Novel Nitro-Heteroaromatic Antimicrobial Agents for the Control and Eradication of Biofilm-Forming Bacteria
Abstract
1. Introduction
1.1. Biofilms in Nosocomial Infections
1.2. Antimicrobial Design
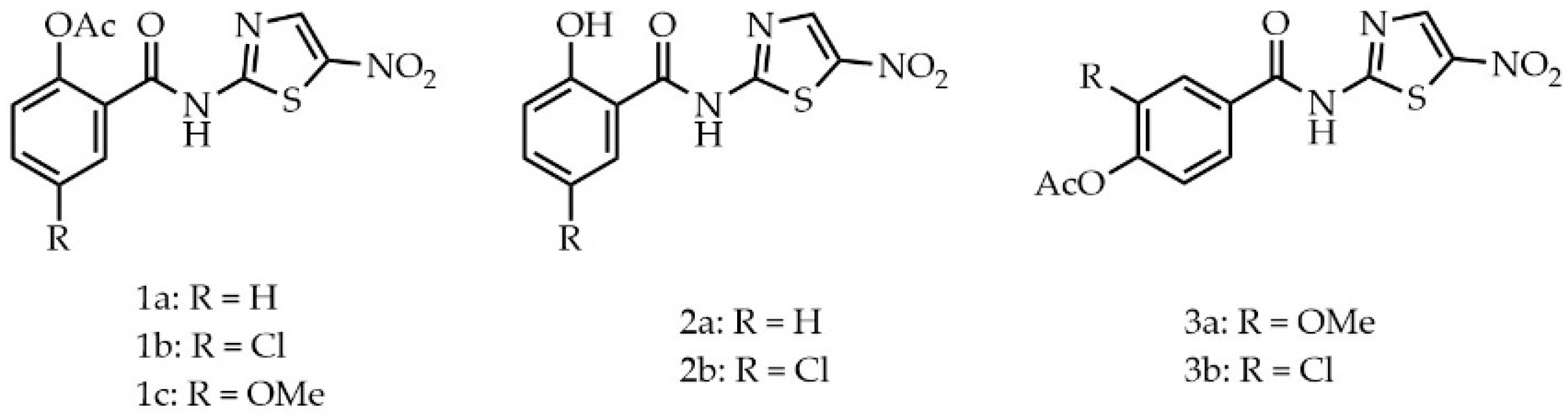
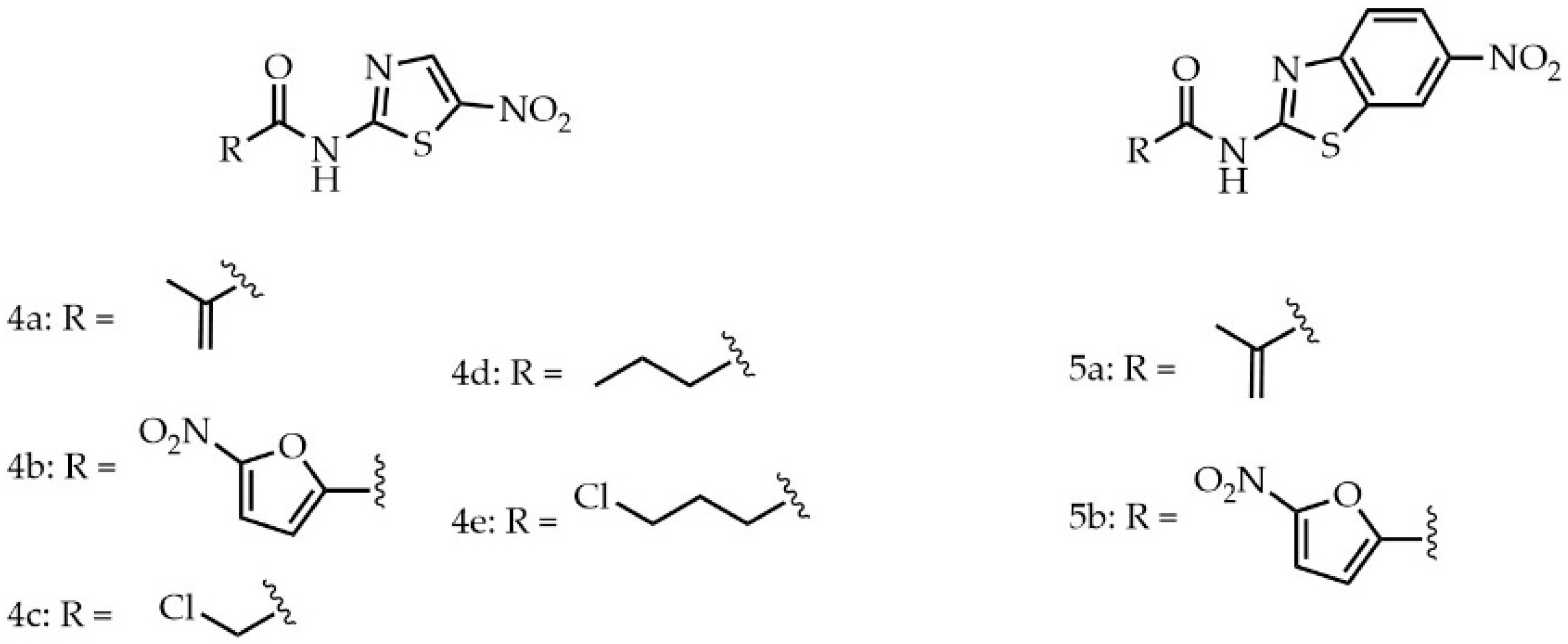
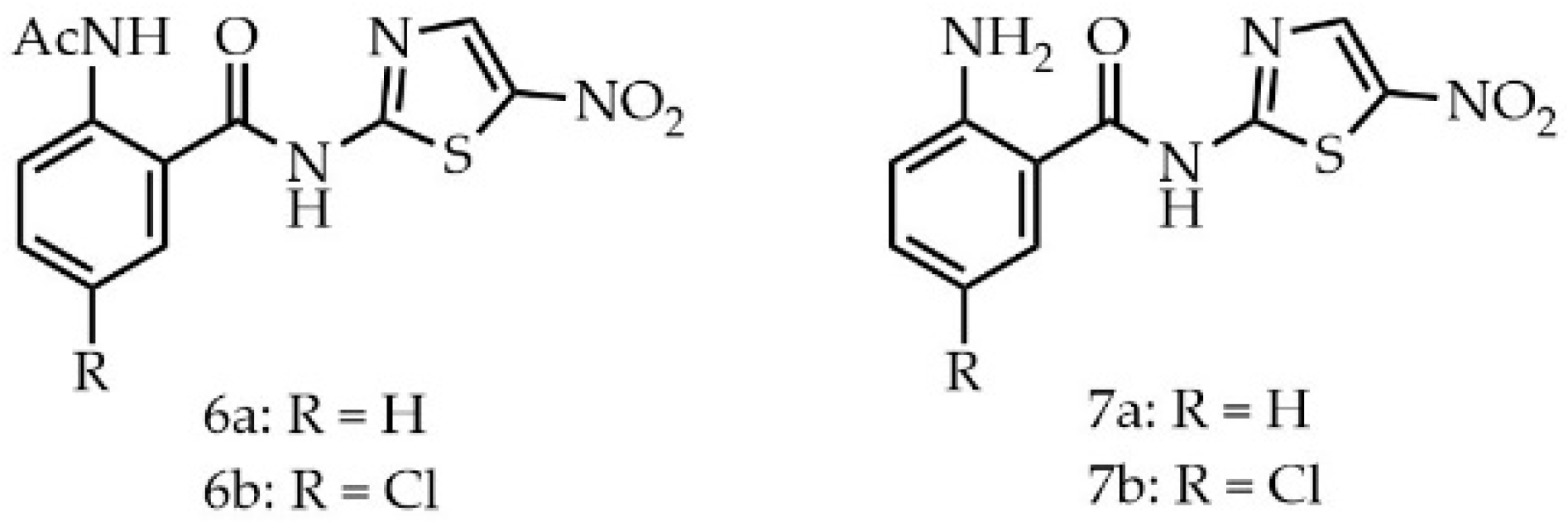
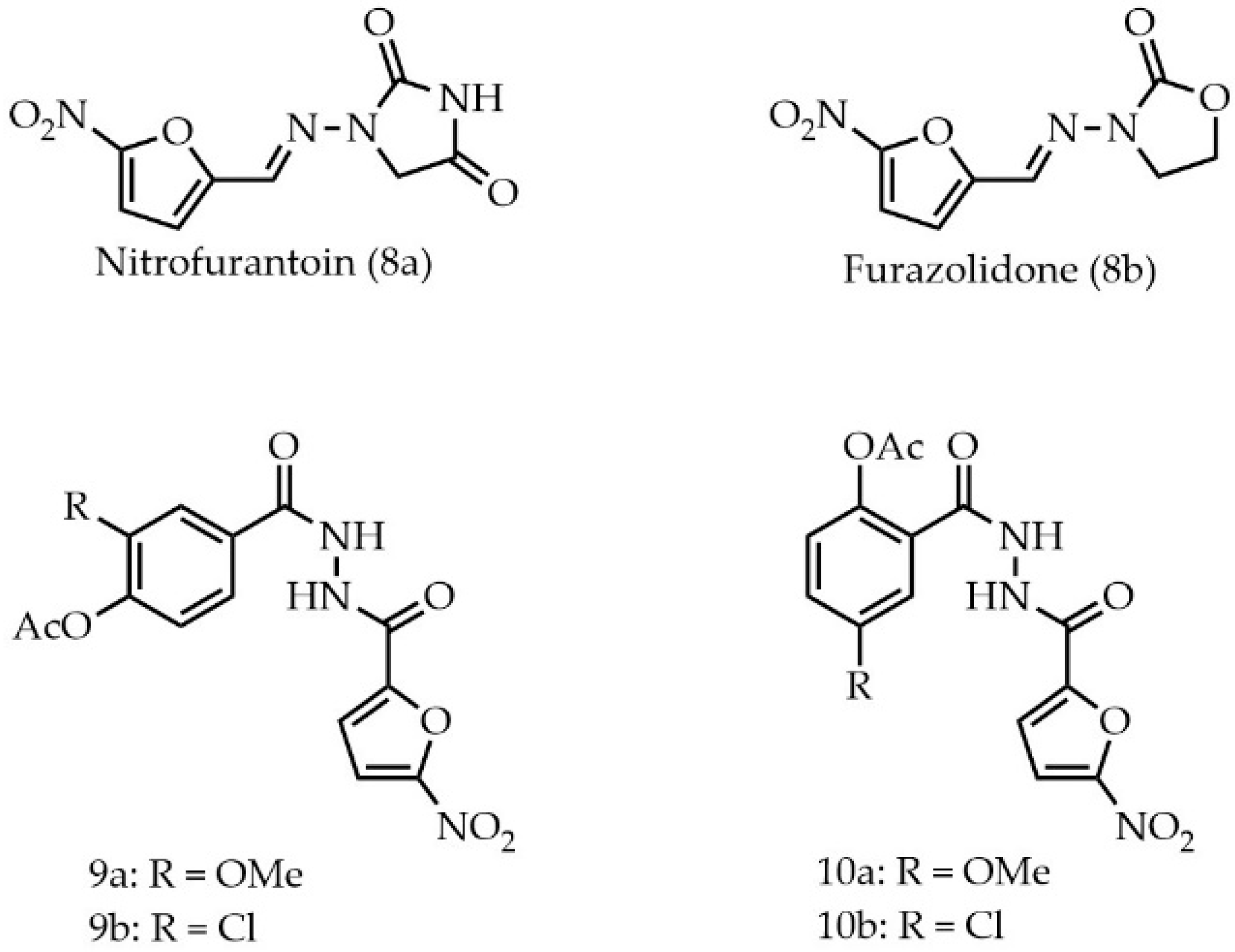
2. Results and Discussion
Biological Evaluation
3. Materials and Methods
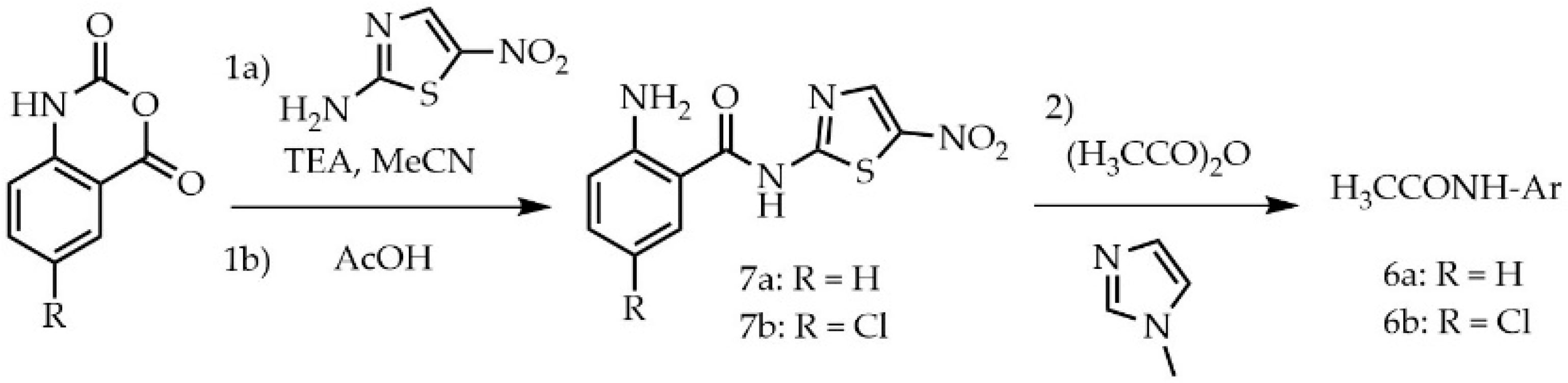
3.1. Chemistry
3.1.1. 2-Acetoxy-5-chloro-N-(5-nitrothiazol-2-yl)benzamide (1b)
3.1.2. 2-Acetoxy-5-methoxy-N-(5-nitrothiazol-2-yl)benzamide (1c)
3.1.3. 2-Hydroxy-N-(5-nitrothiazol-2-yl)benzamide (2a)
3.1.4. 5-Chloro-2-hydroxy-N-(5-nitrothizol-2-yl)benzamide (2b)
3.1.5. 2-Acetoxy-5-methoxy-N-(5-nitrothiazol-2-yl)benzamide (3a)
3.1.6. 4-Acetoxy-3-chloro-N-(5-nitrothiazol-2-yl)benzamide (3b)
3.1.7. N-(5-Nitrothiazol-2-yl)methacrylamide (4a)
3.1.8. 5-Nitro-N-(5-nitrothiazol-2-yl)furan-2-carboxamide (4b)
3.1.9. 2-Chloro-N-(5-nitrothiazol-2-yl)acetamide (4c)
3.1.10. N-(5-nitrothiazol-2-yl)butyramide (4d)
3.1.11. 4-chloro-N-(5-nitrothiazol-2-yl)butyramide (4e)
3.1.12. N-(6-Nitrobenzo[d]thiazol-2-yl)methacrylamide (5a)
3.1.13. 5-Nitro-N-(6-nitrobenzo[d]thiazol-2-yl)furan-2-carboxamide (5b)
3.1.14. 2-Acetamido-N-(5-nitrothizol-2-yl)benzamide (6a)
3.1.15. 2-Acetamido-5-chloro-N-(5-nitrothizol-2-yl)benzamide (6b)
3.1.16. 2-Amino-N-(5-nitrothiazol-2-yl)benzamide (7a)
3.1.17. 2-Amido-5-chloro-N-(5-nitrothizol-2-yl)benzamide (7b)
3.1.18. 4-Acetoxy-3-methoxy-N-(5-nitrofuran-2-carbonyl)benzhydrazide (9a)
3.1.19. 4-Acetoxy-3-chloro-N-(2-(5-nitrofuran-2-carbonyl)benzhydrazide (9b)
