Resistance and Adaptation of Bacteria to Non-Antibiotic Antibacterial Agents: Physical Stressors, Nanoparticles, and Bacteriophages
Abstract
1. Introduction
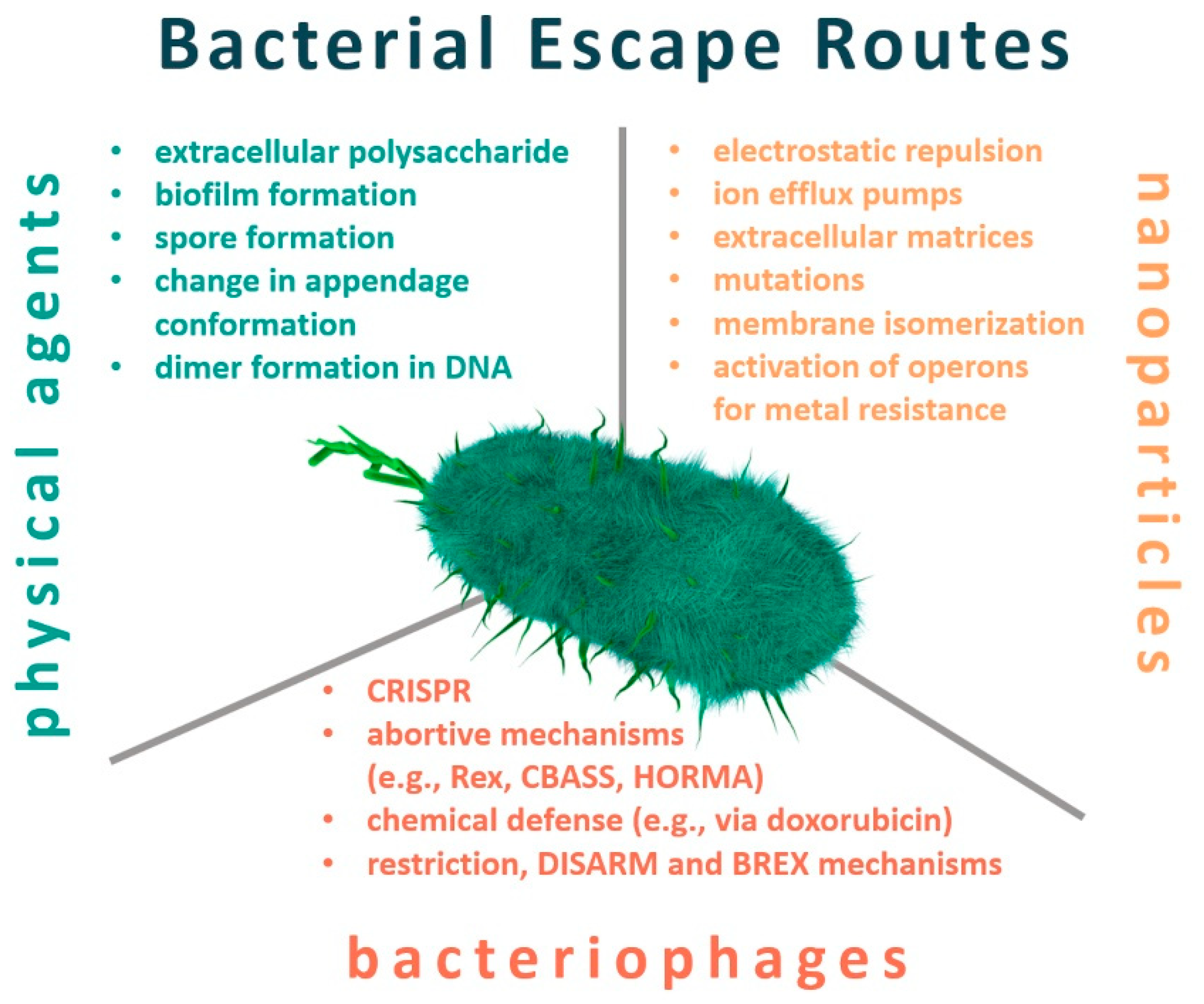
2. Resistance to Physical Factors
2.1. Temperature
2.2. UV Light
2.3. Pressure
2.4. Electric Field
3. Nanotechnology
3.1. Nanoscale Antibacterial Agents
3.1.1. Nanoparticles
3.1.2. Nanozymes—Nanoparticles Mimicking Enzymes
3.1.3. Polymer Nanoparticles
3.1.4. Antibacterial Surfaces
3.2. Adaptation and Resistance of Bacteria to Nanomaterials
4. Bacteriophages
4.1. Phages Against Bacterial Infections
4.2. Bacterial Adaptations against Phages
4.2.1. Physical Mechanisms
4.2.2. Innate Mechanisms
4.2.3. Chemical Defense
4.2.4. Abortive Defense
4.3. Bacterial Resistance Mechanisms
5. Discussion and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pelfrene, E.; Botgros, R.; Cavaleri, M. Antimicrobial multidrug resistance in the era of COVID-19: A forgotten plight? Antimicrob. Resist. Infect. Control 2021, 10, 1–6. [Google Scholar] [CrossRef]
- Capita, R.; Alonso-Calleja, C. Antibiotic-Resistant Bacteria: A Challenge for the Food Industry. Crit. Rev. Food Sci. Nutr. 2013, 53, 11–48. [Google Scholar] [CrossRef] [PubMed]
- Nji, E.; Kazibwe, J.; Hambridge, T.; Joko, C.A.; Larbi, A.A.; Damptey, L.A.O.; Nkansa-Gyamfi, N.A.; Stålsby Lundborg, C.; Lien, L.T.Q. High prevalence of antibiotic resistance in commensal Escherichia coli from healthy human sources in community settings. Sci. Rep. 2021, 11, 1–11. [Google Scholar] [CrossRef]
- Neill, J.O. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. In Review on Antimicrobial Resistance Chaired; Wellcome Trust: UK, London, 2014. [Google Scholar]
