N-Acetylcysteine Protects Bladder Epithelial Cells from Bacterial Invasion and Displays Antibiofilm Activity against Urinary Tract Bacterial Pathogens
Abstract
1. Introduction
2. Results
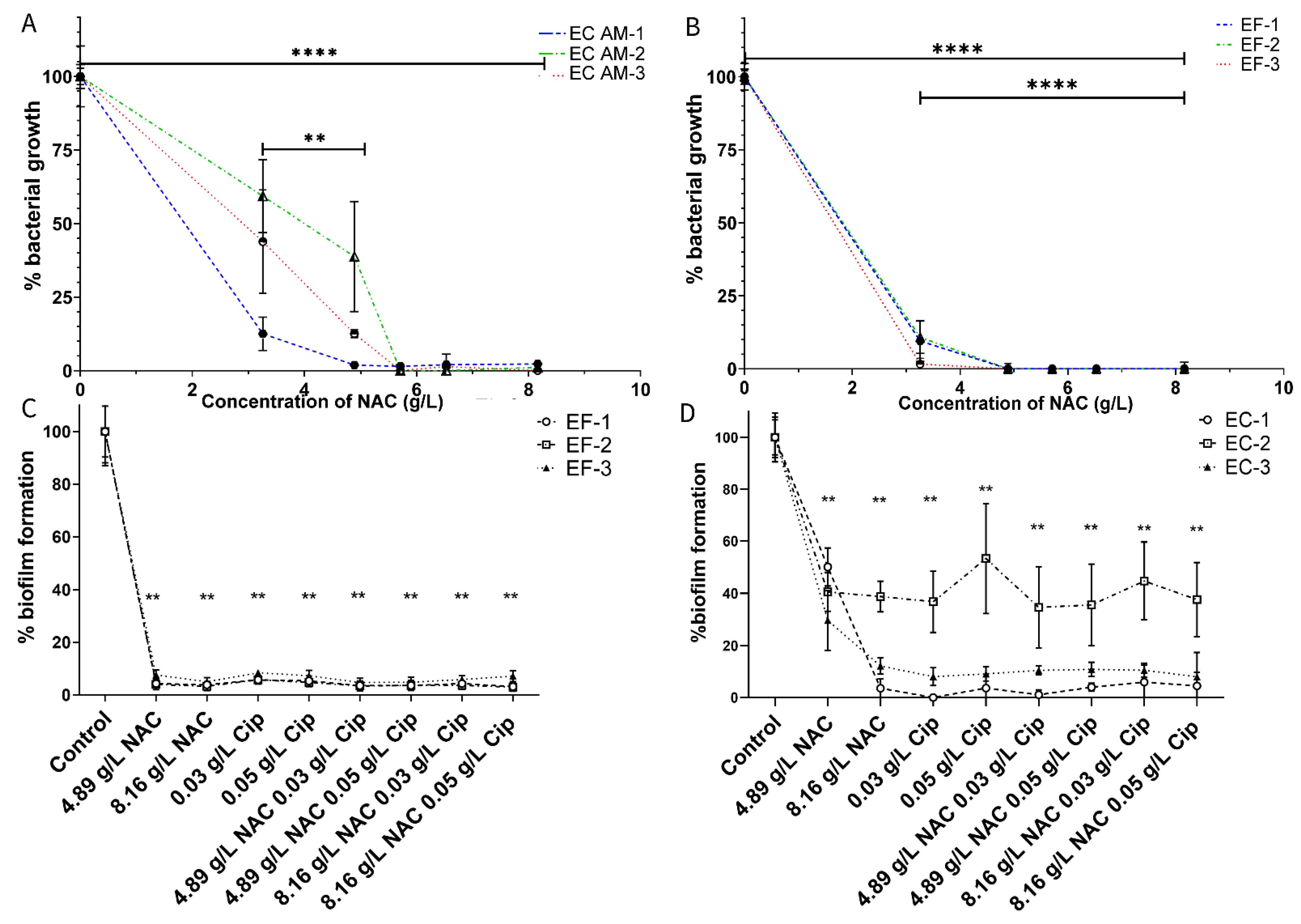
2.1. NAC Inhibits Uropathogenic Growth and Biofilm Formation
2.2. Bacterial Adhesion and Aggregation on BECs Are Inhibited in the Presence of NAC
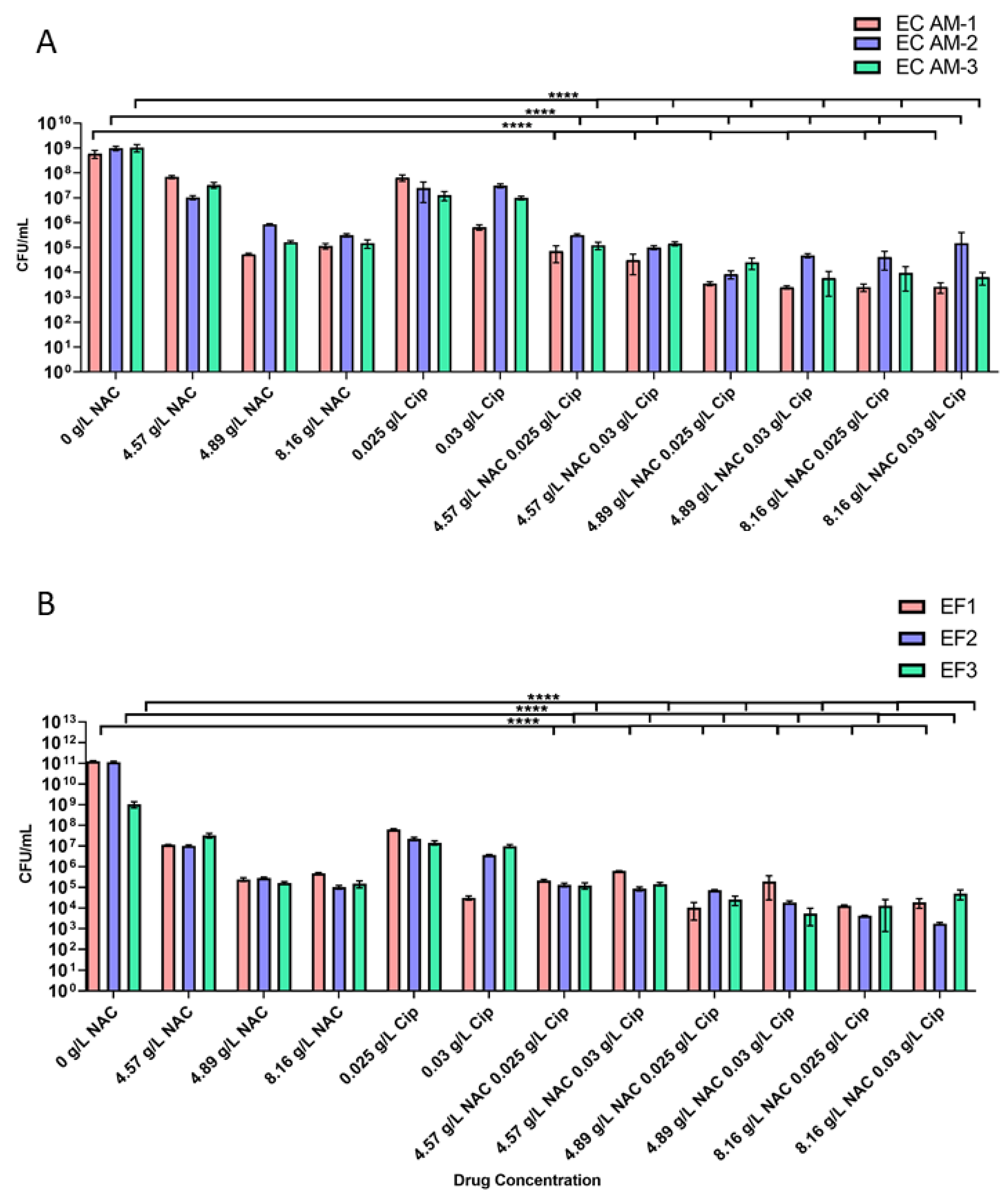
2.3. Treatment with NAC or NAC + Ciprofloxacin Decreased Bacterial Burden in Biofilms
2.4. NAC + Ciprofloxacin Significantly Disrupted E. coli and E. faecalis Biofilm Biomass
2.5. NAC Does Not Display a Concentration-Dependent Cytotoxic Effect on BECs
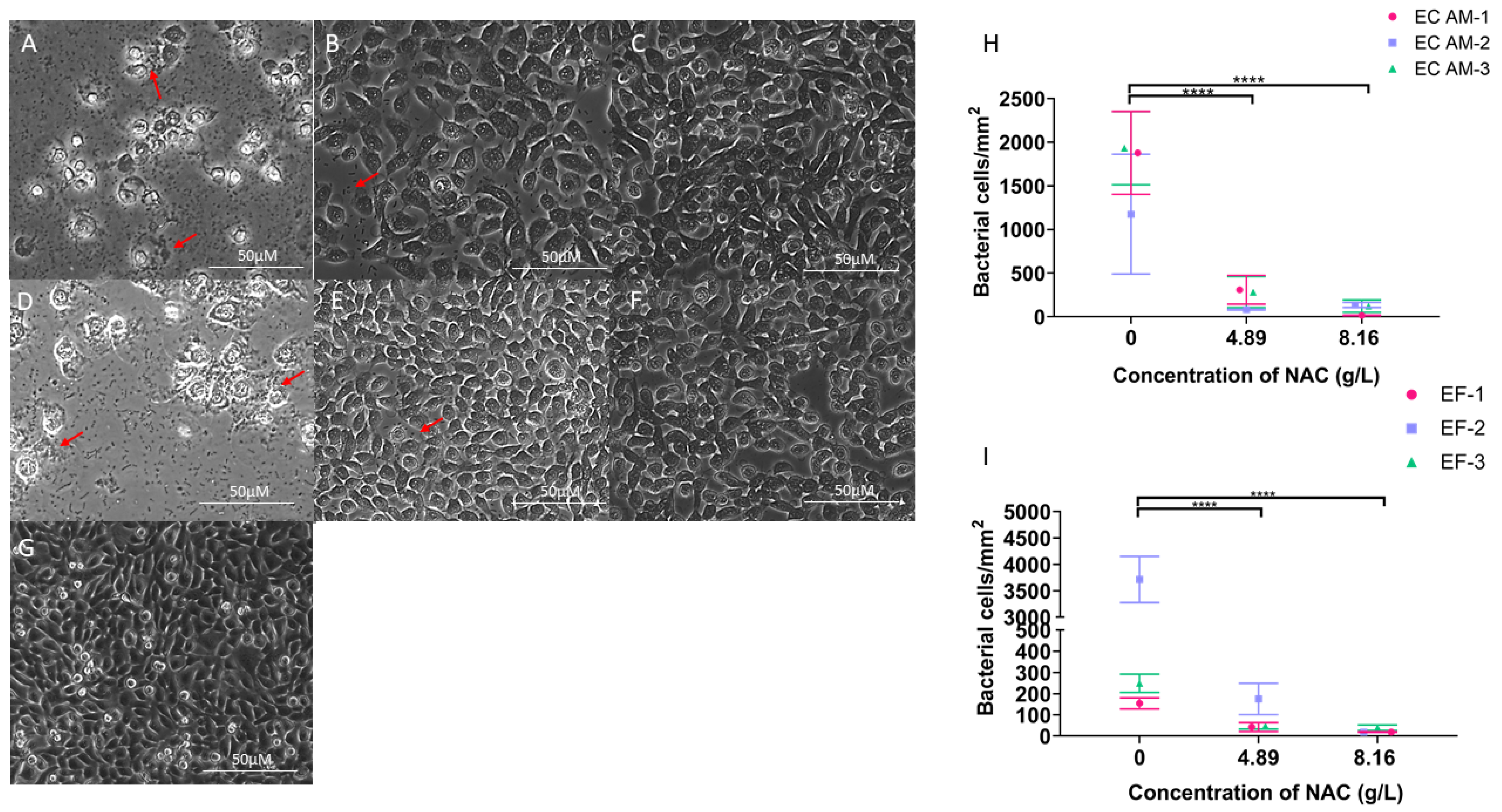
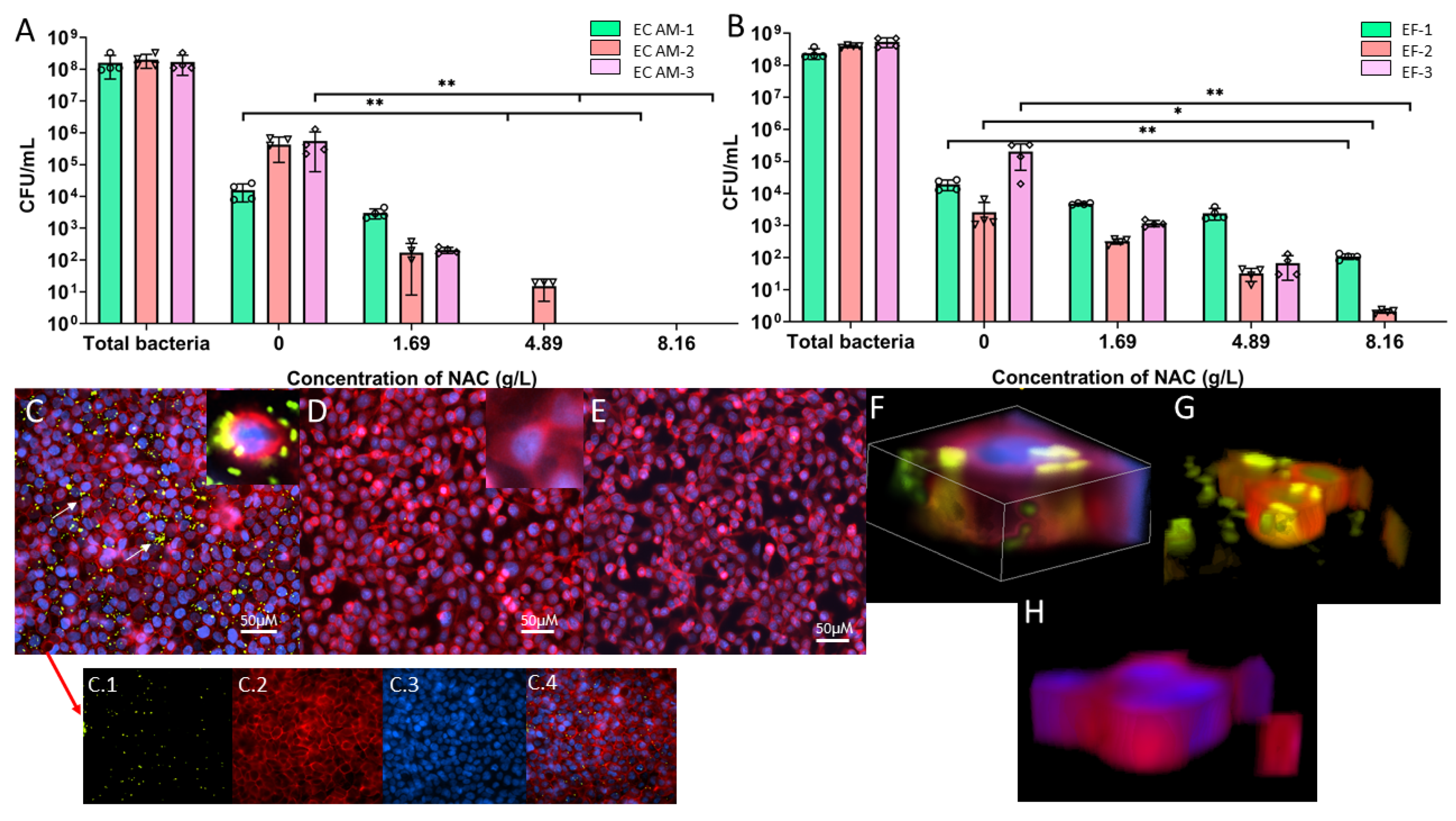
2.6. NAC Protects BECs from Bacterial Invasion In Vitro
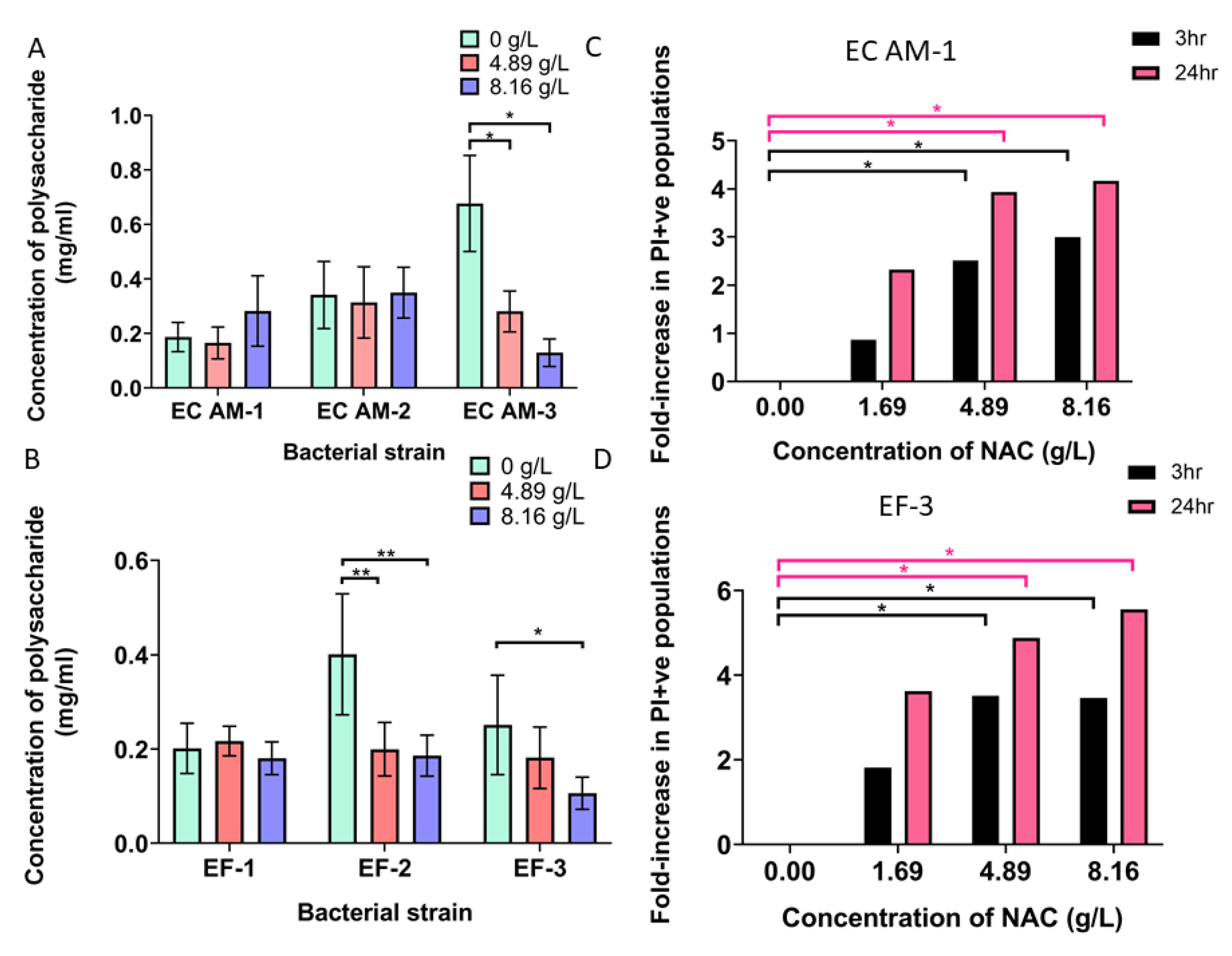
2.7. NAC Damaged Bacterial Membranes and Decreased Polysaccharide Production in a Strain-Dependent Manner
3. Discussion
4. Materials and Methods
4.1. Reagents and Media Used in This Study
4.2. Cell Culture
4.3. Determining the Effect of NAC on Planktonic Growth of UPEC and E. faecalis
4.4. Determining Minimum Biofilm Inhibitory Concentrations for Uropathogenic Strains
4.5. Investigating the Effect of NAC on Initial Adhesion of Uropathogens to Polymer Substratum
4.6. Quantification of Bacterial Load in Biofilms Exposed to Treatment
4.7. Measuring NAC Cytotoxicity on Bladder Epithelial Cells (BECs)
4.8. Determining Inhibition of Bacterial Growth in Presence of NAC on Pre-Confluent BECs
4.9. GFP-Tagging of Clinical Uropathogenic E.coli for Confocal Microscopy Tracking of Uropathogenic Invasion
4.10. Determining Inhibition of Bacterial Cell Invasion in Presence of NAC
4.11. Visualising Inhibition of Uropathogenic E.coli Intracellular Bacterial Colonies in BECs
4.12. Establishing Inhibition of Exopolysaccharide Production by NAC in Biofilm Matrix
4.13. Disruption of Biofilm Architecture Following Treatment with NAC
