Abstract
The choice of an effective therapeutic strategy in the treatment of biofilm-related infections is a significant issue. Amyloids, which have been historically related to human diseases, are now considered to be prevailing structural components of the biofilm matrix in a wide range of bacteria. This assumption creates the potential for an exciting research area, in which functional amyloids are considered to be attractive targets for drug development to dissemble biofilm structures. The present review describes the best-characterized bacterial functional amyloids and focuses on anti-biofilm agents that target intrinsic and facultative amyloids. This study provides a better understanding of the different modes of actions of the anti-amyloid molecules to inhibit biofilm formation. This information can be further exploited to improve the therapeutic strategies to combat biofilm-related infections.
1. Introduction
1.1. Biofilm Related Infections
Nosocomial infections related to the use of medical devices are associated with a high risk of mortality and increased economic costs [1]. These infections are mainly caused by opportunistic bacteria such as Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter species from the ESKAPE group, many of which are resistant to commonly used antibiotics [2]. The rapid appearance of antibiotic resistance in these bacteria is associated with a global increase in antibiotic consumption in the fields of health care, agriculture, and in the environment, in addition to their inappropriate utilization related to inadequate dosing or antibiotic selection [3,4,5]. The difficulty of treating healthcare-associated infections is also intensified by the common trait of the opportunistic bacteria to form biofilms. Bacteria are able to form multicellular communities on the surface of biomaterials, mucosa, tissues, and secretions, and express a self-generated extracellular polymeric matrix in response to myriad signals. The extracellular matrix, composed of polysaccharides, surface proteins, nucleic acids, and amyloid fibers, protects the microbes from external insults and adverse environmental conditions. The biofilm matrix acts as a physical and chemical barrier that reduces the rate of antimicrobial penetration. In addition, it can generate microenvironments that antagonize the action of antibiotics and promote the ability of bacteria to generate persister cells with increased drug tolerance [6,7,8,9]. Thus, bacteria in biofilms are less sensitive to antibiotics than the same strain growing in suspension, resulting in a 10- to 100-fold decrease in their susceptibility [10]. Because conventional antibiotics fail to successfully treat biofilm-associated infections, novel therapeutic solutions are in urgently needed. Efforts are currently underway to develop new therapeutic approaches that focus on preventing the synthesis or the assembly of the biofilm matrix components to render bacteria sensitive to antibiotic treatments.
1.2. Amyloids as Structural Scaffolds of the Biofilm Matrix
Amyloids are traditionally associated with several incurable degenerative human diseases. However, numerous studies have indicated that microorganisms make use of amyloidogenic proteins for a number of non-pathological processes [11]. In recent decades, amyloids have been considered to be essential components of the biofilm, providing consistency and viscoelasticity to the extracellular biofilm matrix [12,13,14,15]. Amyloids are highly ordered fibrillar proteins with a β-sheet secondary structure and a strongly conserved quaternary cross-β structure [16]. To a lesser extent, α-helices can replace the β-strands, thus forming cross-α fibrils [17,18]. The amyloid structure binds a range of specific dyes such as Congo red, Thioflavin T (ThT), and ProteoStat, which, combined with other biophysical techniques, such as solid-state NMR and Fourier-transform infrared spectroscopy (FTIR), are normally used to determine the amyloidogenic features of a protein [19,20,21,22]. Due to the well-ordered structure of β-strands that are aligned perpendicular to a fibril axis, amyloids are an effective extracellular building material. They are mostly resistant to harsh denaturing conditions and proteolytic cleavage. In addition, polymerization of amyloidogenic proteins occurs through a nucleation-dependent self-assembly process, in which starter amyloid aggregates provide a conformational scaffold that facilitates the assembly of polymeric subunits into the amyloid state in the absence of energy. Due to this seeding mechanism, the amyloid conformation is suitable under conditions in which the energy is limited [12,23].
Taking into account that amyloids are widely distributed components of the biofilm matrix in many bacteria, they can be considered excellent targets for anti-amyloid drugs to reduce biofilm formation. This review aims to summarize the current knowledge of the bacterial amyloids and the different mechanisms of action of the anti-amyloid molecules to inhibit biofilm formation.
2. Functional Amyloids of the Biofilm Matrix
In recent years, an increasing number of amyloid components of the biofilm matrix have been identified, purified, and closely investigated [15]. Based on the structural and functional state of the amyloidogenic proteins, it is becoming popular to group them into intrinsic and facultative amyloids (Table 1) [24].

Table 1.
Classification of functional amyloids.
2.1. Intrinsic Amyloids
The polymeric structure of intrinsic amyloids is the result of a dedicated system that controls the secretion and assembly of the monomeric building blocks. Indeed, the amyloidogenic subunits do not have a folded state prior to forming the amyloid fibers. The amyloid conformation represents the primary structural and functional state of the protein. Examples of amyloids from this group are described below.
2.1.1. Curli (csgBAC-csgDEFG Genes)
The curli fimbriae present in E. coli, Salmonella, and other Enterobacteria is one of the most well-characterized functional amyloid systems. The curli-specific genes are disposed in two divergent operons csgBAC and csgDEFG [25]. CsgA is the major curli subunit that structurally forms the amyloid fibers. To achieve this, the minor curli subunit CsgB acts as a nucleator of CsgA assembly (Figure 1a). To avoid the formation of toxic oligomers inside the cell, the periplasmic protein CsgC acts as a chaperone that maintains CsgA as a soluble and unstructured monomer, thus preventing the amyloid state [26]. The csgDEFG operon code for proteins that are involved in the regulation, stabilization, and secretion of CsgA and CsgB subunits [26,27,28]. CsgA and CsgB reach the extracellular milieu, due to a pore-like structure formed by CsgG in the outer membrane [29]. The accessory proteins CsgF and CsgE are associated with CsgG and contribute to the proper assembly of curli fibers [30]. In addition, CsgF interacts with CsgB, ensuring the attachment of the subunit on the cell surface and thus, the fiber formation [30]. CsgD controls at the transcriptional level the expression of the csgBAC operon [31,32] which is triggered by environmental and chemical signals such as osmolality, oxygen, and temperature [33,34]. Curli are implicated in surface colonization, cell–cell contact, biofilm matrix scaffolding, protection against antimicrobial agents and desiccation. Moreover, curli play an important role in the interaction with host cell receptors and the immune system [35,36,37,38,39].
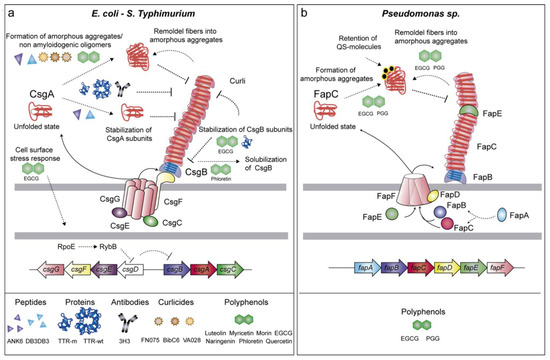
Figure 1.
Schematic representation of the genes involved in the formation of intrinsic amyloids, the drugs targeting such structures and their mechanism of action. (a) The formation of E. coli and S. Typhimurium curli amyloids is affected by several drugs, which act by different mechanisms: (i) stabilization of CsgA monomeric subunits; (ii) formation of amorphous or non-amyloidogenic aggregates; (iii) disaggregation of already-formed fibers; (iv) prevention of CsgB polymerization; (v) solubilization of CsgB; (vi) activation of the cell surface stress response, which reduces the expression of the curli regulator CsgD. (b) Pseudomonas Fap amyloids are inhibited by EGCG and PGG polyphenols, which lead FapC to off-pathway oligomers, remodeling fibers into amorphous aggregates and retaining quorum-sensing molecules.
2.1.2. Fap (fapA-F Genes)
Fap fibers are produced by Pseudomonas spp. as part of the biofilm matrix [52]. The Fap fiber machinery is encoded in the operon fapA-F. The Fap fibers are assembled by the major structural subunit FapC, and the two minor subunits FapB and FapE. FapB is required for fiber polymerization whereas FapE acts as an extracellular chaperon-like protein (Figure 1b). The three proteins are exported outside the cell through the pore-like structure formed by FapF [53]. FapA works as a chaperone of the fiber monomers during the secretion process whereas FapD, a cysteine protease located in the periplasm, is required for FapC externalization [53]. The assembly of Fap amyloids in the biofilm enhances cell aggregation and attachment conferring protection against chemical and mechanical attack [52]. In addition, Fap amyloids increase the overall hydrophobicity and stiffness of the biofilm, thus preventing bacteria desiccation [54].
2.1.3. Chaplins and Rodlins
Filamentous bacteria, such as Streptomyces, grow above humid surfaces forming branching hydrophobic structures called aerial hyphae that ultimately result in hydrophobic spores [55]. The amyloidogenic proteins, chaplins and rodlins are involved in the formation of these [43,44]. There are eight chaplins (ChpA–H) and two rodlins (RdlA and RdlB). The long chaplins (ChpA–C) have a C-terminal sorting signal for attachment to the peptidoglycan via a sortase-mediated covalent bound with the LAXTG motif and two chaplin domains that are highly hydrophobic, whereas the short chaplins (ChpD-H) have one chaplin domain [56]. To date, because long chaplins cannot be extracted from the cell walls of aerial hyphae due to the covalent linkage, only short chaplins have been shown to form β-sheet structures and thus amyloid-like fibers [57]. It is therefore possible that ChpA-C serve as anchoring sites for ChpD-H chaplins to form amyloid fibers, and that ChpE is responsible for the coordination of the polymerization of the fiber subunits in a high pH-dependent mechanism [58]. In addition to chaplins, the RodA and RodB rodlins polymerize into the hydrophobic structure known as the rodlet layer, which covers the surface of aerial hyphae and spores. Amyloid chaplins are aligned into ordered rodlets by the action RdlA and RdlB, only the latter is able to form amyloid fibrils in vitro [59].