3.1.20. 2-Acetoxy-5-methoxy-N-(5-nitrofuran-2-carbonyl)benzhydrazide (10a)
3.1.21. 2-Acetoxy-5-chloro-N-(5-nitrofuran-2-carbonyl)benzhydrazide (10b)
3.2. Microbiology
4. Conclusions
4.1. Key Findings
4.2. Future Research
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hall, C.W.; Mah, T.F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef]
- Bryers, J.D. Medical biofilms. Biotechnol. Bioeng. 2008, 100, 1–18. [Google Scholar] [CrossRef]
- Stewart, P.S. Antimicrobial Tolerance in Biofilms. Microbiol. Spectr. 2015, 3, 13. [Google Scholar] [CrossRef]
- Venkatesan, N.; Perumal, G.; Doble, M. Bacterial resistance in biofilm-associated bacteria. Future Microbiol. 2015, 10, 1743–1750. [Google Scholar] [CrossRef]
- Ball, P. Antibiotic therapy of community respiratory tract infections: Strategies for optimal outcomes and minimized resistance emergence. J. Antimicrob. Chemother. 2002, 49, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.M.; Saravolatz, L.D. Nitazoxanide: A New Thiazolide Antiparasitic Agent. Clin. Infect. Dis. 2005, 40, 1173–1180. [Google Scholar] [CrossRef]
- Dubreuil, L.; Houcke, I.; Mouton, Y.; Rossignol, J.F. In vitro evaluation of activities of nitazoxanide and tizoxanide against anaerobes and aerobic organisms. Antimicrob. Agents Chemother. 1996, 40, 2266–2270. [Google Scholar] [CrossRef] [PubMed]
- Somvanshi, V.S.; Ellis, B.L.; Hu, Y.; Aroian, R.V. Nitazoxanide: Nematicidal mode of action and drug combination studies. Mol. Biochem. Parasitol. 2014, 193, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.W.; Zhang, H.; Yang, Y.J.; Li, J.Y.; Li, B.; Zhou, X.Z.; Zhang, J.Y. Synthesis, Antibacterial Evaluation and Molecular Docking Study of Nitazoxanide Analogues. Asian J. Chem. 2014, 26, 2921–2926. [Google Scholar] [CrossRef]
