Classic vs. Novel Antibacterial Approaches for Eradicating Dental Biofilm as Adjunct to Periodontal Debridement: An Evidence-Based Overview
Abstract
1. Introduction
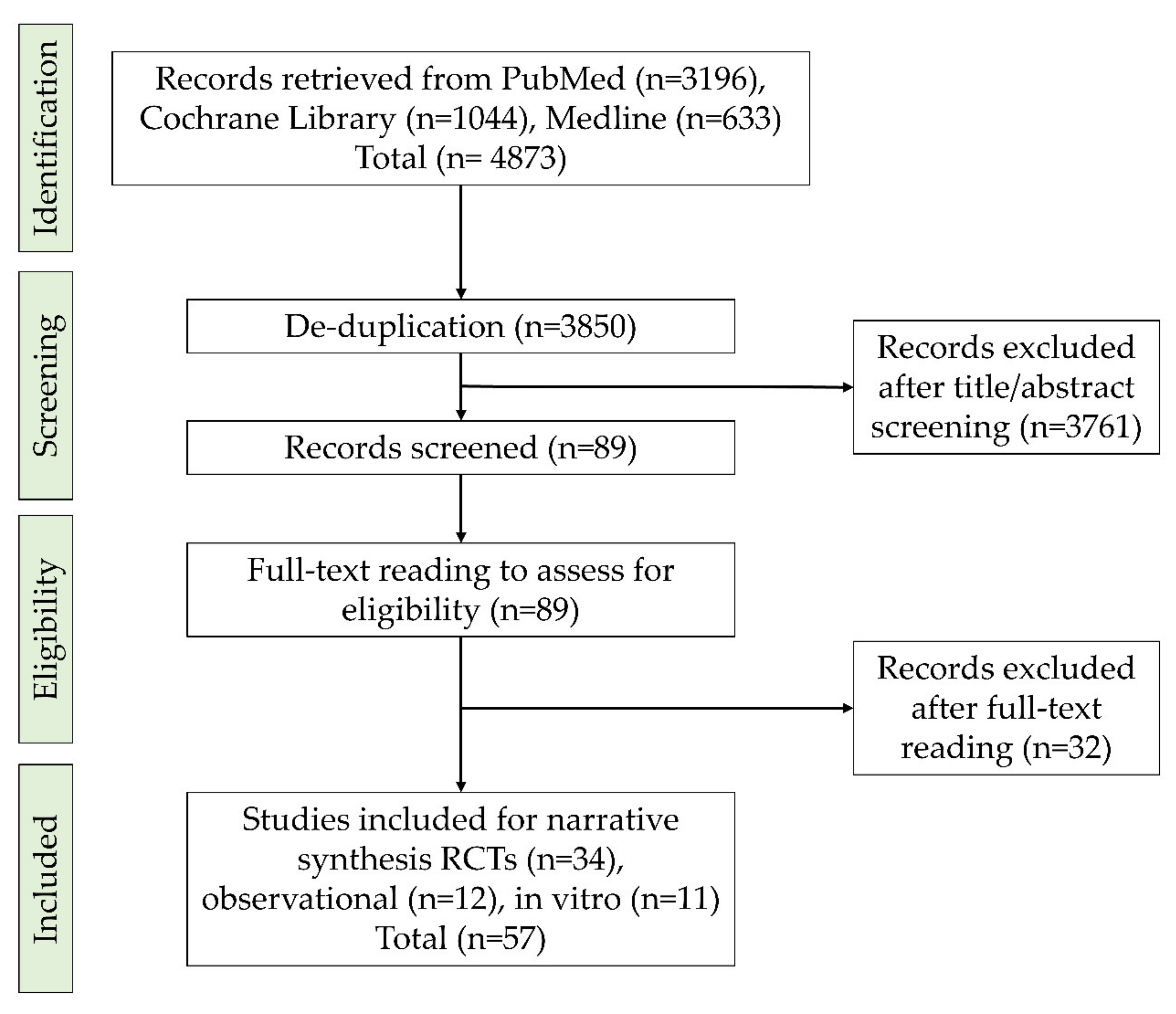
Search Strategy
- Follow-up for at least three months
- One of the arms received subgingival debridement (SD) with adjunctive antimicrobial or photodynamic therapy or probiotics. The other arm (control) should receive SD alone.
- Reporting both microbiological and clinical outcomes
2. Structure of Biofilm
3. Management of Dental Biofilm
3.1. Periodontal Debridement: The Gold Standard for Periodontal Therapy
3.2. Adjunctive Systemic and Local Antimicrobials/Antibiotics in Periodontics
Protocols of Antimicrobials/Antibiotics Prescription during Periodontal Therapy
4. Evidence on Using Antimicrobials/Antibiotics as Adjunct to Periodontal Therapy
4.1. Evidence from In Vitro and Experimental Animal Studies
4.2. Evidence from Observational Studies
4.3. Evidence from Clinical Trials
5. Novel Antibacterial Agents and Strategies to Overcome Bacterial Resistance in Dental Biofilm: Pros and Cons
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mshana, S.E.; Sindato, C.; Matee, M.I.; Mboera, L.E.G. Antimicrobial Use and Resistance in Agriculture and Food Production Systems in Africa: A Systematic Review. Antibiotics 2021, 10, 976. [Google Scholar] [CrossRef] [PubMed]
- Durrance-Bagale, A.; Jung, A.-S.; Frumence, G.; Mboera, L.; Mshana, S.E.; Sindato, C.; Clark, T.G.; Matee, M.; Legido-Quigley, H. Framing the Drivers of Antimicrobial Resistance in Tanzania. Antibiotics 2021, 10, 991. [Google Scholar] [CrossRef]
- Hanna, N.; Sun, P.; Sun, Q.; Li, X.; Yang, X.; Ji, X.; Zou, H.; Ottoson, J.; Nilsson, L.E.; Berglund, B.; et al. Presence of antibiotic residues in various environmental compartments of Shandong province in eastern China: Its potential for resistance development and ecological and human risk. Environ. Int. 2018, 114, 131–142. [Google Scholar] [CrossRef]
- CDC. Untreatable: Report by CDC Details Today’s Drug-Resistant Health Threats: Centers for Disease Control and Prevention. 2013. Available online: https://www.cdc.gov/media/releases/2013/p0916-untreatable.html (accessed on 10 November 2021).
- Neu, H.C. The crisis in antibiotic resistance. Science 1992, 257, 1064–1073. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Spellberg, B.; Guidos, R.; Gilbert, D.; Bradley, J.; Boucher, H.W.; Scheld, W.M.; Bartlett, J.G.; Edwards, J., Jr. The epidemic of antibiotic-resistant infections: A call to action for the medical community from the Infectious Diseases Society of America. Clin. Infect. Dis. 2008, 46, 155–164. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the last 25 years. J. Nat. Prod. 2007, 70, 461–477. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. 2016. Available online: https://amrreview.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 14 November 2021).