2.2. Facultative Amyloids
The facultative amyloids include proteins with a dual function. They are secreted in a functional globular folded state that, under certain conditions, changes their conformation to an amyloid fold [12,24]. Two types of facultative amyloids can be differentiated. One type comprises secreted monomeric proteins, such as S. aureus phenol-soluble modulins (PSMs) and Bacillus subtilis TasA, which assemble into amyloid fibers upon conformational change occurs. The other group includes protein domains with amyloidogenic features, which has been previously processed from a native surface protein (e.g., S. aureus Bap, Enterococcus faecalis Esp, and Streptococcus mutans P1, WapA, and SMU_63C). In their native conformation, the facultative amyloids of the former group can act as antimicrobials and toxins, whereas the latter work as cell surface adhesins. Both turn into matrix scaffolds when they polymerize into amyloid structures.
2.2.1. Phenol-Soluble Modulins (PSMs)
Phenol-soluble modulins (PSMs) are small alpha-helical amphipathic peptides involved in the virulence of S. aureus [60,61]. S. aureus expresses four α-PSMs (PSMα1–PSMα4) encoded in the α-psm operon, two β-PSMs (PSMβ1 and PSMβ2) encoded in the β-psm operon and γ-PSM (δ-toxin) produced from RNAIII, a regulatory RNA of the Agr system [62,63,64]. PSMs lack the typical Sec-signal and their secretion is carried out by an ATP-binding cassette (ABC) transporter [65]. Once at the extracellular media, PSMs may either work as soluble proteins having antimicrobial and immunomodulating activities [66,67,68,69] or change the conformation and polymerize into functional amyloids (Figure 2a). PSMα1 and PSMα4 form aggregates showing a typical amyloid cross-β structure [45] whereas PSMα3 aggregates display an unusual cross-α structure [17]. This structural polymorphism reveals different bacterial functions. Although PSMα1 and PSMα4 participate in biofilm formation, PSMα3 is involved in cytotoxicity of human T cells, probably due to its particular amyloid architecture [17].
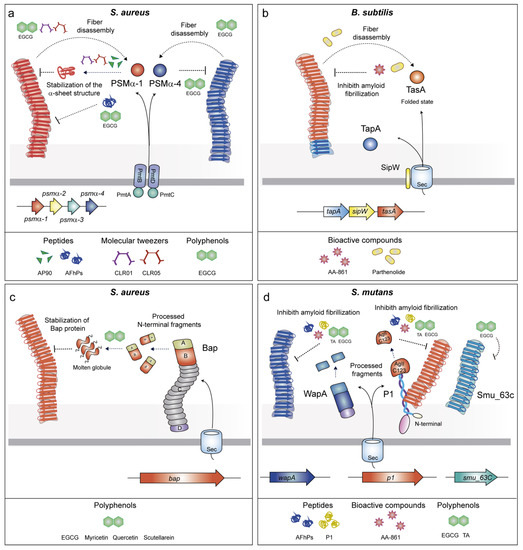
Figure 2.
Schematic representation of the genes involved in the assembly of facultative amyloids, the drugs targeting such structures and their mechanism of action. (a) Drugs targeting S. aureus PSMs act by several mechanisms that involve the stabilization of PSM α-sheet structure and fiber disassembly. (b) B. subtilis TapA polymerization is inhibited by AA-861 and parthenolide. (c) The flavonoids quercetin, myricetin, and scutellarein inhibit polymerization of the Bap amyloid aggregates by stabilization of the Bap protein. (d) Polymerization of the amyloids S. mutants WapA and AgII/C123 is inhibited by AA-861, Tannic acid (TA), and EGCG and indirectly by the AFhPs and P1 peptides. SMU_63c amyloids are inhibited by EGCG.
2.2.2. TasA
The B. subtilis biofilm contains functional fibers with amyloidogenic properties. The main amyloid component is TasA (translocation-dependent antimicrobial spore component), which self-assembles into amyloid fibers (Figure 2b) [46]. The accessory protein TapA (TasA anchoring and assembly protein) assists in the formation of TasA fibrils onto the cell surface [47]. SipW is a membrane-bound peptidase that cleaves the TapA and TasA signal peptides before secretion [70]. Structural analyses have shown that TasA is secreted in a globular state that transits to an amyloid conformation under environmental conditions such as acidic pH and hydrophobicity [71]. In addition to its structural role in the multicellular behavior, TasA maintains the cell membrane stability and prevents excess cell death under biofilm growth conditions [72].
2.2.3. Biofilm Associated Proteins (BAPs)
BAPs are high molecular weight proteins present on bacterial cell surfaces and are characterized by a multi-domain architecture [73,74]. The paradigm S. aureus Bap protein is organized in several domains (Figure 2c) including a putative Sec-dependent signal peptide for their export followed by a N-terminal domain, which comprises region A and B, the latter carries the EF-hand motifs for calcium binding [75,76]. Thereupon, a core domain formed by tandem repeats (C region) and a region D at the C-terminus, which contains the LPXTG motif for anchoring the protein to the cell wall. Bap forms amyloid fibers under specific environmental circumstances [48]. Once the protein is covalently anchored to the peptidoglycan, the N-terminal region is processed and released to the extracellular media. As a result, fragments of Bap adopt an unstable molten globule-like state. When acidic pH and low concentration of Ca2+ are found in the medium, the molten globule switches to an aggregation-prone conformation that enables self-assembly into amyloid fibers that promote biofilm formation (Figure 2c) [48]. In contrast, when Ca2+ is available, it binds to the EF-hand motifs, stabilizing Bap in its native folded state and impairing the polymerization of the amyloid fibers and biofilm formation [48,76]. It is notable that Bap carries out additional functions in its primary globular state. It acts as an adhesin, promoting the primary attachment of S. aureus to abiotic surfaces and thus playing an important role in initial stages of biofilm formation [75]. In addition, Bap is involved in the pathogenesis of S. aureus by improving the adhesion to epithelial cells of mammary glands [77] but exerting a protective role by preventing S. aureus entry into epithelial cell through binding to the Gp96 cell host receptor [78].
The Enterococcal surface protein (Esp) is a Bap-orthologous protein present in E. faecalis [79,80]. The multi-domain configuration of Esp is similar to that of Bap. The Esp N-terminal domain and the region C share 26% and 23% of identity with the corresponding Bap domains, respectively [49]. Analogously, the Esp N-terminal domain is sufficient to induce biofilm formation [81]. Our group has recently described the capacity of the Esp N-terminal region to form amyloid-like fibers in a pH-dependent manner [49]. The similarities observed between Bap and Esp suggest that the dual function of amyloid/adhesin may be widespread among members of the BAP family.
2.2.4. P1 Adhesin/WapA/SMU_63c
Another example of a surface-associated adhesin with amyloidogenic properties is the P1 protein (also known as AgI/II, PAc, SpaP or antigen B) of S. mutans. This P1 is a large protein that is covalently anchored to the cell wall through its C-terminal LPXTG motif. P1 is able to bind extracellular proteins [82,83,84]. The tertiary structure is a prolonged stalk with a globular domain that contains β-sheets. P1 can be processed and its C-terminal domain (AgII or C123 fragment) can be loosely associated with the covalently anchored protein (Figure 2d) [85]. The C123/AgII fragment contains the amyloid-forming moiety that may be implicated in the formation of fibrillar structures with amyloid properties [50,86]. In addition, the wall-associated protein A (WapA) and the secreted protein SMU_63c of S. mutans contribute to the fibrillar structures depending on the pH [51]. As the P1 adhesin, WapA is processed and forms amyloid fibers at neutral pH, whereas SMU_63c does not need to be processed and forms amyloid structures at acidic pH. Overall, this evidence suggests that environmental conditions are crucial for the regulation of S. mutans amyloid fibers.
3. Drugs Targeting Amyloid-Structured Biofilms
Amyloid fibers appear to be common biofilm matrix elements in many pathogenic bacteria. Several approaches have been focused on searching for compounds that either alter the expression of the elements involved in the amyloid production or interrupt their assembly. The identification of the anti-amyloid compounds to disassemble biofilms has been carried out by means of high-throughput screening of active molecule collections, the design of peptides based on the structural biology of the amyloidogenic segments or the repurposing of molecules designed against human amyloids. The most representative drugs active against amyloid-related biofilms and their mechanisms of action are discussed below and shown in Table 2.

Table 2.
Anti-biofilm agents targeting bacterial amyloids.
3.1. Anti-Amyloid Peptides
The design of peptides binding to the domains involved in amyloid fibrillation is one of the most promising strategies used to interrupt amyloid maturation and consequently biofilm formation. Based on the structural similarities existing between the amyloidogenic domains derived from CsgA and those from human amyloids, Perov and collaborators found that the D-enantiomeric peptides (ANK6 and DB3DB3) inhibited the fibrillation of CsgA (Table 2) [87]. ANK6 and DB3DB3 peptides were optimized for their potential to remove amyloid-β (Aβ) oligomers associated with Alzheimer’s disease. The D-peptides bound to CsgA, thus promoting the stabilization of the monomer subunits or inducing the formation of amorphous aggregates, which were unable to assemble into amyloid fibers (Figure 1a). The peptides inhibit the fibrillation of the CsgA spine segments contained at the R1 and R5 repeats. The inhibitory action of the peptides appears to be specific to the secondary structure of the protein because ANK6 and DB3DB3 do not have any effect on cross-α amyloid-like fibrils formed by PSMα3 fibrillation [87]. This distinction was further exemplified in a pioneering study that designed peptides that were complementary to the α-sheet of PSMα1. It is often considered that, because amyloids contain a cross-β-structure, the intermediate aggregative species must also contain a β-structure. However, this is not the case for several amyloids, such as PSMα1, which undergo a transition from α-helix→α-sheet→β-sheet during aggregation [88]. The anti-α-sheet peptide AP90 targets the transitional α-sheet structure of PSMα1, leading to a reduction of amyloid fibrils in vitro (Figure 2a). Consequently, AP90 effectively reduces biofilm formation by S. aureus through the interaction with PSMs [88].