- Devasahayam, G.; Scheld, W.M.; Hoffman, P.S. Newer antibacterial drugs for a new century. Expert Opin. Investig. Drugs 2010, 19, 215–234. [Google Scholar] [CrossRef]
- Hoffman, P.S.; Sisson, G.; Croxen, M.A.; Welch, K.; Harman, W.D.; Cremades, N.; Morash, M.G. Antiparasitic Drug Nitazoxanide Inhibits the Pyruvate Oxidoreductases of Helicobacter pylori, Selected Anaerobic Bacteria and Parasites, and Campylobacter jejuni. Antimicrob. Agents Chemother. 2007, 51, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, A.J.; Bruce, A.M.; Gineste, C.; Ballard, T.E.; Olekhnovich, I.N.; Macdonald, T.L.; Hoffman, P.S. Synthesis and Antimicrobial Evaluation of Amixicile-Based Inhibitors of the Pyruvate-Ferredoxin Oxidoreductases of Anaerobic Bacteria and Epsilonproteobacteria. Antimicrob. Agents Chemother. 2016, 60, 3980–3987. [Google Scholar] [CrossRef]
- Ballard, T.E.; Wang, X.; Olekhnovich, I.; Koerner, T.; Seymour, C.; Salamoun, J.; Warthan, M.; Hoffman, P.S.; Macdonald, T.L. Synthesis and Antimicrobial Evaluation of Nitazoxanide-Based Analogues: Identification of Selective and Broad Spectrum Activity. ChemMedChem 2011, 6, 362–377. [Google Scholar] [CrossRef] [PubMed]
- Ballard, T.E.; Wang, X.; Olekhnovich, I.; Koerner, T.; Seymour, C.; Hoffman, P.S.; Macdonald, T.L. Biological activity of modified and exchanged 2-amino-5-nitrothiazole amide analogues of nitazoxanide. Bioorg. Med. Chem. Lett. 2010, 20, 3537–3539. [Google Scholar] [CrossRef] [PubMed]
