Abstract
In the context of the globally growing problem of resistance to most used antibacterial agents, essential oils offer promising solutions against multidrug-resistant (MDR) bacterial pathogens. The present study aimed to evaluate the prevalence, etiology, and antibiotic-resistance profiles of bacteria responsible for pyogenic infections in Regional Military University Hospital of Constantine. Disc diffusion and broth microdilution (MIC) methods were used to evaluate the antimicrobial activity of essential oils from five Algerian aromatic plants growing wild in the north of Algeria—Salvia officinalis (Sage), Thymus vulgaris (Thyme), Mentha pulegium L. (Mentha), Rosmarinus officinalis (Rosemary), and Pelargonium roseum (Geranium)—against reference and MDR strains. During three months of the prospective study, 112 isolates out of 431 pus samples were identified. Staphylococcus aureus was the most predominant species (25%), followed by Klebsiella pneumoniae (21.42%), Pseudomonas aeruginosa (21%), and Escherichia coli (17.95%). Among pus isolates, 65 were MDR (58.03%). The radial streak-line assay showed that R. officinalis and M. pulegium L. had weak activity against the tested strains, whereas P. roseum showed no activity at all. Meanwhile, T. vulgaris was the most potent, with an inhibition zone of 12–26 mm and an MIC value ranging between 0.25 and 1.25%, followed by S. officinalis with an inhibition zone of 8–12 mm and an MIC value ranging between 0.62 and 2.5%. Generally, A. baumannii and S. aureus ATCC6538P were the most sensitive strains, whereas P. aeruginosa ATCC27853 was the most resistant strain to the oils. Gas chromatography–mass spectrometry analysis of chemical composition revealed the presence of borneol (76.42%) and thymol (17.69%) as predominant in thyme, whereas camphor (36.92%) and α- thujone (34.91%) were the major volatiles in sage. The in-silico study revealed that sesquiterpenes and thymol had the highest binding free energies against the vital enzymes involved in biosynthesis and repair of cell walls, proteins, and nucleic acids compared to monoterpenes. The results demonstrated that T. vulgaris and S. officinalis are ideal candidates for developing future potentially active remedies against MDR strains.
1. Introduction
There is an ever-increasing demand for plant-based therapeutics in both developing and developed countries due to a growing recognition by customers of natural products, which are readily available at affordable prices with almost no complications, and of avoiding the side-effects of chemotherapeutic agents like hypersensitivity, immune-suppression, and allergic reactions [1]. Many studies have reported essential oils’ (EOs) antimicrobial and antifungal activity against various microorganisms, including pathogenic Gram-negative and Gram-positive bacteria. For instance, the presence of long-chain (C6–C10) alcohols and aldehydes in cilantro’s EO made it efficient against Listeria monocytogenes [2]. At the same time, oregano and thyme EO are both effective at reducing or preventing the growth of E. coli O157:H7 in food [3]. Volatile constituents of EOs, like terpenes and phenolic components, e.g., thymol, carvacrol, and eugenol, are responsible for the reported antibacterial activity [4]. The Algerian flora is rich in thousands of wild herbs with medicinal properties like antimicrobial, antifungal, antioxidant, anticancer, and many other properties reported extensively in the literature based on their bioactive EOs and extracts [5,6].
The rise in infections by multidrug-resistant bacteria (MDR) in recent years has increased interest in the possible antibacterial activities of medicinal plants and their metabolites. MDR pathogens like Pseudomonas aeruginosa commonly cause hospital-associated infections, such as Acinetobacter baumannii, extended spectrum-beta-lactamase (ESBL)-producing Escherichia coli, and carbapenemase-producing Klebsiella pneumoniae, not to mention the possibility of transmission in the community [7,8]. Such bacterial isolates may be immune to every antibiotic currently on the market [9]. Creating new antimicrobials is one strategy to combat the medication-resistance issue; in this context, EOs are being researched for their antibacterial properties. To our knowledge, only Origanum glandulosum Desf, Artemisia judaica L. [6,10], and four Lamiaceae aromatic plants (Thymus capitatus L., Lavandula dentata L., Salvia officinalis L., and Mentha rotundifolia L.) [1] from the Algerian flora were screened against MDR. However, most studies dealing with the antibacterial activity of other Algerian EOs were based on non-pathogenic or reference strains [11].
This study aimed to determine the antimicrobial activity of some Algerian EOs, namely, Rosmarinus officinalis, Thymus vulgaris, Salvia officinalis, Pelargonium roseum, and Mentha pulegium, against MDR bacteria isolated from pus samples. The most potent EOs were analyzed using gas chromatography–mass spectrometry (GC–MS). The mechanism of EOs’ antibacterial action against MDR could be elucidated using cheminformatics [12]. Molecular docking is a cheminformatics tool often used to gain valuable insight into the possible molecular mechanisms of pharmacologically active substances. Herein, molecular docking was employed to identify a possible mechanism of action correlated with the recorded antimicrobial effect of the potent EO components against target proteins associated with bactericidal/bacteriostatic effects, such as DHPS, DHFR, Ddl, penicillin-binding protein 1a PBP1a, DNA gyrase, type-IV topoisomerase, and isoleucyl-tRNA synthetase (IARS) [13]. The search for safe botanical materials in this work presents an excellent opportunity to use EOs as antibacterial agents in the pharmaceutical industry and clinical settings to treat or prevent MDR-strain infections.
2. Results and Discussion
2.1. Prevalence of Bacterial Isolates
During the collection period of three months, 112 isolates out of 431 pus samples were identified, which constitute 34.67% of the total isolates detected in different samples collected from the hospital departments (pus, hemoculture, CSL, and urine). S. aureus was the pathogen most commonly isolated from pus specimens with a frequency of 25% (Figure 1). This finding agreed with those of Tchakal-Mesbahi et al. [14], who investigated pus from wound infections in the burn center at Mouhamed Seghir Nekkache Military Hospital of Algiers with a frequency of 28.22%. In this study, K. pneumoniae was the second most predominantly isolated pathogen (21.42%), followed by P. aeruginosa (21%) and E. coli (17.95%). According to Hak-Jae et al. [15], Proteus spp., S. aureus, and K. pneumoniae are the most common bacteria from the pus of wound infections. These bacterial species are abundant in the skin and easily enter the surgical site during hospitalization. At the same time, other species were isolated with less frequency, as presented in Figure 1: Proteus mirabilis (8.92%), Proteus spp., and S. marcescens (4.46%).
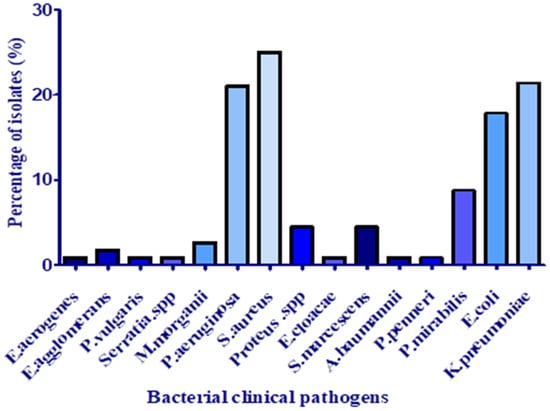
Figure 1.
Distribution of species isolated from pus samples.