Another interesting strategy for treating microbial infections is based on the use of amyloidogenic peptides derived from bacterial amyloids [100]. Synthetic aggregation-prone peptides from Staphylococcus epidermidis (C30, C29, Hit1, Hit50) penetrate bacteria, thus causing toxic protein aggregation of polypeptides in the cytosol, which leads to bacterial death [101]. Interestingly, aggregating peptides are toxic to bacteria, but not to human cells. However, further investigations are needed to test whether these peptides may have an effect on protein aggregation and toxicity in human cells that express amyloids such as α-synuclein or Aβ.
Furthermore, short peptides derived from the amyloidogenic fragment C123 of the adhesin P1 of S. mutans display strong anti-biofilm properties with small or no cytotoxic effect [89]. Simulated amyloid forming peptides predicted from C123 have been synthesized (AFhPs). AFhPs inhibit biofilm formation in a wide range of microorganisms including Gram-positive and Gram-negative bacteria and fungi. These peptides aggregate into rigid amyloid fibers that agglutinate microbial cells into clusters [89], thus leading to the formation of aggregates that impair the establishment of new biofilms. It was proposed that the carbohydrates of the microbial cell wall would be the binding targets of AFhPs rather than the surface-exposed amyloids. Moreover, lipid membranes may accelerate AFhP fibrillation. However, the role of these structures in the anti-biofilm activity of AFhPs remains to be elucidated.
Similarly, an anti-biofilm activity was identified for the P1 peptide, a synthetic peptide of 24 amino acids (different from the P1 protein of S. mutans), which was designed from the repeated motifs of the Ixodes tick antifreeze glycoprotein (IAFGP) sequence [102]. The P1 peptide is able to inhibit biofilm formation of S. mutants and other Gram-positive bacteria but does not possess bactericidal activity [103]. The anti-biofilm effect is associated with P1 aggregation and the formation of antiparallel β-sheets. However, further experiments are needed to determine whether the anti-biofilm effect is caused by the intermolecular interactions of P1 aggregates with bacterial amyloidogenic proteins [103].
3.2. Anti-Amyloid Proteins
The human amyloid precursor transthyretin (TTR) is a 55 kDa homotetramer protein mainly produced by the liver. Interestingly, TTR is structurally similar to the curli inhibitor CsgC, and both share a stable folded β-sheet-rich structure [104,105]. TTR is involved in the transport of thyroxine (T4) and retinol in plasma and cerebrospinal fluid. Misfolded protein monomers, derived from the tetrameric protein TTR, can be accumulated as fibrillar deposits in different organs and cause the most common form of hereditary transthyretin amyloidosis. By comparison, the homotetrameric form of the protein has the ability to inhibit amyloid formation of amyloidogenic proteins, such as Aβ, the N-terminal domain of the bacterial hydrogenase HypF and the human islet amyloid polypeptide [106,107,108]. Taking into account the anti-amyloid properties of TTR, in a remarkable work, Jain et al. examined the effect of TTR on CsgA amyloid assembly [109]. The incubation of CsgA with the tetramer precursor (Wt-TTR) retarded CsgA polymerization, whereas the incubation with the monomeric form (M-TTR) strongly prevented the formation of CsgA protofibrillar and fibrillar structures (Figure 1a). Interestingly, M-TTR also inhibited the assembly of CsgB into amyloid aggregates. Therefore, both Wt-TTR and M-TTR inhibited wrinkled pellicle biofilms formed by E. coli; however, the inhibitory effect of M-TTR was significantly more potent than that produced by Wt-TTR. It is possible that Wt-TTR requires tetramer dissociation to liberate the monomers that are considered the active forms for fibril inhibition. However, the use of a highly stable TTR tetramer form (TTR T119M) inhibited CsgA fibrillogenesis, indicating that the Wt-TTR tetramer dissociation was not required for such inhibition [109].
3.3. Antibodies as Native-State Stabilizing Agents
The administration of antibodies or engineered affibodies has been proposed as one of the most promising therapies to reduce amyloid polymerization [110]. Antibody-based immunotherapy has been used to reduce the amyloid amount by stimulating the clearance of the misfolded aggregates by the immune system cells [111]. In addition, antibodies can be used as native-state stabilizing agents that bind to the aggregation-prone proteins, increasing their stability and impairing the aggregation tendency of human amyloidogenic proteins [112,113]. A recent pioneering study proposed the use of antibodies as native-state stabilizing agents to reduce bacterial amyloid aggregation [90]. The human monoclonal antibody mAB 3H3, which specifically binds Aβ and other human amyloids, such as immunoglobulin light chain, transthyretin, and tau, also binds the curli amyloids. Antibody mAB 3H3 inhibits the initiation of CsgA polymerization and therefore, curli fiber elongation (Figure 1a). The incubation of S. Typhimurium with mAB H3 alters the structure of the biofilm. Consequently, due to changes in the biofilm architecture, antibiotic treatment and macrophage uptake are more effective at removing S. Typhimurium biofilms in vivo from an implanted catheter mouse model. Because mAB 3H3 binds and inhibits polymerization of human and bacterial amyloids with significant differences in the primary amino acid sequence, it has been proposed that mAB H3 should bind to an intermediate conformation that appears to be common in different amyloidogenic proteins [90].
3.4. Molecular Tweezers
Molecular tweezers are supramolecular host molecules comprised of two aromatic arms linked by a spacer that has the capacity to bind guest molecules with high specificity. Therefore, these macromolecules may work as singular molecular receptors for specific targets that can be used in numerous applications in biology and medicine [114]. Among the different applications, it was reported that a lysine-binding tweezer (CLR01) inhibits the self-assembly of different human amyloidogenic proteins, including Aβ protein, tau, and α-synuclein in vitro and in vivo [115,116]. CLR01 alters the assembly process and promotes the formation of non-amyloidogenic structures that can be efficiently removed by host clearance mechanisms (Figure 2a). In addition, CLR01, and its derivative CLR05, reduced biofilm formation of S. aureus [91]. CLR01 and CLR05 inhibit PSMα1 fibrillization and disintegration of mature fibrils. In particular, CLR01 interferes with the secondary structure of PSMα1and inhibits their polymerization, possibly through the enclosure of lysine residues of PSMα1. CLR05, which exhibits less affinity for the lysine residues, inhibits S. aureus biofilm formation more strongly than CLR01. The “truncated” molecular tweezer CLR3, which was used as a negative control, did not affect S. aureus biofilms and had a small impact on PSMα1 assembly. Although these molecules could be promising drugs to treat staphylococcal infections, further characterization of the interactions between CLR01 and CLR05 with PSMα1 is necessary to determine their potential as anti-biofilm molecules in vivo.
3.5. Anti-Amyloids Based on Pilicides and Curlicides
Pilicides have been extensively used as anti-virulence compounds that block the formation of pili in E. coli [117]. Derivatives of the dihydrothiazolo ring-fused 2-pyridone pilicide 1, such as FN075, BibC6, and VA028, gain anti-amyloid properties and inhibit Aβ polymerization in vitro [118]. Thus, FN075, BibC6, and VA028 were tested for their ability to inhibit curli assembly. The compounds inhibited curli biogenesis and CsgA polymerization in vitro [92]. The mechanism by which FN075 inhibits CsgA fibrillization involves the formation of non-amyloidogenic oligomers (Figure 1a) [119]. FN075 and BibC6, at least, prevent amyloid-dependent biofilm formation at the air–liquid interface and all of the compounds inhibit biofilm formation by the uropathogenic E. coli strain UTI89 on PVC microtiter plates [92]. Moreover, FN075 was effective at inhibiting E. coli UTI89 in vivo in a murine model of urinary tract infection [92]. Therefore, a new class of biofilm inhibitors, termed curlicides, was stablished with the capacity to block curli. The double curlicide–pilicide property of FN075 provides additional therapeutic value because it could inhibit the formation of several pili and fibers that are important for virulence and biofilm formation.
3.6. Bioactive Compounds with Anti-Amyloid Properties
Using throughput screening for molecules with anti-biofilm activity, Romero and collaborators found two active molecules, a benzoquinone derivative (AA-861) and a sesquiterpene lactone (parthenolide) with the capacity to alter the wrinkly biofilm phenotype of B. subtilis [93]. The AA-861 and parthenolide did not affect tasA gene expression but they inhibited the polymerization of TasA into amyloid fibers (Figure 2b). AA-861 was more efficient at hindering TasA polymerization, whereas parthenolide was more effective at disrupting already-formed biofilms. These results indicated a synergistic activity of both molecules to inhibit biofilm formation. They also showed that AA-861 and parthenolide were effective at inhibiting biofilm formation of E. coli and B. cereus but did not showed any detectable activity against S. aureus and P. aeruginosa biofilms. However, it was not clarified whether their anti-biofilm effect was via direct inhibition of the CsgA and TasA polymerization, respectively, or due to an alternative mechanism. In a latter work, it was shown that AA-861 and not parthenolide was also effective at inhibiting S. mutans biofilm formation [51]. AA-861 inhibited the fibrillization of the amyloidogenic moieties of the adhesin P1 (C123 and antigen A) and WapA in vitro, whereas SMU_63c was completely unaffected by this molecule (Figure 2d). These results validate the broad-spectrum activity of AA-861 on different amyloids.
