Synthesis and Antimicrobial Activity of Sulfenimines Based on Pinane Hydroxythiols
Abstract
1. Introduction
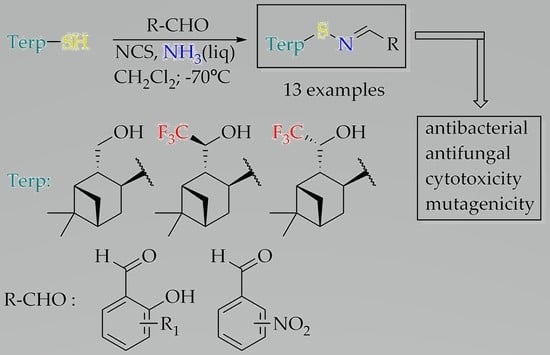
2. Results and Discussion
2.1. Synthesis of Sulfenimines from Pinane Hydroxythiols
2.2. Antimicrobial Activity
3. Conclusions
4. Materials and Methods
4.1. General Information
4.2. General Procedure for the Synthesis of Sulfenimines
4.3. Antibacterial Activity
4.4. Antifungal Activity
4.5. Mutagenicity and Cytotoxicity
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular mechanisms of antibiotic resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Artemova, N.P.; Startseva, V.A. Natural and Thiomodified Monoterpenoids (Russian Edition); LAP LAMBERT: Saarbrücken, Germany, 2011; ISBN 978-3-8484-3023-9. [Google Scholar]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L.; Leach, D.N. The Role of Structure and Molecular Properties of Terpenoids in Determining Their Antimicrobial Activity. Flavour Fragr. J. 1999, 14, 322–332. [Google Scholar] [CrossRef]
- Griffin, S.G.; Wyllie, S.G.; Markham, J.L. Antimicrobially Active Terpenes Cause K+ Leakage in E. coli Cells. J. Essent. Oil Res. 2005, 17, 686–690. [Google Scholar] [CrossRef]
- Gavrilov, V.V.; Startseva, V.; Nikitina, L.; Lodochnikova, O.A.; Gnezdilov, O.; Lisovskaya, S.; Glushko, N.; Klimovitskii, E.N. Synthesis and antifungal activity of sulfides, sulfoxides, and sulfones based on (1S)-(-)-β-pinene. Pharm. Chem. J. 2010, 44, 126–129. [Google Scholar] [CrossRef]
- Mancuso, M.; Catalfamo, M.; Laganà, P.; Rappazzo, A.C.; Raymo, V.; Zampino, D.; Zaccone, R. Screening of antimicrobial activity of citrus essential oils against pathogenic bacteria and Candida strains. Flavour Fragr. J. 2019, 34, 187–200. [Google Scholar] [CrossRef]
- Patil, S.P.; Kumbhar, S.T. Evaluation of terpene-rich extract of Lantana camara L. leaves for antimicrobial activity against mycobacteria using Resazurin Microtiter Assay (REMA). Beni-Suef Univ. J. Basic Appl. Sci. 2018, 7, 511–515. [Google Scholar] [CrossRef]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial Activity of Terpenes and Terpenoids Present in Essential Oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef]
- Kifer, D.; Mužinić, V.; Klarić, M.Š. Antimicrobial Potency of Single and Combined Mupirocin and Monoterpenes, Thymol, Menthol and 1,8-Cineole against Staphylococcus Aureus Planktonic and Biofilm Growth. J. Antibiot. 2016, 69, 689–696. [Google Scholar] [CrossRef]
- Cordeiro, L.; Figueiredo, P.; Souza, H.; Sousa, A.; Andrade-Júnior, F.; Barbosa-Filho, J.; Lima, E. Antibacterial and Antibiofilm Activity of Myrtenol against Staphylococcus aureus. Pharmaceuticals 2020, 13, 133. [Google Scholar] [CrossRef]
- Selvaraj, A.; Valliammai, A.; Sivasankar, C.; Suba, M.; Sakthivel, G.; Pandian, S.K. Antibiofilm and Antivirulence Efficacy of Myrtenol Enhances the Antibiotic Susceptibility of Acinetobacter Baumannii. Sci. Rep. 2020, 10, 21975. [Google Scholar] [CrossRef]
- Zacchino, S.A.; Butassi, E.; Cordisco, E.; Svetaz, L.A. Hybrid combinations containing natural products and antimicrobial drugs that interfere with bacterial and fungal biofilms. Phytomedicine 2017, 37, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Sofronov, A.V.; Nizamov, I.S.; Almetkina, L.A.; Nikitina, L.E.; Fatyhova, D.G.; Zelenikhin, P.V.; Il’Inskaya, O.N.; Cherkasov, R.A. Monoterpenoids dithiophosphates. Synthesis and biological activity. Russ. J. Gen. Chem. 2010, 80, 1267–1271. [Google Scholar] [CrossRef]