- Buonavoglia, A.; Leone, P.; Solimando, A.G.; Fasano, R.; Malerba, E.; Prete, M.; Corrente, M.; Prati, C.; Vacca, A.; Racanelli, V. Antibiotics or No Antibiotics, That Is the Question: An Update on Efficient and Effective Use of Antibiotics in Dental Practice. Antibiotics 2021, 10, 550. [Google Scholar] [CrossRef] [PubMed]
- Lockhart, P.B.; Tampi, M.P.; Abt, E.; Aminoshariae, A.; Durkin, M.J.; Fouad, A.F.; Gopal, P.; Hatten, B.W.; Kennedy, E.; Lang, M.S.; et al. Evidence-based clinical practice guideline on antibiotic use for the urgent management of pulpal- and periapical-related dental pain and intraoral swelling: A report from the American Dental Association. J. Am. Dent. Assoc. 2019, 150, 906–921.e12. [Google Scholar] [CrossRef]
- Wilson, W.; Taubert, K.A.; Gewitz, M.; Lockhart, P.B.; Baddour, L.M.; Levison, M.; Bolger, A.; Cabell, C.H.; Takahashi, M.; Baltimore, R.S.; et al. Prevention of infective endocarditis: Guidelines from the American Heart Association: A guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation 2007, 116, 1736–1754. [Google Scholar] [CrossRef]
- Ndaki, P.M.; Mushi, M.F.; Mwanga, J.R.; Konje, E.T.; Ntinginya, N.E.; Mmbaga, B.T.; Keenan, K.; Sabiiti, W.; Kesby, M.; Benitez-Paez, F.; et al. Dispensing Antibiotics without Prescription at Community Pharmacies and Accredited Drug Dispensing Outlets in Tanzania: A Cross-Sectional Study. Antibiotics 2021, 10, 1025. [Google Scholar] [CrossRef]
- Stein, K.; Farmer, J.; Singhal, S.; Marra, F.; Sutherland, S.; Quiñonez, C. The use and misuse of antibiotics in dentistry: A scoping review. J. Am. Dent. Assoc. 2018, 149, 869–884.e5. [Google Scholar] [CrossRef]
- Kirst, M.E.; Li, E.C.; Alfant, B.; Chi, Y.Y.; Walker, C.; Magnusson, I.; Wang, G.P. Dysbiosis and alterations in predicted functions of the subgingival microbiome in chronic periodontitis. Appl. Environ. Microbiol. 2015, 81, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Kinane, D.F. Causation and pathogenesis of periodontal disease. Periodontology 2000 2001, 25, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Seneviratne, C.J.; Zhang, C.F.; Samaranayake, L.P. Dental plaque biofilm in oral health and disease. Chin. J. Dent. Res. 2011, 14, 87–94. [Google Scholar]
- Buchmann, R.; Nunn, M.E.; Van Dyke, T.E.; Lange, D.E. Aggressive periodontitis: 5-year follow-up of treatment. J. Periodontol. 2002, 73, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Cugini, M.A.; Haffajee, A.D.; Smith, C.; Kent, R.L., Jr.; Socransky, S.S. The effect of scaling and root planing on the clinical and microbiological parameters of periodontal diseases: 12-month results. J. Clin. Periodontol. 2000, 27, 30–36. [Google Scholar] [CrossRef]
- Carvalho, L.H.; D’Avila, G.B.; Leão, A.; Gonçalves, C.; Haffajee, A.D.; Socransky, S.S.; Feres, M. Scaling and root planing, systemic metronidazole and professional plaque removal in the treatment of chronic periodontitis in a Brazilian population II--microbiological results. J. Clin. Periodontol. 2005, 32, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Eick, S.; Seltmann, T.; Pfister, W. Efficacy of antibiotics to strains of periodontopathogenic bacteria within a single species biofilm—An in vitro study. J. Clin. Periodontol. 2004, 31, 376–383. [Google Scholar] [CrossRef]
- Shlezinger, M.; Khalifa, L.; Houri-Haddad, Y.; Coppenhagen-Glazer, S.; Resch, G.; Que, Y.A.; Beyth, S.; Dorfman, E.; Hazan, R.; Beyth, N. Phage Therapy: A New Horizon in the Antibacterial Treatment of Oral Pathogens. Curr. Top. Med. Chem. 2017, 17, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef]
- Huang, R.; Li, M.; Gregory, R.L. Bacterial interactions in dental biofilm. Virulence 2011, 2, 435–444. [Google Scholar] [CrossRef] [PubMed]
- Saini, R.; Saini, S.; Sharma, S. Biofilm: A dental microbial infection. J. Nat. Sci. Biol. Med. 2011, 2, 71–75. [Google Scholar] [CrossRef]
- Miller, M.B.; Bassler, B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001, 55, 165–199. [Google Scholar] [CrossRef]
- Suci, P.A.; Mittelman, M.W.; Yu, F.P.; Geesey, G.G. Investigation of ciprofloxacin penetration into Pseudomonas aeruginosa biofilms. Antimicrob. Agents Chemother. 1994, 38, 2125–2133. [Google Scholar] [CrossRef]
- Sedlacek, M.J.; Walker, C. Antibiotic resistance in an in vitro subgingival biofilm model. Oral Microbiol. Immunol. 2007, 22, 333–339. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef]
- Mombelli, A. Maintenance therapy for teeth and implants. Periodontology 2000 2019, 79, 190–199. [Google Scholar] [CrossRef]
- Suvan, J.E. Effectiveness of mechanical nonsurgical pocket therapy. Periodontology 2000 2005, 37, 48–71. [Google Scholar] [CrossRef]
- Jakubovics, N.S.; Goodman, S.D.; Mashburn-Warren, L.; Stafford, G.P.; Cieplik, F. The dental plaque biofilm matrix. Periodontology 2000 2021, 86, 32–56. [Google Scholar] [CrossRef]
- Haffajee, A.D.; Teles, R.P.; Socransky, S.S. The effect of periodontal therapy on the composition of the subgingival microbiota. Periodontology 2000 2006, 42, 219–258. [Google Scholar] [CrossRef]
- Socransky, S.S.; Haffajee, A.D. Dental biofilms: Difficult therapeutic targets. Periodontology 2000 2002, 28, 12–55. [Google Scholar] [CrossRef]
- Gul, S.S.; Griffiths, G.S.; Stafford, G.P.; Al-Zubidi, M.I.; Rawlinson, A.; Douglas, C.W.I. Investigation of a Novel Predictive Biomarker Profile for the Outcome of Periodontal Treatment. J. Periodontol. 2017, 88, 1135–1144. [Google Scholar] [CrossRef] [PubMed]
- Saglie, F.R.; Marfany, A.; Camargo, P. Intragingival occurrence of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis in active destructive periodontal lesions. J. Periodontol. 1988, 59, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Wikström, M.; Dahlén, G.; Slots, J.; Egelberg, J. Effect of root debridement on the elimination of Actinobacillus actinomycetemcomitans and Bacteroides gingivalis from periodontal pockets. J. Clin. Periodontol. 1990, 17, 345–350. [Google Scholar] [CrossRef]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontology 2000 2015, 67, 131–186. [Google Scholar] [CrossRef]
- Graziani, F.; Karapetsa, D.; Alonso, B.; Herrera, D. Nonsurgical and surgical treatment of periodontitis: How many options for one disease? Periodontology 2000 2017, 75, 152–188. [Google Scholar] [CrossRef]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef]
- Sanz, M.; Teughels, W. Innovations in non-surgical periodontal therapy: Consensus Report of the Sixth European Workshop on Periodontology. J. Clin. Periodontol. 2008, 35, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Cionca, N.; Almaghlouth, A.; Décaillet, F.; Courvoisier, D.S.; Giannopoulou, C. Are there specific benefits of amoxicillin plus metronidazole in Aggregatibacter actinomycetemcomitans-associated periodontitis? Double-masked, randomized clinical trial of efficacy and safety. J. Periodontol. 2013, 84, 715–724. [Google Scholar] [CrossRef]