4.14. Quantification of Bacterial Membrane Damage by NAC
4.15. Statistical Analysis of Data
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stamm, W.; Norby, S. Urinary Tract Infection Disease: Phenomena and Challenges. J. Infect. Dis. 2001, 183, 51–54. [Google Scholar] [CrossRef]
- Mittal, R.; Aggarwal, S.; Sharma, S.; Chhibber, S.; Harjai, K. Urinary tract infections caused by Pseudomonas aeruginosa: A minireview. J. Infect. Public Heal. 2009, 2, 101–111. [Google Scholar] [CrossRef]
- Derington, C.G.; Benavides, N.; Delate, T.; Fish, D.N. Multiple-Dose Oral Fosfomycin for Treatment of Complicated Urinary Tract Infections in the Outpatient Setting. Open Forum Infect. Dis. 2020, 7, ofaa034. [Google Scholar] [CrossRef]
- Care ACoSaQiH. The Fourth Australian Atlas of Healthcare Variation; Care ACoSaQiH: Sydney, Australia, 2021. [Google Scholar]
- McLellan, L.K.; Hunstad, D.A. Urinary tract infection: Pathogenesis and outlook. Trends Mol. Med. 2016, 22, 946–957. [Google Scholar] [CrossRef]
- Horsley, H.; Malone-Lee, J.; Holland, D.; Tuz, M.; Hibbert, A.; Kelsey, M.; Kupelian, A.; Rohn, J.L. Enterococcus faecalis Subverts and Invades the Host Urothelium in Patients with Chronic Urinary Tract Infection. PLoS ONE 2013, 8, e83637. [Google Scholar] [CrossRef]
- Kucheria, R.; Dasgupta, P.; Sacks, S.H.; Khan, M.S.; Sheerin, N.S. Urinary tract infections: New insights into a common problem. Postgrad. Med. J. 2005, 81, 83–86. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Leslie, S.W. Cystitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Eberly, A.R.; Floyd, K.A.; Beebout, C.J.; Colling, S.J.; Fitzgerald, M.J.; Stratton, C.W.; Schmitz, J.E.; Hadjifrangiskou, M. Biofilm Formation by Uropathogenic Escherichia coli Is Favored under Oxygen Conditions That Mimic the Bladder Environment. Int. J. Mol. Sci. 2017, 18, 2077. [Google Scholar] [CrossRef] [PubMed]