2.2. Distribution of Bacterial Isolates According to the Hospital Department
Pus samples were recuperated mainly from wound, post-surgical, ear, and ODP (obstructive pulmonary disease) infections. Most of the samples were collected from the orthopedic department and general medicine, followed by general surgery, with frequencies of 31.25, 20.53, and 14.28%, respectively (Figure 2). According to Uçkay et al. [16], in the orthopedic department, surgical site and osteoarticular infections, in addition to bone trauma, are frequent and challenging to treat and associated with lifelong recurrence risks of around 10–20%.
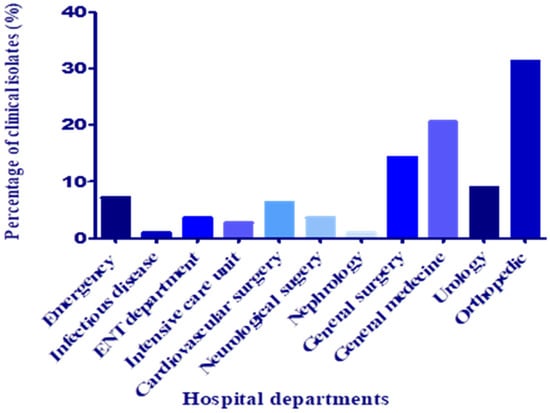
Figure 2.
Distribution of clinical isolates according to the hospital department.
2.3. Distribution of Bacterial Isolates According to Sex
The distribution of the total isolates (112) according to sex showed a vast difference between the frequency of isolation from male samples (84.82%) and that derived from female samples (15.17%), with a sex ratio of 5.58 male/female. These results are consistent with those obtained by Lye et al. [17], whose study showed a predominance of isolates derived from males (57%) hospitalized for a longer duration before bacteremia or lodged in the ICU and who were more acutely ill.
2.4. Antibiotics-Susceptibility Testing
The testing of methicillin-resistant S. aureus (MRSA) susceptibility to most used antibiotics showed a very high resistance frequency to penicillin G (50%), oxacillin, and cefoxitin (46.4%), whereas they were less resistant to erythromycin and spiramycin (10.71%) (Table 1). Lincomycin, clindamycin, amikacin, and ofloxacin were the most effective antibiotics. The present study’s resistance frequencies were lower than those obtained by Feleke et al. [18] at Gondar Specialized Hospital in Ethiopia, where S. aureus isolates (35.6%) were the most common isolates, with an MRSA-isolation rate of 67.5%. The most common clinical samples were wounds/pus (63%), which agrees with the present study. They reported very high resistance rates to penicillin (96.1%), amoxicillin (50.6%), and clindamycin (36.4%) [18]. The variations in resistance-frequency rates may be due to host, microbial, and environmental factors. This major human pathogen mostly comes from the patient themself, but it is mainly transported to infectious sites by personnel or medical devices. Therefore, researchers continue to seek to understand these infections’ dynamics and their resistance mechanisms to prevent and cure them [19]. In the current study, Enterobacteriaceae members were mainly resistant to ampicillin, amoxicillin, amoxicillin/clavulanate, and third-generation cephalosporins (cefotaxime and ceftriaxone), whereas imipenem and amikacin were active on all isolates (0%). Many studies reported a high resistance level against penicillins, cephalosporins, and trimethoprim/sulfamethoxazole [20]. Whereas P. aeruginosa demonstrated increased susceptibility to the most used antibiotics, including ticarcillin/clavulanate, piperacillin, and ceftazidime (8.33%), all isolates were susceptible to colistin (0%). In contrast, isolates of A. baumannii were the most resistant. They were fully resistant to all tested antibiotics (100%) except for amikacin and colistin (0%). These findings align with those of Tchakal-Mesbahi et al. [14], who reported that A. baumannii isolates were highly resistant to ceftazidime and imipenem (90%), and more than 80% were resistant to amikacin, gentamicin, and ciprofloxacin. According to Lin and Lan [21], A. baumannii is known for its intrinsic resistance to antibiotics and ability to acquire gene-encoding resistance determinants.

Table 1.
Antimicrobial-susceptibility profile of multidrug-resistant bacteria (MDR) against most commonly used antibiotics.
2.5. Phenotypic Detection of Multidrug-Resistant Bacteria
The analysis of susceptibility results by antibiogram showed that 65 out of 112 tested isolates (58.03%) were resistant to at least three antibiotics from different classes (MDR phenotype), which indicates an alarming frequency. Dessie et al. [22] reported similar results with a high frequency of MDR (75.2%) in a study conducted in Addis Ababa. The increase in the MDR phenotype greatly impacts public health by increasing treatment failure, which requires approaches to be developed for rational use of antimicrobials; improving hand hygiene and infection control; and developing new alternatives for commonly used antibiotics. The difference in the prevalence of MDR is associated with many factors, including long-term antimicrobial therapy, cross-transmission, length of hospital stays, invasive procedures, differences in environmental conditions, and policy of antibiotics use [23,24]. Antimicrobial susceptibility patterns often differ between geographical regions, populations, and hospital types/units [25,26].
Resistance to third-generation cephalosporins is mainly due to ESBL production; in this study, 22.61% were Enterobacteriaceae-producing ESBL. This frequency was less than that reported by Elmekes et al. [27]. They showed that 48% of Enterobacteriaceae were ESBL producers in a study conducted at the intensive care unit of the University Hospital Center in Marrakesh, Morocco. Resistance to cephalosporins also may be due to AmpC cephalosporinase production; in this study, only 2.38% of Enterobacteriaceae-producing cephalosporinases were identified. The incidence of MRSA was also alarming, with a frequency of 46.42 %. Similar results were obtained by Nigussie et al. and Latif et al. [28,29], of 38.5% and 31.25%, respectively. These variations may be due to the type of assessment used for the diagnosis of MRSA and the status of the hospital [30]. Therefore, it is essential to confront the rapid increase in infections caused by MRSA and Enterobacteriaceae-producing ESBLs in Algeria.
2.6. Antibacterial Activity
2.6.1. The Radial Streak-Line Method
Analysis of radial streak-line assay results showed that, among five tested essential oils, T. vulgaris oil was the most active, followed by S. officinalis (Table 2). However, P. roseum, M. pulegium, and R. officinalis showed weak activity. These findings led to the selection of T. vulgaris and S. officinalis essential oils for further investigation; disk diffusion assay, GC/MS, and in silico study revealed the volatile constituents responsible for the activity against enzymes involved in biosynthesis and repair of cell walls, proteins, and nucleic acids of the MDR pathogens.

Table 2.
Effect of tested essential oils on radial bacterial growth.
2.6.2. Evaluation of the Antibacterial Activity of Potent EOs
The antibacterial activity of T. vulgaris and S. officinalis essential oils (EOs) were evaluated by the disc-diffusion method, minimum inhibitory concentration (MIC), and minimal bactericidal concentration (MBC), as shown in Table 3. The results show that T. vulgaris EO was very effective against S. aureus ATCC 6538P and A. baumannii with inhibition-zone diameters of 26 mm and 22 mm, respectively. It was also active on MRSA, S. marcescens (17 mm), and E. coli ESBL (15 mm). The previous results indicate that T. vulgaris EO had a broad spectrum against Gram-negative and Gram-positive bacteria, except for P. aeruginosa. MIC values ranged from 0.07 to 1.25% and for MBC values between 0.07 and 2.5%. The highest antibacterial activity of T. vulgaris EO is mainly due to the presence of phenolic compounds and monoterpene hydrocarbons [31].

Table 3.
Antibacterial activity of T. vulgaris and S. officinalis EOs against multidrug-resistant isolates using three methods (disk-diffusion method, MIC, and MBC).
