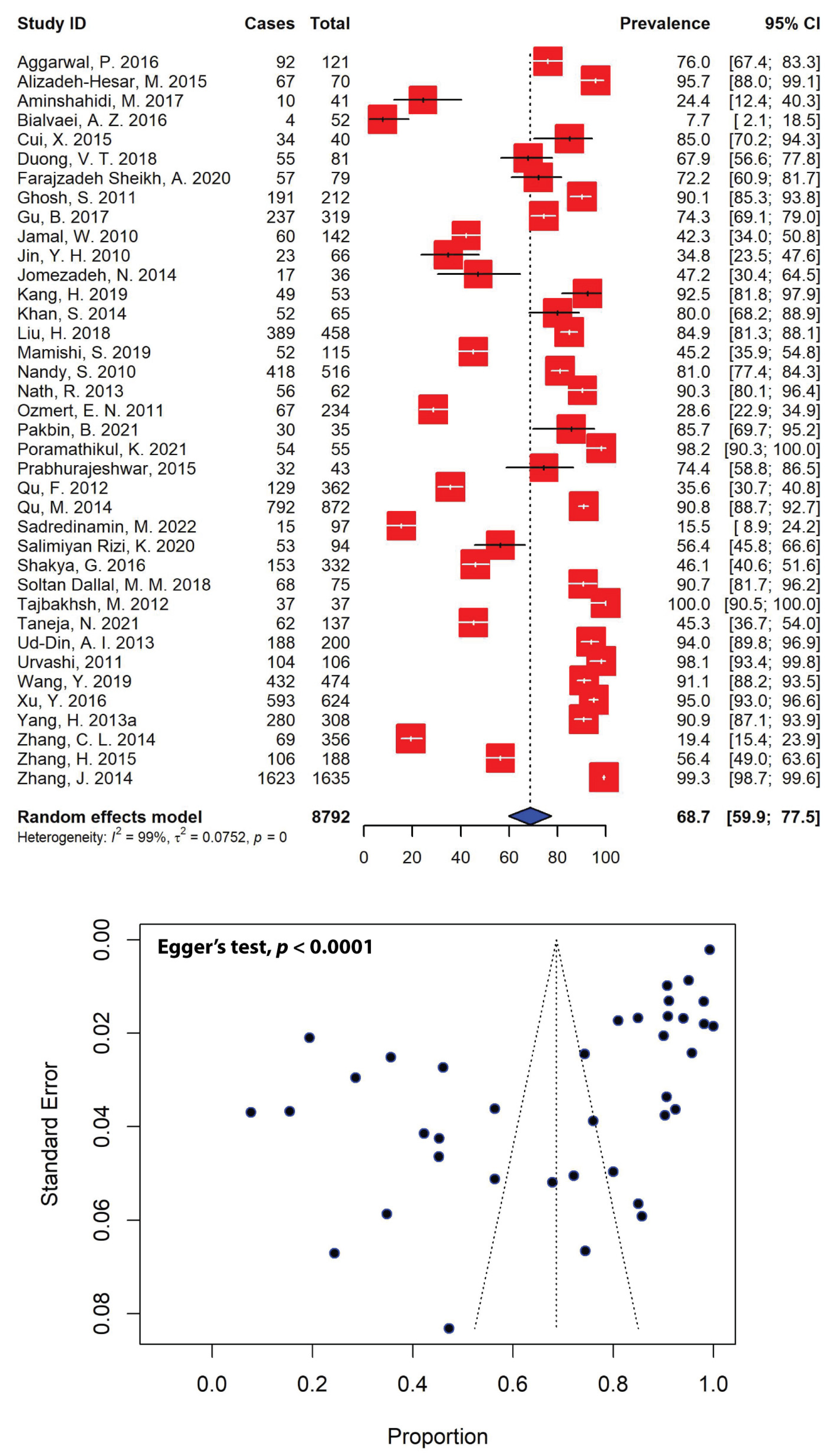
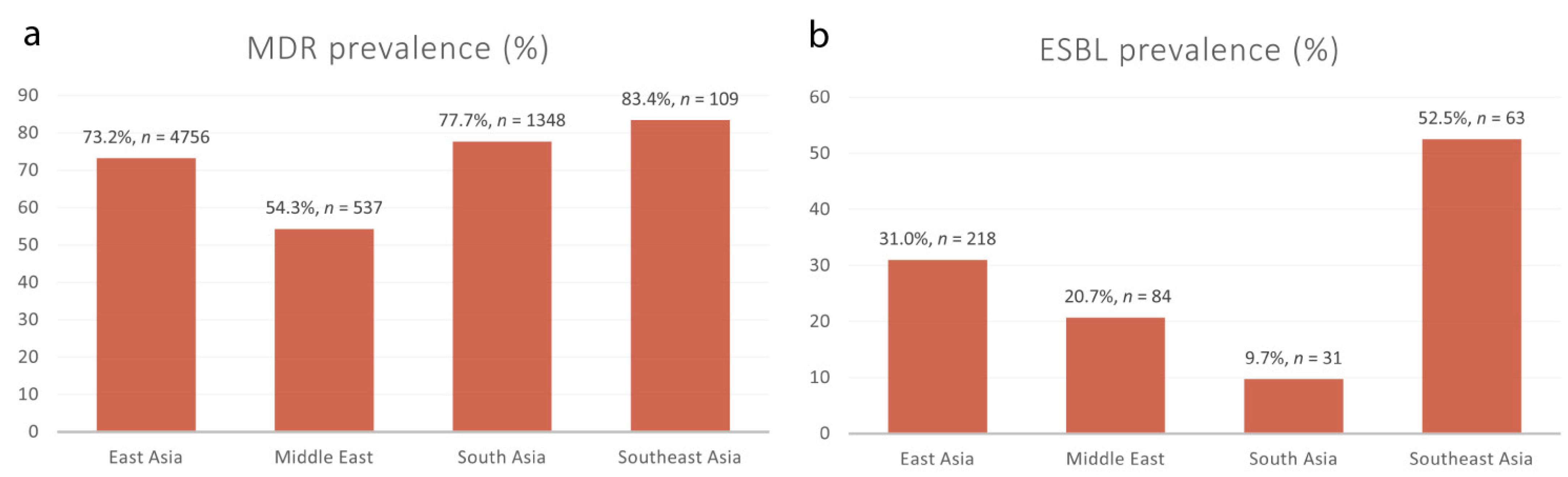
2.4. Prevalence of Multidrug-Resistant Shigella spp. in Asia
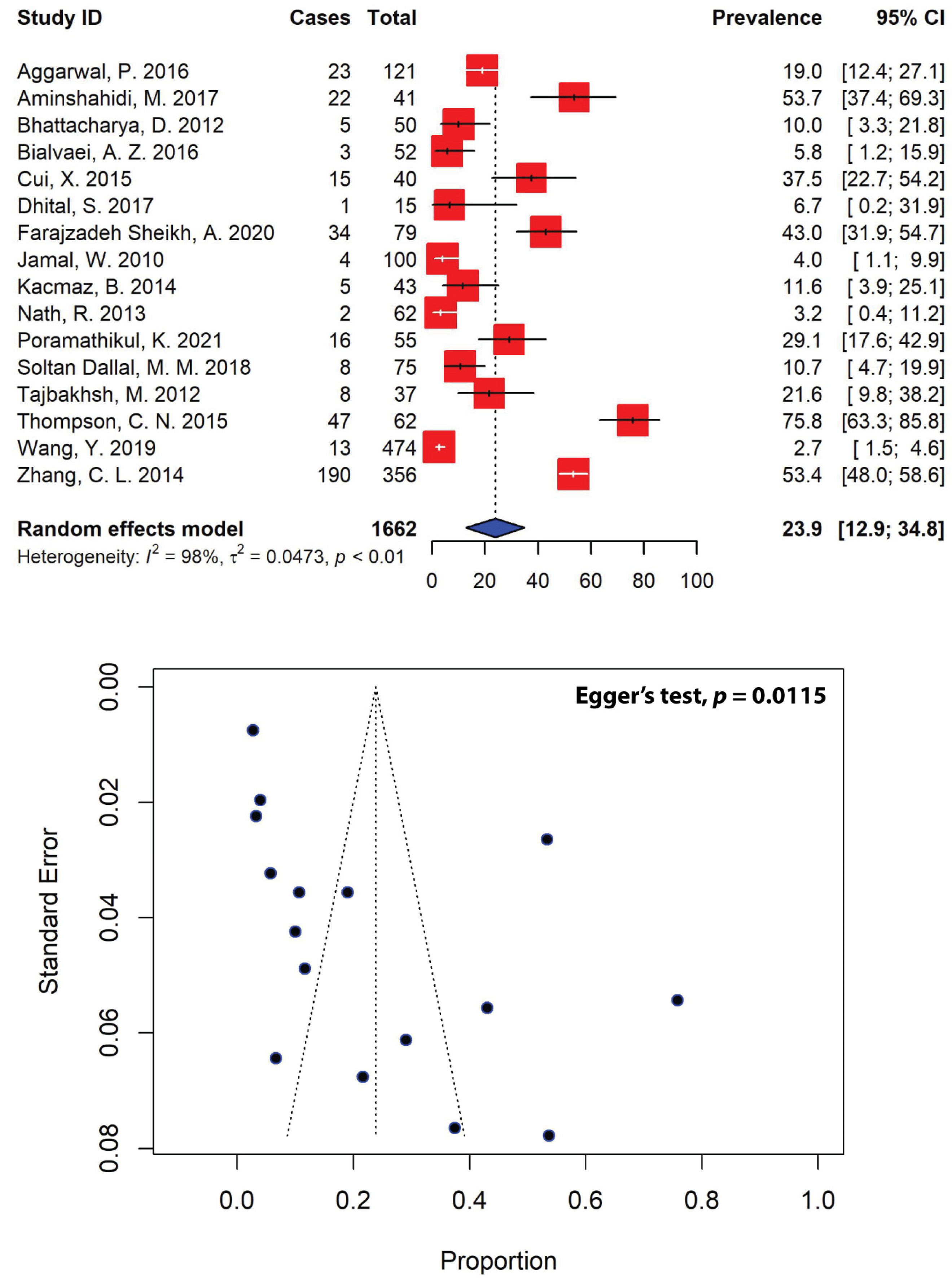
2.5. Patterns of Extended-Spectrum β-Lactamase-Producing Shigella spp.
3. Discussion
4. Methodology
4.1. Literature Search Strategy and Selection
4.2. Inclusion and Exclusion Criteria
4.3. Data Extraction and Quality Control
4.4. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung The, H.; Rabaa, M.A.; Pham Thanh, D.; De Lappe, N.; Cormican, M.; Valcanis, M.; Howden, B.P.; Wangchuk, S.; Bodhidatta, L.; Mason, C.J.; et al. South Asia as a Reservoir for the Global Spread of Ciprofloxacin-Resistant Shigella sonnei: A Cross-Sectional Study. PLoS Med. 2016, 13, e1002055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, B.; Cao, Y.; Pan, S.; Zhuang, L.; Yu, R.; Peng, Z.; Qian, H.; Wei, Y.; Zhao, L.; Liu, G.; et al. Comparison of the prevalence and changing resistance to nalidixic acid and ciprofloxacin of Shigella between Europe-America and Asia-Africa from 1998 to 2009. Int. J. Antimicrob. Agents 2012, 40, 9–17. [Google Scholar] [CrossRef]
- Thompson, C.N.; Duy, P.T.; Baker, S. The Rising Dominance of Shigella sonnei: An Intercontinental Shift in the Etiology of Bacillary Dysentery. PLoS Negl. Trop. Dis. 2015, 9, e0003708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ud-Din, A.I.M.S.; Wahid, S.U.H.; Latif, H.A.; Shahnaij, M.; Akter, M.; Azmi, I.J.; Hasan, T.N.; Ahmed, D.; Hossain, M.A.; Faruque, A.S.G.; et al. Changing Trends in the Prevalence of Shigella species: Emergence of Multi-Drug Resistant Shigella sonnei Biotype g in Bangladesh. PLoS ONE 2013, 8, 82601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The, H.C.; Baker, S. Out of Asia: The independent rise and global spread of fluoroquinolone-resistant Shigella. Microb. Genomics 2018, 4, e000171. [Google Scholar] [CrossRef] [Green Version]
- Kotloff, K.L.; Riddle, M.S.; Platts-Mills, J.A.; Pavlinac, P.; Zaidi, A.K.M. Shigellosis. Lancet 2018, 391, 801–812. [Google Scholar] [CrossRef]
- Khalil, I.A.; Troeger, C.; Blacker, B.F.; Rao, P.C.; Brown, A.; Atherly, D.E.; Brewer, T.G.; Engmann, C.M.; Houpt, E.R.; Kang, G.; et al. Morbidity and mortality due to shigella and enterotoxigenic Escherichia coli diarrhoea: The Global Burden of Disease Study 1990–2016. Lancet Infect. Dis. 2018, 18, 1229–1240. [Google Scholar] [CrossRef] [Green Version]
- WHO Guidelines for the Control of Shigellosis, Including Epidemics Due to Shigella Dysenteriae Type 1. Available online: https://apps.who.int/iris/handle/10665/43252 (accessed on 15 September 2022).
- Chung The, H.; Boinett, C.; Pham Thanh, D.; Jenkins, C.; Weill, F.X.; Howden, B.P.; Valcanis, M.; De Lappe, N.; Cormican, M.; Wangchuk, S.; et al. Dissecting the molecular evolution of fluoroquinolone-resistant Shigella sonnei. Nat. Commun. 2019, 10, 4828. [Google Scholar] [CrossRef] [Green Version]
- Chung The, H.; Bodhidatta, L.; Pham, D.T.; Mason, C.J.; Ha Thanh, T.; Voong Vinh, P.; Turner, P.; Hem, S.; Dance, D.A.B.; Newton, P.N.; et al. Evolutionary histories and antimicrobial resistance in Shigella flexneri and Shigella sonnei in Southeast Asia. Commun. Biol. 2021, 4, 353. [Google Scholar] [CrossRef]
- Von Seidlein, L.; Deok, R.K.; Ali, M.; Lee, H.; Wang, X.Y.; Vu, D.T.; Do, G.C.; Chaicumpa, W.; Agtini, M.D.; Hossain, A.; et al. A multicentre study of Shigella diarrhoea in six Asian countries: Disease burden, clinical manifestations, and microbiology. PLoS Med. 2006, 3, 1556–1569. [Google Scholar] [CrossRef]
- Baker, K.S.; Dallman, T.J.; Field, N.; Childs, T.; Mitchell, H.; Day, M.; Weill, F.X.; Lefèvre, S.; Tourdjman, M.; Hughes, G.; et al. Horizontal antimicrobial resistance transfer drives epidemics of multiple Shigella species. Nat. Commun. 2018, 9, 1462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjbar, R.; Farahani, A. Shigella: Antibiotic-Resistance Mechanisms and New Horizons for Treatment. Infect. Drug Resist. 2019, 12, 3137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nhu, N.T.K.; Vinh, H.; Nga, T.V.T.; Stabler, R.; Duy, P.T.; Vien, L.T.M.; Van Doorn, H.R.; Cerdeño-Tárraga, A.M.; Thomson, N.; Campbell, J.; et al. The Sudden Dominance of blaCTX–M Harbouring Plasmids in Shigella spp. Circulating in Southern Vietnam. PLoS Negl. Trop. Dis. 2010, 4, e702. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, P.; Uppal, B.; Ghosh, R.; Krishna Prakash, S.; Chakravarti, A.; Jha, A.K.; Rajeshwari, K. Multi drug resistance and Extended Spectrum Beta Lactamases in clinical isolates of Shigella: A study from New Delhi, India. Travel Med. Infect. Dis. 2016, 14, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, D.; Hoque, A.; Elahi, M.S.B.; Endtz, H.P.; Hossain, M.A. Bacterial aetiology of diarrhoeal diseases and antimicrobial resistance in Dhaka, Bangladesh, 2005–2008. Epidemiol. Infect. 2012, 140, 1678–1684. [Google Scholar] [CrossRef] [Green Version]
- Alizadeh-Hesar, M.; Bakhshi, B.; Najar-Peerayeh, S. Clonal dissemination of a single Shigella sonnei strain among Iranian children during Fall 2012 in Tehran, I.R. Iran. Infect. Genet. Evol. 2015, 34, 260–266. [Google Scholar] [CrossRef]
- Aminshahidi, M.; Arastehfar, A.; Pouladfar, G.; Arman, E.; Fani, F. Diarrheagenic Escherichia coli and Shigella with High Rate of Extended-Spectrum Beta-Lactamase Production: Two Predominant Etiological Agents of Acute Diarrhea in Shiraz, Iran. Microb. Drug Resist. 2017, 23, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Behruznia, P.; Sadredinamin, M.; Hashemi, A.; Hajikhani, B.; Yousefi Nojookambari, N.; Behruznia, M.; Ghalavand, Z. Decreased Susceptibility of Shigella isolates to Azithromycin in Children in Tehran, Iran. Can. J. Infect. Dis. Med. Microbiol. 2022, 2022, 4503964. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Bhattacharya, H.; Sayi, D.S.; Bharadwaj, A.P.; Singhania, M.; Sugunan, A.P.; Roy, S. Changing patterns and widening of antibiotic resistance in Shigella spp. over a decade (2000-2011), Andaman Islands, India. Epidemiol. Infect. 2015, 143, 470–477. [Google Scholar] [CrossRef]