- Kolakovic, M.; Held, U.; Schmidlin, P.R.; Sahrmann, P. An estimate of pocket closure and avoided needs of surgery after scaling and root planing with systemic antibiotics: A systematic review. BMC Oral Health 2014, 14, 159. [Google Scholar] [CrossRef]
- Guerrero, A.; Griffiths, G.S.; Nibali, L.; Suvan, J.; Moles, D.R.; Laurell, L.; Tonetti, M.S. Adjunctive benefits of systemic amoxicillin and metronidazole in non-surgical treatment of generalized aggressive periodontitis: A randomized placebo-controlled clinical trial. J. Clin. Periodontol. 2005, 32, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Keestra, J.A.; Grosjean, I.; Coucke, W.; Quirynen, M.; Teughels, W. Non-surgical periodontal therapy with systemic antibiotics in patients with untreated chronic periodontitis: A systematic review and meta-analysis. J. Periodontal Res. 2015, 50, 294–314. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S.; Gunsolley, J.C. Systemic anti-infective periodontal therapy. A systematic review. Ann. Periodontol. 2003, 8, 115–181. [Google Scholar] [CrossRef]
- Teughels, W.; Feres, M.; Oud, V.; Martín, C.; Matesanz, P.; Herrera, D. Adjunctive effect of systemic antimicrobials in periodontitis therapy: A systematic review and meta-analysis. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 257–281. [Google Scholar] [CrossRef] [PubMed]
- Cionca, N.; Giannopoulou, C.; Ugolotti, G.; Mombelli, A. Amoxicillin and metronidazole as an adjunct to full-mouth scaling and root planing of chronic periodontitis. J. Periodontol. 2009, 80, 364–371. [Google Scholar] [CrossRef]
- Cionca, N.; Giannopoulou, C.; Ugolotti, G.; Mombelli, A. Microbiologic testing and outcomes of full-mouth scaling and root planing with or without amoxicillin/metronidazole in chronic periodontitis. J. Periodontol. 2010, 81, 15–23. [Google Scholar] [CrossRef]
- Feres, M.; Soares, G.M.; Mendes, J.A.; Silva, M.P.; Faveri, M.; Teles, R.; Socransky, S.S.; Figueiredo, L.C. Metronidazole alone or with amoxicillin as adjuncts to non-surgical treatment of chronic periodontitis: A 1-year double-blinded, placebo-controlled, randomized clinical trial. J. Clin. Periodontol. 2012, 39, 1149–1158. [Google Scholar] [CrossRef]
- Feres-Filho, E.J.; Silva, C.M.; Giovannetti-Menezes, N.; Torres, M.C.; Leão, A.T.; Sansone, C. Treatment of chronic periodontitis with systemic antibiotics only. J. Clin. Periodontol. 2006, 33, 936–937, author reply 931–940. [Google Scholar] [CrossRef] [PubMed]
- Mombelli, A.; Almaghlouth, A.; Cionca, N.; Courvoisier, D.S.; Giannopoulou, C. Differential benefits of amoxicillin-metronidazole in different phases of periodontal therapy in a randomized controlled crossover clinical trial. J. Periodontol. 2015, 86, 367–375. [Google Scholar] [CrossRef]
- Lollobrigida, M.; Pingitore, G.; Lamazza, L.; Mazzucchi, G.; Serafini, G.; De Biase, A. Antibiotics to Prevent Surgical Site Infection (SSI) in Oral Surgery: Survey among Italian Dentists. Antibiotics 2021, 10, 949. [Google Scholar] [CrossRef]
- Rabelo, C.C.; Feres, M.; Gonçalves, C.; Figueiredo, L.C.; Faveri, M.; Tu, Y.K.; Chambrone, L. Systemic antibiotics in the treatment of aggressive periodontitis. A systematic review and a Bayesian Network meta-analysis. J. Clin. Periodontol. 2015, 42, 647–657. [Google Scholar] [CrossRef] [PubMed]
- Sgolastra, F.; Petrucci, A.; Ciarrocchi, I.; Masci, C.; Spadaro, A. Adjunctive systemic antimicrobials in the treatment of chronic periodontitis: A systematic review and network meta-analysis. J. Periodontal Res. 2021, 56, 236–248. [Google Scholar] [CrossRef]
- McGowan, K.; McGowan, T.; Ivanovski, S. Optimal dose and duration of amoxicillin-plus-metronidazole as an adjunct to non-surgical periodontal therapy: A systematic review and meta-analysis of randomized, placebo-controlled trials. J. Clin. Periodontol. 2018, 45, 56–67. [Google Scholar] [CrossRef]
- Souto, M.L.S.; Rovai, E.S.; Ganhito, J.A.; Holzhausen, M.; Chambrone, L.; Pannuti, C.M. Efficacy of systemic antibiotics in nonsurgical periodontal therapy for diabetic subjects: A systematic review and meta-analysis. Int. Dent. J. 2018, 68, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Slama, T.G.; Amin, A.; Brunton, S.A.; File, T.M., Jr.; Milkovich, G.; Rodvold, K.A.; Sahm, D.F.; Varon, J.; Weiland, D., Jr. A clinician’s guide to the appropriate and accurate use of antibiotics: The Council for Appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am. J. Med. 2005, 118 (Suppl. 7A), 1s–6s. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.L. The rationale for the daily use of an antimicrobial mouthrinse. J. Am. Dent. Assoc. 2006, 137 (Suppl. 3), 16s–21s. [Google Scholar] [CrossRef] [PubMed]
- Gunsolley, J.C. Clinical efficacy of antimicrobial mouthrinses. J. Dent. 2010, 38 (Suppl. 1), S6–S10. [Google Scholar] [CrossRef]
- Hu, D.; Li, X.; Sreenivasan, P.K.; DeVizio, W. A randomized, double-blind clinical study to assess the antimicrobial effects of a cetylpyridinium chloride mouth rinse on dental plaque bacteria. Clin. Ther. 2009, 31, 2540–2548. [Google Scholar] [CrossRef]
- Guggenheim, B.; Giertsen, E.; Schüpbach, P.; Shapiro, S. Validation of an in vitro biofilm model of supragingival plaque. J. Dent. Res. 2001, 80, 363–370. [Google Scholar] [CrossRef]
- Shapiro, S.; Giertsen, E.; Guggenheim, B. An in vitro oral biofilm model for comparing the efficacy of antimicrobial mouthrinses. Caries Res. 2002, 36, 93–100. [Google Scholar] [CrossRef]
- Foster, J.S.; Pan, P.C.; Kolenbrander, P.E. Effects of antimicrobial agents on oral biofilms in a saliva-conditioned flowcell. Biofilms 2004, 1, 5–12. [Google Scholar] [CrossRef][Green Version]
- Sánchez, M.C.; Alonso-Español, A.; Ribeiro-Vidal, H.; Alonso, B.; Herrera, D.; Sanz, M. Relevance of Biofilm Models in Periodontal Research: From Static to Dynamic Systems. Microorganisms 2021, 9, 428. [Google Scholar] [CrossRef] [PubMed]
- Astasov-Frauenhoffer, M.; Braissant, O.; Hauser-Gerspach, I.; Weiger, R.; Walter, C.; Zitzmann, N.U.; Waltimo, T. Microcalorimetric determination of the effects of amoxicillin, metronidazole, and their combination on in vitro biofilm. J. Periodontol. 2014, 85, 349–357. [Google Scholar] [CrossRef]