- Mathur, H.; Rea, M.C.; Cotter, P.D.; Hill, C.; Ross, R.P. The efficacy of thuricin CD, tigecycline, vancomycin, teicoplanin, rifampicin and nitazoxanide, independently and in paired combinations against Clostridium difficile biofilms and planktonic cells. Gut Pathog. 2016, 8, 20. [Google Scholar] [CrossRef]
- Gau, J.S.; Lin, W.P.; Kuo, L.C.; Hu, M.K. Nitazoxanide Analogues as Antimicrobial Agents against Nosocomial Pathogens. Med. Chem. 2016, 12, 544–552. [Google Scholar] [CrossRef]
- Herrlich, P.; Schweiger, M. Nitrofurans, a group of synthetic antibiotics, with a new mode of action: Discrimination of specific messenger RNA classes. Proc. Natl. Acad. Sci. USA 1976, 73, 3386–3390. [Google Scholar] [CrossRef]
- Yempalla, K.R.; Munagala, G.; Singh, S.; Magotra, A.; Kumar, S.; Rajput, V.S.; Bharate, S.S.; Tikoo, M.; Singh, G.D.; Khan, I.A.; et al. Nitrofuranyl Methyl Piperazines as New Anti-TB Agents: Identification, Validation, Medicinal Chemistry, and PK Studies. ACS Med. Chem. Lett. 2015, 6, 1041–1046. [Google Scholar] [CrossRef]
- Wang, A.P.; Yang, Y.; Jun, Y.S.; Wang, B.; Lv, K.; Liu, M.L.; Guo, H.Y.; Lu, Y. Synthesis, evaluation and CoMFA/CoMSIA study of nitrofuranyl methyl N-heterocycles as novel antitubercular agents. Bioorg. Med. Chem. 2018, 26, 2073–2084. [Google Scholar] [CrossRef] [PubMed]
- Goldman, R.C. Maximizing bactericidal activity with combinations of bioreduced drugs. Future Med. Chem. 2010, 2, 1253–1271. [Google Scholar] [CrossRef] [PubMed]
- Moraski, G.C.; Thanassi, J.A.; Podos, S.D.; Pucci, M.J.; Miller, M.J. One-step syntheses of nitrofuranyl benzimidazoles that are active against multidrug-resistant bacteria. J. Antibiot. 2011, 64, 667–671. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mcosker, C.C.; Fitzpatrick, P.M. Nitrofurantoin: Mechanism of action and implications for resistance development in common uropathogens. J. Antimicrob. Chemother. 1994, 33, 23–30. [Google Scholar] [CrossRef]