- Kunin, C.M. Urinary tract infections in females. Clin. Infect. Dis. 1994, 18, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Orenstein, R.; Wong, E.S. Urinary tract infections in adults. Am. Fam. Physician 1999, 59, 1225. [Google Scholar]
- Higuita, N.I.A.; Huycke, M.M. Enterococcal disease, epidemiology, and implications for treatment. In Enterococci: From Commensals to Leading Causes of Drug Resistant Infection; Gilmore, M.S., Clewell, D.B., Ike, Y., Shankar, N., Eds.; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- Terlizzi, M.E.; Gribaudo, G.; Maffei, M.E. UroPathogenic Escherichia coli (UPEC) Infections: Virulence Factors, Bladder Responses, Antibiotic, and Non-antibiotic Antimicrobial Strategies. Front. Microbiol. 2017, 8, 1566. [Google Scholar] [CrossRef] [PubMed]
- Kai-Larsen, Y.; Lüthje, P.; Chromek, M.; Peters, V.; Wang, X.; Holm, Å.; Kádas, L.; Hedlund, K.-O.; Johansson, J.; Chapman, M.; et al. Uropathogenic Escherichia coli Modulates Immune Responses and Its Curli Fimbriae Interact with the Antimicrobial Peptide LL-37. PLoS Pathog. 2010, 6, e1001010. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.L.; Martin, S.M.; Lyon, W.; Hayes, E.; Caparon, M.G.; Hultgren, S.J. Enterococcus faecalis Tropism for the Kidneys in the Urinary Tract of C57BL/6J Mice. Infect. Immun. 2005, 73, 2461–2468. [Google Scholar] [CrossRef] [PubMed]
- Delcaru, C.; Alexandru, I.; Podgoreanu, P.; Grosu, M.; Stavropoulos, E.; Chifiriuc, M.C.; Lazar, V. Microbial Biofilms in Urinary Tract Infections and Prostatitis: Etiology, Pathogenicity, and Combating strategies. Pathogens 2016, 5, 65. [Google Scholar] [CrossRef]
- Niveditha, S.; Pramodhini, S.; Umadevi, S.; Kumar, S.; Stephen, S. The Isolation and the Biofilm Formation of Uropathogens in the Patients with Catheter Associated Urinary Tract Infections (UTIs). J. Clin. Diagn. Res. 2012, 6, 1478–1482. [Google Scholar] [CrossRef]