- Belibasakis, G.N.; Thurnheer, T. Validation of antibiotic efficacy on in vitro subgingival biofilms. J. Periodontol. 2014, 85, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Soares, G.M.; Teles, F.; Starr, J.R.; Feres, M.; Patel, M.; Martin, L.; Teles, R. Effects of azithromycin, metronidazole, amoxicillin, and metronidazole plus amoxicillin on an in vitro polymicrobial subgingival biofilm model. Antimicrob. Agents Chemother. 2015, 59, 2791–2798. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, K.A.; da Silva, H.D.P.; Miranda, S.L.F.; Gonçalves, F.; de Sousa, A.P.; de Figueiredo, L.C.; Feres, M.; Bueno-Silva, B. Brazilian Red Propolis Is as Effective as Amoxicillin in Controlling Red-Complex of Multispecies Subgingival Mature Biofilm In Vitro. Antibiotics 2020, 9, 432. [Google Scholar] [CrossRef]
- Dabija-Wolter, G.; Al-Zubaydi, S.S.; Mohammed, M.M.A.; Bakken, V.; Bolstad, A.I. The effect of metronidazole plus amoxicillin or metronidazole plus penicillin V on periodontal pathogens in an in vitro biofilm model. Clin. Exp. Dent. Res. 2018, 4, 6–12. [Google Scholar] [CrossRef]
- Ong, H.S.; Oettinger-Barak, O.; Dashper, S.G.; Darby, I.B.; Tan, K.H.; Reynolds, E.C. Effect of azithromycin on a red complex polymicrobial biofilm. J. Oral Microbiol. 2017, 9, 1339579. [Google Scholar] [CrossRef]
- Schmid, J.L.; Kirchberg, M.; Sarembe, S.; Kiesow, A.; Sculean, A.; Mäder, K.; Buchholz, M.; Eick, S. In Vitro Evaluation of Antimicrobial Activity of Minocycline Formulations for Topical Application in Periodontal Therapy. Pharmaceutics 2020, 12, 352. [Google Scholar] [CrossRef]
- Sánchez, M.C.; Llama-Palacios, A.; Marín, M.J.; Figuero, E.; León, R.; Blanc, V.; Herrera, D.; Sanz, M. Validation of ATP bioluminescence as a tool to assess antimicrobial effects of mouthrinses in an in vitro subgingival-biofilm model. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e86–e92. [Google Scholar] [CrossRef]
- Miranda, S.L.F.; Damaceno, J.T.; Faveri, M.; Figueiredo, L.C.; Soares, G.M.S.; Feres, M.; Bueno-Silva, B. In Vitro Antimicrobial Effect of Cetylpyridinium Chloride on Complex Multispecies Subgingival Biofilm. Braz. Dent. J. 2020, 31, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Vidal, H.; Sánchez, M.C.; Alonso-Español, A.; Figuero, E.; Ciudad, M.J.; Collado, L.; Herrera, D.; Sanz, M. Antimicrobial Activity of EPA and DHA against Oral Pathogenic Bacteria Using an In Vitro Multi-Species Subgingival Biofilm Model. Nutrients 2020, 12, 2812. [Google Scholar] [CrossRef] [PubMed]
- Ceri, H.; Olson, M.E.; Stremick, C.; Read, R.R.; Morck, D.; Buret, A. The Calgary Biofilm Device: New technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 1999, 37, 1771–1776. [Google Scholar] [CrossRef]
- Fux, C.A.; Shirtliff, M.; Stoodley, P.; Costerton, J.W. Can laboratory reference strains mirror “real-world” pathogenesis? Trends Microbiol. 2005, 13, 58–63. [Google Scholar] [CrossRef]
- Roy, R.; Tiwari, M.; Donelli, G.; Tiwari, V. Strategies for combating bacterial biofilms: A focus on anti-biofilm agents and their mechanisms of action. Virulence 2018, 9, 522–554. [Google Scholar] [CrossRef]
- John, M.T.; Michalowicz, B.S.; Kotsakis, G.A.; Chu, H. Network meta-analysis of studies included in the Clinical Practice Guideline on the nonsurgical treatment of chronic periodontitis. J. Clin. Periodontol. 2017, 44, 603–611. [Google Scholar] [CrossRef]
- Ali, M.; Walboomers, X.F.; Jansen, J.A.; Yang, F. Influence of formulation parameters on encapsulation of doxycycline in PLGA microspheres prepared by double emulsion technique for the treatment of periodontitis. J. Drug Deliv. Sci. Technol. 2019, 52, 263–271. [Google Scholar] [CrossRef]
- Serrano, C.; Torres, N.; Valdivieso, C.; Castaño, C.; Barrera, M.; Cabrales, A. Antibiotic resistance of periodontal pathogens obtained from frequent antibiotic users. Acta Odontol. Latinoam. 2009, 22, 99–104. [Google Scholar] [PubMed]
- Jepsen, K.; Falk, W.; Brune, F.; Fimmers, R.; Jepsen, S.; Bekeredjian-Ding, I. Prevalence and antibiotic susceptibility trends of periodontal pathogens in the subgingival microbiota of German periodontitis patients: A retrospective surveillance study. J. Clin. Periodontol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, A.; Blanc, V.; Mor, C.; Nart, J.; León, R. Resistance to β-lactams and distribution of β-lactam resistance genes in subgingival microbiota from Spanish patients with periodontitis. Clin. Oral Investig. 2020, 24, 4639–4648. [Google Scholar] [CrossRef] [PubMed]
- Ardila, C.-M.; Bedoya-García, J.-A. Antimicrobial resistance of Aggregatibacter actinomycetemcomitans, Porphyromonas gingivalis and Tannerella forsythia in periodontitis patients. J. Glob. Antimicrob. Resist. 2020, 22, 215–218. [Google Scholar] [CrossRef] [PubMed]
- Bhardwaj, S.B.; Mehta, M.; Sood, S.; Sharma, J. Biofilm Formation by Drug Resistant Enterococci Isolates Obtained from Chronic Periodontitis Patients. J. Clin. Diagn. Res. 2017, 11, dc01–dc03. [Google Scholar] [CrossRef] [PubMed]
- Akrivopoulou, C.; Green, I.M.; Donos, N.; Nair, S.P.; Ready, D. Aggregatibacter actinomycetemcomitans serotype prevalence and antibiotic resistance in a UK population with periodontitis. J. Glob. Antimicrob. Resist. 2017, 10, 54–58. [Google Scholar] [CrossRef]
- Rams, T.E.; Feik, D.; Mortensen, J.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic susceptibility of periodontal Enterococcus faecalis. J. Periodontol. 2013, 84, 1026–1033. [Google Scholar] [CrossRef]
- Ardila, C.M.; López, M.A.; Guzmán, I.C. High resistance against clindamycin, metronidazole and amoxicillin in Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans isolates of periodontal disease. Med. Oral Patol. Oral Cir. Bucal 2010, 15, e947–e951. [Google Scholar] [CrossRef] [PubMed]
- Arredondo, A.; Blanc, V.; Mor, C.; Nart, J.; León, R. Azithromycin and erythromycin susceptibility and macrolide resistance genes in Prevotella from patients with periodontal disease. Oral Dis. 2019, 25, 860–867. [Google Scholar] [CrossRef]
- Almeida, V.S.M.; Azevedo, J.; Leal, H.F.; Queiroz, A.T.L.; da Silva Filho, H.P.; Reis, J.N. Bacterial diversity and prevalence of antibiotic resistance genes in the oral microbiome. PLoS ONE 2020, 15, e0239664. [Google Scholar] [CrossRef]
- Collins, J.R.; Arredondo, A.; Roa, A.; Valdez, Y.; León, R.; Blanc, V. Periodontal pathogens and tetracycline resistance genes in subgingival biofilm of periodontally healthy and diseased Dominican adults. Clin. Oral Investig. 2016, 20, 349–356. [Google Scholar] [CrossRef]
- Aguilar-Luis, M.A.; Casas Apayco, L.; Tinco Valdez, C.; De Lama-Odría, M.D.C.; Weilg, C.; Mazulis, F.; Silva-Caso, W.G.; Del Valle-Mendoza, J.M. Screening and Assessment of Antimicrobial Susceptibility of Periodontopathic Bacteria in Peruvian Patients with Periodontitis: A Pilot Study. Int. J. Dent. 2021, 2021, 2695793. [Google Scholar] [CrossRef] [PubMed]