- Chatterjee, S.N.; Ghosh, S. Mechanism of action of furazolidone: Inter-strand cross-linking in DNA & liquid holding recovery of Vibrio cholerae cells. Indian J. Biochem. Biophys. 1979, 16, 125–130. [Google Scholar]
- Walsh, D.J.; Livinghouse, T.; Goeres, D.M.; Mettler, M.; Stewart, P.S. Antimicrobial Activity of Naturally Occurring Phenols and Derivatives Against Biofilm and Planktonic Bacteria. Front. Chem. 2019, 7, 653. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Durling, G.M.; Chase-Bayless, Y.; Arnold, A.D.; Stewart, P.S. Sulfenate Esters of Simple Phenols Exhibit Enhanced Activity against Biofilms. ACS Omega 2020, 5, 6010–6020. [Google Scholar] [CrossRef]
- Walsh, D.J.; Livinghouse, T.; Durling, G.M.; Arnold, A.D.; Brasier, W.; Berry, L.; Goeres, D.M.; Stewart, P.S. Novel phenolic antimicrobials enhanced activity of iminodiacetate prodrugs against biofilm and planktonic bacteria. Chem. Biol. Drug Des. 2021, 97, 134–147. [Google Scholar] [CrossRef]
- Flemming, H.C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef]
- Donlan, R.M.; Costerton, J.W. Biofilms: Survival mechanisms of clinically relevant microorganisms. Clin. Microbiol. Rev. 2002, 15, 167–193. [Google Scholar] [CrossRef] [PubMed]
- Campanac, C.; Pineau, L.; Payard, A.; Baziard-Mouysset, G.; Roques, C. Interactions between biocide cationic agents and bacterial biofilms. Antimicrob. Agents Chemother. 2002, 46, 1469–1474. [Google Scholar] [CrossRef]