- Nizamov, I.S.; Al’metkina, L.A.; Gabdullina, G.T.; Shamilov, R.R.; Sofronov, A.V.; Nikitina, L.E.; Lisovskaya, S.A.; Glushko, N.I.; Cherkasov, R.A. Chiral Phosphorus Dithio Acids Derived from (1S,2S,3S,5R)-(+)-Isopinocampheol. Synthesis and Fungicidal Activity. Russ. Chem. Bull. 2012, 61, 2370–2371. [Google Scholar] [CrossRef]
- Sikkema, J.; de Bont, J.A.; Poolman, B. Mechanisms of Membrane Toxicity of Hydrocarbons. Microbiol. Rev. 1995, 59, 201–222. [Google Scholar] [CrossRef]
- Uribe, S.; Pena, A. Toxicity of allelopathic monoterpene suspensions on yeast dependence on droplet size. J. Chem. Ecol. 1990, 16, 1399–1408. [Google Scholar] [CrossRef] [PubMed]
- Šturm, L.; Poklar Ulrih, N. Propolis Flavonoids and Terpenes, and Their Interactions with Model Lipid Membranes: A Review. In Advances in Biomembranes and Lipid Self-Assembly; Elsevier: Amsterdam, The Netherlands, 2020; Volume 32, pp. 25–52. ISBN 978-0-12-820968-4. [Google Scholar]
- Nogueira, J.O.E.; Campolina, G.A.; Batista, L.R.; Alves, E.; Caetano, A.R.S.; Brandão, R.M.; Nelson, D.L.; Cardoso, M.D.G. Mechanism of Action of Various Terpenes and Phenylpropanoids against Escherichia coli and Staphylococcus aureus. FEMS Microbiol. Lett. 2021, 368, fnab052. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.-K.; Yusoff, K.; Ajat, M.; Yap, W.-S.; Lim, S.-H.E.; Lai, K.-S. Antimicrobial Activity and Mode of Action of Terpene Linalyl Anthranilate against Carbapenemase-Producing Klebsiella Pneumoniae. J. Pharm. Anal. 2021, 11, 210–219. [Google Scholar] [CrossRef]
- Nikitina, L.E.; Artemova, N.P.; Startseva, V.A.; Fedyunina, I.V.; Klochkov, V.V. Biological Activity of S-Containing Monoterpenoids. Chem. Nat. Compd. 2017, 53, 811–819. [Google Scholar] [CrossRef]
- Mendanha, S.; Moura, S.S.; Anjos, J.L.; Valadares, M.C.; Alonso, A. Toxicity of terpenes on fibroblast cells compared to their hemolytic potential and increase in erythrocyte membrane fluidity. Toxicol. In Vitro 2013, 27, 323–329. [Google Scholar] [CrossRef]
- Grebyonkina, O.N.; Lezina, O.M.; Izmest’Ev, E.S.; Sudarikov, D.V.; Pestova, S.V.; Rubtsova, S.A.; Kutchin, A.V. Synthesis of new monoterpene sulfonic acids and their derivatives. Russ. J. Org. Chem. 2017, 53, 860–868. [Google Scholar] [CrossRef]
- Sudarikov, D.V.; Krymskaya, Y.V.; Il’chenko, N.O.; Slepukhin, P.A.; Rubtsova, S.A.; Kutchin, A.V. Synthesis and Biological Activity of Fluorine-Containing Amino Derivatives Based on 4-Caranethiol. Russ. Chem. Bull. 2018, 67, 731–742. [Google Scholar] [CrossRef]
- Zhang, K.; Ding, H.-W.; Ju, H.; Huang, Q.; Zhang, L.-J.; Song, H.-R.; Fu, D.-C. Design, Synthesis and Biological Evaluation of Sulfenimine Cephalosporin Sulfoxides as β-Lactamase Inhibitors. Chin. Chem. Lett. 2015, 26, 801–803. [Google Scholar] [CrossRef]
- Xu, S.-P.; Lv, P.-C.; Shi, L.; Zhu, H.-L. Design, Synthesis, and Pharmacological Investigation of Iodined Salicylimines, New Prototypes of Antimicrobial Drug Candidates. Arch. Pharm. Chem. Life Sci. 2010, 343, 282–290. [Google Scholar] [CrossRef]