3.7. Polyphenols
Polyphenols are naturally occurring secondary metabolites, and are widely spread in fruits, vegetables, seeds and plant-derived oils. They are considered to be powerful anti-amyloidogenic compounds due to their physicochemical features. Polyphenols can affect the fibrillation of amyloidogenic proteins involved in neurodegenerative diseases by inhibiting nucleation and elongation or redirecting to “off-pathway” aggregation [120,121,122]. The structural similarities between human and bacterial amyloids suggest that polyphenols may disturb the biofilm matrix by interfering with the bacterial amyloids.
A potent anti-amyloidogenic polyphenol is epigallocatechin gallate (EGCG), which is the major active polyphenol in green tea (Camellia sinensis). EGCG has an indiscriminate protein binding and inhibits the function of a variety of proteins. Its effect on the oligomerization of multiple human amyloids such as Aβ [123,124,125], α-synuclein [124,126,127,128], islet amyloid polypeptide [129,130], huntingtin [131], and tau protein [132] has been extensively demonstrated. In these proteins, EGCG induces the formation of partially stable oligomers, which are unable to form amyloid fibers [125]. EGCG can also reorganize preformed amyloid fibrils into amorphous aggregates [133]. The anti-amyloidogenic properties of EGCG have been also extended to bacterial amyloids. Serra and collaborators showed that EGCG interfered with the assembly of curli amyloid subunits, and found that EGCG prevented CsgB from adopting an amyloid conformation and prevented CsgA from polymerization into amyloid fibers (Figure 1a) [94]. In addition to its direct effect on curli polymerization, EGCG triggers the cell surface stress response governed by the alternative sigma factor RpoE, which finally induces the downregulation of the biofilm regulator CsgD [94].
At the same time, it was shown that the EGCG acts against Fap amyloid fibers in P. aeruginosa [96]. EGCG both remodels already-formed Fap fibrils into amorphous aggregates and inhibits FapC fibrillation by redirecting FapC to off-pathway oligomers (Figure 1b) [96]. EGCG can inhibit FapC fibril formation, even in the presence of bacterial amphiphiles, such as rhamnolipid and LPS, otherwise they promote fibril formation [134]. In addition to EGCG, the gallotannin penta-O-galloyl-d-glucose (PGG) was found to be a strong inhibitor of Fap fibrillization [134]. PGG induced the formation of oligomers and larger non-fibrillar aggregates, that had low β-sheet content and that were remarkably stable. EGCG and PGG reduce the amyloid content in P. aeruginosa biofilms, leading to a higher antibiotic susceptibility of bacterial cells [95,96].
An alternative mechanism of biofilm inhibition by EGCG involved the bacterial quorum-sensing system [96]. It was speculated that the FapC amorphous aggregates formed by EGCG increased the binding and retention of the quorum sensing molecule pyocyanin, thereby reducing the autoinducer concentration [96]. Therefore, the administration of the polyphenols EGCG and PGG was proposed as a promising treatment for cystic fibrosis patients in which Fap fibers play an important role during P. aeruginosa biofilm-mediated infections.
The anti-amyloid effect of EGCG was also extended to other biofilm-associated amyloids including S. mutans P1 as the main target and WapA and SMU_63c as secondary targets and S. aureus PSMα1 and PSMα4 (Figure 2a,d) [51,97]. Due to the extensive anti-amyloid properties of EGCG, in vivo studies will be needed to determine its potential as a therapeutic compound against biofilm-related infections.
Polyphenols of the flavonoid group, such as luteolin, myricetin, morin, quercetin, phloretin and, to a lesser degree, naringenin, also inhibited the assembly of the curli amyloid fibers. Most of these flavonoids drive CsgA into an off-pathway by the formation of insoluble oligomers. In contrast, phloretin affected the solubility of CsgB, leading it in a soluble form and, therefore, compromising CsgA polymerization (Figure 1a) [98]. In a parallel work, it was reported that the flavonoids quercetin, myricetin, and scutellarein specifically inhibited biofilm formation of S. aureus, S. hyicus, S. simiae, and S. saprophyticus, which expressed Bap [99]. The flavonoids did not affect bap expression but reduced the formation of Bap amyloid-like aggregates (Figure 2c). Because the Bap N-terminal region adopts an unstable molten globule-like state, it was proposed that flavonoids bind this region, thus stabilizing the Bap native folded state and avoiding the polymerization of the amyloid aggregates. Interestingly, quercetin and myricetin, at least, were effective under in vivo conditions because they reduced S. aureus biofilm formation on catheters implanted in mice. Since BAPs are present in many pathogenic bacteria, the use of polyphenols to fight against biofilm associated infections could be widely extended [135].
4. Final Remarks
Numerous compounds with anti-amyloid properties have been identified to date. Some of them are effective at reducing biofilm formation in many bacteria. However, future directions of research on anti-biofilm molecules that target functional amyloids should include several aspects. First, it is necessary to consider that anti-amyloid compounds can have species-specific effects. They can inhibit biofilm formation of a certain bacteria but could have the opposite effect on the biofilm of other bacteria. This is the case for the plant flavonoids luteolin, myricetin, and quercetin, which inhibit the assembly of curli of E. coli and Bap amyloids of S. aureus, strongly reducing the extracellular biofilm matrix; in contrast, they enhance P. aeruginosa macrocolony biofilm formation and have no effect on B. subtilis biofilm formation [98]. In addition, EGCG was found to promote biofilm formation of P. aeruginosa and increase its resistance to tobramycin [136]. Under certain conditions, EGCG reduced the susceptibility to vancomycin, oxacillin, and ampicillin of S. aureus [137]. The cell envelope stress generated by some compounds could lead to an induced biofilm development and an increase in anti-microbial resistance.
A second intriguing aspect is the cross-reactivity effect that the anti-amyloid molecules could have on human amyloids. Although many of the compounds able to inhibit bacterial functional amyloids also actively reduce human aberrant amyloids, it is conceivable that they could have a contrary effect. It is important to highlight that FN075 displays cross-reactivity with diverging activity. It inhibits CsgA fibril formation but increases the rate of α-synuclein fibrillation. This discovery reveals the need to assess cross-reactivity with several amyloidogenic human proteins when anti-amyloid molecules are designed [119].
Bacterial amyloids, such as curli, are major compounds of the extracellular matrix of gut microbiomes, which have been linked to autoimmune diseases, neurodegenerative diseases, and cancer [138]. However, bacterial amyloids of the gut may benefit the host by educating the immune system and reinforcing the epithelial barrier. This raises an intriguing question as whether the oral administration of anti-amyloid compounds would influence gut amyloids and therefore, have any influence on health and disease. The specific processes underlying the beneficial or detrimental effects of anti-amyloid drugs on gut amyloids require thorough analysis.
Finally, many of the compounds identified to date have been tested for their anti-amyloid and anti-biofilm effects using in vitro models. Biofilm formation is a multifactorial process in which many different molecular processes are involved. This means that not all of the components that are expressed in vitro will be produced in vivo. Therefore, future research should focus on confirming the anti-amyloid activity of the drug candidates using in vivo models.
Author Contributions
J.V. conceptualized the content of the article. J.V., L.M.-C., and A.T.-A. contributed to writing and revision of the work. All authors have read and agreed to the published version of the manuscript.
Funding
Work in the laboratory of J.V. is funded by grant RTI2018-096011-B-I00 from the Spanish Ministry of Science, Innovation and Universities.