- Davenport, E.K.; Call, D.R.; Beyenal, H. Differential protection from tobramycin by extracellular polymeric substances from Acinetobacter baumannii and Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2014, 58, 4755–4761. [Google Scholar] [CrossRef]
- Stewart, P.S. Mechanisms of antibiotic resistance in bacterial biofilms. Int. J. Med. Microbiol. IJMM 2002, 292, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Conlon, B.P.; Nakayasu, E.S.; Fleck, L.E.; LaFleur, M.D.; Isabella, V.M.; Coleman, K.; Leonard, S.N.; Smith, R.D.; Adkins, J.N.; Lewis, K. Activated ClpP kills persisters and eradicates a chronic biofilm infection. Nature 2013, 503, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Saleh, Y.R.H.; Saadeh, H.A.; Kaur, H.; Goyal, K.; Sehgal, R.; Mubarak, M.S. The synthesis of novel hybrid compounds containing 5-nitrothiazole moiety as potential antiparasitic agents. Mon. Chem.-Chem. Mon. 2015, 146, 2087–2095. [Google Scholar] [CrossRef]
| Compound | S. epidermidis | P. aeruginosa |
|---|---|---|
| Nitazoxanide (1a) | 0.24 | 0.06 |
| 1b | 0.019 | 1.9 |
| 1c | 0.39 | 3.125 |
| Tizoxanide (2a) | 0.39 | 0.195 |
| 2b | 3.9 | 7.8 |
| 3a | 0.012 | 0.049 |
| 3b | 0.006 | 0.195 |
| 4a | 0.049 | 0.049 |
| 4c | 0.012 | 0.39 |
| 4d | 0.012 | 0.097 |
| 4e | 0.098 | 3.125 |
| 5a | 0.039 | 0.195 |
| 5b | <0.0015 | 0.098 |
| 6a | 0.098 | 1.56 |
| 6b | 0.78 | 1.56 |
| 7a | 0.024 | 1.56 |
| 7b | 0.003 | 0.78 |
| Nitrofurantoin (8a) | 0.049 | 0.098 |
| Furazolidone (8b) | 0.098 | 0.195 |
| 9a | 0.39 | 0.78 |
| 9b | 0.012 | 0.78 |
| 10a | 0.0015 | 3.125 |
| 10b | 0.006 | 0.195 |
| Compound | S. epidermidis | P. aeruginosa |
|---|---|---|