- Ceramella, J.; Iacopetta, D.; Catalano, A.; Cirillo, F.; Lappano, R.; Sinicropi, M.S. A Review on the Antimicrobial Activity of Schiff Bases: Data Collection and Recent Studies. Antibiotics 2022, 11, 191. [Google Scholar] [CrossRef]
- Qiu, Y.; Chan, S.T.; Lin, L.; Shek, T.L.; Tsang, T.F.; Barua, N.; Zhang, Y.; Ip, M.; Chan, P.K.-S.; Blanchard, N.; et al. Design, synthesis and biological evaluation of antimicrobial diarylimine and –amine compounds targeting the interaction between the bacterial NusB and NusE proteins. Eur. J. Med. Chem. 2019, 178, 214–231. [Google Scholar] [CrossRef]
- da Silva, C.M.; da Silva, D.L.; Modolo, L.; Alves, R.B.; de Resende, M.A.; Martins, C.V.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Sayyed, M.; Mokle, S.; Bokhare, M.; Mankar, A.; Surwase, S.; Bhusare, S.; Vibhute, Y. Synthesis of Some New 2,3-Diaryl-1,3-Thiazolidin-4-Ones as Antibacterial Agents. Arkivoc 2006, 2006, 187–192. [Google Scholar] [CrossRef]
- Johnson, B.M.; Shu, Y.-Z.; Zhuo, X.; Meanwell, N.A. Metabolic and Pharmaceutical Aspects of Fluorinated Compounds. J. Med. Chem. 2020, 63, 6315–6386. [Google Scholar] [CrossRef]
- Meanwell, N.A. Synopsis of Some Recent Tactical Application of Bioisosteres in Drug Design. J. Med. Chem. 2011, 54, 2529–2591. [Google Scholar] [CrossRef]
- Isanbor, C.; O’Hagan, D. Fluorine in Medicinal Chemistry: A Review of Anti-Cancer Agents. J. Fluor. Chem. 2006, 127, 303–319. [Google Scholar] [CrossRef]
- Hagmann, W.K. The Many Roles for Fluorine in Medicinal Chemistry. J. Med. Chem. 2008, 51, 4359–4369. [Google Scholar] [CrossRef] [PubMed]
- Ilchenko, N.O.; Sudarikov, D.V.; Slepukhin, P.A.; Rubtsova, S.A.; Kutchin, A.V. Synthesis of Chiral CF3-Contaning Pinane-Type Hydroxythiols. ChemistrySelect 2021, 6, 1710–1714. [Google Scholar] [CrossRef]
- Martínez-Ramos, F.; Vargas-Díaz, M.E.; Chacón-García, L.; Tamariz, J.; Joseph-Nathan, P.; Zepeda, L.G. Highly Diastereoselective Nucleophilic Additions Using a Novel Myrtenal-Derived Oxathiane as a Chiral Auxiliary. Tetrahedron Asymmetry 2001, 12, 3095–3103. [Google Scholar] [CrossRef]
- Sudarikov, D.V.; Krymskaya, Y.V.; Melekhin, A.K.; Shevchenko, O.G.; Rubtsova, S.A. Synthesis and Antioxidant Activity of Monoterpene Nitrobenzylidenesulfenimines. Chem. Pap. 2021, 75, 2957–2963. [Google Scholar] [CrossRef]
- Sudarikov, D.V.; Krymskaya, Y.V.; Shevchenko, O.G.; Slepukhin, P.A.; Rubtsova, S.A.; Kutchin, A.V. Synthesis and Antioxidant Activity of Carane and Pinane Based Sulfenimines and Sulfinimines. Chem. Biodivers. 2019, 16, e1900413. [Google Scholar] [CrossRef]
- Yang, T.-K.; Chen, R.-Y.; Lee, D.-S.; Peng, W.-S.; Jiang, Y.-Z.; Mi, A.-Q.; Jong, T.-T. Application of New Camphor-Derived Mercapto Chiral Auxiliaries to the Synthesis of Optically Active Primary Amines. J. Org. Chem. 1994, 59, 914–921. [Google Scholar] [CrossRef]
- Mloston, G.; Romanski, J.; Linden, A.; Heimgartner, H. Erstes Beispiel einer H-Verschiebung in ‘Thiocarbonyl-aminiden’ (N-(Alkylidensulfonio)aminiden). Helv. Chim. Acta 1995, 78, 1067–1078. [Google Scholar] [CrossRef]
- Andrade, L.A.F.; Silla, J.M.; Freitas, M.P. The Gauche Effect Is Governed by Internal Hydrogen Bond in 2-Amino-2-Methyl-Propanol. J. Mol. Struct. 2014, 1072, 203–207. [Google Scholar] [CrossRef]