Acknowledgments
We acknowledge support of the publication fee by the CSIC Open Access Publication Support Initiative through its Unit of Information Resources for Research (URICI).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sikora, A.; Zahra, F. Nosocomial Infections; StatPearls Publishing: Bethesda, MD, USA; Treasure Island, FL, USA, 2020. [Google Scholar]
- Ma, Y.X.; Wang, C.Y.; Li, Y.Y.; Li, J.; Wan, Q.Q.; Chen, J.H.; Tay, F.R.; Niu, L.N. Considerations and caveats in combating ESKAPE pathogens against nosocomial infections. Adv. Sci. 2020, 7, 1901872. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Matsoso, P.; Pant, S.; Brower, C.; Rottingen, J.-A.; Klugman, K.; Davies, S. Access to effective antimicrobials: A worldwide challenge. Lancet 2016, 387, 168–175. [Google Scholar] [CrossRef]
- Holmes, A.H.; Moore, L.S.P.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J.V. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef]
- Prestinaci, F.; Pezzotti, P.; Pantosti, A. Antimicrobial resistance: A global multifaceted phenomenon. Pathog. Glob. Health 2015, 109, 309–318. [Google Scholar] [CrossRef]
- Jefferson, K.K.; Goldmann, D.A.; Pier, G.B. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Antimicrob. Agents Chemother. 2005, 49, 2467–2473. [Google Scholar] [CrossRef] [PubMed]
- Lewis, K. Persister cells, dormancy and infectious disease. Nat. Rev. Micro. 2007, 5, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J. The biofilm matrix. Nat. Rev. Micro. 2010, 8, 623–633. [Google Scholar] [CrossRef]
- Hall, C.W.; Mah, T.-F. Molecular mechanisms of biofilm-based antibiotic resistance and tolerance in pathogenic bacteria. FEMS Microbiol. Rev. 2017, 41, 276–301. [Google Scholar] [CrossRef] [PubMed]
- Stewart, P.S.; Costerton, J.W. Antibiotic resistance of bacteria in biofilms. Lancet 2001, 358, 135–138. [Google Scholar] [CrossRef]
- Blanco, L.P.; Evans, M.L.; Smith, D.R.; Badtke, M.P.; Chapman, M.R. Diversity, biogenesis and function of microbial amyloids. Trends Microbiol. 2012, 20, 66–73. [Google Scholar] [CrossRef]
- Taglialegna, A.; Lasa, I.; Valle, J. Amyloid structures as biofilm matrix scaffolds. J. Bacteriol. 2016, 198, 2579–2588. [Google Scholar] [CrossRef]
- Álvarez-Mena, A.; Cámara-Almirón, J.; de Vicente, A.; Romero, D. Multifunctional Amyloids in the Biology of Gram-Positive Bacteria. Microorganisms 2020, 8, 2020. [Google Scholar] [CrossRef]
- Erskine, E.; Macphee, C.E.; Stanley-Wall, N.R. Functional Amyloid and Other Protein Fibers in the Biofilm Matrix. J. Mol. Biol. 2018, 430, 3642–3656. [Google Scholar] [CrossRef]
- Levkovich, S.A.; Gazit, E.; Bar-Yosef, D.L. Two Decades of Studying Functional Amyloids in Microorganisms. Trends Microbiol. 2020, 1–15. [Google Scholar] [CrossRef]
- Uversky, V.N.; Fink, A.L. Conformational constraints for amyloid fibrillation: The importance of being unfolded. Biochim. Biophys Acta 2004, 1698, 131–153. [Google Scholar] [CrossRef]
- Tayeb-Fligelman, E.; Tabachnikov, O.; Moshe, A.; Goldshmidt-Tran, O.; Sawaya, M.R.; Coquelle, N.; Colletier, J.-P.; Landau, M. The cytotoxic Staphylococcus aureus PSMα3 reveals a cross-α amyloid-like fibril. Science 2017, 355, 831–833. [Google Scholar] [CrossRef] [PubMed]
- Salinas, N.; Tayeb-Fligelman, E.; Sammito, M.D.; Bloch, D.; Jelinek, R.; Noy, D.; Usón, I.; Landau, M. The amphibian antimicrobial peptide uperin 3.5 is a cross-α/cross-β chameleon functional amyloid. Proc. Natl Acad Sci USA 2021, 118. [Google Scholar] [CrossRef] [PubMed]
- Groenning, M. Binding mode of Thioflavin T and other molecular probes in the context of amyloid fibrils-current status. J. Chem. Biol 2010, 3, 1–18. [Google Scholar] [CrossRef]
- Howie, A.J.; Brewer, D.B.; Howell, D.; Jones, A.P. Physical basis of colors seen in Congo red-stained amyloid in polarized light. Lab. Investig. 2008, 88, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Navarro, S.; Ventura, S. Fluorescent dye ProteoStat to detect and discriminate intracellular amyloid-like aggregates in Escherichia coli. Biotechnol. J. 2014, 9, 1259–1266. [Google Scholar] [CrossRef] [PubMed]
- Shewmaker, F.; McGlinchey, R.P.; Wickner, R.B. Structural insights into functional and pathological amyloid. J. Biol. Chem. 2011, 286, 16533–16540. [Google Scholar] [CrossRef]
- Hufnagel, D.A.; DePas, W.H.; Chapman, M.R. The biology of the Escherichia coli extracellular matrix. MicroBiol. Spectr. 2015, 3, 23. [Google Scholar] [CrossRef]
- Van Gerven, N.; Van der Verren, S.E.; Reiter, D.M.; Remaut, H. The role of functional amyloids in bacterial virulence. J. Mol. Biol. 2018, 430, 3657–3684. [Google Scholar] [CrossRef]
- Hammar, M.; Arnqvist, A.; Bian, Z.; Olsen, A.; Normark, S. Expression of two csg operons is required for production of fibronectin- and congo red-binding curli polymers in Escherichia coli K-12. Mol. Microbiol. 1995, 18, 661–670. [Google Scholar] [CrossRef]
- Hammer, N.D.; McGuffie, B.A.; Zhou, Y.; Badtke, M.P.; Reinke, A.A.; Brännström, K.; Gestwicki, J.E.; Olofsson, A.; Almqvist, F.; Chapman, M.R. The C-terminal repeating units of CsgB direct bacterial functional amyloid nucleation. J. Mol. Biol. 2012, 422, 376–389. [Google Scholar] [CrossRef][Green Version]
- Hobley, L.; Harkins, C.; Macphee, C.E.; Stanley-Wall, N.R. Giving structure to the biofilm matrix: An overview of individual strategies and emerging common themes. FEMS MicroBiol. Rev. 2015, 39, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Van Gerven, N.; Klein, R.D.; Hultgren, S.J.; Remaut, H. Bacterial amyloid formation: Structural insights into curli biogensis. Trends Microbiol. 2015, 23, 693–706. [Google Scholar] [CrossRef]
- Robinson, L.S.; Ashman, E.M.; Hultgren, S.J.; Chapman, M.R. Secretion of curli fibre subunits is mediated by the outer membrane-localized CsgG protein. Mol. Microbiol. 2006, 59, 870–881. [Google Scholar] [CrossRef]
- Nenninger, A.A.; Robinson, L.S.; Hammer, N.D.; Epstein, E.A.; Badtke, M.P.; Hultgren, S.J.; Chapman, M.R. CsgE is a curli secretion specificity factor that prevents amyloid fibre aggregation. Mol. Microbiol. 2011, 81, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Brombacher, E.; Baratto, A.; Dorel, C.; Landini, P. Gene expression regulation by the Curli activator CsgD protein: Modulation of cellulose biosynthesis and control of negative determinants for microbial adhesion. J. Bacteriol. 2006, 188, 2027–2037. [Google Scholar] [CrossRef]
- Zakikhany, K.; Harrington, C.R.; Nimtz, M.; Hinton, J.C.D.; Römling, U. Unphosphorylated CsgD controls biofilm formation in Salmonella enterica serovar Typhimurium. Mol. Microbiol. 2010, 77, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Jubelin, G.; Vianney, A.; Beloin, C.; Ghigo, J.-M.; Lazzaroni, J.-C.; Lejeune, P.; Dorel, C. CpxR/OmpR interplay regulates curli gene expression in response to osmolarity in Escherichia coli. J. Bacteriol. 2005, 187, 2038–2049. [Google Scholar] [CrossRef]
- Khambhati, K.; Patel, J.; Saxena, V.; Jain, N. Gene regulation of biofilm-associated functional amyloids. Pathogens 2021, 10, 490. [Google Scholar] [CrossRef]
- Gophna, U.; Oelschlaeger, T.A.; Hacker, J.; Ron, E.Z. Role of fibronectin in curli-mediated internalization. FEMS MicroBiol. Lett. 2002, 212, 55–58. [Google Scholar] [CrossRef] [PubMed]
- Pawar, D.M.; Rossman, M.L.; Chen, J. Role of curli fimbriae in mediating the cells of enterohaemorrhagic Escherichia coli to attach to abiotic surfaces. J. Appl. Microbiol. 2005, 99, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Saldaña, Z.; Xicohténcatl-Cortes, J.; Avelino, F.; Phillips, A.D.; Kaper, J.B.; Puente, J.L.; Girón, J.A. Synergistic role of curli and cellulose in cell adherence and biofilm formation of attaching and effacing Escherichia coli and identification of Fis as a negative regulator of curli. Environ. Microbiol. 2009, 11, 992–1006. [Google Scholar] [CrossRef]
- Goulter-Thorsen, R.M.; Taran, E.; Gentle, I.R.; Gobius, K.S.; Dykes, G.A. CsgA production by Escherichia coli O157:H7 alters attachment to abiotic surfaces in some growth environments. Appl. Environ. Microbiol. 2011, 77, 7339–7344. [Google Scholar] [CrossRef] [PubMed]