- Marchese, A.; Bozzolasco, M.; Gualco, L.; Debbia, E.A.; Schito, G.C.; Schito, A.M. Effect of fosfomycin alone and in combination with N-acetylcysteine on E. coli biofilms. Int. J. Antimicrob. Agents 2003, 22, 95–100. [Google Scholar] [CrossRef]
- McDermott, C.; Chess-Williams, R.; Mills, K.; Kang, S.; Farr, S.; Grant, G.; Perkins, A.; Davey, A.; Anoopkumar-Dukie, S. Alterations in acetylcholine, PGE2 and IL6 release from urothelial cells following treatment with pyocyanin and lipopolysaccharide. Toxicol. Vitr. 2013, 27, 1693–1698. [Google Scholar] [CrossRef]
- Martinez, J.J.; Mulvey, M.; Schilling, J.D.; Pinkner, J.S.; Hultgren, S.J. Type 1 pilus-mediated bacterial invasion of bladder epithelial cells. EMBO J. 2000, 19, 2803–2812. [Google Scholar] [CrossRef]
- Bishop, B.L.; Duncan, M.J.; Song, J.; Li, G.; Zaas, D.; Abraham, S.N. Cyclic AMP–regulated exocytosis of Escherichia coli from infected bladder epithelial cells. Nat. Med. 2007, 13, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Kau, A.; Hunstad, D.; Hultgren, S.J. Interaction of uropathogenic Escherichia coli with host uroepithelium. Curr. Opin. Microbiol. 2005, 8, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Rosen, D.; Hooton, T.M.; Stamm, W.E.; Humphrey, P.A.; Hultgren, S.J. Detection of Intracellular Bacterial Communities in Human Urinary Tract Infection. PLoS Med. 2007, 4, e329. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.; Schilling, J.D.; Hultgren, S.J. Establishment of a Persistent Escherichia coli Reservoir during the Acute Phase of a Bladder Infection. Infect. Immun. 2001, 69, 4572–4579. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, M.A.; Lopez-Boado, Y.S.; Wilson, C.L.; Roth, R.; Parks, W.C.; Heuser, J.; Hultgren, S.J. Induction and evasion of host defenses by type 1-piliated uropathogenic Escherichia coli. Science 1998, 282, 1494–1497. [Google Scholar] [CrossRef] [PubMed]
- Flores-Mireles, A.L.; Walker, J.N.; Caparon, M.; Hultgren, S.J. Urinary tract infections: Epidemiology, mechanisms of infection and treatment options. Nat. Rev. Microbiol. 2015, 13, 269–284. [Google Scholar] [CrossRef]