- Harks, I.; Koch, R.; Eickholz, P.; Hoffmann, T.; Kim, T.S.; Kocher, T.; Meyle, J.; Kaner, D.; Schlagenhauf, U.; Doering, S.; et al. Is progression of periodontitis relevantly influenced by systemic antibiotics? A clinical randomized trial. J. Clin. Periodontol. 2015, 42, 832–842. [Google Scholar] [CrossRef]
- Griffiths, G.S.; Ayob, R.; Guerrero, A.; Nibali, L.; Suvan, J.; Moles, D.R.; Tonetti, M.S. Amoxicillin and metronidazole as an adjunctive treatment in generalized aggressive periodontitis at initial therapy or re-treatment: A randomized controlled clinical trial. J. Clin. Periodontol. 2011, 38, 43–49. [Google Scholar] [CrossRef]
- Cosgarea, R.; Eick, S.; Jepsen, S.; Arweiler, N.B.; Juncar, R.; Tristiu, R.; Salvi, G.E.; Heumann, C.; Sculean, A. Microbiological and host-derived biomarker evaluation following non-surgical periodontal therapy with short-term administration of systemic antimicrobials: Secondary outcomes of an RCT. Sci. Rep. 2020, 10, 16322. [Google Scholar] [CrossRef]
- Mombelli, A.; Brochut, P.; Plagnat, D.; Casagni, F.; Giannopoulou, C. Enamel matrix proteins and systemic antibiotics as adjuncts to non-surgical periodontal treatment: Clinical effects. J. Clin. Periodontol. 2005, 32, 225–230. [Google Scholar] [CrossRef]
- Pietruska, M.; Dolińska, E.; Milewski, R.; Sculean, A. Effect of systemic antibiotics on the outcomes of regenerative periodontal surgery in intrabony defects: A randomized, controlled, clinical study. Clin. Oral Investig. 2021, 25, 2959–2968. [Google Scholar] [CrossRef]
- Loos, B.G.; Louwerse, P.H.; Van Winkelhoff, A.J.; Burger, W.; Gilijamse, M.; Hart, A.A.; van der Velden, U. Use of barrier membranes and systemic antibiotics in the treatment of intraosseous defects. J. Clin. Periodontol. 2002, 29, 910–921. [Google Scholar] [CrossRef]
- Morales, A.; Contador, R.; Bravo, J.; Carvajal, P.; Silva, N.; Strauss, F.J.; Gamonal, J. Clinical effects of probiotic or azithromycin as an adjunct to scaling and root planning in the treatment of stage III periodontitis: A pilot randomized controlled clinical trial. BMC Oral Health 2021, 21, 12. [Google Scholar] [CrossRef]
- Čuk, K.; Povšič, K.; Milavec, S.; Seme, K.; Gašperšič, R. Influence of adjunctive azithromycin on microbiological and clinical outcomes in periodontitis patients: 6-month results of randomized controlled clinical trial. BMC Oral Health 2020, 20, 241. [Google Scholar] [CrossRef]
- Qureshi, A.; Bokhari, S.A.H.; Haque, Z.; Baloch, A.A.; Zaheer, S. Clinical efficacy of scaling and root planing with and without metronidazole on glycemic control: Three-arm randomized controlled trial. BMC Oral Health 2021, 21, 253. [Google Scholar] [CrossRef]
- Cruz, D.F.D.; Duarte, P.M.; Figueiredo, L.C.; da Silva, H.D.P.; Retamal-Valdes, B.; Feres, M.; Miranda, T.S. Metronidazole and amoxicillin for patients with periodontitis and diabetes mellitus: 5-year secondary analysis of a randomized controlled trial. J. Periodontol. 2021, 92, 479–487. [Google Scholar] [CrossRef]
- Trajano, V.; Brasileiro, C.B.; Henriques, J.A.S.; Cota, L.M.; Lanza, C.R.; Cortés, M.E. Doxycycline encapsulated in β-cyclodextrin for periodontitis: A clinical trial. Braz. Oral Res. 2020, 33, e112. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Saliem, S.; Abdulkareem, A.; Radhi, H.; Gul, S. Evaluation of the efficacy of lycopene gel compared with minocycline hydrochloride microspheres as an adjunct to nonsurgical periodontal treatment: A randomised clinical trial. J. Dent. Sci. 2021, 16, 691–699. [Google Scholar] [CrossRef]
- Choi, E.; Um, H.S.; Chang, B.S.; Lee, S.Y.; Lee, J.K. Clinical and microbiological effects of adjunctive local delivery of minocycline (Periocline®) in patients receiving supportive periodontal therapy: A pilot study. J. Periodontal Implant. Sci. 2021, 51, 53–62. [Google Scholar] [CrossRef]
- Serino, G.; Rosling, B.; Ramberg, P.; Hellström, M.K.; Socransky, S.S.; Lindhe, J. The effect of systemic antibiotics in the treatment of patients with recurrent periodontitis. J. Clin. Periodontol. 2001, 28, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Cosgarea, R.; Eick, S.; Batori-Andronescu, I.; Jepsen, S.; Arweiler, N.B.; Rößler, R.; Conrad, T.; Ramseier, C.A.; Sculean, A. Clinical and Microbiological Evaluation of Local Doxycycline and Antimicrobial Photodynamic Therapy during Supportive Periodontal Therapy: A Randomized Clinical Trial. Antibiotics 2021, 10, 277. [Google Scholar] [CrossRef]
- Caffesse, R.G.; Sweeney, P.L.; Smith, B.A. Scaling and root planing with and without periodontal flap surgery. J. Clin. Periodontol. 1986, 13, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Oda, S.; Ishikawa, I. In vitro effectiveness of a newly-designed ultrasonic scaler tip for furcation areas. J. Periodontol. 1989, 60, 634–639. [Google Scholar] [CrossRef]
- Stamatova, I.; Meurman, J.H. Probiotics and periodontal disease. Periodontology 2000 2009, 51, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Morozova, L.V.; Pozharitskaia, M.M.; Mel’nichuk, G.M. The therapeutic efficacy of probiotics for the correction of microfloral imbalance in periodontitis. Stomatologiia 1996, 68–69. [Google Scholar]
- Cobb, C.M. Lasers and the treatment of periodontitis: The essence and the noise. Periodontology 2000 2017, 75, 205–295. [Google Scholar] [CrossRef]
- Dobson, J.; Wilson, M. Sensitization of oral bacteria in biofilms to killing by light from a low-power laser. Arch. Oral Biol. 1992, 37, 883–887. [Google Scholar] [CrossRef]
- Giannelli, M.; Lasagni, M.; Bani, D. Photonic Therapy in Periodontal Diseases an Overview with Appraisal of the Literature and Reasoned Treatment Recommendations. Int. J. Mol. Sci. 2019, 20, 4741. [Google Scholar] [CrossRef]
- Wadhwa, A.; Mallapragada, S.; Sharma, P. Novel indocyanine green mediated antimicrobial photodynamic therapy in the management of chronic periodontitis—A randomized controlled clinico-microbiological pilot study. J. Oral Biol. Craniofacial Res. 2021, 11, 57–62. [Google Scholar] [CrossRef]
- Gandhi, K.K.; Pavaskar, R.; Cappetta, E.G.; Drew, H.J. Effectiveness of Adjunctive Use of Low-Level Laser Therapy and Photodynamic Therapy After Scaling and Root Planing in Patients with Chronic Periodontitis. Int. J. Periodontics Restor. Dent. 2019, 39, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Annaji, S.; Sarkar, I.; Rajan, P.; Pai, J.; Malagi, S.; Bharmappa, R.; Kamath, V. Efficacy of Photodynamic Therapy and Lasers as an Adjunct to Scaling and Root Planing in the Treatment of Aggressive Periodontitis—A Clinical and Microbiologic Short Term Study. J. Clin. Diagn. Res. 2016, 10, ZC08–ZC12. [Google Scholar] [CrossRef] [PubMed]