The antibacterial activity of S. officinalis essential oil showed a moderate effect against clinical isolates and S. aureus ATCC 6538P with an inhibition zone ranging from 8 to 12 mm. The results obtained are different from those reported by Moumni et al. [32], who found a more potent antimicrobial activity against E. coli (11.3 mm), B. subtilis (17.3 mm), and P. aeruginosa (7 mm). This variation may be attributed to many factors, including species, plant origin, climatic conditions, distillation method and conditions, chemical composition, time of harvesting and storage conditions, and bacterial-strain susceptibility [33,34,35]. MIC values ranged between 0.62 and 2.5%, whereas MBC values varied from 2.5 to 5%. In previous studies, Delamare et al. [36] attributed the antibacterial activity of S. officinalis EO against E. coli, P. aeruginosa, B. subtilis, and S. aureus to high concentrations of thujone, 1,8-cineole, and camphor.
2.7. Chemical Composition of the Potent EOs
The chemical composition of volatile oils from aerial parts of S. officinalis and T. vulgaris was characterized by GC-MS (Table 4, Figure 3A,B). A total of 14 and nine components were identified, representing 99.56 and 97.83% of the total oils, respectively. Camphor (39.62%), α-thujone (34.91%), 1,8-cineole (13.20%), and viridiflorol (5.84%) were the dominant compounds in the S. officinalis oil (Table 4, Figure 3A). The previous findings are consistent with Dob et al. [37], Boutebouhart et al. [38], and Mahdjoubi et al. [39], who identified the same trend in oils extracted from plants cultivated in the Algiers and Laghouat regions, Algeria, despite remarkable quantitative differences due to geographical variation. The chemotaxonomy of familiar sage (Salvia officinalis) based on the volatile constituents studied by Craft et al. [40] revealed five major chemotypes, with the most common being an α-thujone/camphor/1,8-cineole, α-humulene, β-thujone, 1,8-cineole/camphor, and sclareol/α-thujone. The S. officinalis under investigation and most of the Algerian oils reported in the literature belonged to the typical α-thujone/camphor/1,8-cineole chemotype.

Table 4.
Volatile constituents identified from Algerian S. officinalis and T. vulgaris using GC-MS.
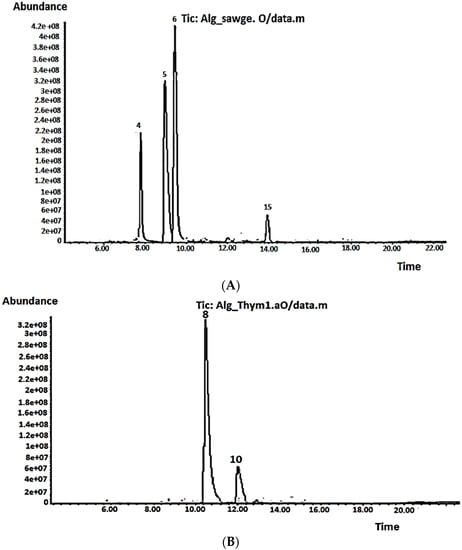
Figure 3.
Volatile chromatograms for (A) S. officinalis and (B) T. vulgaris.
In the volatile oil extracted from T. vulgaris, nine compounds were identified, representing 97.83% of the total oil, with the major constituents being borneol (76.42%) and thymol (17.69%) (Table 4, Figure 3B). According to Benaliouche et al. [41], the genus Thymus contains more than 300 species, 11 of which are located in Algeria. Based on the chemotype, each species showed variability in the chemical profile of their EOs, which affected their physicochemical and biological properties. Several chemotypes have been characterized based on the terpenes, including linalool, borneol, geraniol, sabinene hydrate, thymol, and carvacrol [42]. Consequently, to our knowledge, the Algerian thyme oil under investigation in the current study represents the first reported one to belong to the borneol chemotype. Quantitative and qualitative variations in the T. vulgaris EOs obtained from different regions in Algeria have been reported in the literature based on their chemotype. For example, Benaliouche et al. [41] identified thyme oil extracted from Annaba as linalool chemotype, whereas Abdelli et al. [43] showed that EOs extracted from T. vulgaris cultivated in Tlemcen and Mostaganem belong to the carvacrol chemotype. Interestingly, in a previous study by Abdelli et al. [44], thyme EOs from the same regions, Tlemcen and Mostaganem, had thymol as the predominant EO, at 59.5 and 67.3%, respectively. Generally, the variations in the chemical composition of thyme EO may be attributed to many factors, such as climatic change, environmental conditions, plant age, plant part, development stage, growing place, harvesting period, or principal chemotype, as they all affect plant biosynthetic pathways and consequently the relative proportion of the main ingredients [45].
2.8. Molecular-Docking Study
In silico assessment employing a molecular-docking approach was used to better understand the molecular basis of the antibacterial activity provided by the major volatile components of the potent EOs (S. officinalis and T. vulgaris), as antibiotics target the microbial metabolism and restrict their growth by deactivating the vital enzymes involved in biosynthesis and repair of cell walls, proteins, and nucleic acids such as isoleucyl-tRNA synthetase, DNA gyrase, dihydropteroate synthase, D-alanine ligase, topoisomerase 4, dihydrofolate reductase, and penicillin-binding protein (Table 5).

Table 5.
Binding free-energy values calculated through the molecular docking of the major volatile constituents of S. officinalis and T. vulgaris EOs and the bacterial key metabolic enzymes as receptors.
Aminoacyl-tRNA synthetases have been identified as possible drug targets for several infectious diseases. They are responsible for charging a specific tRNA with its cognate amino acid, which is essential for protein synthesis. Inhibition of tRNA aminoacylation has proven to be an effective antimicrobial strategy, impeding a necessary step of protein synthesis. The design of novel aminoacylation inhibitors is complicated by either the presence of MDR clinical isolates or the steadfast requirement to avoid off-target inhibition of protein synthesis in human cells [46]. The major sesquiterpenes in both potent EOs are β- caryophyllene (−6.8 kcal/mol), α-humulene (−7.0 kcal/mol), and viridoflorol (−7.0 kcal/mol), and revealed the highest binding affinity against isoleucyl-tRNA synthetase (PDB ID: 1JZQ) compared to monoterpenes (Table 5). The previous findings agree with Jianu et al. [13], where caryophyllene oxide showed the highest affinity toward 1JZQ during in silico and in vitro evaluation of the antimicrobial and antioxidant potential of Romanian Mentha × smithiana R. Graham EO.
DNA gyrase controls the topology of DNA during transcription and replication by introducing transient breaks to both DNA strands. Since DNA gyrase is pivotal for bacterial survival, it is essential to exploit bacterial DNA gyrase as a critical target for the antibacterial agents. Consequently, a molecular-docking study was carried out to examine the binding interactions of the major volatile constituents with the pocket of DNA gyrase (PDB ID: 1KZN) (Table 5). The compounds displayed binding energy ranging from −4.5 to −6.3 kcal/mol for thymol, one of the major constituents in T. vulgaris EO (Table 5). The extract components of Ocimum cufodontii used traditionally in Ethiopia as an antibacterial agent shown a comparable binding affinity toward DNA gyrase (−6.1 to −6.9 kcal/mol), which is in line with the results of the present study [47]. The inhibition of dihydrofolic acid-formation affects DNA synthesis. Therefore, antibiotic agents compete with p-amino benzoic acid to bind with the dihydropteroate synthetase (PDB ID: 2VEG) with much greater affinity and inhibit the formation of dihydrofolic acid. In line with the affinity showed by β-cubebene towards 2VEG (−6.1 kcal/mol) reported by Salvi et al. [48], the sesquiterpenes in the current study—α-humulene and viridoflorol—showed similar affinity (Table 5).