- Bhattacharya, D.; Sugunan, A.P.; Bhattacharjee, H.; Thamizhmani, R.; Sayi, D.S.; Thanasekaran, K.; Manimunda, S.P.; Ghosh, A.R.; Bharadwaj, A.P.; Singhania, M.; et al. Antimicrobial resistance in Shigella—Rapid increase & widening of spectrum in Andaman Islands, India. Indian J. Med. Res. 2012, 135, 365. [Google Scholar]
- Bialvaei, A.Z.; Kafil, H.S.; Asgharzadeh, M.; Aghazadeh, M.; Yousefi, M. CTX-M extended-spectrum β-lactamase-producing Klebsiella spp, Salmonella spp, Shigella spp and Escherichia coli isolates in Iranian hospitals. Braz. J. Microbiol. 2016, 47, 706–711. [Google Scholar] [CrossRef] [PubMed]
- Chamoun, K.; Farah, M.; Araj, G.; Daoud, Z.; Moghnieh, R.; Salameh, P.; Saade, D.; Mokhbat, J.; Abboud, E.; Hamze, M.; et al. Surveillance of antimicrobial resistance in Lebanese hospitals: Retrospective nationwide compiled data. Int. J. Infect. Dis. 2016, 46, 64–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.; Yang, C.; Wang, J.; Liang, B.; Yi, S.; Li, H.; Liu, H.; Li, P.; Wu, Z.; Xie, J.; et al. Antimicrobial Resistance of Shigella flexneri Serotype 1b Isolates in China. PLoS ONE 2015, 10, e0129009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, A.; Natarajan, M.; Mandal, J. The Emergence of Quinolone Resistant Shigella sonnei, Pondicherry, India. PLoS ONE 2016, 11, e0160290. [Google Scholar] [CrossRef] [Green Version]
- Dhital, S.; Sherchand, J.B.; Pokharel, B.M.; Parajuli, K.; Mishra, S.K.; Sharma, S.; Kattel, H.P.; Khadka, S.; Khatiwada, S.; Rijal, B. Antimicrobial susceptibility pattern of Shigella spp. isolated from children under 5 years of age attending tertiary care hospitals, Nepal along with first finding of ESBL-production. BMC Res. Notes 2017, 10, 192. [Google Scholar] [CrossRef]
- Duong, V.T.; Tuyen, H.T.; Van Minh, P.; Campbell, J.I.; Le Phuc, H.; Nhu, T.D.H.; Tu, L.T.P.; Chau, T.T.H.; Nhi, L.T.Q.; Hung, N.T.; et al. No Clinical Benefit of Empirical Antimicrobial Therapy for Pediatric Diarrhea in a High-Usage, High-Resistance Setting. Clin. Infect. Dis. 2018, 66, 504. [Google Scholar] [CrossRef] [Green Version]
- Farajzadeh Sheikh, A.; Moradi Bandbal, M.; Saki, M. Emergence of multidrug-resistant Shigella species harboring extended-spectrum beta-lactamase genes in pediatric patients with diarrhea from southwest of Iran. Mol. Biol. Rep. 2020, 47, 7097–7106. [Google Scholar] [CrossRef]
- Ghosh, S.; Pazhani, G.P.; Chowdhury, G.; Guin, S.; Dutta, S.; Rajendran, K.; Bhattacharya, M.K.; Takeda, Y.; Niyogi, S.K.; Balakrish Nair, G.; et al. Genetic characteristics and changing antimicrobial resistance among Shigella spp. isolated from hospitalized diarrhoeal patients in Kolkata, India. J. Med. Microbiol. 2011, 60, 1460–1466. [Google Scholar] [CrossRef] [Green Version]
- Gu, B.; Xu, T.; Kang, H.; Xu, Y.; Liu, G.; Pan, S.; Qian, H.; Ma, P. A 10-year surveillance of antimicrobial susceptibility patterns in Shigella sonnei isolates circulating in Jiangsu Province, China. J. Glob. Antimicrob. Resist. 2017, 10, 29–34. [Google Scholar] [CrossRef]
- Iqbal, M.S.; Rahman, M.; Islam, R.; Banik, A.; Amin, M.B.; Akter, F.; Talukder, K.A. Plasmid-Mediated Sulfamethoxazole Resistance Encoded by the sul2 Gene in the Multidrug-Resistant Shigella flexneri 2a Isolated from Patients with Acute Diarrhea in Dhaka, Bangladesh. PLoS ONE 2014, 9, 85338. [Google Scholar] [CrossRef]
- Jain, P.; Kulkarni, R.; Dutta, S.; Ganavali, A.; Kalabhavi, A.; Shetty, P.; Shubhada, C.; Hosamani, M.; Appannanavar, S.; Hanamaraddi, D. Prevalence and antimicrobial profile of Shigella isolates in a tertiary care hospital of North Karnataka: A 12-year study. Indian J. Med. Microbiol. 2020, 38, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Jamal, W.; Rotimi, V.O.; Pal, T.; Sonnevend, A.; Dimitrov, T.S. Comparative in vitro activity of tigecycline and other antimicrobial agents against Shigella species from Kuwait and the United Arab of Emirates. J. Infect. Public Health 2010, 3, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jean, S.S.; Lu, M.C.; Shi, Z.Y.; Tseng, S.H.; Wu, T.S.; Lu, P.L.; Shao, P.L.; Ko, W.C.; Wang, F.-D.; Hsueh, P.R. In vitro activity of ceftazidime–avibactam, ceftolozane–tazobactam, and other comparable agents against clinically important Gram-negative bacilli: Results from the 2017 Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART). Infect. Drug Resist. 2018, 11, 1983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Oh, Y.; Jung, J.; Kim, S.; Kim, J.; Han, K.; Kim, M.; Park, S.; Lee, Y. Antimicrobial resistance patterns and characterization of integrons of Shigella sonnei isolates in Seoul, 1999–2008. J. Microbiol. 2010, 48, 236–242. [Google Scholar] [CrossRef]
- Jomezadeh, N.; Babamoradi, S.; Kalantar, E.; Javaherizadeh, H. Isolation and antibiotic susceptibility of Shigella species from stool samples among hospitalized children in Abadan, Iran. Gastroenterol. Hepatol. Bed Bench 2014, 7, 218. [Google Scholar]
- Kacmaz, B.; Unaldi, O.; Sultan, N.; Durmaz, R. Drug resistance profiles and clonality of sporadic Shigella sonnei isolates in Ankara, Turkey. Braz. J. Microbiol. 2014, 45, 845. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.; Wang, L.; Li, Y.; Lu, Y.; Fan, W.; Bi, R.; Qian, H.; Gu, B. Dissemination of Multidrug-Resistant Shigella flexneri and Shigella sonnei with Class 1, Class 2, and Atypical Class 1 Integrons in China. Microb. Drug Resist. 2019, 25, 1465–1474. [Google Scholar] [CrossRef]
- Kansakar, P.; Baral, P.; Malla, S.; Ghimire, G.R. Antimicrobial susceptibilities of enteric bacterial pathogens isolated in Kathmandu, Nepal, during 2002–2004. J. Infect. Dev. Ctries. 2011, 5, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Singh, P.; Ansari, M.; Asthana, A. Isolation of Shigella species and their resistance patterns to a panel of fifteen antibiotics in mid and far western region of Nepal. Asian Pac. J. Trop. Dis. 2014, 4, 30. [Google Scholar] [CrossRef]