- DeBenedictis, E.P.; Liu, J.; Keten, S. Adhesion mechanisms of curli subunit CsgA to abiotic surfaces. Sci. Adv. 2016, 2. [Google Scholar] [CrossRef]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Dueholm, M.S.; Petersen, S.V.; Sønderkaer, M.; Larsen, P.; Christiansen, G.; Hein, K.L.; Enghild, J.J.; Nielsen, J.L.; Nielsen, K.L.; Nielsen, P.H.; et al. Functional amyloid in Pseudomonas. Mol. Microbiol. 2010, 77, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Alteri, C.J.; Xicohténcatl-Cortes, J.; Hess, S.; Caballero-Olín, G.; Girón, J.A.; Friedman, R.L. Mycobacterium tuberculosis produces pili during human infection. Proc. Natl. Acad. Sci. USA 2007, 104, 5145–5150. [Google Scholar] [CrossRef] [PubMed]
- Elliot, M.A.; Talbot, N.J. Building filaments in the air: Aerial morphogenesis in bacteria and fungi. Curr. Opin. MicroBiol. 2004, 7, 594–601. [Google Scholar] [CrossRef]
- Willey, J.M.; Willems, A.; Kodani, S.; Nodwell, J.R. Morphogenetic surfactants and their role in the formation of aerial hyphae in Streptomyces coelicolor. Mol. Microbiol. 2006, 59, 731–742. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Syed, A.K.; Stephenson, R.E.; Rickard, A.H.; Boles, B.R. Functional amyloids composed of phenol soluble modulins stabilize Staphylococcus aureus biofilms. PLoS Pathogens 2012, 8, e1002744. [Google Scholar] [CrossRef]
- Romero, D.; Aguilar, C.; Losick, R.; Kolter, R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA 2010, 107, 2230–2234. [Google Scholar] [CrossRef]
- Romero, D.; Vlamakis, H.; Losick, R.; Kolter, R. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol. Microbiol. 2011, 80, 1155–1168. [Google Scholar] [CrossRef]
- Taglialegna, A.; Navarro, S.; Ventura, S.; Garnett, J.A.; Matthews, S.; Penadés, J.R.; Lasa, I.; Valle, J. Staphylococcal Bap proteins build amyloid scaffold biofilm matrices in response to environmental signals. PLoS Pathog 2016, 12, e1005711. [Google Scholar] [CrossRef] [PubMed]
- Taglialegna, A.; Matilla-Cuenca, L.; Dorado-Morales, P.; Navarro, S.; Ventura, S.; Garnett, J.A.; Lasa, I.; Valle, J. The biofilm-associated surface protein Esp of Enterococcus faecalis forms amyloid-like fibers. NPJ Biofilms Microbiomes 2020, 6, 1–12. [Google Scholar] [CrossRef]
- Oli, M.W.; Otoo, H.N.; Crowley, P.J.; Heim, K.P.; Nascimento, M.M.; Ramsook, C.B.; Lipke, P.N.; Brady, L.J. Functional amyloid formation by Streptococcus mutans. Microbiology 2012, 158, 2903–2916. [Google Scholar] [CrossRef]
- Besingi, R.N.; Wenderska, I.B.; Senadheera, D.B.; Cvitkovitch, D.G.; Long, J.R.; Wen, Z.T.; Brady, L.J. Functional amyloids in Streptococcus mutans, their use as targets of biofilm inhibition and initial characterization of SMU_63c. Microbiology 2017, 163, 488–501. [Google Scholar] [CrossRef]
- Dueholm, M.S.; Søndergaard, M.T.; Nilsson, M.; Christiansen, G.; Stensballe, A.; Overgaard, M.T.; Givskov, M.; Tolker-Nielsen, T.; Otzen, D.E.; Nielsen, P.H. Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. Microbiologyopen 2013, 2, 365–382. [Google Scholar] [CrossRef]
- Rouse, S.L.; Hawthorne, W.J.; Berry, J.-L.; Chorev, D.S.; Ionescu, S.A.; Lambert, S.; Stylianou, F.; Ewert, W.; Mackie, U.; Morgan, R.M.L.; et al. A new class of hybrid secretion system is employed in Pseudomonas amyloid biogenesis. Nat. Commun. 2017, 8, 263. [Google Scholar] [CrossRef] [PubMed]
- Zeng, G.; Vad, B.S.; Dueholm, M.S.; Christiansen, G.; Nilsson, M.; Tolker-Nielsen, T.; Nielsen, P.H.; Meyer, R.L.; Otzen, D.E. Functional bacterial amyloid increases Pseudomonas biofilm hydrophobicity and stiffness. Front. Microbiol. 2015, 6, 1099. [Google Scholar] [CrossRef] [PubMed]
- Perrett, S.; Buell, A.K.; Knowles, T.P.J. Biological and Bio-Inspired Nanomaterials; Springer Nature: Singapore, 2020. [Google Scholar]
- Sawyer, E.B.; Claessen, D.; Haas, M.; Hurgobin, B.; Gras, S.L. The assembly of individual chaplin peptides from Streptomyces coelicolor into functional amyloid fibrils. PLoS ONE 2011, 6, e18839. [Google Scholar] [CrossRef] [PubMed]
- Gebbink, M.F.B.G.; Claessen, D.; Bouma, B.; Dijkhuizen, L.; Wösten, H.A.B. Amyloids—A functional coat for microorganisms. Nat. Rev. Micro 2005, 3, 333–341. [Google Scholar] [CrossRef] [PubMed]
- Dokouhaki, M.; Hung, A.; Day, L.; Gras, S.L. The pH-dependent assembly of Chaplin E from Streptomyces coelicolor. J. Struct. Biol. 2017, 198, 82–91. [Google Scholar] [CrossRef]
- Yang, W.; Willemse, J.; Sawyer, E.B.; Lou, F.; Gong, W.; Zhang, H.; Gras, S.L.; Claessen, D.; Perrett, S. The propensity of the bacterial rodlin protein RdlB to form amyloid fibrils determines its function in Streptomyces coelicolor. Sci. Rep. 2017, 7, 42867. [Google Scholar] [CrossRef]
- Periasamy, S.; Chatterjee, S.S.; Cheung, G.Y.C.; Otto, M. Phenol-soluble modulins in staphylococci: What are they originally for? Commun. Integr. Biol. 2012, 5, 275–277. [Google Scholar] [CrossRef]
- Peschel, A.; Otto, M. Phenol-soluble modulins and staphylococcal infection. Nat. Rev. Micro. 2013, 11, 667–673. [Google Scholar] [CrossRef]
- Cheung, G.Y.C.; Kretschmer, D.; Queck, S.Y.; Joo, H.-S.; Wang, R.; Duong, A.C.; Nguyen, T.H.; Bach, T.-H.L.; Porter, A.R.; DeLeo, F.R.; et al. Insight into structure-function relationship in phenol-soluble modulins using an alanine screen of the phenol-soluble modulin (PSM) α3 peptide. FASEB J. 2014, 28, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Queck, S.Y.; Jameson-Lee, M.; Villaruz, A.E.; Bach, T.-H.L.; Khan, B.A.; Sturdevant, D.E.; Ricklefs, S.M.; Li, M.; Otto, M. RNAIII-independent target gene control by the agr quorum-sensing system: Insight into the evolution of virulence regulation in Staphylococcus aureus. Mol. Cell 2008, 32, 150–158. [Google Scholar] [CrossRef]
- Wang, R.; Braughton, K.R.; Kretschmer, D.; Bach, T.-H.L.; Queck, S.Y.; Li, M.; Kennedy, A.D.; Dorward, D.W.; Klebanoff, S.J.; Peschel, A.; et al. Identification of novel cytolytic peptides as key virulence determinants for community-associated MRSA. Nat. Med. 2007, 13, 1510–1514. [Google Scholar] [CrossRef]
- Chatterjee, S.S.; Joo, H.-S.; Duong, A.C.; Dieringer, T.D.; Tan, V.Y.; Song, Y.; Fischer, E.R.; Cheung, G.Y.C.; Li, M.; Otto, M. Essential Staphylococcus aureus toxin export system. Nat. Med. 2013, 19, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Cogen, A.L.; Yamasaki, K.; Sanchez, K.M.; Dorschner, R.A.; Lai, Y.; MacLeod, D.T.; Torpey, J.W.; Otto, M.; Nizet, V.; Kim, J.E.; et al. Selective antimicrobial action is provided by phenol-soluble modulins derived from Staphylococcus epidermidis, a normal resident of the skin. J. Invest. Dermatol. 2010, 130, 192–200. [Google Scholar] [CrossRef]
- Joo, H.-S.; Cheung, G.Y.C.; Otto, M. Antimicrobial activity of community-associated methicillin-resistant Staphylococcus aureus is caused by phenol-soluble modulin derivatives. J. Biol. Chem. 2011, 286, 8933–8940. [Google Scholar] [CrossRef]
- Nakamura, Y.; Oscherwitz, J.; Cease, K.B.; Chan, S.M.; Muñoz-Planillo, R.; Hasegawa, M.; Villaruz, A.E.; Cheung, G.Y.C.; McGavin, M.J.; Travers, J.B.; et al. Staphylococcus δ-toxin induces allergic skin disease by activating mast cells. Nature 2013, 503, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.R.; Armbruster, N.S.; Günter, M.; Biljecki, M.; Klenk, J.; Heumos, S.; Autenrieth, S.E. PSM Peptides from community-associated methicillin-resistant Staphylococcus aureus impair the adaptive immune response via modulation of dendritic cell subsets in vivo. Front. Immunol. 2019, 10, 995. [Google Scholar] [CrossRef]
- Terra, R.; Stanley-Wall, N.R.; Cao, G.; Lazazzera, B.A. Identification of Bacillus subtilis SipW as a bifunctional signal peptidase that controls surface-adhered biofilm formation. J. Bacteriol. 2012, 194, 2781–2790. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.; Romero, D.; Kayatekin, C.; Akabayov, B.; Vlamakis, H.; Losick, R.; Kolter, R. Isolation, characterization, and aggregation of a structured bacterial matrix precursor. J. Biol. Chem. 2013, 288, 17559–17568. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Almirón, J.; Navarro, Y.; Díaz-Martínez, L.; Magno-Pérez-Bryan, M.C.; Molina-Santiago, C.; Pearson, J.R.; de Vicente, A.; Pérez-García, A.; Romero, D. Dual functionality of the amyloid protein TasA in Bacillus physiology and fitness on the phylloplane. Nat. Commun. 2020, 11, 1–21. [Google Scholar] [CrossRef]