Regarding the target-protein structures involved in antimicrobial activity, the results indicate an increased affinity of thymol (−7.7 kcal/mol) towards the D-alanine–D-alanine ligase (PDB ID: 2ZDQ). The enzyme 2ZDQ catalyzes the condensation of two D-Ala molecules using ATP to produce D-Ala–D-Ala, the terminal peptide of a peptidoglycan monomer. The cell-wall peptidoglycan polymer is produced through cross-linking peptidoglycan monomer units [49]. The highest affinity of thymol among the examined volatiles (Table 5), which seems to have been responsible for the higher antibacterial activity of T. vulgaris EO, is due to its bactericidal effect by bacterial cell-wall denaturation, causing leakage of essential nutrients [50]. Therefore, the assumption that one of the antibacterial mechanisms of the EO’s monoterpene components is the suppression of bacterial-wall synthesis by inhibiting the D-alanine–d-alanine ligase enzyme is highly plausible. The binding-affinity energy of thymol recorded in the current study was superior compared to the literature [13,48] and opens the prospects for using EOs rich in thymol as antibacterial agents against MDR isolates. In Gram-positive bacteria, topoisomerase 4 catalyzes the separation of daughter strands following replication. The molecular-docking analysis for the major volatiles in the potent antibacterial EOs of the present study revealed that 1,8-cineole and sesquiterpenes showed the highest binding affinity towards topoisomerase 4 (PDB ID: 3RAE), with −6.0 to −6.5 kcal/mol (Table 5), which is comparable to the binding affinity of O. gratissimum, O. tenuiflorum, and O. sanctum EOs volatiles reported by Salvi et al. [48] and higher than the volatiles of Mentha × smithiana essential oil [13].
Dihydrofolate reductase (PDB ID: 3SRW) is an enzyme in the thymidine synthesis pathway. Antibiotics bind to 3SRW and inhibit thymidine synthesis, which eventually affects DNA synthesis. In agreement with Jianu et al. [49] and Salvi et al. [48], sesquiterpenes exhibited higher in silico binding affinity towards 3SRW than well-known antibiotics used against enzymes such as trimethoprim. Viridiflorol had the highest binding energy (−8.1 kcal/mol) among all the examined volatiles towards all receptors included in the study. An essential part of the bacterial cell wall, cross-linked peptidoglycan, is created by penicillin-binding proteins (PDB ID: 3UDI). These proteins feature penicillin-sensitive C-terminal transpeptidase and N-terminal trans-glycosylase domains. Therefore, the inactivation of 3UDI proteins limits the development of cell walls, which eventually causes bacterial growth to cease [51]. In silico analysis revealed that the three phytoconstituents—viridoflorol (6.5 kcal/mol), α-humulene (6.0 kcal/mol), and β-caryophyllene (5.9 kcal/mol)—had a comparatively similar binding affinity towards 3UDI to that of β-cubebene, caryophyllene oxide, and ylangene, as reported by Salvi et al. [48].
Since thymol (−7.7 kcal/mol) and viridoflorol (−8.1 kcal/mol) were recorded, the best docking scores among all other volatiles examined were towards 2ZDQ and 3SRW; therefore, binding analysis was performed to reveal the interaction between ligands and protein-binding sites (Figure 4A,B). The hydroxyl group of thymol was very well oriented, forming two conventional hydrogen bonds with TYR A:218 and GLU A:197 of 2ZDQ. In addition, thymol showed other pi-alkyl interactions with PHE A:151, 222, 272; pi-pi stacked with PHE A:151, 272; and finally, alkyl binding with protein moieties like VAL A:195, LEU A:192, and others (Figure 4A). On the other hand, the interaction of viridoflorol with 3SRW involved the amino acids residues VAL7, ALA8, ILE15, GLY16, PHE17, ASN19, GLN20, LEU21, LYS46, THR47, ILE51, PHE93, GLY94, GLY95, GLN96, PHE99, and THR122 through alkyl and pi-alkyl interactions. A conventional H-bonding with SER50 was detected through in-silico analysis (Figure 4B). The previous findings revealed the responsibility of different binding interactions for the higher docking scores of both thymol and viridoflorol, and consequently the antibacterial activity of both S. officinalis and T. vulgaris against MDR clinical isolates.
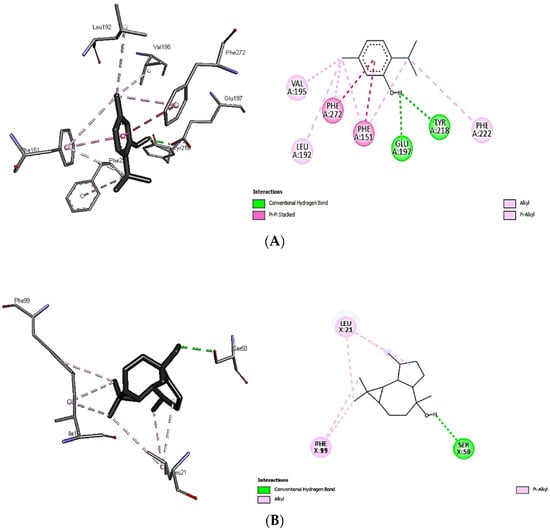
Figure 4.
Interactions of thymol with D-alanine: D-alanine ligase 2ZDQ (A) and viridoflorol with dihydrofolate reductase 3SRW (B).
3. Materials and Methods
3.1. Study Design
During three months of a prospective study conducted at the central microbiology laboratory in the Regional Military University Hospital of Constantine, a total of 112 isolates from 431 pus samples of hospitalized patients was obtained and analyzed.
3.2. Identification of Clinical Isolates
Identification of isolated bacteria from pus samples was conducted according to their morphological and biochemical characteristics. Pus smeared on a clean, grease-free slide showing Gram-positive staining and occurring as clusters was subjected to growth on mannitol salt agar, coagulase, and catalase tests [52,53], whereas that showing Gram-negative stains was purified on differential and selective media. The species were identified using a qualitative micro-method employing conventional and chromogenic substrates to identify clinically important selected oxidase-negative. Identification kit RapID™ ONE system tests and Remel™ RapID™ ERIC™ software v.1.0.771 (Thermo Scientific, Waltham, MA, USA) were also used to identify isolates according to the recorded database.
3.3. Antibiotic-Susceptibility Test
Susceptibility to antibiotics was carried out using the disk-diffusion method on Muller Hinton Agar at 37 °C for 18 h. Results were interpreted according to CLSI breakpoint criteria (2014). Three reference isolates (Staphylococcus aureus ATCC 25923, Escherichia coli ATCC 25922, and Pseudomonas aeruginosa ATCC 27853) were used for quality control of the purchased disks (Oxoid, Basingstoke, Hampshire, UK). S. aureus isolates were tested on a total of 10 different antibiotic disks: oxacillin (OX5), cefoxitin (FOX30), penicillin G (P10), spiramycin (SP100), clindamycin (DA2), lincomycin (L15), erythromycin (E15 (RD5), vancomycin (VA30), amikacin (AK30), and ofloxacin (OFX5), whereas Enterobacteriaceae members were tested for amoxicillin (AMX25), amoxicillin/clavulanate (AMC10), cefotaxime (CTX30), ceftriaxone (CRO 30), ampicillin (AMP10), cefazolin (CF30), imipenem (IMP10), ofloxacin (OFX5), amikacin (AK10), and trimethoprim/sulfamethoxazole (SXT1.25/23.75). Non-fermentative bacilli (P. aeruginosa and A. baumannii) were tested for the following antibiotics: piperacillin (PIP100), ticarcillin (TIC75), ticarcillin–clavulanate (TCC75), aztreonam (ATM30), ceftazidime (CAZ30), piperacillin (PRL100), levofloxacin (LEV5), amikacin (AK30), colistin (CT10), and trimethoprim/sulfamethoxazole (SXT1.25/23.75).