- Lee, Y.L.; Lu, M.C.; Shao, P.L.; Lu, P.L.; Chen, Y.H.; Cheng, S.H.; Ko, W.C.; Lin, C.Y.; Wu, T.S.; Yen, M.Y.; et al. Nationwide surveillance of antimicrobial resistance among clinically important Gram-negative bacteria, with an emphasis on carbapenems and colistin: Results from the Surveillance of Multicenter Antimicrobial Resistance in Taiwan (SMART) in 2018. Int. J. Antimicrob. Agents 2019, 54, 318–328. [Google Scholar] [CrossRef]
- Liu, H.; Zhu, B.; Qiu, S.; Xia, Y.; Liang, B.; Yang, C.; Dong, N.; Li, Y.; Xiang, Y.; Wang, S.; et al. Dominant serotype distribution and antimicrobial resistance profile of Shigella spp. in Xinjiang, China. PLoS ONE 2018, 13, e0195259. [Google Scholar] [CrossRef] [Green Version]
- Maharjan, S.; Rayamajhee, B.; Shreshtha, A.; Acharya, J. Serotyping and antibiotic susceptibility patterns of Vibrio and Shigella isolates from diarrheal patients visiting a Tropical and Infectious Diseases Hospital in central Nepal. BMC Res. Notes 2017, 10, 626. [Google Scholar] [CrossRef] [PubMed]
- Mamishi, S.; Arab Yazdi, Z.; Mahmoudi, S.; Moradzadeh, M.; Taghi Haghi Ashtiani, M.; Pourakbari, B. Antimicrobial-resistance pattern of Shigella species in children: A six-year study in an Iranian referral Hospital. Ann. Ig. 2019, 31, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.Y.; Smith, B.L.; Bodhidatta, L.; Richard, S.A.; Vansith, K.; Thy, B.; Srijan, A.; Serichantalergs, O.; Mason, C.J. Etiology of diarrhea in young children and patterns of antibiotic resistance in Cambodia. Pediatr. Infect. Dis. J. 2011, 30, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Mohebi, S.; Nave, H.H.; Javadi, K.; Amanati, A.; Kholdi, S.; Hadadi, M.; Hashemizadeh, Z.; Motamedifar, M. Evaluate the distribution of virulence genes and to investigate antibiotic resistance pattern among Shigella species isolated from children with shigellosis in Iran. Gene Rep. 2021, 23, 101189. [Google Scholar] [CrossRef]
- Nandy, S.; Mitra, U.; Rajendran, K.; Dutta, P.; Dutta, S. Subtype prevalence, plasmid profiles and growing fluoroquinolone resistance in Shigella from Kolkata, India (2001–2007): A hospital-based study. Trop. Med. Int. Health 2010, 15, 1499–1507. [Google Scholar] [CrossRef]
- Nath, R.; Saikia, L.; Choudhury, G.; Sharma, D. Drug resistant Shigella flexneri in & around Dibrugarh, north-east India. Indian J. Med. Res. 2013, 137, 183. [Google Scholar]
- Özmert, E.N.; Ince, O.T.; Örün, E.; Yalçin, S.; Yurdakök, K.; Gür, D. Clinical characteristics and antibiotic resistance of Shigella gastroenteritis in Ankara, Turkey between 2003 and 2009, and comparison with previous reports. Int. J. Infect. Dis. 2011, 15, e849–e853. [Google Scholar] [CrossRef] [Green Version]
- Pakbin, B.; Didban, A.; Monfared, Y.K.; Mahmoudi, R.; Peymani, A.; Modabber, M.R. Antibiotic susceptibility and genetic relatedness of Shigella species isolated from food and human stool samples in Qazvin, Iran. BMC Res. Notes 2021, 14, 144. [Google Scholar] [CrossRef]
- Peleg, I.; Givon-Lavi, N.; Leibovitz, E.; Broides, A. Epidemiological trends and patterns of antimicrobial resistance of Shigella spp. isolated from stool cultures in two different populations in Southern Israel. Diagn. Microbiol. Infect. Dis. 2014, 78, 287–291. [Google Scholar] [CrossRef]
- Poramathikul, K.; Wojnarski, M.; Sok, S.; Sokh, V.; Chiek, S.; Seng, H.; Krang, S.; Ly, S.; Nou, S.; Chann, S.; et al. Update on Shigella and Nontyphoidal Salmonella Antimicrobial Drug Resistance: Implications on Empirical Treatment of Acute Infectious Diarrhea in Cambodia. Antimicrob. Agents Chemother. 2021, 65, e00671-21. [Google Scholar] [CrossRef] [PubMed]
- Pourakbari, B.; Charati, M.G.; Mahmoudi, S.; Abdolsalehi, M.R.; Sadeghi, R.H.; Mamishi, S. High frequency of antimicrobial resistance and virulence gene in Shigella species isolated from pediatric patients in an Iranian Referral Hospital. Acta Bio Med. Atenei Parm. 2022, 93, 2022027. [Google Scholar] [CrossRef]
- Prabhurajeshwar; Oli, A.K.; Ashajyothi, C.; Chandrakanth, R.K. Prevalence and antibiotic susceptibility pattern of fluoroquinolone resistant Shigella species isolated from infants stool in Gulbarga district, Karnataka, India. Asian Pac. J. Trop. Dis. 2015, 5, S116–S120. [Google Scholar] [CrossRef]
- Qu, F.; Bao, C.; Chen, S.; Cui, E.; Guo, T.; Wang, H.; Zhang, J.; Wang, H.; Tang, Y.W.; Mao, Y. Genotypes and antimicrobial profiles of Shigella sonnei isolates from diarrheal patients circulating in Beijing between 2002 and 2007. Diagn. Microbiol. Infect. Dis. 2012, 74, 166. [Google Scholar] [CrossRef] [PubMed]
- Qu, M.; Zhang, X.; Liu, G.; Huang, Y.; Jia, L.; Liang, W.; Li, X.; Wu, X.; Li, J.; Yan, H.; et al. An eight-year study of Shigella species in Beijing, China: Serodiversity, virulence genes, and antimicrobial resistance. J. Infect. Dev. Ctries. 2014, 8, 904–908. [Google Scholar] [CrossRef] [Green Version]
- Rajpara, N.; Nair, M.; Chowdhury, G.; Mukhopadhyay, A.K.; Ramamurthy, T.; Niyogi, S.K.; Bhardwaj, A.K. Molecular analysis of multidrug resistance in clinical isolates of Shigella spp. from 2001-2010 in Kolkata, India: Role of integrons, plasmids, and topoisomerase mutations. Infect. Drug Resist. 2018, 11, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Sadredinamin, M.; Shabani, M.; Karimi, A.; Sohrabi, M.R.; Karimi-Yazdi, M.; Ghalavand, Z.; Alebouyeh, M. Virulence genes expression profiling of different Shigella flexneri serotypes in response to sub-inhibitory concentrations of azithromycin and ciprofloxacin. Gut Pathog. 2022, 14, 10. [Google Scholar] [CrossRef]
- Sah, S.K.; Basnet, S.; Shrestha, S.; Ghale, K.; Tamang, S.; Mandal, D.K.; Pun, S.B. Burden of Shigella spp and Vibrio spp, and their antibiotic sensitivity pattern in the patients with acute gastroenteritis in tertiary care hospital in Nepal. BMC Res. Notes 2019, 12, 699. [Google Scholar] [CrossRef] [Green Version]