- McCann, J.; Ames, B.N. A Simple Method for Detecting Environmental Carcinogens as Mutagens. Ann. N. Y. Acad. Sci. 1976, 271, 5–13. [Google Scholar] [CrossRef]
- SAINT. Data Reduction and Correction Program; Bruker AXS: Madison, WI, USA, 2014. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, C71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R.; Cantón, R.; Brown, D.F.J.; Giske, C.G.; Heisig, P.; MacGowan, A.P.; Mouton, J.W.; Nordmann, P.; Rodloff, A.C.; Rossolini, G.M.; et al. EUCAST expert rules in antimicrobial susceptibility testing. Clin. Microbiol. Infect. 2013, 19, 141–160. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeats: Approved Standard, 3rd ed.; Rex, J.H., Clinical and Laboratory Standards Institute, Eds.; Clinical and Laboratory Standards Institute CLSI: Wayne, PA, USA, 2008; ISBN 978-1-56238-666-5. [Google Scholar]


| Compound | MIC, µg/mL | IC50 EBL, μg/mL | Mutagenicity in the Ames Test | |||
|---|---|---|---|---|---|---|
| S. aureus ATCC 29213 (MSSA) | S. aureus Clinical Isolate (MRSA) | P. aeruginosa ATCC 27853 | C. albicans 703 Clinical Isolate | |||
| 7a | 8 | 8 | 8 | 64 | 21 ± 2.7 | NF * |
| 8a | 16 | 16 | 32 | 32 | 23 ± 3.8 | NF |
| 9a | 256 | 8 | 1024 | 8 | 14 ± 3.5 | NF |
| 7b | 64 | >1024 | >1024 | 64 | 20 ± 2.8 | NF |
| 8b | >1024 | >1024 | >1024 | >1024 | ND | ND |
| 7c | 32 | 32 | 32 | 32 | 45 ± 10.1 | NF |
| 8c | 512 | >1024 | 512 | >1024 | ND | ND |
| 7d | 32 | 32 | >1024 | 512 | 18 ± 4.5 | TA 102 |
| 8d | >1024 | >1024 | >1024 | 1024 | ND | ND |
| 7e | 32 | 64 | 32 | >1024 | 14 ± 4.6 | NF |
| 8e | 16 | 32 | 32 | 16 | 35 ± 9.1 | NF |
| 7f | 32 | 32 | 1024 | 64 | 9 ± 1.9 | TA 102 |
| 8f | 256 | 64 | 1024 | 16 | 14 ± 3.1 | NF |
| Amikacin | 4 | 4 | 4 | ND | ND | ND |
| Ampicillin | 0.5 | >1024 | >1024 | ND | ND | ND |
| Ciprofloxacin | 2 | 4 | 4 | ND | ND | ND |
| Fluconazole | ND * | ND | ND | 16 | ND | ND |
| Benzalkonium chloride | 1 | 1 | 4 | 0.5 | 1 ± 0.3 | ND |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilchenko, N.O.; Sudarikov, D.V.; Rumyantcev, R.V.; Baidamshina, D.R.; Zakarova, N.D.; Yahia, M.N.; Kayumov, A.R.; Kutchin, A.V.; Rubtsova, S.A. Synthesis and Antimicrobial Activity of Sulfenimines Based on Pinane Hydroxythiols. Antibiotics 2022, 11, 1548. https://doi.org/10.3390/antibiotics11111548
Ilchenko NO, Sudarikov DV, Rumyantcev RV, Baidamshina DR, Zakarova ND, Yahia MN, Kayumov AR, Kutchin AV, Rubtsova SA. Synthesis and Antimicrobial Activity of Sulfenimines Based on Pinane Hydroxythiols. Antibiotics. 2022; 11(11):1548. https://doi.org/10.3390/antibiotics11111548
Chicago/Turabian StyleIlchenko, Nikita O., Denis V. Sudarikov, Roman V. Rumyantcev, Diana R. Baidamshina, Nargiza D. Zakarova, Monyr Nait Yahia, Airat R. Kayumov, Aleksandr V. Kutchin, and Svetlana A. Rubtsova. 2022. "Synthesis and Antimicrobial Activity of Sulfenimines Based on Pinane Hydroxythiols" Antibiotics 11, no. 11: 1548. https://doi.org/10.3390/antibiotics11111548
APA StyleIlchenko, N. O., Sudarikov, D. V., Rumyantcev, R. V., Baidamshina, D. R., Zakarova, N. D., Yahia, M. N., Kayumov, A. R., Kutchin, A. V., & Rubtsova, S. A. (2022). Synthesis and Antimicrobial Activity of Sulfenimines Based on Pinane Hydroxythiols. Antibiotics, 11(11), 1548. https://doi.org/10.3390/antibiotics11111548