- Lasa, I.; Penadés, J.R. Bap: A family of surface proteins involved in biofilm formation. Res. Microbiol. 2006, 157, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Fang, X.; Lasa, I. Revisiting Bap multidomain protein: More than sticking bacteria together. Front. Microbiol. 2020, 11, 7490–7499. [Google Scholar] [CrossRef]
- Cucarella, C.; Solano, C.; Valle, J.; Amorena, B.; Lasa, I.; Penadés, J.R. Bap, a Staphylococcus aureus surface protein involved in biofilm formation. J. Bacteriol. 2001, 183, 2888–2896. [Google Scholar] [CrossRef]
- Arrizubieta, M.J.; Toledo-Arana, A.; Amorena, B.; Penadés, J.R.; Lasa, I. Calcium inhibits bap-dependent multicellular behavior in Staphylococcus aureus. J. Bacteriol. 2004, 186, 7490–7498. [Google Scholar] [CrossRef] [PubMed]
- Cucarella, C.; Tormo, M.A.; Ubeda, C.; Trotonda, M.P.; Monzón, M.; Peris, C.; Amorena, B.; Lasa, I.; Penadés, J.R. Role of biofilm-associated protein Bap in the pathogenesis of bovine Staphylococcus aureus. Infect. Immun. 2004, 72, 2177–2185. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.; Latasa, C.; Gil, C.; Toledo-Arana, A.; Solano, C.; Penadés, J.R.; Lasa, I. Bap, a biofilm matrix protein of Staphylococcus aureus prevents cellular internalization through binding to GP96 host receptor. PLoS Pathog. 2012, 8, e1002843. [Google Scholar] [CrossRef]
- Toledo-Arana, A.; Valle, J.; Solano, C.; Arrizubieta, M.J.; Cucarella, C.; Lamata, M.; Amorena, B.; Leiva, J.; Penadés, J.R.; Lasa, I. The enterococcal surface protein, Esp, is involved in Enterococcus faecalis biofilm formation. Appl. Environ. Microbiol. 2001, 67, 4538–4545. [Google Scholar] [CrossRef]
- Shankar, V.; Baghdayan, A.S.; Huycke, M.M.; Lindahl, G.; Gilmore, M.S. Infection-derived Enterococcus faecalis strains are enriched in esp, a gene encoding a novel surface protein. Infect. Immun. 1999, 67, 193–200. [Google Scholar] [CrossRef]
- Tendolkar, P.M.; Baghdayan, A.S.; Shankar, N. The N-terminal domain of enterococcal surface protein, Esp, is sufficient for Esp-mediated biofilm enhancement in Enterococcus faecalis. J. Bacteriol. 2005, 187, 6213–6222. [Google Scholar] [CrossRef]
- Petersen, F.C.; Assev, S.; van der Mei, H.C.; Busscher, H.J.; Scheie, A.A. Functional variation of the antigen I/II surface protein in Streptococcus mutans and Streptococcus intermedius. Infect. Immun. 2002, 70, 249–256. [Google Scholar] [CrossRef]
- Kelemen, L.; Rizk, S.; Debreczeny, M.; Ogier, J.; Szalontai, B. Streptococcal antigen I/II binds to extracellular proteins through intermolecular beta-sheets. FEBS Lett. 2004, 566, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Nobbs, A.H.; Lamont, R.J.; Jenkinson, H.F. Streptococcus adherence and colonization. MicroBiol. Mol. Biol. Rev. 2009, 73, 407–450. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.P.; Sullan, R.M.A.; Crowley, P.J.; El-Kirat-Chatel, S.; Beaussart, A.; Tang, W.; Besingi, R.; Dufrêne, Y.F.; Brady, L.J. Identification of a supramolecular functional architecture of Streptococcus mutans adhesin P1 on the bacterial cell. J. Biol. Chem. 2015, 290, 9002–9019. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Bhatt, A.; Smith, A.N.; Crowley, P.J.; Brady, L.J.; Long, J.R. Specific binding of a naturally occurring amyloidogenic fragment of Streptococcus mutans adhesin P1 to intact P1 on the cell surface characterized by solid state NMR spectroscopy. J. BioMol. NMR 2016, 64, 153–164. [Google Scholar] [CrossRef]
- Perov, S.; Lidor, O.; Salinas, N.; Golan, N.; Tayeb-Fligelman, E.; Deshmukh, M.; Willbold, D.; Landau, M. Structural Insights into Curli CsgA cross-β fibril architecture inspire repurposing of anti-amyloid compounds as anti-biofilm agents. PLoS Pathog. 2019, 15, e1007978-31. [Google Scholar] [CrossRef] [PubMed]
- Bleem, A.; Francisco, R.; Bryers, J.D.; Daggett, V. Designed α-sheet peptides suppress amyloid formation in Staphylococcus aureus biofilms. NPJ Biofilms Microbiomes 2017, 3, 16. [Google Scholar] [CrossRef]
- Chen, D.; Li, J.; Pan, T.; Wu, R.; Tao, Y.; Lin, H. The broad-spectrum antibiofilm activity of amyloid-forming hexapeptides. Microb. Biotechnol. 2021, 14, 656–667. [Google Scholar] [CrossRef]
- Tursi, S.A.; Puligedda, R.D.; Szabo, P.; Nicastro, L.K.; Miller, A.L.; Qiu, C.; Gallucci, S.; Relkin, N.R.; Buttaro, B.A.; Dessain, S.K.; et al. Salmonella Typhimurium biofilm disruption by a human antibody that binds a pan-amyloid epitope on curli. Nat. Commun. 2020, 11, 1–13. [Google Scholar] [CrossRef]
- Malishev, R.; Salinas, N.; Gibson, J.; Eden, A.B.; Mieres-Perez, J.; Ruiz-Blanco, Y.B.; Malka, O.; Kolusheva, S.; Klärner, F.-G.; Schrader, T.; et al. Inhibition of Staphylococcus aureus biofilm-forming functional amyloid by molecular tweezers. Cell Chemical. Biol. 2021, 1–17. [Google Scholar] [CrossRef]
- Cegelski, L.; Pinkner, J.S.; Hammer, N.D.; Cusumano, C.K.; Hung, C.S.; Chorell, E.; Aberg, V.; Walker, J.N.; Seed, P.C.; Almqvist, F.; et al. Small-molecule inhibitors target Escherichia coli amyloid biogenesis and biofilm formation. Nat. Chem. Biol. 2009, 5, 913–919. [Google Scholar] [CrossRef]
- Romero, D.; Sanabria-Valentín, E.; Vlamakis, H.; Kolter, R. Biofilm inhibitors that target amyloid proteins. Chem. Biol. 2013, 20, 102–110. [Google Scholar] [CrossRef]
- Serra, D.O.; Mika, F.; Richter, A.M.; Hengge, R. The green tea polyphenol EGCG inhibits E. coli biofilm formation by impairing amyloid curli fibre assembly and down-regulating the biofilm regulator CsgD via the σ E-dependent sRNA RybB. Mol. Microbiol. 2016. [Google Scholar] [CrossRef]
- Najarzadeh, Z.; Mohammad-Beigi, H.; Nedergaard Pedersen, J.; Christiansen, G.; Sønderby, T.V.; Shojaosadati, S.A.; Morshedi, D.; Strømgaard, K.; Meisl, G.; Sutherland, D.; et al. Plant polyphenols inhibit functional amyloid and biofilm formation in Pseudomonas strains by directing monomers to off-pathway oligomers. Biomolecules 2019, 9, 659. [Google Scholar] [CrossRef] [PubMed]
- Stenvang, M.; Dueholm, M.S.; Vad, B.S.; Seviour, T.W.; Zeng, G.; Geifman-Shochat, S.; Søndergaard, M.T.; Christiansen, G.; Meyer, R.L.; Kjelleberg, S.; et al. Epigallocatechin gallate remodels overexpressed functional amyloids in Pseudomonas aeruginosa and increases biofilm susceptibility to antibiotic treatment. J. Biol. Chem. 2016. [Google Scholar] [CrossRef] [PubMed]
- Marinelli, P.; Pallares, I.; Navarro, S.; Ventura, S. Dissecting the contribution of Staphylococcus aureus α-phenol-soluble modulins to biofilm amyloid structure. Sci. Rep. 2016, 6, 34552. [Google Scholar] [CrossRef]
- Pruteanu, M.; Hernández Lobato, J.I.; Stach, T.; Hengge, R. Common plant flavonoids prevent the assembly of amyloid curli fibres and can interfere with bacterial biofilm formation. Environ. Microbiol. 2020, 22, 5280–5299. [Google Scholar]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapun-Araiz, B.; Žiemytė, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm activity of flavonoids on staphylococcal biofilms through targeting BAP amyloids. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef]
- Ventura, S. Curing bacterial infections with protein aggregates. Mol. Microbiol. 2016, 99, 827–830. [Google Scholar] [CrossRef]
- Bednarska, N.G.; Van Eldere, J.; Gallardo, R.; Ganesan, A.; Ramakers, M.; Vogel, I.; Baatsen, P.; Staes, A.; Goethals, M.; Hammarström, P.; et al. Protein aggregation as an antibiotic design strategy. Mol. Microbiol. 2016, 99, 849–865. [Google Scholar] [CrossRef] [PubMed]
- Heisig, M.; Abraham, N.M.; Liu, L.; Neelakanta, G.; Mattessich, S.; Sultana, H.; Shang, Z.; Ansari, J.M.; Killiam, C.; Walker, W.; et al. Antivirulence properties of an antifreeze protein. Cell Rep. 2014, 9, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.M.; Abraham, N.M.; Massaro, J.; Murphy, K.; Smith-Carpenter, J.; Fikrig, E. Anti-biofilm activity of a self-aggregating peptide against Streptococcus mutans. Front. Microbiol. 2017, 8, 488. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, J.A.; Benson, M.D. Transthyretin: A review from a structural perspective. Cell Mol. Life Sci. 2001, 58, 1491–1521. [Google Scholar] [CrossRef]