- Rizi, K.S.; Farsiani, H.; Sasan, M.S. High rate of resistance to ceftriaxone and azithromycin among Shigella spp. isolates at three children’s referral hospitals in Northeast Iran. J. Infect. Chemother. 2020, 26, 955–958. [Google Scholar] [CrossRef]
- Shakya, G.; Acharya, J.; Adhikari, S.; Rijal, N. Shigellosis in Nepal: 13 years review of nationwide surveillance. J. Health Popul. Nutr. 2016, 35, 36. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Qian, H.; Gong, J.; Deng, F.; Dong, C.; Zhou, L.; Guo, H. High Prevalence of Antibiotic Resistance and Molecular Characterization of Integrons among Shigella isolates in Eastern China. Antimicrob. Agents Chemother. 2013, 57, 1549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soltan Dallal, M.M.; Ranjbar, R.; Yaghoubi, S.; Rajabi, Z.; Aminharati, F.; Adeli Behrooz, H. Molecular epidemiology and genetic characterization of Shigella in pediatric patients in Iran. Le Infez. Med. 2018, 26, 321–328. [Google Scholar]
- Tajbakhsh, M.; García Migura, L.; Rahbar, M.; Svendsen, C.A.; Mohammadzadeh, M.; Zali, M.R.; Aarestrup, F.M.; Hendriksen, R.S. Antimicrobial-resistant Shigella infections from Iran: An overlooked problem? J. Antimicrob. Chemother. 2012, 67, 1128–1133. [Google Scholar] [CrossRef]
- Taneja, N.; Tiewsoh, J.B.A.; Gupta, S.; Mohan, B.; Verma, R.; Shankar, P.; Narayan, C.; Yadav, V.K.; Jayashree, M.; Singh, S. Antimicrobial resistance in Shigella species: Our five years (2015–2019) experience in a tertiary care center in north India. Indian J. Med. Microbiol. 2021, 39, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Teimourpour, R.; Babapour, B.; Esmaelizad, M.; Arzanlou, M.; Peeri-Doghaheh, H. Molecular characterization of quinolone resistant Shigella spp. isolates from patients in Ardabil, Iran. Iran. J. Microbiol. 2019, 11, 496. [Google Scholar] [CrossRef]
- Thompson, C.N.; Phan, M.V.T.; Van Minh Hoang, N.; Van Minh, P.; Vinh, N.T.; Thuy, C.T.; Nga, T.T.T.; Rabaa, M.A.; Duy, P.T.; Dung, T.T.N.; et al. A Prospective Multi-Center Observational Study of Children Hospitalized with Diarrhea in Ho Chi Minh City, Vietnam. Am. J. Trop. Med. Hyg. 2015, 92, 1045. [Google Scholar] [CrossRef] [PubMed]
- Urvashi; Saxena, S.; Dutta, R. Antimicrobial Resistance Pattern of Shigella species Over Five Years at a Tertiary-care Teaching Hospital in North India. J. Health Popul. Nutr. 2011, 29, 292. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Q.; Hao, R.; Zhang, Q.; Yao, S.; Han, J.; Ren, B.; Fan, T.; Chen, L.; Xu, X.; et al. Antimicrobial resistance and genetic characterization of Shigella spp. in Shanxi Province, China, during 2006–2016. BMC Microbiol. 2019, 19, 116. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Zhuang, L.; Kang, H.; Ma, P.; Xu, T.; Pan, S.; Gu, B. Prevalence, resistance patterns, and characterization of integrons of Shigella flexneri isolated from Jiangsu Province in China, 2001–2011. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1347–1353. [Google Scholar] [CrossRef]
- Yang, H.; Chen, G.; Zhu, Y.; Liu, Y.; Cheng, J.; Hu, L.; Ye, Y.; Li, J. Surveillance of antimicrobial susceptibility patterns among Shigella species isolated in China during the 7-year period of 2005–2011. Ann. Lab. Med. 2013, 33, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Yang, H.; Sun, W.; Duan, G.; Zhu, J.; Zhang, W.; Xi, Y.; Fan, Q. Serotype distribution and characteristics of antimicrobial resistance in Shigella isolated from Henan province, China, 2001–2008. Epidemiol. Infect. 2013, 141, 1946–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.L.; Liu, Q.Z.; Wang, J.; Chu, X.; Shen, L.M.; Guo, Y.Y. Epidemic and virulence characteristic of Shigella spp. with extended-spectrum cephalosporin resistance in Xiaoshan District, Hangzhou, China. BMC Infect. Dis. 2014, 14, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Pan, F.; Zhao, X.; Wang, G.; Tu, Y.; Fu, S.; Wang, J.; Pan, J.; Song, J.; Wang, W.; et al. Distribution and antimicrobial resistance of enteric pathogens in Chinese paediatric diarrhoea: A multicentre retrospective study, 2008–2013. Epidemiol. Infect. 2015, 143, 2512–2519. [Google Scholar] [CrossRef]
- Zhang, J.; Jin, H.; Hu, J.; Yuan, Z.; Shi, W.; Yang, X.; Xu, X.; Meng, J. Antimicrobial resistance of Shigella spp. from humans in Shanghai, China, 2004–2011. Diagn. Microbiol. Infect. Dis. 2014, 78, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.X.; Chen, H.Y.; Tu, L.H.; Xi, M.F.; Chen, M.; Zhang, J. Fluoroquinolone Resistance Mechanisms in Shigella isolates in Shanghai, China, Between 2010 and 2015. Microb. Drug Resist. 2019, 25, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Abbafati, C.; Abbas, K.M.; Abbasi-Kangevari, M.; Abd-Allah, F.; Abdelalim, A.; Abdollahi, M.; Abdollahpour, I.; Abegaz, K.H.; Abolhassani, H.; Aboyans, V.; et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2020, 396, 1204–1222. [Google Scholar] [CrossRef]
- Troeger, C.; Forouzanfar, M.; Rao, P.C.; Khalil, I.; Brown, A.; Reiner, R.C.; Fullman, N.; Thompson, R.L.; Abajobir, A.; Ahmed, M.; et al. Estimates of global, regional, and national morbidity, mortality, and aetiologies of diarrhoeal diseases: A systematic analysis for the Global Burden of Disease Study 2015. Lancet Infect. Dis. 2017, 17, 909. [Google Scholar] [CrossRef] [Green Version]
- Muthuirulandi Sethuvel, D.P.; Devanga Ragupathi, N.K.; Anandan, S.; Veeraraghavan, B. Update on: Shigella new serogroups/serotypes and their antimicrobial resistance. Lett. Appl. Microbiol. 2017, 64, 8–18. [Google Scholar] [CrossRef]
- Williams, E.; Lew, T.E.; Fuller, A.; Spelman, D.W.; Jenney, A.W. A case of multi-drug resistant ESBL-producing Shigella sonnei acute acalculous cholecystitis and gastroenteritis in a returned traveller. J. Travel Med. 2018, 25, tay029. [Google Scholar] [CrossRef]
- Barrantes, K.; Achí, R. The importance of integrons for development and propagation of resistance in Shigella: The case of Latin America. Braz. J. Microbiol. 2016, 47, 800–806. [Google Scholar] [CrossRef] [Green Version]