3.4. Phenotypic Detection of MDR-Strain Resistance Mechanisms
Resistance mechanisms were detected phenotypically for MDR isolates based on the analysis of susceptibility to antibiotics by antibiogram and the use of inhibitors (clavulanic acid and cloxacillin). Enterobacteriaceae and non-fermentative gram-negative bacilli were selected based on their resistance to third-generation cephalosporins (CTX and CAZ) to detect extended-spectrum beta-lactamase (ESBL) and cephalosporinase production. A double-disk synergy test (DDST) was used to detect ESBL production; increasing inhibition-zone diameter (synergy) indicates a positive test. Positive results were confirmed by the double-disk-diffusion test (DDDT) [54], whereas AmpC cephalosporinase detection was performed for isolates with a negative synergy test using cloxacillin as an inhibitor (200 μg/mL) and cefoxitin (FOX30), as described by Tan et al. [55]. Disks of ox (5 µg) and fox (30 µg) were used for screening methicillin-resistant S. aureus (MRSA) [56].
3.5. Plant Material and Extraction
Five aromatic plants growing wild in different regions of North Algeria were collected during the flowering season (June 2019). Thymus vulgaris was collected from Setif, Mentha pulegium and Pelargonium roseum were collected from Constantine, and Salvia officinalis and Rosmarinus officinalis were obtained from the Bejaia region. Identification was performed by an ecologist at the biology laboratories at the Faculty of Sciences of Bejaia University. The plant’s aerial parts (leaves, flowers, and stems) were dried in the dark at room temperature (25–26 °C). The plant material (100 g) was supported above boiling water on a perforated grid for 3 hours. The condensate was recuperated from the condenser, and a glass decanter separated essential oils from the condensate. The purified oils were stored in brown glass bottles at 4 °C until their use.
3.6. Bacterial Strains
Four reference isolates (P. aeruginosa ATCC2525, S. aureus ATCC6538P, K. pneumoniae ATCC700603, E. coli ATCC8739) and a selection of five MDR clinical isolates from the military hospital (MRSA, E. coli ESBL, K. pneumoniae ESBL, S. marcescens ESBL, and A. baumannii) were used to evaluate the effectiveness of the extracted essential oils.
3.7. Antimicrobial Activity
3.7.1. The Radial Streak-Line Method
This is a preliminary qualitative test generally used to check and select between efficient pure essential oils. Sterile filter discs (Whatman n°1, 6 mm diameter) were impregnated with 10 µL of stock solutions of essential oils and placed at the center of the Muller Hinton agar plate surface. Plates were inoculated with bacterial suspensions adjusted to 0.5 McF (≈1.5 × 108 UFC/mL) by radial lines of inoculums from the border to the center of the plate. The antimicrobial activity was evaluated by analyzing the size in mm of the inhibition zone [57].
3.7.2. Agar Diffusion Method
The antimicrobial activity of two-fold serial diluted essential oils (in dimethyl sulfoxide) was determined as previously described [17]. Briefly, a bacterial suspension (106 CFU/mL) was inoculated on MH agar, then 20 μL of each oil dilution were poured into wells of 6 mm diameter, and DMSO was used as a negative control. To allow oil diffusion onto agar plates, they were incubated at 4 °C for two hours, then incubated at 37 °C overnight (24 h); the mean of inhibition-zone diameters was determined for each dilution. Each assay was repeated three times [58].
3.7.3. Broth Microdilution Assay
A microdilution test was performed using 96-well microplates. The serial dilutions of the essential oils dissolved in DMSO were prepared in BHI broth with a concentration ranging from 0.04 to 5% (v/v). Each well containing 50 µL of diluted essential oil was inoculated with 50 µL of bacterial suspension in BHI Broth (106 CFU/mL). A well containing 100 μL of BHI broth was used as a negative control and a well containing 100 μL of BHI broth with a bacterial suspension without essential oil as a positive control. After that, incubation was carried out at 37 °C for 24 h. Tetrazolium chloride (2,3,5-triphenyl-2H-tetrazolium chloride TTC) was used as an indicator of viability to determine values of minimum inhibitory concentrations (MICs). A volume of 10 μL of broth from each well with no visible growth (≥MICs) was subcultured onto fresh MH agar plates, then incubated at 37 °C for 24 h. Concentration in which there is no colony growth is considered the minimum bactericidal concentration (MBC) [59].
3.8. Chemical Composition of Potent EOs
Potent steam-distilled oils (SD) were analyzed using gas chromatography–mass spectrometry (GC-MS); separation was carried out on a Trace GC Ultra Chromatography system (Thermo Scientific, Waltham, MA, USA) outfitted with an ISQ mass spectrometer and a 60 m 0.25 mm 0.25 μm TG-5MS capillary column (Thermo Scientific, Waltham, MA, USA). The column-separation-program temperature began at 50 °C with a holding time of 3 min, then was raised by 4 °C per minute to 140 °C with a holding period of 5 min. After that, the temperature rose at a rate of 6 °C per minute before reaching 260 °C for an isothermal holding period of 5 min. The injector temperature was 180 °C, the ion source temperature was 200 °C, and the transition-line temperature was 250 °C. Helium flowed at a steady rate of 1.0 mL/min as the carrier gas. The mass spectrometer had an ionization energy of 70 eV and a scan range of 40–450 m/z. The MS computer library (NIST library, 2005 edition), raw chemicals, and published data were used to identify compounds [40,60]. Integration by GC was used to compute the area percentage of the detected components. By comparing the values with those reported in the literature and utilizing the retention durations of a homologous series of C6–C26 n-alkanes, the Kovats index was determined for each compound [61].
3.9. Molecular Docking
The crystal structures of isoleucyl-tRNA synthetase (PDB ID: 1JZQ), DNA gyrase (PDB ID: 1KZN), dihydropteroate synthase (PDB ID: 2VEG), D-alanine: D-alanine ligase (PDB ID: 2ZDQ), IV topoisomerase (PDB ID: 3RAE), dihydrofolate reductase (PDB ID: 3SRW), and penicillin-binding protein 1a (PDB ID: 3UDI) were obtained from the Protein DataBank (https://www.rcsb.org/, accessed on 16 August 2022). It was prepared as a receptor by removing water and co-crystallized ligands and ions, then protonated using the Pymol software ver. 2.5.1 (Supplementary files as receptors in pdb format). Meanwhile, the 3D structures of the ligands, which were downloaded from the PubChem database (http://pubchem.ncbi.nlm.nih.gov/, accessed on 16 August 2022), were optimized by using the MMFF94 force field by Avogadro Software ver. 1.2.0 (Supplementary files as ligands in sdf and mol2 formats). Blind docking was performed using a web-based program called CB-DOCK2 (http://clab.labshare.cn/cb-dock/php/, accessed on 16 August 2022). After submission, CB-Dock2 checks the input files and converts them to pdbqt-formatted files using OpenBabel and MGL Tools. Next, CB-Dock predicts cavities of the protein and calculates the centers and sizes of the top N (n = 5 by default) cavities. Each center and size and the pdbqt files are submitted to AutoDock Vina for docking. The final results are displayed after the computation of N rounds (Supplementary docking files in pdb format). The benchmarks performed by Liu et al. [62] illustrated that, in terms of success rates for top-ranking poses whose root-mean-squared deviation (RMSD) was within 2 Å from the position in the X-ray crystal structure, CB-Dock2 outperformed other blind-docking tools. The profiles of interaction and visualization were performed for the best-docked complexes using Discovery Studio software (Ver. 21.1.0.20298) [63].