- Mysorekar, I.U.; Hultgren, S.J. Mechanisms of uropathogenic Escherichia coli persistence and eradication from the urinary tract. Proc. Natl. Acad. Sci. USA 2006, 103, 14170–14175. [Google Scholar] [CrossRef] [PubMed]
- Das, T.; Paino, D.; Manoharan, A.; Farrell, J.; Whiteley, G.; Kriel, F.H.; Glasbey, T.; Manos, J. Conditions Under Which Glutathione Disrupts the Biofilms and Improves Antibiotic Efficacy of Both ESKAPE and Non-ESKAPE Species. Front. Microbiol. 2019, 10, 2000. [Google Scholar] [CrossRef] [PubMed]
- Zafarullah, M.; Li, W.; Sylvester, J.; Ahmad, M. Molecular mechanisms of N -acetylcysteine actions. Cell. Mol. Life Sci. 2003, 60, 6–20. [Google Scholar] [CrossRef] [PubMed]
- Costa, F.; Sousa, D.M.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-H.; Choi, Y.-S.; Lee, H.-W.; Heo, J.S.; Chang, S.W.; Lee, J.-Y. Antibacterial effects of N-acetylcysteine against endodontic pathogens. J. Microbiol. 2016, 54, 322–329. [Google Scholar] [CrossRef]
- Manoharan, A.; Das, T.; Whiteley, G.S.; Glasbey, T.; Kriel, F.H.; Manos, J. The effect of N-acetylcysteine in a combined antibiofilm treatment against antibiotic-resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2020, 75, 1787–1798. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Tran, C.; Whiteley, G.S.; Glasbey, T.; Kriel, F.H.; McKenzie, D.R.; Manos, J.; Das, T. Covalent Immobilization of N-Acetylcysteine on a Polyvinyl Chloride Substrate Prevents Bacterial Adhesion and Biofilm Formation. Langmuir 2020, 36, 13023–13033. [Google Scholar] [CrossRef]
- Aiyer, A.; Manoharan, A.; Paino, D.; Farrell, J.; Whiteley, G.S.; Kriel, F.H.; Glasbey, T.O.; Manos, J.; Das, T. Disruption of biofilms and killing of Burkholderia cenocepacia from cystic fibrosis lung using an antioxidant-antibiotic combination therapy. Int. J. Antimicrob. Agents 2021, 58, 106372. [Google Scholar] [CrossRef] [PubMed]