- Moreira, A.L.; Novaes, A.B., Jr.; Grisi, M.F.; Taba, M., Jr.; Souza, S.L.; Palioto, D.B.; de Oliveira, P.G.; Casati, M.Z.; Casarin, R.C.; Messora, M.R. Antimicrobial photodynamic therapy as an adjunct to non-surgical treatment of aggressive periodontitis: A split-mouth randomized controlled trial. J. Periodontol. 2015, 86, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Pu, R.; Qian, Y.; Shi, J.; Si, M. Antimicrobial photodynamic therapy versus antibiotics as an adjunct in the treatment of periodontitis and peri-implantitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2021, 34, 102231. [Google Scholar] [CrossRef] [PubMed]
- Muzaheed Acharya, S.; Hakami, A.R.; Allemailem, K.S.; Alqahtani, K.; Al Saffan, A.; Aldakheel, F.M.; Divakar, D.D. Effectiveness of single versus multiple sessions of photodynamic therapy as adjunct to scaling and root planing on periodontopathogenic bacteria in patients with periodontitis. Photodiagnosis. Photodyn. Ther. 2020, 32, 102035. [Google Scholar] [CrossRef]
- Chondros, P.; Nikolidakis, D.; Christodoulides, N.; Rössler, R.; Gutknecht, N.; Sculean, A. Photodynamic therapy as adjunct to non-surgical periodontal treatment in patients on periodontal maintenance: A randomized controlled clinical trial. Lasers Med. Sci. 2009, 24, 681–688. [Google Scholar] [CrossRef]
- Hill, G.; Dehn, C.; Hinze, A.V.; Frentzen, M.; Meister, J. Indocyanine green-based adjunctive antimicrobial photodynamic therapy for treating chronic periodontitis: A randomized clinical trial. Photodiagnosis. Photodyn. Ther. 2019, 26, 29–35. [Google Scholar] [CrossRef]
- Tabenski, L.; Moder, D.; Cieplik, F.; Schenke, F.; Hiller, K.A.; Buchalla, W.; Schmalz, G.; Christgau, M. Antimicrobial photodynamic therapy vs. local minocycline in addition to non-surgical therapy of deep periodontal pockets: A controlled randomized clinical trial. Clin. Oral Investig. 2017, 21, 2253–2264. [Google Scholar] [CrossRef] [PubMed]
- Pulikkotil, S.J.; Toh, C.G.; Mohandas, K.; Leong, K. Effect of photodynamic therapy adjunct to scaling and root planing in periodontitis patients: A randomized clinical trial. Aust. Dent. J. 2016, 61, 440–445. [Google Scholar] [CrossRef]
- Queiroz, A.C.; Suaid, F.A.; de Andrade, P.F.; Oliveira, F.S.; Novaes, A.B., Jr.; Taba, M., Jr.; Palioto, D.B.; Grisi, M.F.; Souza, S.L. Adjunctive effect of antimicrobial photodynamic therapy to nonsurgical periodontal treatment in smokers: A randomized clinical trial. Lasers Med. Sci. 2015, 30, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, A.C.; Suaid, F.A.; de Andrade, P.F.; Novaes, A.B., Jr.; Taba, M., Jr.; Palioto, D.B.; Grisi, M.F.; Souza, S.L. Antimicrobial photodynamic therapy associated to nonsurgical periodontal treatment in smokers: Microbiological results. J. Photochem. Photobiol. B Biol. 2014, 141, 170–175. [Google Scholar] [CrossRef]
- Chitsazi, M.T.; Shirmohammadi, A.; Pourabbas, R.; Abolfazli, N.; Farhoudi, I.; Daghigh Azar, B.; Farhadi, F. Clinical and Microbiological Effects of Photodynamic Therapy Associated with Non-surgical Treatment in Aggressive Periodontitis. J. Dent. Res. Dent. Clin. Dent. Prospect. 2014, 8, 153–159. [Google Scholar] [CrossRef]
- Rühling, A.; Fanghänel, J.; Houshmand, M.; Kuhr, A.; Meisel, P.; Schwahn, C.; Kocher, T. Photodynamic therapy of persistent pockets in maintenance patients-a clinical study. Clin. Oral Investig. 2010, 14, 637–644. [Google Scholar] [CrossRef]
- Ozen, M.; Dinleyici, E.C. The history of probiotics: The untold story. Benef. Microbes 2015, 6, 159–165. [Google Scholar] [CrossRef]
- Guarner, F.; Perdigon, G.; Corthier, G.; Salminen, S.; Koletzko, B.; Morelli, L. Should yoghurt cultures be considered probiotic? Br. J. Nutr. 2005, 93, 783–786. [Google Scholar] [CrossRef]
- Saito, T. Selection of useful probiotic lactic acid bacteria from the Lactobacillus acidophilus group and their applications to functional foods. Anim. Sci. J. 2004, 75, 1–13. [Google Scholar] [CrossRef]
- Meurman, J.H. Probiotics: Do they have a role in oral medicine and dentistry? Eur. J. Oral Sci. 2005, 113, 188–196. [Google Scholar] [CrossRef]
- Invernici, M.M.; Furlaneto, F.A.C.; Salvador, S.L.; Ouwehand, A.C.; Salminen, S.; Mantziari, A.; Vinderola, G.; Ervolino, E.; Santana, S.I.; Silva, P.H.F.; et al. Bifidobacterium animalis subsp lactis HN019 presents antimicrobial potential against periodontopathogens and modulates the immunological response of oral mucosa in periodontitis patients. PLoS ONE 2020, 15, e0238425. [Google Scholar] [CrossRef]
- Invernici, M.M.; Salvador, S.L.; Silva, P.H.F.; Soares, M.S.M.; Casarin, R.; Palioto, D.B.; Souza, S.L.S.; Taba, M., Jr.; Novaes, A.B., Jr.; Furlaneto, F.A.C.; et al. Effects of Bifidobacterium probiotic on the treatment of chronic periodontitis: A randomized clinical trial. J. Clin. Periodontol. 2018, 45, 1198–1210. [Google Scholar] [CrossRef]
- Laleman, I.; Pauwels, M.; Quirynen, M.; Teughels, W. A dual-strain Lactobacilli reuteri probiotic improves the treatment of residual pockets: A randomized controlled clinical trial. J. Clin. Periodontol. 2020, 47, 43–53. [Google Scholar] [CrossRef]
- Dhaliwal, P.K.; Grover, V.; Malhotra, R.; Kapoor, A. Clinical and Microbiological Investigation of the Effects of Probiotics Combined with Scaling and Root Planing in the Management of Chronic Periodontitis: A Randomized, Controlled Study. J. Int. Acad. Periodontol. 2017, 19, 101–108. [Google Scholar]
- Tekce, M.; Ince, G.; Gursoy, H.; Dirikan Ipci, S.; Cakar, G.; Kadir, T.; Yılmaz, S. Clinical and microbiological effects of probiotic lozenges in the treatment of chronic periodontitis: A 1-year follow-up study. J. Clin. Periodontol. 2015, 42, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Durukan, A.; Ozcelik, O.; Pauwels, M.; Quirynen, M.; Haytac, M.C. Clinical and microbiological effects of Lactobacillus reuteri probiotics in the treatment of chronic periodontitis: A randomized placebo-controlled study. J. Clin. Periodontol. 2013, 40, 1025–1035. [Google Scholar] [CrossRef]
- Pudgar, P.; Povšič, K.; Čuk, K.; Seme, K.; Petelin, M.; Gašperšič, R. Probiotic strains of Lactobacillus brevis and Lactobacillus plantarum as adjunct to non-surgical periodontal therapy: 3-month results of a randomized controlled clinical trial. Clin. Oral Investig. 2021, 25, 1411–1422. [Google Scholar] [CrossRef]
- Morales, A.; Gandolfo, A.; Bravo, J.; Carvajal, P.; Silva, N.; Godoy, C.; Garcia-Sesnich, J.; Hoare, A.; Diaz, P.; Gamonal, J. Microbiological and clinical effects of probiotics and antibiotics on nonsurgical treatment of chronic periodontitis: A randomized placebo- controlled trial with 9-month follow-up. J. Appl. Oral Sci. 2018, 26, e20170075. [Google Scholar] [CrossRef]