4. Conclusions
The preliminary antibacterial examination of many Algerian essential oils growing wild in the northern regions against MDR bacterial pathogens revealed the efficacy of S. officinalis and T. vulgaris, which was assured in vitro using further advanced assays. The higher antibacterial activities of the previous essential oils aligned with their higher concentrations of camphor, α-thujone, viridoflorol, borneol, and thymol. The in-silico study showed the higher affinity of sesquiterpenes and thymol toward vital enzymes involved in the biosynthesis and repair of cell walls, proteins, and nucleic acids of the MDR bacteria of S. officinalis and T. vulgaris compared to monoterpenes. The outcomes showed that T. vulgaris and S. officinalis are excellent prospects for creating potential future treatments that are active against MDR strains as alternatives for the standard used antibiotics.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/antibiotics11101317/s1, File S1: Molecular docking results in pdb format, ligands in sdf and mol2 formats, and receptors in pdb format.
Author Contributions
Conceptualization, project administration, investigation, and writing—original draft, A.A. and A.F.; extraction, methodology, formal analysis, statistical analysis, and data curation, S.B., A.A. and A.F.; software and docking, A.F.; revision and editing, A.F.; funding acquisition, M.A.A. and M.A.S.A. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Deanship of Scientific Research at Umm Al-Qura University, program No. (22UQU4290565DSR84).
Institutional Review Board Statement
The study was conducted following the Declaration of Helsinki, and approved by the Institutional Review Board of Ferhat Abbas University of Setif, Faculty of Nature and Life Sciences (ECA/001/22, 28 June 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in this article.
Acknowledgments
The authors would like to thank the Deanship of Scientific Research at UmmAl-Qura University for supporting this work with grant code 22UQU4290565DSR84.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Mohamed, S.B.; Eddine, A.D. Antibacterial Activity of Essential Oils of Some Algerian Aromatic Plants Against Multidrug Resistant Bacteria. J. Essent. Oil-Bear. Plants 2010, 13, 362–370. [Google Scholar] [CrossRef]
- Delaquis, P.J.; Stanich, K.; Girard, B.; Mazza, G. Antimicrobial activity of individual and mixed fractions of dill, cilantro, coriander and eucalyptus essential oils. Int. J. Food Microbiol. 2002, 74, 101–109. [Google Scholar] [CrossRef]
- Burt, S.A.; Reinders, R.D. Antimicrobial activity of selected plante essential oils against Escherichia coli O157:H7. Lett. Appl. Microbiol. 2003, 36, 162–167. [Google Scholar] [CrossRef]
- Man, A.; Santacroce, L.; Jacob, R.; Mare, A.; Man, L. Antimicrobial Activity of Six Essential Oils Against a Group of Human Pathogens: A Comparative Study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef]
- Aouf, A.; Ali, H.; Al-Khalifa, A.R.; Mahmoud, K.F.; Farouk, A. Influence of Nanoencapsulation Using High-Pressure Homogenization on the Volatile Constituents and Anticancer and Antioxidant Activities of Algerian Saccocalyx satureioides Coss. et Durieu. Molecules 2020, 25, 4756. [Google Scholar] [CrossRef]
- Bouaouina, S.; Aouf, A.; Touati, A.; Ali, H.; Elkhadragy, M.; Yehia, H.; Farouk, A. Effect ofNanoencapsulation on theAntimicrobial and Antibiofilm Activities of Algerian Origanum glandulosum Desf. Against Multidrug-Resistant Clinical Isolates. Nanomaterials 2022, 12, 2630. [Google Scholar] [CrossRef]
- Lee, N.Y.; Lee, C.C.; Huang, W.H.; Tsui, K.C.; Hsueh, P.R.; Ko, W.C. Carbapenemtherapy for bacteremia due to extended-spectrum-ß-lactamaseproducing Escherichia coli or Klebsiella pneumoniae: Implications of ertapenem susceptibility. Antimicrob. Agents Chemother. 2012, 56, 2888–2893. [Google Scholar] [CrossRef]
- Lucena, A.; Costa, D.L.M.; Nogueira, K.S.; Matos, A.P.; Gales, A.C.; Paganini, M.C.; Castro, M.E.S.; Raboni, S.M. Nosocomial infections with metallo-betalactamase-producing Pseudomonas aeruginosa: Molecularepidemiology, risk factors, clinical features and outcomes. J. Hosp. Infect. 2014, 87, 234–240. [Google Scholar] [CrossRef]
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef]
- Benmansour, N.; Benmansour, A.; El Hanbali, F.; González-Mas, M.C.; Blázquez, M.A.; El Hakmaoui, A.; Akssira, M. Antimicrobial activity of essential oil of Artemisia judaica L. from Algeria against Multi-Drug Resistant bacteria from clinical origin. Flavour Fragr. J. 2016, 31, 137–142. [Google Scholar] [CrossRef]
- Mehalaine, S.; Belfadel, O.; Menasria, T.; Messaili, A. Chemical composition and antibacterial activity of essential oils of three medicinal plants from Algerian semi-arid climatic zone. Phytothérapie 2017, 16, S155–S163. [Google Scholar] [CrossRef]
- Miladinović, D.L.; Ilić, B.S.; Kocić, B.D. Chemoinformatics Approach to Antibacterial Studies of Essential Oils. Nat. Prod. Commun. 2015, 10, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Jianu, C.; Stoin, D.; Cocan, I.; David, I.; Pop, G.; Lukinich-Gruia, A.T.; Mioc, M.; Mioc, A.; Șoica, C.; Muntean, D.; et al. In Silico and In Vitro Evaluation of the Antimicrobial and Antioxidant Potential of Mentha × smithiana R. GRAHAM Essential Oil from Western Romania. Foods 2021, 10, 815. [Google Scholar] [CrossRef] [PubMed]
- Tchakal-Mesbahi, A.; Abdouni, M.A.; Metref, M. Prevalence of Multidrug-Resistant Bacteria Isolated from Burn Wounds In Algeria. Ann. Burn. Fire Disasters 2021, 34, 150–156. [Google Scholar]
- Hak-Jae, K.; Sae, W.N.; Hissah, A.A.; Munirah, A.A.D.; Nandhakumar, P.; Dyona, L. Prevalence of multidrug-resistant bacteria associated with polymicrobial infections. J. Infect. Public Health 2021, 14, 1864–1869. [Google Scholar]
- Uçkay, I.; Hoffmeyer, P.; Lew, D.; Pittet, D. Prevention of surgical site infections in orthopaedic surgery and bone trauma: State-of-the-art update. J. Hosp. Infect. 2013, 84, 5–12. [Google Scholar] [CrossRef]