- Kundukad, B.; Schussman, M.; Yang, K.; Seviour, T.; Yang, L.; Rice, S.A.; Kjelleberg, S.; Doyle, P.S. Mechanistic action of weak acid drugs on biofilms. Sci. Rep. 2017, 7, 4783. [Google Scholar] [CrossRef]
- Olofsson, A.-C.; Hermansson, M.; Elwing, H. N-Acetyl- l -Cysteine Affects Growth, Extracellular Polysaccharide Production, and Bacterial Biofilm Formation on Solid Surfaces. Appl. Environ. Microbiol. 2003, 69, 4814–4822. [Google Scholar] [CrossRef] [PubMed]
- Anderson, G.G.; Palermo, J.J.; Schilling, J.D.; Roth, R.; Heuser, J.; Hultgren, S.J. Intracellular Bacterial Biofilm-Like Pods in Urinary Tract Infections. Science 2003, 301, 105–107. [Google Scholar] [CrossRef] [PubMed]
- Scott, V.C.; Haake, D.A.; Churchill, B.M.; Justice, S.S.; Kim, J.-H. Intracellular Bacterial Communities: A Potential Etiology for Chronic Lower Urinary Tract Symptoms. Urology 2015, 86, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Gamaley, I.; Efremova, T.; Kirpichnikova, K.; Kever, L.; Komissarchik, Y.; Polozov, Y.; Khaitlina, S. N-Acetylcysteine-induced changes in susceptibility of transformed eukaryotic cells to bacterial invasion. Cell Biol. Int. 2006, 30, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Fasugba, O.; Gardner, A.; Mitchell, B.G.; Mnatzaganian, G. Ciprofloxacin resistance in community- and hospital-acquired Escherichia coli urinary tract infections: A systematic review and meta-analysis of observational studies. BMC Infect. Dis. 2015, 15, 545. [Google Scholar] [CrossRef]
- El-Rehewy, M.S.K.; El-Feky, M.A.; Hassan, M.A.; Abolella, H.A.; Abolyosr, A.; El-Baky, R.M.A.; Gad, G.F. In vitro Efficacy of Ureteral Catheters Impregnated with Ciprofloxacin, N-acetylcysteine and their Combinations on Microbial Adherence. Clin. Med. Urol. 2009, 3, CMU–S3367. [Google Scholar]