- Hussen, S.; Mulatu, G.; Yohannes Kassa, Z. Prevalence of Shigella species and its drug resistance pattern in Ethiopia: A systematic review and meta-analysis. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 22. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.R.; Reddy, V.; Hanson, H.; Johnson, K.M.; Tsoi, B.; Cokes, C.; Gallagher, L.; Lee, L.; Plentsova, A.; Dang, T.; et al. Antimicrobial resistance trends of Shigella serotypes in New York City, 2006–2009. Microb. Drug Resist. 2010, 16, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.Z.; Zuraina, N.M.N.N.; Hajissa, K.; Ilias, M.I.; Deris, Z.Z. Prevalence of Multidrug-Resistant Diarrheagenic Escherichia coli in Asia: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 1333. [Google Scholar] [CrossRef] [PubMed]
- Imrey, P.B. Limitations of Meta-analyses of Studies with High Heterogeneity. JAMA Netw. Open 2020, 3, e1919325. [Google Scholar] [CrossRef] [PubMed]
- Moradi, F.; Hadi, N.; Akbari, M.; Hashemizadeh, Z.; Jahromi, R.R. Frequency and Antimicrobial Resistance of Shigella species in Iran During 2000–2020. Jundishapur J. Health Sci. 2021, 13, 114902. [Google Scholar] [CrossRef]
- Chang, Z.; Lu, S.; Chen, L.; Jin, Q.; Yang, J. Causative Species and Serotypes of Shigellosis in Mainland China: Systematic Review and Meta-Analysis. PLoS ONE 2012, 7, e52515. [Google Scholar] [CrossRef]
- Salleh, M.Z.; Banga Singh, K.K.; Deris, Z.Z. Structural Insights into Substrate Binding and Antibiotic Inhibition of Enterobacterial Penicillin-Binding Protein 6. Life 2022, 12, 1022. [Google Scholar] [CrossRef]
- Ma, Q.; Huang, Y.; Wang, J.; Xu, X.; Hawkey, J.; Yang, C.; Liang, B.; Hu, X.; Wu, F.; Yang, X.; et al. Multidrug-resistant Shigella sonnei carrying the plasmid-mediated mcr-1 gene in China. Int. J. Antimicrob. Agents 2018, 52, 14–21. [Google Scholar] [CrossRef]
- Li, J.; Li, B.; Ni, Y.; Sun, J. Molecular characterization of the extended-spectrum beta-lactamase (ESBL)-producing Shigella spp. in Shanghai. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 447–451. [Google Scholar] [CrossRef]
- Gonzales, J.C.; Seribelli, A.A.; Gomes, C.N.; dos Prazeres Rodrigues, D.; Campioni, F.; Passaglia, J.; da Silva, P.; Falcão, J.P. A high number of multidrug-resistant and predominant genetically related cluster of Shigella flexneri strains isolated over 34 years in Brazil. Braz. J. Microbiol. 2020, 51, 1563–1571. [Google Scholar] [CrossRef]
- Abd-Elmeged, G.M.; Khairy, R.M.; Abo-Eloyoon, S.M.; Abdelwahab, S.F. Changing patterns of drug-resistant Shigella isolates in egypt. Microb. Drug Resist. 2015, 21, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Izquierdo, M.; Benavente-Fernández, A.; López-Gómez, J.; Láinez-Ramos-bossini, A.J.; Rodríguez-Camacho, M.; Valero-Ubierna, M.D.C.; Martín-Delosreyes, L.M.; Jiménez-Mejías, E.; Moreno-Roldán, E.; Lardelli-Claret, P.; et al. Prevalence of Multi-Resistant Microorganisms and Antibiotic Stewardship among Hospitalized Patients Living in Residential Care Homes in Spain: A Cross-Sectional Study. Antibiotics 2020, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Beyene, A.M.; Gezachew, M.; Mengesha, D.; Yousef, A.; Gelaw, B. Prevalence and drug resistance patterns of Gram-negative enteric bacterial pathogens from diarrheic patients in Ethiopia: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0265271. [Google Scholar] [CrossRef] [PubMed]
- Ingle, D.J.; Andersson, P.; Valcanis, M.; Barnden, J.; da Silva, A.G.; Horan, K.A.; Seemann, T.; Easton, M.; Williamson, D.A.; Sherry, N.L.; et al. Prolonged Outbreak of Multidrug-Resistant Shigella sonnei Harboring blaCTX-M-27 in Victoria, Australia. Antimicrob. Agents Chemother. 2020, 64, e01518-20. [Google Scholar] [CrossRef] [PubMed]
- Reuland, E.A.; Overdevest, I.T.M.A.; al Naiemi, N.; Kalpoe, J.S.; Rijnsburger, M.C.; Raadsen, S.A.; Ligtenberg-Burgman, I.; van der Zwaluw, K.W.; Heck, M.; Savelkoul, P.H.M.; et al. High prevalence of ESBL-producing Enterobacteriaceae carriage in Dutch community patients with gastrointestinal complaints. Clin. Microbiol. Infect. 2013, 19, 542–549. [Google Scholar] [CrossRef] [Green Version]
- Hajissa, K.; Marzan, M.; Idriss, M.I.; Islam, M.A. Prevalence of drug-resistant tuberculosis in Sudan: A systematic review and meta-analysis. Antibiotics 2021, 10, 932. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Viechtbauer, W. Conducting Meta-Analyses in R with the metafor Package. J. Stat. Softw. 2010, 36, 1–48. [Google Scholar] [CrossRef] [Green Version]
- Huedo-Medina, T.B.; Sánchez-Meca, J.; Marín-Martínez, F.; Botella, J. Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychol. Methods 2006, 11, 193–206. [Google Scholar] [CrossRef]



| No | Study ID (Author, Year) | Ref. | Study Period | Country | Sample Population | Age | Gender | Sample Size | Bacterial Species | Study Methods | Tested Antibiotics 1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Aggarwal, P., 2016 | [15] | 2009 to 2012 | India | 6339 | All age groups | ND | 121 | SF, SB, SS, SD | SAT, KB, PCR, DDST | AMP, SAM, GEN, AMK, DOX, CHL, SXT, NAL, CIP, OFX, CTR, CAZ, CTX, TZP, IMI, MEM, ETP |
| 2 | Ahmed, D., 2012 | [16] | 2005 to 2008 | Bangladesh | 14,428 | All age groups | ND | 2925 | SF, SB, SS, SD | KB | AMP, CIP, NAL, SXT |
| 3 | Alizadeh-Hesar, M., 2015 | [17] | 11/2012 to 10/2013 | Iran | 5291 | 0–5 years | ND | 70 | SS, SF, SB | SAT, PCR, KB | STR, SXT, TET, MIN, GEN, NAL, CIP |
| 4 | Aminshahidi, M., 2017 | [18] | 8/2014 to 2/2015 | Iran | 269 | 0–18 years | 141 M, 128 F | 41 | SF, SS | KB, PCR | MEM, CAZ, CTX, CTR, CIP, AMK, AMP, SXT, GEN |
| 5 | Behruznia, P., 2022 | [19] | 3/2017 to 9/2019 | Iran | ND | 0–14 years | 72 M, 48 F | 120 | SS, SF, SB | SAT, PCR, KB | AMP, NAL, CTX, CFM, MIN, CIP, LEV |
| 6 | Bhattacharya, D., 2015 | [20] | 2000 to 2011 | India | 1886 | 0–14 years | 1245 M, 641 F | 162 | SF, SB, SS, SD | KB, DDST | AMP, NAL, TET, NOR, SXT, CIP, OFX, CAR, AZI, AMK, CEP, CHL, CFX, GEN, CFM, CTR, CTX, CAZ, AMC, IMI |