- Lye, D.C.; Earnest, A.; Ling, M.L.; Lee, T.-E.; Yong, H.-C.; Fisher, D.A.; Krishnan, P.; Hsu, L.-Y. The impact of multidrug resistance in healthcare-associated and nosocomial Gram-negative bacteraemia on mortality and length of stay: Cohort study. Clin. Microbiol. Infect. 2012, 8, 502–508. [Google Scholar] [CrossRef]
- Feleke, T.; Eshetie, S.; Dagnew, M.; Endris, M.; Abebe, W.; Tiruneh, M.; Moges, F. Multidrug-resistant bacterial isolates from patients suspected of Nosocomial infections at the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. BMC Res. Notes 2018, 11, 602–608. [Google Scholar] [CrossRef]
- Tong, S.Y.C.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G., Jr. Staphylococcus aureus Infections: Epidemiology, Pathophysiology, Clinical Manifestations, and Management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef]
- Zeynudin, A.; Pritsch, M.; Schubert, S.; Messerer, M.; Liegl, G.; Belachew, M.H.T.; Wieser, A. Prevalence and antibiotic susceptibility pattern of CTX-M type extended-spectrum β-lactamases among clinical isolates of gram-negative bacilli in Jimma, Ethiopia. BMC Infect. Dis. 2018, 18, 524–533. [Google Scholar] [CrossRef]
- Lin, M.F.; Lan, C.Y. Antimicrobial resistance in Acinetobacter baumannii: From bench to bedside. World J. Clin. Cases 2014, 2, 787–814. [Google Scholar] [CrossRef] [PubMed]
- Dessie, W.; Mulugeta, G.; Fentaw, S.; Mihret, A.; Hassen, M.; Abebe, E. Pattern of bacterial pathogens and their susceptibility isolated from surgical site infections at selected referral hospitals, Addis Ababa, Ethiopia. Int. J. Microbiol. 2016, 2016, 2418902. [Google Scholar] [CrossRef] [PubMed]
- Agodi, A.; Zarrilli, R.; Barchitta, M.; Anzaldi, A.; Di Popolo, A.; Mattaliano, A.; Ghiraldi, E.; Travali, S. Alert surveillance of intensive care unit-acquired Acinetobacter infections in a Sicilian hospital. Clin. Microbiol. Infect. 2006, 12, 241–247. [Google Scholar] [CrossRef][Green Version]
- Ylipalosaari, P.; Ala-Kokko, T.I.; Laurila, J.; Ohtonen, P.; Syrjala, H. Intensive care acquired infection is an independent risk factor for hospital mortality: A prospective cohort study. Crit. Care 2006, 10, R66–R71. [Google Scholar] [CrossRef] [PubMed]
- Gomila, M.; Del Carmen, G.M.; Fernandez-Baca, V.; Pareja, A.; Pascual, M.; Diaz-Antolin, P.; García-Valdés, E.; Lalucat, J. Genetic diversity of clinical Pseudomonas aeruginosa isolates in a public hospital in Spain. BMC Microbiol. 2013, 13, 1471–2180. [Google Scholar] [CrossRef]
- Werarak, P.; Kiratisin, P.; Thamlikitkul, V. Hospital-acquired pneumonia and ventilator-associated pneumonia in adults at Siriraj Hospital: Etiology, clinical outcomes, and impact of antimicrobial resistance. J. Med. Assoc. Thai. 2010, 93, S126–S138. [Google Scholar] [PubMed]
- Zahlane, K.; Ouafi, A.T.; Barakate, M. The clinical and epidemiological risk factors of infections due to multidrug resistant bacteria in an adult intensive care unit of University Hospital Center in Marrakesh-Morocco. J. Infect. Public Health 2020, 13, 637–643. [Google Scholar]
- Negussie, A.; Mulugeta, G.; Bedru, A.; Ali, I.; Shimeles, D.; Lema, T.; Aseffa, A. Bacteriological profile and antimicrobial susceptibility pattern of blood culture isolates among septicemia suspected children in selected hospitals Addis Ababa, Ethiopia. Int. J. Biol. Med. Res. 2015, 6, 4709–4717. [Google Scholar]
- Latif, S.; Anwar, M.S.; Ahmad, I. Bacterial pathogens responsible for blood stream infection (BSI) and pattern of drug resistance in a tertiary care hospital of Lahore. Biomedica 2009, 25, 101–105. [Google Scholar]
- Dilnessa, T.; Bitew, A. Prevalence and antimicrobial susceptibility pattern of methicillin resistant Staphylococcus aureus isolated from clinical samples at Yekatit 12 Hospital Medical College, Addis Ababa, Ethiopia. BMC Infect. Dis. 2016, 16, 398–406. [Google Scholar] [CrossRef]
- Džamić, A.M.; Nikolić, B.J.; Giweli, A.A.; Mitić-Ćulafić, D.S.; Soković, M.D.; Ristić, M.S.; Knežević-Vukčević, J.B.; Marin, P.D. Libyan Thymus capitatus essential oil: Antioxidant, antimicrobial cytotoxic and colon pathogen adhesion-inhibition properties. J. Appl. Microbiol. 2015, 119, 389–399. [Google Scholar] [CrossRef]
- Moumni, S.; Elaissi, A.; Trabelsi, A.; Merghni, A.; Chraief, I.; Jelassi, B.; Chemli, R.; Ferchich, S. Correlation between chemical composition and antibacterial activity of some Lamiaceae species essential oils from Tunisia. BMC Complement. Altern. Med. 2020, 20, 103–117. [Google Scholar] [CrossRef]
- Ezzeddine, N.B.H.B.; Abdelkéfi, M.M.; Aissa, R.B.; Chaabouni, M.M. Antibacterial screening of Origanum majorana L. oil from Tunisia. J. Essent. Oil Res. 2001, 13, 295–297. [Google Scholar] [CrossRef]
- Sellami, I.H.; Maamouri, E.; Chahed, T.; Wannes, W.A.; Kchouk, M.E.; Marzouk, B. Effect of growth stage on the content and composition of the essential and phenolic fraction of sweet marjoram (Origanum majorana L.). Ind. Crop. Prod. 2009, 30, 395–402. [Google Scholar] [CrossRef]
- Elaissi, A.; Rouis, Z.; Mabrouk, S.; Salah, K.B.H.; Aouni, M.; Khouja, M.L.; Farhat, F.; Chemli, R.; Harzallah-Skhiri, F. Correlation between chemical compostion and antibacterial activity of essential oils from fifteen eucalyptus species growing in Korbous and Jbel Abderrahmam arboreta north East Tunisia. Molecules 2012, 17, 3044–3057. [Google Scholar] [CrossRef]
- Delamare, L.A.P.; Pisterello, M.I.T.; Artico, L.; Atti-Serafini, L.; Echeverrigary, S. Antibqcterial activity of the essential oils of Salvia officinalis L.and Salvia triloba L.cultivated in south Brazil. Food Chem. 2007, 100, 603–608. [Google Scholar]
- Dob, T.; Berramdane, T.; Dahmane, D.; Benabdelkader, T.; Chelghoum, C. Chemical composition of the essential oil of Salvia officinalis from Algeria. Chem. Nat. Compd. 2007, 43, 491–494. [Google Scholar] [CrossRef]
- Boutebouhart, H.; Didaoui, L.; Tata, S.; Sabaou, N. Effect of Extraction and Drying Method on Chemical Composition, and Evaluation of Antioxidant and Antimicrobial Activities of Essential Oils from Salvia officinalis L. J. Essent. Oil-Bear. Plants 2019, 22, 717–727. [Google Scholar] [CrossRef]