- Cai, T.; Gallelli, L.; Meacci, F.; Brugnolli, A.; Prosperi, L.; Roberta, S.; Eccher, C.; Mazzoli, S.; Lanzafame, P.; Caciagli, P.; et al. The Efficacy of Umbelliferone, Arbutin, and N-Acetylcysteine to Prevent Microbial Colonization and Biofilm Development on Urinary Catheter Surface: Results from a Preliminary Study. J. Pathog. 2016, 2016, 1590952. [Google Scholar] [CrossRef] [PubMed]
- Harmon, C.; Hassoun, A. Antibiotic Bladder Irrigation in Preventing and Reducing Chronic Urinary Catheter-Related Urinary Tract Infections (UTI). Open Forum Infect. Dis. 2017, 4, S347. [Google Scholar] [CrossRef]
- Wu, J.; Miao, Y.; Abraham, S.N. The multiple antibacterial activities of the bladder epithelium. Ann. Transl. Med. 2017, 5, 35. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Liu, Y. N-acetylcysteine inhibit biofilms produced by Pseudomonas aeruginosa. BMC Microbiol. 2010, 10, 140. [Google Scholar] [CrossRef] [PubMed]
- Makipour, K.; Friedenberg, F.K. The potential role of N-acetylcysteine for the treatment of Helicobacter pylori. J. Clin. Gastroenterol. 2011, 45, 841. [Google Scholar] [CrossRef] [PubMed]
- Hancock, V.; Ferrières, L.; Klemm, P. Biofilm formation by asymptomatic and virulent urinary tract infectious Escherichia coli strains. FEMS Microbiol. Lett. 2007, 267, 30–37. [Google Scholar] [CrossRef]
- Blango, M.G.; Mulvey, M.A. Persistence of Uropathogenic Escherichia coli in the Face of Multiple Antibiotics. Antimicrob. Agents Chemother. 2010, 54, 1855–1863. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, N.M.; Martinez-Garcia, E.; Xavier, J.; Durham, W.M.; Kolter, R.; Kim, W.; Foster, K.R. Biofilm formation as a response to ecological competition. PLoS Biol. 2015, 13, e1002191. [Google Scholar] [CrossRef]
- Abebe, G.M. The Role of Bacterial Biofilm in Antibiotic Resistance and Food Contamination. Int. J. Microbiol. 2020, 2020, 1705814. [Google Scholar] [CrossRef] [PubMed]
- Coleman, N. Preparation of electrocompetent cells of Pseudomonas aeruginosa or E.coli. Available online: http://coleman-lab.org/lab-protocols/ (accessed on 31 March 2021).