| Nitazoxanide (1a) | 50 | 3.125 |
| 1b | 0.16 | 12.5 |
| 1c | 1.56 | 25 |
| Tizoxanide (2a) | >100 | >100 |
| 2b | 6.25 | 12.5 |
| 3a | 1.56 | 25 |
| 3b | 6.25 | 100 |
| 4a | 1.56 | 3.125 |
| 4b | 3.125 | 6.25 |
| 4c | 3.125 | 50 |
| 4d | 3.125 | 25 |
| 4e | 12.5 | 50 |
| 5a | 25 | 50 |
| 5b | 3.125 | 25 |
| 6a | 1.56 | 50 |
| 6b | 1.56 | 25 |
| 7a | 3.125 | 12.5 |
| 7b | 0.39 | 3.125 |
| Nitrofurantoin (8a) | 3.125 | 12.5 |
| Furazolidone (8b) | 3.125 | 6.25 |
| 9a | 3.125 | 12.5 |
| 9b | 3.125 | 25 |
| 10a | 12.5 | 25 |
| 10b | 3.125 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koenig, H.N.; Durling, G.M.; Walsh, D.J.; Livinghouse, T.; Stewart, P.S. Novel Nitro-Heteroaromatic Antimicrobial Agents for the Control and Eradication of Biofilm-Forming Bacteria. Antibiotics 2021, 10, 855. https://doi.org/10.3390/antibiotics10070855
Koenig HN, Durling GM, Walsh DJ, Livinghouse T, Stewart PS. Novel Nitro-Heteroaromatic Antimicrobial Agents for the Control and Eradication of Biofilm-Forming Bacteria. Antibiotics. 2021; 10(7):855. https://doi.org/10.3390/antibiotics10070855
Chicago/Turabian StyleKoenig, Heidi N., Gregory M. Durling, Danica J. Walsh, Tom Livinghouse, and Philip S. Stewart. 2021. "Novel Nitro-Heteroaromatic Antimicrobial Agents for the Control and Eradication of Biofilm-Forming Bacteria" Antibiotics 10, no. 7: 855. https://doi.org/10.3390/antibiotics10070855
APA StyleKoenig, H. N., Durling, G. M., Walsh, D. J., Livinghouse, T., & Stewart, P. S. (2021). Novel Nitro-Heteroaromatic Antimicrobial Agents for the Control and Eradication of Biofilm-Forming Bacteria. Antibiotics, 10(7), 855. https://doi.org/10.3390/antibiotics10070855