- Gravitz, L. Turning a new phage. Nat. Med. 2012, 18, 1318–1320. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, C.; Sarkar, P.; Issa, R.; Haldar, J. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019, 27, 323–338. [Google Scholar] [CrossRef] [PubMed]
- Steier, L.; de Oliveira, S.D.; de Figueiredo, J.A.P. Bacteriophages in Dentistry-State of the Art and Perspectives. Dent. J. 2019, 7, 6. [Google Scholar] [CrossRef]
- Adam, F.A.; Rani, H.; Baharin, B.; Yusof, M.M.; Mohd, N. Salvadora persica L. chewing sticks and standard toothbrush as anti-plaque and anti-gingivitis tool: A systematic review and meta-analysis. J. Ethnopharmacol. 2021, 274, 113882. [Google Scholar] [CrossRef]
- Jassoma, E.; Baeesa, L.; Sabbagh, H. The antiplaque/anticariogenic efficacy of Salvadora persica (Miswak) mouthrinse in comparison to that of chlorhexidine: A systematic review and meta-analysis. BMC Oral Health 2019, 19, 64. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Cardoso, V.F.; Roppa, R.H.A.; Antunes, C.; Moraes, A.N.S.; Santi, L.; Konrath, E.L. Efficacy of medicinal plant extracts as dental and periodontal antibiofilm agents: A systematic review of randomized clinical trials. J. Ethnopharmacol. 2021, 281, 114541. [Google Scholar] [CrossRef]
- Janakiram, C.; Venkitachalam, R.; Fontelo, P.; Iafolla, T.J.; Dye, B.A. Effectiveness of herbal oral care products in reducing dental plaque & gingivitis–a systematic review and meta-analysis. BMC Complementary Med. Ther. 2020, 20, 43. [Google Scholar]
- Martins, M.L.; Ribeiro-Lages, M.B.; Masterson, D.; Magno, M.B.; Cavalcanti, Y.W.; Maia, L.C.; Fonseca-Gonçalves, A. Efficacy of natural antimicrobials derived from phenolic compounds in the control of biofilm in children and adolescents compared to synthetic antimicrobials: A systematic review and meta-analysis. Arch. Oral Biol. 2020, 118, 104844. [Google Scholar] [CrossRef]
- Moro, M.G.; Silveira Souto, M.L.; Franco, G.C.N.; Holzhausen, M.; Pannuti, C.M. Efficacy of local phytotherapy in the nonsurgical treatment of periodontal disease: A systematic review. J. Periodontal Res. 2018, 53, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, K. Aloe vera herbal dentifrices for plaque and gingivitis control: A systematic review. Oral Dis. 2014, 20, 254–267. [Google Scholar] [CrossRef] [PubMed]
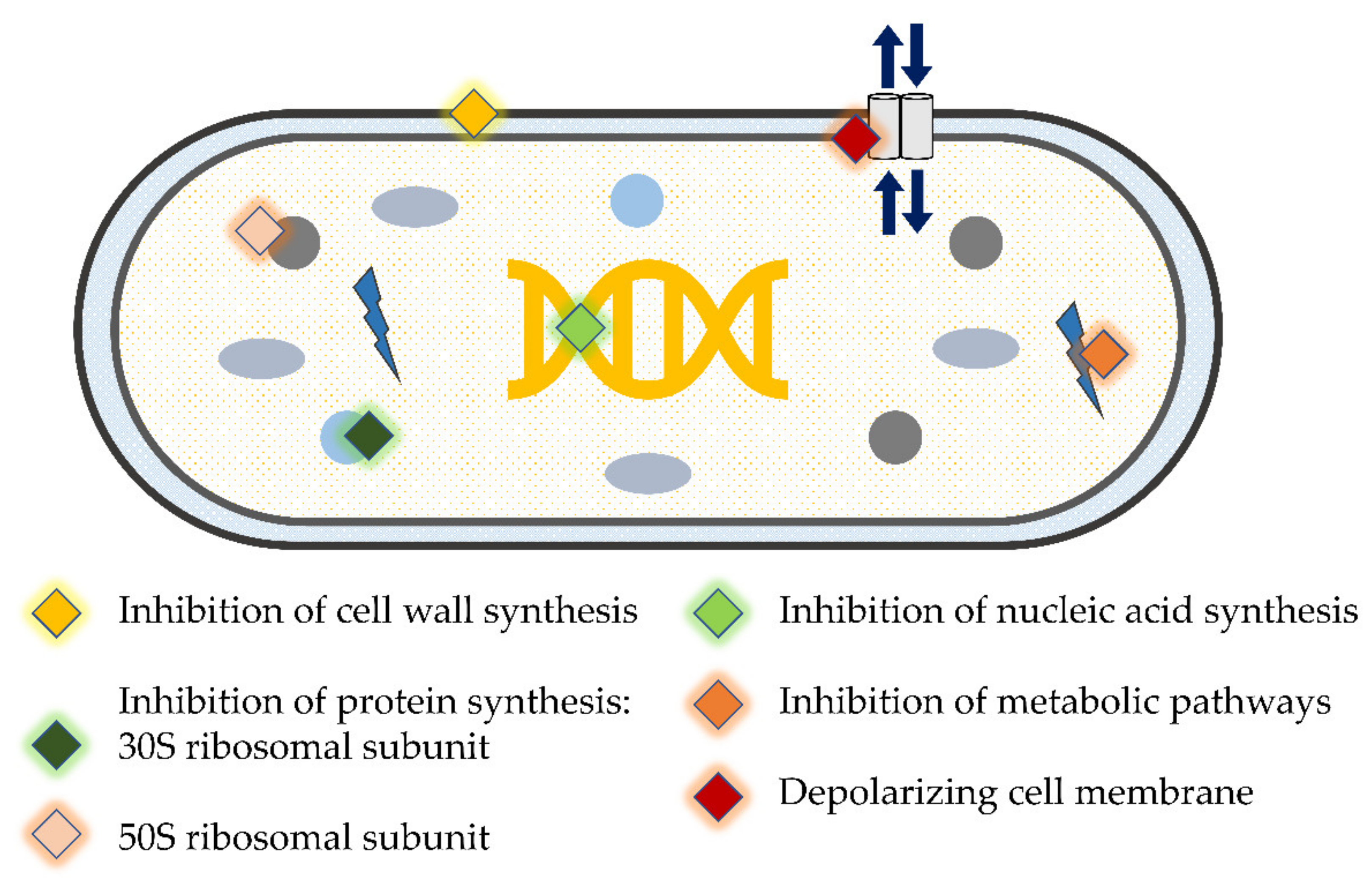
| Antimicrobial | Bacterial Species * | Outcome | Publications |
|---|---|---|---|
| AMX, MET, or their combination | Ag, Ai, Ao, Aod, Vp, Sg, Si, Sm, So, Ss, Sa, Smu, Aa, Cg, Co, Cs, Ec, Cc, Cgr, Cr, Csh, En, Es, Fn, Fnp, Fnv, Fp, Pm, Pi, Pn, Pme, Sn, Tf, Pg, Gm, Lb, Nm, Td, Pa, and Sno | Combination of AMX and MET exhibited greater antimicrobial effects than using each antibiotic seperately. | [68,69,70,71] |
| Ss, Fn, and Pg | Growth rate was reduced in response to either AMX or MET but not their combination. | [66] | |
| To, Sa, Ao, Fn, Vd, Cr, Pi, Pg, Tf and Td | Antibiotics caused species-specific reductions, but not total bacterial loads | [67] | |
| AZM | Pg, Td, Tf, | AZM was ineffective in preventing biofilm formation within a clinically achievable concentration. | [68,71] |
| So, Sa, Ao, Fn, Vd, Cr, Pi, Pg, Tf, and Td. | Total bacterial counts were significantly reduced | [67] | |
| MNO | Pg, Fn, Tf, Sg, An, and Pm | The antimicrobial activity of MNO reduced total cfu of examined species. | [72] |
| DOX | Pg and Fn | Substantial antimicrobial activity of DOX against periodontal pathogens. | [80] |
| CHX and CPC | An, Ao, Ag, Ai, Vp, Aod, Ss, So, Si, Sg, Sm, Aa, Co, Cg, Ec, Cs, Sc, En, Fnv, Pm, Fnp, Csh, Fn, Fp, Pi, Pg, Tf, Es, Sa, Sno, Pa, and Gm. |
| [73,74,75] |
| Antibiotic | Resistant Bacteria | Publications |
|---|---|---|
| Amoxicillin | Rd, Fn, Tf, Aa, Pg, Pi, Streptococcus spp., Enterococci spp. | [81,82,83,84,85,86,88,92] |
| Metronidazole | Rd, Ga, An, Aa, Pg, Tf, Pi, Fn | [81,84,86,88,92] |
| Penicillin | An, Aa | [86,92] |
| Amoxicillin/clavulanic acid | Rd, Fn, Aa | [82,86,92] |
| Azithromycin | Ec, An, Pg, Pi, Fn, Aa, Tf | [82,84,89,92] |
| Tetracyclin | An, Aa, Ef | [86,87,92] |
| Erythromycin | Pi, Streptococcus spp., EF, | [83,85,87,89] |
| Ciprofloxacin | Enterococci spp. | [85] |
| Clindamycin | Enterococci spp., Aa, Pg | [85,86,88] |
| Author, Year | Type of Treatment | Sample (n) | Antibiotic Dose/Frequency | Follow-Up | Periodontal Parameters |
|---|---|---|---|---|---|
| No improvement in clinical parameters | |||||
| Morales et al., 2021 [99] | SD for stage III periodontitis patients | control: SD (n = 15); test: SD+ probiotics (n = 16) test: SD + AB (n = 16) | 500 mg of AZM 1/day for 5 days | 12-months | PI, BOP, PPD, and CAL |
| Qureshi et al., 2021 [101] | SD and OHI for T2DM patients with periodontitis | control: OHI (n = 50) control: SD + OHI (n = 50) test: AB + SD + OHI (n = 50) | 400 mg of MET 3/day for 10 days | 3- and 6-months | BOP, PPD and CAL |
| Serino et al., 2001 [106] | SD for patients with recurrent advanced periodontitis | 17 received SD + AB | 400 mg of MET 3/day + 750 mg AMX 2/day for 2 weeks | 1, 3, 5 years | PI, BOP, PPD, PAL and radiographic bone level |
| Choi et al., 2021 [105] | SD periodontitis patients | control: SD (n = 12) test: SD + 2% minocycline | microcapsule gel containing 2% minocycline HCl ointment | 1- and 3-months | PI, BOP, PPD, CAL and relative ratios of periodontal pathogens |
| Harks et al., 2015 [93] | SD + maintenance therapy at 3 months intervals. | control: SD (n = 200) test: SD+AB (n = 206) | 500 mg AMX + 400 mg MET 3/day for 7 days | 27.5-months | percentage of sites showing further attachment loss, measurements from occlusal surface to the pocket bottom |
| Improvement in clinical parameters only | |||||
| Cosgarea et al., 2020 [95] | SD for severe periodontitis patients | control: (n = 26) test: AMX + MET for first 3 days: (n = 24); AMX+MET for 7 days: (n = 25) | 500 mg of AMX thrice a day 500 mg of MET 3/day | 3-, 6- and 12-months | PI, BOP, PPD, CAL and number of deep sites with PPD ≥ 6 mm, |
| Mombelli et al., 2013 [42] | full-mouth SD within 48 hrs for moderate to advanced periodontitis patients | control: only SD (n = 38) test: SD+AB (n = 44) | 375 mg of AMX + 500 mg of MET, 3/day for 7 days | 3-months | Persistence of sites with a probing depth (PD) >4 mm and BOP |
| Trajano et al., 2020 [103] | SD | control: SD test: 10% doxycycline in β-cyclodextrin +SD test: 10% doxycycline +SD | gel of 10% doxycycline in β-cyclodextrin or alone applied at baseline and after a month | 30 and 60 days | PI, BOP, PPD, and CAL |
| Mombelli et al., 2005 [96] | SD + enamel matrix derivatives for periodontitis patients | control: SD (n = 8) test: AB+SD (n = 8) | 375 mg of AMX + 250 mg of MET 3/day for 7 days | 6- and 12-months | PPD and CAL |