| 7 | Bhattacharya, D., 2012 | [21] | 2006 to 2009 | India | 412 | 6 months to 14 years | ND | 50 | SF, SS, SD | KB, E-test, DDST | AMP, NAL, TET, CIP, OFX, SXT, NOR, CAR, AZI, AMK, CHL, GEN, LEX, CFX, CFM, CTR, CTX, CAZ, AMC, IMI |
| 8 | Bialvaei, A. Z., 2016 | [22] | 1/2012 to 12/2013 | Iran | ND | ND | ND | 52 | Shigella | KB, PCR, E-test, DDST | AMP, AMX, CEP, GEN, CAZ, TET, CIP, IMI |
| 9 | Chamoun, K., 2016 | [23] | 1/2011 to 12/2013 | Lebanon | ND | ND | ND | 164 | Shigella | KB, DDST | AMP, AMC, CTX, CTR, CPM, SXT, CIP, NOR |
| 10 | Cui, X., 2015 | [24] | 2005 to 2013 | China | ND | 0–4 years | ND | 40 | SF | Broth microdilution, PCR | CAZ, CTR, CPM, CFP, CFZ, FOX, IMI, PIP, AMP, TIC, TET, TOB, GEN, AMK, AZT, CHL, TCC, LEV, NOR, SXT |
| 11 | Das, A., 2016 | [25] | 2012 to 2015 | India | ND | ND | ND | 184 | SF, SB, SS, SD | KB, E-test, SAT, PCR | CIP, LEV, OFX |
| 12 | Dhital, S., 2017 | [26] | 1/2014 to 12/2014 | Nepal | 717 | 0–5 years | ND | 15 | SF, SS | KB, SAT, DDST, Agar dilution | AMX, NAL, SXT, CIP, TET, AZI, CFM, CAZ, CTR, SAM |
| 13 | Duong, V. T., 2018 | [27] | 5/2014 to 4/2016 | Vietnam | 3166 | 1 month to 15 years | 1945 M, 1221 F | 81 | Shigella | KB | NAL, CIP, CTR, CAZ, AMC, AMP, SXT, AZI, CHL, IMI |
| 14 | Farajzadeh Sheikh, A., 2020 | [28] | 3/2019 to 1/2020 | Iran | 1100 | 0–15 years | 650 M, 450 F | 79 | SF, SS | SAT, KB, PCR, DDST | IMI, SXT, AMP, FOX, CTR, CAZ, CTX, CPM, ERY, CIP, AMK, GEN |
| 15 | Ghosh, S., 2011 | [29] | 11/2007 to 10/2010 | India | 3262 | All age groups | ND | 212 | SF, SB, SS, SD | SAT, PCR, KB | SXT, NAL, TET, CIP, NOR, OFX, CHL, AMP, AZI, CTR |
| 16 | Gu, B., 2017 | [30] | 2006 to 2011 | China | ND | ND | ND | 319 | SS | SAT, KB, PCR | AMP, AMC, CEP, CTX, GEN, NAL, NOR, TET, SXT |
| 17 | Iqbal, M. S., 2014 | [31] | 2006 to 2011 | Bangladesh | ND | ND | ND | 200 | SF | KB, PCR, Agar dilution | SXT, NAL, AMP, ERY, TET, CIP, CTR |
| 18 | Jain, P. A., 2020 | [32] | 1/2006 to 12/2017 | India | 4578 | All age groups | 110 M, 79F | 189 | SF, SB, SS, SD | KB, SAT | AMP, SXT, CIP, CTR |
| 19 | Jamal, W., 2010 | [33] | 4/2003 to 3/2005 | Kuwait | ND | ND | ND | 42 | SF, SB, SS, SD | E-test, SAT | AMK, AMP, AMC, CTX, CTR, CFX, CHL, CIP, GEN, IMI, MEM, TZP, TET, SXT |
| 1/2003 to 12/2004 | United Arab Emirates | ND | ND | ND | 100 | ||||||
| 20 | Jean, S. S., 2018 | [34] | 1/2017 to 12/2017 | Taiwan | ND | ND | ND | 21 | SF, SS | Broth microdilution, PCR | AMP, CTR, ETP, CIP, LEV, SXT, AZI |
| 21 | Jin, Y. H., 2010 | [35] | 1999 to 2008 | South Korea | ND | ND | ND | 66 | SS | KB, PCR | AMP, SAM, CEP, STR, KAN, CTR, CIP, CHL, GEN, NAL, TET, SXT |
| 22 | Jomezadeh, N., 2014 | [36] | 6/2011 to 5/2013 | Iran | 705 | 0–5 years | 313 M, 392 F | 36 | SF, SB, SS, SD | KB, SAT | AMP, TET, SXT, NAL, CTR, CHL, CIP, CFM, GEN |
| 23 | Kacmaz, B., 2014 | [37] | 2004 to 2006 | Turkey | ND | 5–75 years | 23 M, 20 F | 43 | SS | KB, PCR, DDST | AMP, CHL, CIP, SAM, AMC, CTR, CFM, SXT |
| 24 | Kang, H., 2019 | [38] | 12/2010 | China | ND | ND | ND | 53 | SF, SS | KB, SAT, PCR | CAZ, AZT, AMP, AMX, PIP, CPM, AMC, IMI, MEM, MIN, CHL, TET, GEN, CTX, CIP, LEV, SXT |
| 25 | Kansakar, P., 2011 | [39] | 1/2002 to 12/2004 | Nepal | 877 | All age groups | ND | 41 | Shigella | KB | AMP, CIP, NAL, SXT, CTR, MEC |
| 26 | Khan, S., 2014 | [40] | 9/2011 to 3/2013 | Nepal | 458 | All age groups | 36 M, 39 F | 65 | SF, SB, SS, SD | KB, SAT | AMK, AMP, AMC, CTX, CAZ, CTR, CHL, CIP, SXT, DOX, GEN, IMI, NAL, NOR, OFX |
| 27 | Lee, Y. L., 2019 | [41] | 2018 | Taiwan | 1184 | ND | ND | 18 | SF | Broth microdilution, PCR | SAM, CTX, CAZ, CPM, TZP, ETP, IMI, MEM, CIP, LEV, SXT |
| 28 | Liu, H., 2018 | [42] | 2008 to 2014 | China | ND | All age groups | 252 M, 206 F | 458 | SF, SS | Broth microdilution, PCR | AMK, AMP, AZT, CFZ, CPM, CFP, FOX, CAZ, CTR, CHL, GEN, IMI, LEV, NIT, NOR, PIP, SXT, TET, TIC, TCC, TOB |
| 29 | Maharjan, S., 2017 | [43] | 6/2014 to 12/2014 | Nepal | 650 | All age groups | 28 M, 22 F | 29 | SF, SB, SS, SD | KB, SAT | AMP, SXT, NAL, CTX, CHL, CIP, OFX |
| 30 | Mamishi, S., 2019 | [44] | 3/2011 to 3/2016 | Iran | 46,795 | 3–7 years | 290 M, 283 F | 573 | SF, SB, SS, SD | KB, SAT | AMP, CTX, SXT, NAL, GEN, AMK, CIP |
| 31 | Meng, C. Y., 2011 | [45] | 11/2004 to 10/2006 | Cambodia | 1178 | 3 months to 5 years | 664 M, 514 F | 41 | Shigella | KB | AZI, ERY, NAL, CIP, AMP, GEN, SXT, TET |
| 32 | Mohebi, S., 2021 | [46] | 10/2018 to 3/2019 | Iran | 309 | 0–18 years | 162 M, 147 F | 73 | SF, SS, SB | KB, SAT, PCR | AMP, SXT, NAL, CTR, CTX, AZI, CFM, AZT, CHL, CAZ, CIP, SAM, AMK, IMI, GEN |
| 33 | Nandy, S., 2010 | [47] | 1/2001 to 12/2007 | India | 4478 | 0–5 years | 273 M, 243 F | 516 | SF, SB, SS, SD | KB, SAT | AMP, TET, CHL, SXT, NAL, CIP, NOR, GEN, AMK, CTX, OFX |
| 34 | Nath, R., 2013 | [48] | 1/2008 to 11/2010 | India | 1411 | All age groups | ND | 62 | SF | KB, SAT, DDST | AMP, SXT, TET, CHL, NAL, CIP, NOR, OFX, CTR, AZI, CTX, IMI, GEN, AMK, AMC, SAM, FUR, TZP |
| 35 | Ozmert, E. N., 2011 | [49] | 2003 to 2009 | Turkey | ND | All age groups | 136 M, 102 F | 238 | SF, SS, SB | KB, SAT | SXT, NAL, AMP, CIP |
| 36 | Pakbin, B., 2021 | [50] | 11/2019 to 7/2020 | Iran | 453 | 2–5 years | 242 M, 211 F | 35 | SF, SS, SB | KB, PCR | IMI, AMP, TET, AMK, CHL, NAL, AZI |
| 37 | Peleg, I., 2014 | [51] | 2005 to 2009 | Israel | ND | ND | ND | 3295 | SF, SB, SS, SD | KB | AMP, SXT, NAL, CTR, OFX |
| 38 | Poramathikul, K., 2021 | [52] | 7/2014 to 6/2019 | Cambodia | 1985 | All age groups | ND | 55 | SF, SS | KB, DDST | AMP, AZI, CTX, CAZ, CTR, CIP, NAL, SXT, TET |
| 39 | Pourakbari, B., 2022 | [53] | 9/2018 to 2/2020 | Iran | 7121 | 1–16 years | 93 M, 90 F | 183 | SF, SS | KB, PCR | NAL, AMK, AMP, GEN, CTX, CIP, SXT |
| 40 | Prabhurajeshwar,, 2015 | [54] | 7/2013 to 3/2014 | India | 334 | 1 month to 5 years | 26 M, 17 F | 43 | SF, SS, SD | KB | AMP, AMK, GEN, IMI, TET, LEX, CPM, CIP, CTR, SXT, CAZ, NAL, CTX, CHL, OFX, OXA |
| 41 | Qu, F., 2012 | [55] | 1/2002 to 12/2007 | China | ND | All age groups | 197 M, 165 F | 362 | SS | KB, SAT | AMP, PIP, CTR, CPM, CIP, NOR, OFX, LEV, CHL, SXT, FOS |