- Chiba, A.; Sugimoto, S.; Sato, F.; Hori, S.; Mizunoe, Y. A refined technique for extraction of extracellular matrices from bacterial biofilms and its applicability. Microb. Biotechnol. 2015, 8, 392–403. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, M.; Wingender, J.; Flemming, H.-C. Application of fluorescently labelled lectins for the visualization and biochemical characterization of polysaccharides in biofilms of Pseudomonas aeruginosa. J. Microbiol. Methods 2002, 50, 237–248. [Google Scholar] [CrossRef]
- Rasband, W. ImageJ NIH; Bethesda: Rockville, MD, USA, 1997–2018. [Google Scholar]


| Bacterial Species | Source | Antibiotics | ||||
|---|---|---|---|---|---|---|
| CIP | AMK | AMC | NF | TC | ||
| E. faecalis-1 | St George Hospital, Kogarah, NSW, Australia; urine isolate | S | S | S | S | S |
| E. faecalis-2 | St George Hospital, Kogarah, NSW, Australia; indwelling catheter isolate | S | S | S | S | S |
| E. faecalis-3 | St George Hospital, Kogarah, NSW Australia; suprapubic catheter isolate | S | S | S | S | NT |
| E. coliAM-1 | Microbiology department Royal Prince Alfred Hospital (RPAH), Sydney, NSW, Australia; urine isolate | S | S | S | S | R |
| E. coliAM-2 | Microbiology department RPAH, Sydney, NSW, Australia; urine isolate | S | S | S | S | R |
| E. coliAM-3 | Microbiology department RPAH, Sydney, NSW, Australia; urine isolate | S | S | S | S | R |
| Combinations of Treatment Tested | NAC (g/L) | Ciprofloxacin (g/L) |
|---|---|---|
| 1.63 | - | |
| 4.57 | - | |
| 4.89 | - | |
| 8.16 | - | |
| - | 0.025 | |
| - | 0.03 | |
| 4.57 | 0.025 | |
| 4.57 | 0.03 | |
| 8.16 | 0.025 | |
| 8.16 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manoharan, A.; Ognenovska, S.; Paino, D.; Whiteley, G.; Glasbey, T.; Kriel, F.H.; Farrell, J.; Moore, K.H.; Manos, J.; Das, T. N-Acetylcysteine Protects Bladder Epithelial Cells from Bacterial Invasion and Displays Antibiofilm Activity against Urinary Tract Bacterial Pathogens. Antibiotics 2021, 10, 900. https://doi.org/10.3390/antibiotics10080900
Manoharan A, Ognenovska S, Paino D, Whiteley G, Glasbey T, Kriel FH, Farrell J, Moore KH, Manos J, Das T. N-Acetylcysteine Protects Bladder Epithelial Cells from Bacterial Invasion and Displays Antibiofilm Activity against Urinary Tract Bacterial Pathogens. Antibiotics. 2021; 10(8):900. https://doi.org/10.3390/antibiotics10080900
Chicago/Turabian StyleManoharan, Arthika, Samantha Ognenovska, Denis Paino, Greg Whiteley, Trevor Glasbey, Frederik H. Kriel, Jessica Farrell, Kate H. Moore, Jim Manos, and Theerthankar Das. 2021. "N-Acetylcysteine Protects Bladder Epithelial Cells from Bacterial Invasion and Displays Antibiofilm Activity against Urinary Tract Bacterial Pathogens" Antibiotics 10, no. 8: 900. https://doi.org/10.3390/antibiotics10080900
APA StyleManoharan, A., Ognenovska, S., Paino, D., Whiteley, G., Glasbey, T., Kriel, F. H., Farrell, J., Moore, K. H., Manos, J., & Das, T. (2021). N-Acetylcysteine Protects Bladder Epithelial Cells from Bacterial Invasion and Displays Antibiofilm Activity against Urinary Tract Bacterial Pathogens. Antibiotics, 10(8), 900. https://doi.org/10.3390/antibiotics10080900