| Cruz et al., 2021 [102] | SD for T2DM patients with periodontitis. None had received SD from 2 to 5 years post-treatment | control: SD (n = 10) test: SD+AB (n = 15) | 400 gm MET+500 mg AMX 3/day for 14 days and started at the first SD session | up to 5 years | PI, BOP, PPD, CAL and number of sites with PD ≥ 5 mm |
| Ali et al., 2021 [104] | SD for mild to moderate periodontitis patients | control: SD (n = 24) test: SD+ Lycopene (n = 24) test: SD + minocycline HCL (n = 24) | minocycline HCL microspheres and lycopene gel | 30 days | PI, BOP, PPD and CAL |
| Improvement in microbilogical parameters only | |||||
| Cosgarea et al., 2021 [107] | SD for periodontitis patients | control: SD (n = 35) test: SD + LDD (n = 35) | LDD | 3- and 6-months | PI, BOP, PPD, CAL, number of treated sites with BOP and 8 periodontopathogens levels |
| Čuk et al., 2020 [100] | SD for periodontitis patients | control: SD (n = 20) test: SD + AB (n = 20) | AZM 500 mg/day for 3 days | 6-months | N of sites with PD ≥ 5 mm and BOP, changes in numbers of periodontal pathogens in pockets |
| Author, Year | Study Design, Follow-Up | Study Population | Clinical/Microbiological Parameters | aPDT Treatment Modalities |
|---|---|---|---|---|
| Improvement in microbiological and clinical parameters § | ||||
| Moreira et al., 2015 [118] | Split-mouth RCT, 3-months | Patients with generalized AgP (n = 20) |
| SD + Diode laser (670 nm)/phenothiazine chloride (10 mg/mL) photosensitizer |
| Gandhi et al., 2019 [116] | Split-mouth, RCT, 9-months | Periodontitis patients (n = 26) |
| SD + Diode laser (810 nm)/ICG photosensitizer |
| Annaji et al., 2016 [117] | Split-mouth RCT, 3-months | Patients with AgP (n = 15) |
| SD+ Diode Laser (810 nm) |
| Wadhwa et al., 2021 [115] | Split-mouth RCT, 6-months | Chronic periodontitis patients (n = 30) | Total viable anaerobic count | SD + Diode laser (810 nm)/ICG photosensitizer |
| Improvement in microbiological parameters only § | ||||
| Muzaheed et al., 2020 [120] | Parallel arm RCT, 3-months | Periodontitis patients (n = 45) |
| SD + Diode laser (660 nm)/methylene-blue (0.005%) photosensitizer |
| Chondros et al., 2009 [121] | Parallel arm RCT, 6-months | Periodontitis patients (n = 24) |
| SD + Diode Laser (670 nm)/phenothiazine chloride (10 mg/mL) photosensitizer |
| No improvement in microbiological and clinical parameters § | ||||
| Chitsazi et al., 2014 [127] | Split-mouth RCT, 3-months | Patients with AgP (n = 24) |
| SD + Diode Laser (670–690 nm) |
| Rühling et al., 2010 [128] | Parallel arm RCT, 3-months | Periodontitis patients (n = 54) |
| SD + Diode Laser (635 nm)/5% tolonium chloride photosensitizer |
| Queiroz et al., 2015 [125] Queiroz et al., 2014 [126] | Parallel arm RCT, 3-months | Periodontitis smoker patients (n = 20) |
| SD + Diode Laser (660 nm)/phenothiazine chloride (10 mg/mL) photosensitizer |
| Tabenski et al., 2017 [123] | Parallel arm RCT, 12-months | Periodontitis patients (n = 45) |
| SD + Diode Laser (670 nm)/phenothiazine chloride photosensitizer |
| Hill et al., 2019 [122] | Split-mouth RCT, 6-months | Periodontitis patients (n = 20) |
| SD + Diode laser (808 nm)/ICG photosensitizer |
| Pulikkotil et al., 2016 [124] | Split-mouth RCT, 3-months | Periodontitis patients (n = 20) |
| SD + LED lamp (red spectrum, 628 Hz)/methylene blue photosensitizer |
| Author, Year | Study Design, Follow-Up | Study Population | Strain of Probiotic | Mode/Frequency of Administration | Clinical/Microbiological Parameters |
|---|---|---|---|---|---|
| Improvement in microbiological and clinical parameters § | |||||
| Invernici et al., 2018 [134] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 41) | Bl (HN019) 1 × 109 CFU | Lozenges (10 mg) 2×/day for 30-days |
|
| Invernici et al., 2020 [133] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 30) | Bl (HN019) 1 × 109 CFU | Lozenges 2×/day in the morning and before bedtime for 30-days |
|
| Improvement in clinical parameters only § | |||||
| Laleman et al., 2020 [135] | Parallel arm RCT, 6-months | Chronic periodontitis patients (n = 39) | Lr (DSM 17,938 and ATCC PTA 5289) 2 × 108 CFU each | Five probiotic drops applied to residual pocket immediately after SD. Then each patient instructed to use lozenges 2×/day after brushing for 3-months |
|
| Tekce et al., 2015 [137] | Parallel arm RCT, 12-months | Chronic periodontitis patients (n = 30) | Lr (DSM 17,938 and ATCC PTA 5289) 2 × 108 CFU each | Lozenges 2×/day after brushing for 3-weeks |
|
| Improvement in microbiological parameters only § | |||||
| Dhaliwal et al., 2017 [136] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 30) | Sf (T-110 JPC), 30 × 107 CFU, Cb (TO-A HIS), 2 × 106 CFU, Bm (TO-A JPC), 1 × 106 CFU and Ls (HIS), 5 × 107 CFU | Bifilac lozenges 2×/day or 21-days |
|
| Teughels et al., 2013 [138] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 30) | Lr (DSM17938 and ATCC PTA5289) 9 × 108 CFU each | Lozenges 2×/day for 3-months |
|
| No improvement in microbiological and clinical parameters § | |||||
| Pudgar et al., 2021 [139] | Parallel arm RCT, 3-months | Chronic periodontitis patients (n = 40) | Lb (CECT7480) and Lp (CECT7481), 6.0 × 109 CFU/mL each | One lozenge/day |
|
| Morales et al., 2018 [140] | Parallel arm RCT, 9-months | Chronic periodontitis patients (n = 47) | Lrh (SP1) 2 × 107 CFU | One sachet in water (150 mL) and ingest it once a day after brushing for 3-months |
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdulkareem, A.; Abdulbaqi, H.; Gul, S.; Milward, M.; Chasib, N.; Alhashimi, R. Classic vs. Novel Antibacterial Approaches for Eradicating Dental Biofilm as Adjunct to Periodontal Debridement: An Evidence-Based Overview. Antibiotics 2022, 11, 9. https://doi.org/10.3390/antibiotics11010009
Abdulkareem A, Abdulbaqi H, Gul S, Milward M, Chasib N, Alhashimi R. Classic vs. Novel Antibacterial Approaches for Eradicating Dental Biofilm as Adjunct to Periodontal Debridement: An Evidence-Based Overview. Antibiotics. 2022; 11(1):9. https://doi.org/10.3390/antibiotics11010009
Chicago/Turabian StyleAbdulkareem, Ali, Hayder Abdulbaqi, Sarhang Gul, Mike Milward, Nibras Chasib, and Raghad Alhashimi. 2022. "Classic vs. Novel Antibacterial Approaches for Eradicating Dental Biofilm as Adjunct to Periodontal Debridement: An Evidence-Based Overview" Antibiotics 11, no. 1: 9. https://doi.org/10.3390/antibiotics11010009
APA StyleAbdulkareem, A., Abdulbaqi, H., Gul, S., Milward, M., Chasib, N., & Alhashimi, R. (2022). Classic vs. Novel Antibacterial Approaches for Eradicating Dental Biofilm as Adjunct to Periodontal Debridement: An Evidence-Based Overview. Antibiotics, 11(1), 9. https://doi.org/10.3390/antibiotics11010009