| 42 | Qu, M., 2014 | [56] | 2004 to 2011 | China | ND | ND | ND | 1652 | SF, SB, SS, SD | KB, PCR | NAL, TET, AMP, SXT, GEN, CEP, CFZ, AMC, CTX, CIP, NOR, OFX |
| 43 | Rajpara, N., 2018 | [57] | 2001 to 2010 | India | ND | ND | ND | 95 | SF, SB, SS, SD | KB | AMP, AZI, CTR, CHL, CIP, SXT, CFX, GEN, KAN, NAL, NOR, STR, TET, TMP |
| 44 | Sadredinamin, M., 2022 | [58] | 3/2016 to 9/2018 | Iran | ND | 2 months to 14 years | 185 M, 148 F | 333 | SF, SS, SB | KB, SAT, PCR | AMP, CTX, CPM, SXT, AZI, CIP, NAL, TET, MIN |
| 45 | Sah, S. K., 2019 | [59] | 4/2016 to 9/2017 | Nepal | 153 | Above 14 years | 80 M, 73 F | 17 | SF, SS | KB | TET, CIP, SXT, CHL, NAL, NOR, AMP, OFX, CTR |
| 46 | Salimiyan Rizi, K., 2020 | [60] | 2/2018 to 9/2019 | Iran | 233 | 0–14 years | ND | 94 | SF, SS, SD | KB, SAT | AZI, CTR, CIP, SXT, NAL, GEN, AMX, AMP, DOX, CFM |
| 47 | Shakya, G., 2016 | [61] | 2003 to 2015 | Nepal | ND | All age groups | ND | 332 | SF, SB, SS, SD | KB | AMP, CIP, NAL, SXT, MEC, CTR |
| 48 | Shen, Y., 2013 | [62] | 1/2008 to 11/2010 | China | ND | 1–88 years | ND | 716 | SF, SB, SS, SD | KB, SAT | AMP, AMC, CEP, CTX, GEN, NAL, NOR, TET, SXT |
| 49 | Soltan Dallal, M. M., 2018 | [63] | 5/2015 to 10/2016 | Iran | 946 | Pediatrics | ND | 75 | SF, SB, SS, SD | KB, DDST, SAT, PCR | GEN, IMI, CHL, NAL, CIP, TET, AMP, SXT, CTX, CAZ, CTR, AZI |
| 50 | Tajbakhsh, M., 2012 | [64] | 9/2008 to 3/2010 | Iran | 848 | 2–56 years | ND | 37 | SF, SB, SS, SD | KB, SAT, PCR | AMC, AMP, TIO, CHL, CIP, GEN, CTX, NAL, TET, TMP |
| 51 | Taneja, N., 2021 | [65] | 2015 to 2019 | India | 10,456 | All age groups | 82 M, 55 F | 137 | SF, SB, SS, SD | KB, SAT | AMP, AMX, CTR, CTX, CIP, SXT, AMK, GEN, CHL, TET |
| 52 | Teimourpour, R., 2019 | [66] | 2015 to 2017 | Iran | 1280 | 0–10 years | ND | 113 | SF, SB, SS, SD | KB, PCR | CIP, NAL, NOR, PER, GEN, CTR, SXT, IMI, AMK, AZI |
| 53 | Thompson, C. N., 2015 | [67] | 5/2009 to 4/2010 | Vietnam | 1419 | 0–5 years | 908 M, 511 F | 48 | SF, SS | KB, SAT, DDST | AMP, AMC, CAZ, CIP, GAT, OFX, CHL, TMP, NAL, CTR |
| 54 | Ud-Din, A. I., 2013 | [4] | 2001 to 2011 | Bangladesh | ND | All age groups | ND | 200 | SS | KB, SAT, PCR | AMP, STR, TET, CIP, NAL, MEC, SXT, CTR, CTX, CAZ, IMI |
| 55 | Urvashi, 2011 | [68] | 2004 to 2008 | India | 12,983 | All age groups | ND | 106 | SF, SB, SS, SD | SF, SB, SS, SD | AMP, SXT, TET, CHL, GEN, NAL, CIP, FUR, CTX, CTR |
| 56 | Wang, Y., 2019 | [69] | 2006 to 2016 | China | ND | All age groups | 278 M, 196 F | 474 | SF, SS | E-test, SAT, PCR | CAZ, CTR, CPM, CFP, CFZ, FOX, IMI, NIT, PIP, AMP, TIC, TET, TOB, GEN, AMK, CHL, TCC, LEV, NOR, SXT, AZT |
| 57 | Xu, Y., 2016 | [70] | 1/2001 to 12/2011 | China | ND | ND | ND | 624 | SF | KB, PCR | AMP, AMC, CEP, CTX, GEN, NAL, NOR, TET, SXT |
| 58 | Yang, H., 2013a | [71] | 2005 to 2011 | China | ND | All age groups | 163 M, 145 F | 308 | SF, SB, SS, SD | Agar dilution | AMP, PIP, CTX, CTR, CAZ, CPM, FOX, AZT, NAL, CIP, LEV, NOR, GAT, GEN, AMK, CHL, SXT, TET, IMI |
| 59 | Yang, H., 2013b | [72] | 2001 to 2008 | China | 2485 | All age groups | 287 M, 239 F | 526 | SF, SS | KB, SAT | CFZ, CTX, AMC, AMP, GEN, TOB, CHL, TET, SXT, NAL, PIM, CIP, FUR |
| 60 | Zhang, C. L., 2014 | [73] | 1/2008 to 12/2012 | China | ND | All age groups | ND | 356 | SF, SS | KB, PCR | AMP, PIP, SAM, TZP, CAZ, CPM, FOX, IMI, SXT, CIP, LEV |
| 61 | Zhang, H., 2015 | [74] | 1/2008 to 12/2013 | China | 1577 | 0–14 years | 897 M, 680 F | 207 | SF, SB, SS, SD | KB, SAT | AMP, SAM, CTR, CIP, SXT, CHL |
| 62 | Zhang, J., 2014 | [75] | 1/2004 to 12/2011 | China | 77,600 | All age groups | ND | 1638 | SF, SS, SB | KB, SAT | TET, TMP, SXT, AMP, AMC, CPM, CTX, CAZ, NAL, CIP, OFX, GEN, STR, CHL |
| 63 | Zhang, W. X., 2019 | [76] | 2010 to 2015 | China | ND | ND | ND | 402 | SF, SS | KB, PCR | AMP, CTX, CPM, STR, CHL, AMC, TET, NAL, CIP, NOR, LEV, FOX, SXT |
| Subgroup | Prevalence (%) [95% CIs 1] | No. of Studies | Sample Size (Shigella Isolates) | Sample Population | I2 | p-Value |
|---|---|---|---|---|---|---|
| Regions | ||||||
| East Asia | 9.5 [0.2–18.7] | 4 | 2389 | 82,846 | 100% | <0.01 |
| Middle East | 10.2 [4.0–16.3] | 12 | 1409 | 65,350 | 98% | <0.01 |
| South Asia | 7.4 [4.7–10.2] | 16 | 4690 | 63,422 | 100% | 0 |
| Southeast Asia | 2.9 [2.5–3.3] | 4 | 225 | 7748 | 20% | 0.29 |
| Countries | ||||||
| Bangladesh | 20.3 [19.6–20.9] | 1 | 2925 | 14,428 | NA 2 | NA |
| Cambodia | 3.0 [2.4–3.7] | 2 | 96 | 3163 | 16% | 0.27 |
| China | 12.1 [1.3–23.0] | 3 | 2371 | 81,662 | 100% | <0.01 |
| India | 6.2 [3.4–9.0] | 10 | 1598 | 46,139 | 99% | <0.01 |
| Iran | 10.2 [4.0–16.3] | 12 | 1409 | 65,350 | 98% | <0.01 |
| Nepal | 7.0 [2.6–11.4] | 5 | 167 | 2855 | 93% | <0.01 |
| Taiwan | 1.5 [0.9–2.4] | 1 | 18 | 1184 | NA | NA |
| Vietnam | 2.9 [2.1–3.7] | 2 | 129 | 4585 | 55% | 0.14 |
| Antibiotics | Prevalence (%) [95% CIs 1] | No. of Resistant Isolates | No. of Studies | I2 | p-Value |
|---|---|---|---|---|---|
| First Generation Cephalosporins | |||||
| Cephalothin (CEP) | 33.6 [10.7–56.5] | 780 | 7 | 100% | 0 |
| Cefazolin (CFZ) | 20.4 [11.8–28.9] | 452 | 5 | 97% | <0.01 |
| Cefalexin (LEX) | 56.4 [0.0–100.0] | 40 | 2 | 99% | <0.01 |
| Second Generation Cephalosporins | |||||
| Cefuroxime (CFX) | 10.9 [2.0–19.7] | 44 | 4 | 94% | <0.01 |
| Cefoxitin (FOX) | 1.8 [0.4–3.1] | 36 | 7 | 78% | <0.01 |
| Third Generation Cephalosporins | |||||
| Ceftazidime (CAZ) | 20.3 [8.8–31.7] | 464 | 23 | 100% | 0 |
| Cefixime (CFM) | 27.6 [7.6–47.7] | 199 | 8 | 99% | <0.01 |
| Cefoperazone (CFP) | 20.0 [9.8–30.2] | 162 | 3 | 79% | <0.01 |
| Ceftriaxone (CTR) | 23.8 [16.1–31.6] | 1035 | 39 | 98% | 0 |
| Cefotaxime (CTX) | 28.6 [19.7–37.5] | 1938 | 34 | 99% | 0 |
| Ceftiofur (TIO) | 21.6 [9.8–38.2] | 8 | 1 | NA 2 | NA |
| Fourth Generation Cephalosporins | |||||
| Cefepime (CPM) | 19.2 [6.4–32.1] | 645 | 14 | 99% | <0.01 |
| Aminoglycosides | |||||