- Mahdjoubi, H.; Bakchiche, B.; Gherib, A.; Boudjelal, F.; Bardaweel, S.K. Essential Oil of Salvia officinalis L. from the Algerian Saharan Atlas: Chemical Composition and Biological Evaluation. Jordan J. Pharm. Sci. 2020, 13, 415–423. [Google Scholar]
- Craft, J.D.; Satyal, P.; Setzer, W.N. The Chemotaxonomy of Common Sage (Salvia officinalis) Based on the Volatile Constituents. Medicines 2017, 4, 47. [Google Scholar] [CrossRef]
- Benaliouche, F.; Sbartai, H.; Meraghni, M.; Hadj-Moussa, H.; Sbartai, I. Chemical characterization of the essential oil of Thymus vulgaris and evaluation of its antifungal activity on the apple scab pathogen (Venturia inaequalis L.). CATRINA 2021, 21, 57–65. [Google Scholar]
- Satyal, P.; Murray, B.L.; McFeeters, R.L.; Setzer, W.N. Essential Oil Characterization of Thymus vulgaris from Various Geographical Locations. Foods 2016, 5, 70. [Google Scholar] [CrossRef]
- Abdelli, W.; Sysak, A.; Bahri, F.; Szumny, A.; Pawlak, A.; Obmiska-Mrukowicz, B. Chemical composition, antimicrobial and cytotoxic activity of essential oils of Algerian Thymus vulgaris L. Acta Pol. Pharm. 2019, 76, 1051–1059. [Google Scholar] [CrossRef]
- Abdelli, W.; Bahri, F.; Romane, A.; Höferl, M.; Wanner, J.; Schmidt, E.; Jirovetz, L. Chemical Composition and Anti-inflammatory Activity of Algerian Thymus vulgaris Essential Oil. Nat. Prod. Commun. 2017, 12, 611–614. [Google Scholar] [CrossRef]
- Oliva, M.M.; Beltramino, E.; Gallucci, N.; Casero, C.; Zygadlo, J.; Demo, M. Antimicrobial activity of essential oils of Aloysia triphylla (L’Her.) Britton from different regions of Argentina. Bol. Latinoam. Caribe. Plant Med. Aromat. 2010, 9, 21–37. [Google Scholar]
- Ho, M.J.; Bakkalbasi, E.; Söll, D.; Miller, A.C. Drugging tRNA aminoacylation. RNA Biol. 2018, 15, 667–677. [Google Scholar] [CrossRef]
- Aliye, M.; Dekebo, A.; Tesso, H.; Abdo, T.; Eswaramoorthy, R.; Melaku, Y. Molecular docking analysis and evaluation of the antibacterial and antioxidant activities of the constituents of Ocimum cufodontii. Sci. Rep. 2021, 11, 10101–10111. [Google Scholar] [CrossRef]
- Salvi, P.; Kumar, G.; Gandass, N.; Kajal; Verma, A.; Rajarammohan, S.; Rai, N.; Gautam, V. Antimicrobial Potential of Essential Oils from Aromatic Plant Ocimum sp.; A Comparative Biochemical Profiling and In-SilicoAnalysis. Agronomy 2022, 12, 627. [Google Scholar] [CrossRef]
- Kitamura, Y.; Ebihara, A.; Agari, Y.; Shinkai, A.; Hirotsu, K.; Kuramitsu, S. Structure of d-alanine-d-alanine ligase from Thermusthermophilus HB8: Cumulative conformational change and enzyme–ligand interactions. Acta Crystallogr. Sect. D Biol. Crystallogr. 2009, 65, 1098–1106. [Google Scholar] [CrossRef]
- Trombetta, D.; Castelli, F.; Sarpietro, M.G.; Venuti, V.; Cristani, M.; Daniele, C.; Saija, A.; Mazzanti, G.; Bisignano, G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005, 49, 2474–2478. [Google Scholar] [CrossRef]
- Basu, J.; Chattopadhyay, R.; Kundu, M.; Chakrabarti, P. Purification and partial characterization of a penicillin-binding proteinfrom Mycobacterium smegmatis. J. Bacteriol. 1992, 174, 4829–4832. [Google Scholar] [CrossRef]
- Cappuccino, G.J.; Sherman, N. Biochemical activities of microorganisms. In Microbiology: A Laboratory Manual, 6th ed.; Pearson Education USA: Upper Saddle River, NJ, USA, 2001; Volume 5, pp. 133–194. [Google Scholar]
- Cheesbrough, M. Microbiological test. In District Laboratory Practice in Tropical Countries, 2nd ed.; Cambridge University Press: Cambridge, UK, 2009; Volume 7, pp. 1–226. [Google Scholar] [CrossRef]
- Jonathan, N. Screening for extended-spectrum beta-lactamase-producing pathogenic enterobacteria in district general hospitals. J. Clin. Microbiol. 2005, 43, 1488–1490. [Google Scholar] [CrossRef]
- Tan, T.Y.; Yong Ng, L.S.; He, J.; Koh, T.H.; Hsu, L.Y. Evaluation of screening methods to detect plasmid-mediated AmpC in Escherichia coli, Klebsiella pneumoniae, and Proteus mirabilis. Antimicrob. Agents Chemother. 2009, 53, 146–149. [Google Scholar] [CrossRef] [PubMed]
- Hidri, N.; Ploy, M.C. Instauration et surveillance d’un traitement antibiotique. In Bacteriologiemedicale, 2nd ed.; Denis, F., Ploy, M.C., Martin, C., Bingen, E., Quentin, R., Eds.; Elsevier Masson: Paris, France, 2016; Volume 42, pp. 585–594. [Google Scholar]
- Bosch, M.; Nart, J.; Audivert, S.; Bonachera, M.A.; Alemany, A.S.; Fuentes, M.C.; Cuné, J. Isolation and characterization of probiotic strains for improving oral health. Arch. Oral Biol. 2012, 57, 539–549. [Google Scholar] [CrossRef]
- Natarajan, D.; Britto, S.J.; Srinivasan, K.; Nagamurugan, N.; Mohanasundari, C.; Perumal, G. Anti-bacterial activity of Euphorbia fusiformis a rare medicinal herb. J. Ethnopharmacol. 2005, 102, 123–126. [Google Scholar] [CrossRef]
- Taleb, M.H.; Abdeltawab, N.F.; Shamma, R.N.; Abdelgayed, S.S.; Mohamed, S.S.; Farag, M.A.; Ramadan, M.A. Origanum vulgare L. Essential Oil as a Potential Anti-Acne Topical Nanoemulsion—In Vitro and In Vivo Study. Molecules 2018, 23, 2164. [Google Scholar] [CrossRef]
- Kaloustian, J.; Abou, L.; Mikail, C.; Amiot, M.J.; Portugal, H. Southern French thyme oils: Chromatographic study of chemotypes. J. Sci. Food Agric. 2005, 85, 2437–2444. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas. In Chromatography/Mass Spectroscopy, 4th ed.; Allured Publishing Corporation: Carol Stream, IL, USA, 2007. [Google Scholar]
- Liu, Y.; Yang, X.; Gan, J.; Chen, S.; Xiao, Z.-X.; Cao, Y. CB-Dock2: Improved protein–ligand blind docking by integrating cavity detection, docking and homologous template fitting. Nucleic Acids Res. 2022, 50, 159–164. [Google Scholar] [CrossRef]
- Farouk, A.; Mohsen, M.; Ali, H.; Shaaban, H.; Albaridi, N. Antioxidant Activity and Molecular Docking Study of Volatile Constituents from Different Aromatic Lamiaceous Plants Cultivated in Madinah Monawara, Saudi Arabia. Molecules 2021, 26, 4145. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).