- Taylor, J.D.; Hawthorne, W.J.; Lo, J.; Dear, A.; Jain, N.; Meisl, G.; Andreasen, M.; Fletcher, C.; Koch, M.; Darvill, N.; et al. Electrostatically-guided inhibition of curli amyloid nucleation by the CsgC-like family of chaperones. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, X.; Ladiwala, A.R.A.; Du, D.; Yadav, J.K.; Tessier, P.M.; Wright, P.E.; Kelly, J.W.; Buxbaum, J.N. Mechanisms of transthyretin inhibition of β-amyloid aggregation in vitro. J. Neurosci. 2013, 33, 19423–19433. [Google Scholar] [CrossRef]
- Cascella, R.; Conti, S.; Mannini, B.; Li, X.; Buxbaum, J.N.; Tiribilli, B.; Chiti, F.; Cecchi, C. Transthyretin suppresses the toxicity of oligomers formed by misfolded proteins in vitro. BBA Mol. Basis Dis. 2013, 1832, 2302–2314. [Google Scholar] [CrossRef] [PubMed]
- Wasana Jayaweera, S.; Surano, S.; Pettersson, N.; Oskarsson, E.; Lettius, L.; Gharibyan, A.L.; Anan, I.; Olofsson, A. Mechanisms of transthyretin inhibition of IAPP amyloid formation. Biomolecules 2021, 11, 411. [Google Scholar] [CrossRef]
- Jain, N.; Åden, J.; Nagamatsu, K.; Evans, M.L.; Li, X.; McMichael, B.; Ivanova, M.I.; Almqvist, F.; Buxbaum, J.N.; Chapman, M.R. Inhibition of curli assembly and Escherichia coli biofilm formation by the human systemic amyloid precursor transthyretin. Proc. Natl. Acad. Sci. USA 2017, 114, 12184–12189. [Google Scholar] [CrossRef] [PubMed]
- Panza, F.; Lozupone, M.; Seripa, D.; Imbimbo, B.P. Amyloid-β immunotherapy for Alzheimer disease: Is it now a long shot? Ann. Neurol. 2019, 85, 303–315. [Google Scholar] [CrossRef]
- Fu, H.J.; Liu, B.; Frost, J.L.; Lemere, C.A. Amyloid-β immunotherapy for Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2010, 9, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Härd, T.; Lendel, C. Inhibition of amyloid formation. J. Mol. Biol. 2012, 421, 441–465. [Google Scholar] [CrossRef]
- Knowles, T.P.J.; Vendruscolo, M.; Dobson, C.M. The amyloid state and its association with protein misfolding diseases. Nat. Rev. Mol. Cell Biol. 2014, 15, 384–396. [Google Scholar] [CrossRef]
- Mbarek, A.; Moussa, G.; Chain, J.L. Pharmaceutical applications of molecular tweezers, clefts and clips. Molecules 2019, 24, 1803. [Google Scholar] [CrossRef] [PubMed]
- Hadrovic, I.; Rebmann, P.; Klärner, F.-G.; Bitan, G.; Schrader, T. Molecular lysine tweezers counteract aberrant protein aggregation. Front. Chem. 2019, 7, 657. [Google Scholar] [CrossRef] [PubMed]
- Di, J.; Siddique, I.; Li, Z.; Malki, G.; Hornung, S.; Dutta, S.; Hurst, I.; Ishaaya, E.; Wang, A.; Tu, S.; et al. The molecular tweezer CLR01 improves behavioral deficits and reduces tau pathology in P301S-tau transgenic mice. Alzheimer’s Res. Ther. 2020, 13, 1–20. [Google Scholar]
- Pinkner, J.S.; Remaut, H.; Buelens, F.; Miller, E.; Aberg, V.; Pemberton, N.; Hedenström, M.; Larsson, A.; Seed, P.; Waksman, G.; et al. Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 17897–17902. [Google Scholar] [CrossRef]
- Aberg, V.; Norman, F.; Chorell, E.; Westermark, A.; Olofsson, A.; Elisabeth Sauer-Eriksson, A.; Almqvist, F. Microwave-assisted decarboxylation of bicyclic 2-pyridone scaffolds and identification of Aβ-peptide aggregation inhibitors. Org. BioMol. Chem. 2005, 3, 2817–2823. [Google Scholar] [CrossRef] [PubMed]
- Horvath, I.; Weise, C.F.; Andersson, E.K.; Chorell, E.; Sellstedt, M.; Bengtsson, C.; Olofsson, A.; Hultgren, S.J.; Chapman, M.; Wolf-Watz, M.; et al. Mechanisms of protein oligomerization: Inhibitor of functional amyloids templates α-synuclein fibrillation. J. Am. Chem. Soc. 2012, 134, 3439–3444. [Google Scholar] [CrossRef] [PubMed]
- Velander, P.; Wu, L.; Henderson, F.; Zhang, S.; Bevan, D.R.; Xu, B. Natural product-based amyloid inhibitors. Biochem. Pharmacol. 2017, 139, 40–55. [Google Scholar] [CrossRef]
- Freyssin, A.; Page, G.; Fauconneau, B.; Rioux Bilan, A. Natural polyphenols effects on protein aggregates in Alzheimer’s and Parkinson’s prion-like diseases. Neural. Regen Res. 2018, 13, 955–961. [Google Scholar]
- Kobayashi, H.; Murata, M.; Kawanishi, S.; Oikawa, S. Polyphenols with anti-amyloid β aggregation show potential risk of toxicity via pro-oxidant properties. Int. J. Mol. Sci. 2020, 21, 3561. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wang, S.; Dong, S.; Chang, P.; Jiang, Z. Structural characteristics of (−)-epigallocatechin-3-gallate inhibiting amyloid Aβ42 aggregation and remodeling amyloid fibers. RSC Adv. 2015, 5, 62402–62413. [Google Scholar] [CrossRef]
- Bieschke, J.; Russ, J.; Friedrich, R.P.; Ehrnhoefer, D.E.; Wobst, H.; Neugebauer, K.; Wanker, E.E. EGCG remodels mature alpha-synuclein and amyloid-beta fibrils and reduces cellular toxicity. Proc. Natl. Acad. Sci. USA 2010, 107, 7710–7715. [Google Scholar] [CrossRef] [PubMed]
- Ehrnhoefer, D.E.; Bieschke, J.; Boeddrich, A.; Herbst, M.; Masino, L.; Lurz, R.; Engemann, S.; Pastore, A.; Wanker, E.E. EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008, 15, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Zhang, Y.; Quan, Z.; Wong, W.; Guo, J.; Zhang, R.; Yang, Q.; Dai, R.; McGeer, P.L.; Qing, H. Epigallocatechin gallate (EGCG) inhibits alpha-synuclein aggregation: A potential agent for Parkinson’s disease. Neurochem. Res. 2016, 41, 2788–2796. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Liang, Q.; Sun, Q.; Chen, C.; Xu, L.; Ding, Y.; Zhou, P. (−)-Epigallocatechin-3-gallate (EGCG) inhibits fibrillation, disaggregates amyloid fibrils of α-synuclein, and protects PC12 cells against α-synuclein-induced toxicity. RSC Adv. 2017, 7, 32508–32517. [Google Scholar] [CrossRef]
- Roy, S.; Bhat, R. Suppression, disaggregation, and modulation of γ-Synuclein fibrillation pathway by green tea polyphenol EGCG. Protein Sci. 2019, 28, 382–402. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Lin, Y.; Cox, S.J.; Kinoshita, M.; Sahoo, B.R.; Ivanova, M.; Ramamoorthy, A. Zinc boosts EGCG’s hIAPP amyloid inhibition both in solution and membrane. BBA Proteins Proteom. 2019, 1867, 529–536. [Google Scholar] [CrossRef]
- Xu, Z.-X.; Ma, G.-L.; Zhang, Q.; Chen, C.-H.; He, Y.-M.; Xu, L.-H.; Zhou, G.-R.; Li, Z.-H.; Yang, H.-J.; Zhou, P. Inhibitory mechanism of epigallocatechin gallate on fibrillation and aggregation of amidated human islet amyloid polypeptide. Chem. Phys. Chem. 2017, 18, 1611–1619. [Google Scholar] [CrossRef]
- Ehrnhoefer, D.E.; Duennwald, M.; Markovic, P.; Wacker, J.L.; Engemann, S.; Roark, M.; Legleiter, J.; Marsh, J.L.; Thompson, L.M.; Lindquist, S.; et al. Green tea (−)-epigallocatechin-gallate modulates early events in huntingtin misfolding and reduces toxicity in Huntington’s disease models. Hum. Mol. Genet. 2006, 15, 2743–2751. [Google Scholar] [CrossRef]
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (−)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2014, 589, 77–83. [Google Scholar] [CrossRef]
- Palhano, F.L.; Lee, J.; Grimster, N.P.; Kelly, J.W. Toward the molecular mechanism(s) by which EGCG treatment remodels mature amyloid fibrils. J. Am. Chem. Soc. 2013, 135, 7503–7510. [Google Scholar] [CrossRef] [PubMed]
- Najarzadeh, Z.; Pedersen, J.N.; Christiansen, G.; Shojaosadati, S.A.; Pedersen, J.S.; Otzen, D.E. Bacterial amphiphiles as amyloid inducers_ effect of rhamnolipid and lipopolysaccharide on FapC fibrillation. BBA Proteins Proteom. 2019, 1867, 140263. [Google Scholar] [CrossRef]
- Bamunuarachchi, N.I.; Khan, F.; Kim, Y.M. Inhibition of virulence factors and biofilm formation of Acinetobacter baumannii by naturally-derived and synthetic drugs. Curr. Drug Targets 2021, 22, 734–759. [Google Scholar] [CrossRef] [PubMed]
- O’May, C.; Ciobanu, A.; Lam, H.; Tufenkji, N. Tannin derived materials can block swarming motility and enhance biofilm formation in Pseudomonas aeruginosa. Biofouling 2012, 28, 1063–1076. [Google Scholar] [CrossRef] [PubMed]
- Bikels-Goshen, T.; Landau, E.; Saguy, S.; Shapira, R. Staphylococcal strains adapted to epigallocathechin gallate (EGCG) show reduced susceptibility to vancomycin, oxacillin and ampicillin, increased heat tolerance, and altered cell morphology. Int. J. Food Microbiol. 2010, 138, 26–31. [Google Scholar] [CrossRef]
- Miller, A.L.; Bessho, S.; Grando, K.; Tükel, C. Microbiome or infections: Amyloid-containing biofilms as a trigger for complex human diseases. Front. Immunol. 2021, 12, 638867. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).