| Amikacin (AMK) | 15.9 [6.9–24.8] | 234 | 20 | 95% | <0.01 |
| Gentamicin (GEN) | 21.7 [14.7–28.6] | 2494 | 36 | 99% | 0 |
| Kanamycin (KAN) | 51.9 [0.8–100.0] | 91 | 2 | 98% | <0.01 |
| Streptomycin (STR) | 98.4 [96.9–100.0] | 2279 | 6 | 78% | <0.01 |
| Tobramycin (TOB) | 4.7 [0.7–8.6] | 91 | 4 | 91% | <0.01 |
| Carbapenems | |||||
| Ertapenem (ETP) | 0.0 [0.0–1.1] | 0 | 3 | 0% | 1.00 |
| Imipenem (IMI) | 0.1 [0.0–0.2] | 45 | 21 | 80% | <0.01 |
| Meropenem (MEM) | 0.0 [0.0–0.7] | 0 | 5 | 0% | 1.00 |
| Macrolides | |||||
| Azithromycin (AZI) | 29.2 [20.8–37.6] | 389 | 17 | 95% | <0.01 |
| Erythromycin (ERY) | 57.4 [51.6–63.2] | 160 | 2 | 0% | 0.73 |
| Monobactams | |||||
| Aztreonam (AZT) | 15.0 [5.6–24.5] | 172 | 6 | 94% | <0.01 |
| Penicillins | |||||
| Amoxicillin + clavulanic acid (AMC) | 23.4 [12.4–34.4] | 1687 | 17 | 99% | 0 |
| Amoxicillin (AMX) | 73.2 [52.4–94.1] | 240 | 5 | 98% | <0.01 |
| Ampicillin (AMP) | 72.6 [66.4–78.7] | 12,667 | 57 | 99% | 0 |
| Ampicillin + sulbactam (SAM) | 27.5 [15.1–39.9] | 307 | 9 | 97% | <0.01 |
| Carbenicillin (CAR) | 49.9 [15.4–84.5] | 87 | 2 | 95% | <0.01 |
| Mecillinam (MEC) | 37.8 [8.8–66.7] | 191 | 3 | 98% | <0.01 |
| Oxacillin (OXA) | 71.9 [53.3–86.3] | 23 | 1 | NA | NA |
| Piperacillin (PIP) | 43.5 [22.8–64.2] | 740 | 7 | 99% | <0.01 |
| Piperacillin + tazobactam (TZP) | 0.2 [0.0–0.6] | 1 | 5 | 0% | 0.98 |
| Ticarcillin (TIC) | 90.5 [84.7–96.3] | 884 | 3 | 89% | <0.01 |
| Ticarcillin + clavulanic acid (TCC) | 11.7 [0.0–27.1] | 55 | 3 | 94% | <0.01 |
| Phenicols | |||||
| Chloramphenicol (CHL) | 36.3 [27.0–45.6] | 2905 | 32 | 100% | 0 |
| Quinolones | |||||
| Ciprofloxacin (CIP) | 29.8 [22.4–37.1] | 2476 | 54 | 99% | 0 |
| Gatifloxacin (GAT) | 4.6 [0.0–13.9] | 29 | 2 | 95% | <0.01 |
| Levofloxacin (LEV) | 14.7 [6.1–23.3] | 212 | 12 | 92% | <0.01 |
| Nalidixic acid (NAL) | 68.0 [58.6–77.4] | 9656 | 44 | 100% | 0 |
| Norfloxacin (NOR) | 24.4 [14.4–34.4] | 1287 | 20 | 99% | 0 |
| Ofloxacin (OFX) | 31.3 [16.9–45.7] | 839 | 18 | 99% | 0 |
| Tetracyclines | |||||
| Doxycycline (DOX) | 48.0 [7.0–89.0] | 154 | 3 | 99% | <0.01 |
| Minocycline (MIN) | 57.1 [23.5–90.6] | 202 | 4 | 98% | <0.01 |
| Tetracycline (TET) | 78.3 [70.4–86.3] | 7037 | 35 | 99% | 0 |
| Others | |||||
| Fosfomycin (FOS) | 1.4 [0.4–3.2] | 5 | 1 | NA | NA |
| Furazolidone (FUR) | 9.1 [0.0–23.9] | 21 | 3 | 92% | <0.01 |
| Nitrofurantoin (NIT) | 0.0 [0.0–0.2] | 0 | 2 | 0% | 1.00 |
| Sulfamethoxazole + trimethoprim (SXT) | 78.6 [74.4–82.7] | 13,991 | 56 | 98% | 0 |
| Trimethoprim (TMP) | 95.5 [92.3–98.8] | 1780 | 4 | 55% | 0.08 |
| Subgroup | Prevalence (%) [95% CIs 1] | No. of MDR 2 Isolates | Total Shigella Isolates | No. of Studies | I2 | p-Value | |
|---|---|---|---|---|---|---|---|
| Regions | |||||||
| East Asia | 73.2 [58.5–87.9] | 4756 | 5755 | 13 | 100% | 0 | |
| Middle East | 54.3 [38.1–70.4] | 537 | 1107 | 13 | 99% | <0.01 | |
| South Asia | 77.7 [66.1–89.3] | 1348 | 1794 | 10 | 98% | <0.01 | |
| Southeast Asia | 83.4 [53.8–100.0] | 109 | 136 | 2 | 97% | <0.01 | |
| Countries | |||||||
| Bangladesh | 94.0 [89.8–96.9] | 188 | 200 | 1 | NA 3 | NA | |
| Cambodia | 98.2 [90.3–100.0] | 54 | 55 | 1 | NA | NA | |
| China | 76.3 [61.8–90.8] | 4733 | 5689 | 12 | 100% | 0 | |
| India | 79.6 [66.8–92.4] | 955 | 1197 | 7 | 97% | <0.01 | |
| Iran | 58.4 [38.7–78.1] | 410 | 731 | 11 | 99% | <0.01 | |
| Kuwait * | 50.0 [34.2–65.8] | 21 | 42 | 1 | NA | NA | |
| Nepal | 62.8 [29.6–96.0] | 205 | 397 | 2 | 97% | <0.01 | |
| South Korea | 34.8 [23.5–47.6] | 23 | 66 | 1 | NA | NA | |
| Turkey | 28.6 [22.9–34.9] | 67 | 234 | 1 | NA | NA | |
| U.A.E * | 39.0 [29.4–49.3] | 39 | 100 | 1 | NA | NA | |
| Vietnam | 67.9 [56.6–77.8] | 55 | 81 | 1 | NA | NA | |
| Subgroup | Prevalence (%) [95% CIs 1] | No. of ESBL 2 Isolates | Total Shigella Isolates | No. of Studies | I2 | p-Value | |
|---|---|---|---|---|---|---|---|
| Regions | |||||||
| East Asia | 31.0 [0.9–61.0] | 218 | 870 | 3 | 99% | <0.01 | |
| Middle East | 20.7 [6.7–34.7] | 84 | 427 | 7 | 93% | <0.01 | |
| South Asia | 9.7 [2.3–17.1] | 31 | 248 | 4 | 79% | <0.01 | |
| Southeast Asia | 52.5 [6.8–98.3] | 63 | 117 | 2 | 97% | <0.01 | |
| Countries | |||||||
| Cambodia | 29.1 [17.6–42.9] | 16 | 55 | 1 | NA 3 | NA | |
| China | 31.0 [0.9–61.0] | 218 | 870 | 3 | 99% | <0.01 | |
| India | 10.5 [1.2–19.8] | 30 | 233 | 3 | 86% | <0.01 | |
| Iran | 26.3 [8.3–44.3] | 75 | 284 | 5 | 93% | <0.01 | |
| Nepal | 6.7 [0.2–31.9] | 1 | 15 | 1 | NA | NA | |
| Turkey | 11.6 [3.9–25.1] | 5 | 43 | 1 | NA | NA | |
| U.A.E | 4.0 [1.1–9.9] | 4 | 100 | 1 | NA | NA | |
| Vietnam | 75.8 [63.3–85.8] | 47 | 62 | 1 | NA | NA | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salleh, M.Z.; Nik Zuraina, N.M.N.; Hajissa, K.; Ilias, M.I.; Banga Singh, K.K.; Deris, Z.Z. Prevalence of Multidrug-Resistant and Extended-Spectrum Beta-Lactamase-Producing Shigella Species in Asia: A Systematic Review and Meta-Analysis. Antibiotics 2022, 11, 1653. https://doi.org/10.3390/antibiotics11111653
Salleh MZ, Nik Zuraina NMN, Hajissa K, Ilias MI, Banga Singh KK, Deris ZZ. Prevalence of Multidrug-Resistant and Extended-Spectrum Beta-Lactamase-Producing Shigella Species in Asia: A Systematic Review and Meta-Analysis. Antibiotics. 2022; 11(11):1653. https://doi.org/10.3390/antibiotics11111653
Chicago/Turabian StyleSalleh, Mohd Zulkifli, Nik Mohd Noor Nik Zuraina, Khalid Hajissa, Mohamad Ikram Ilias, Kirnpal Kaur Banga Singh, and Zakuan Zainy Deris. 2022. "Prevalence of Multidrug-Resistant and Extended-Spectrum Beta-Lactamase-Producing Shigella Species in Asia: A Systematic Review and Meta-Analysis" Antibiotics 11, no. 11: 1653. https://doi.org/10.3390/antibiotics11111653
APA StyleSalleh, M. Z., Nik Zuraina, N. M. N., Hajissa, K., Ilias, M. I., Banga Singh, K. K., & Deris, Z. Z. (2022). Prevalence of Multidrug-Resistant and Extended-Spectrum Beta-Lactamase-Producing Shigella Species in Asia: A Systematic Review and Meta-Analysis. Antibiotics, 11(11), 1653. https://doi.org/10.3390/antibiotics11111653