- Smith, M. Antibiotic Resistance Mechanisms. Journeys Med. Res. Three Cont. Over 2017, 95–99. [Google Scholar] [CrossRef]
- Ventola, C.L. The antibiotic resistance crisis: Part 1: Causes and threats. Pharm. Ther. 2015, 40, 277–283. [Google Scholar]
- Wang, W.; Arshad, M.I.; Khurshid, M.; Rasool, M.H.; Nisar, M.A.; Aslam, M.A.; Qamar, M.U. Antibiotic resistance: A rundown of a global crisis. Infect. Drug Resist. 2018, 11, 1645–1658. [Google Scholar]
- Liu, Y.-Y.; Wang, Y.; Walsh, T.R.; Yi, L.-X.; Zhang, R.; Spencer, J.; Doi, Y.; Tian, G.; Dong, B.; Huang, X.; et al. Emergence of plasmid-mediated colistin resistance mechanism MCR-1 in animals and human beings in China: A microbiological and molecular biological study. Lancet Infect. Dis. 2015. [Google Scholar] [CrossRef]
- Wang, R.; Van Dorp, L.; Shaw, L.P.; Bradley, P.; Wang, Q.; Wang, X.; Jin, L.; Zhang, Q.; Liu, Y.; Rieux, A.; et al. The global distribution and spread of the mobilized colistin resistance gene mcr-1. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Otto, C.; Zahn, S.; Rost, F.; Zahn, P.; Jaros, D.; Rohm, H. Physical Methods for Cleaning and Disinfection of Surfaces. Food Eng. Rev. 2011, 3, 171–188. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Piddock, L.J.V. Non-traditional Antibacterial Therapeutic Options and Challenges. Cell Host Microbe 2019, 26, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative antimicrobial approach: Nano-antimicrobial materials. Evid. Based Complement. Altern. Med. 2015, 2015. [Google Scholar] [CrossRef]
- Wybrańska, K.; Paczesny, J.; Serejko, K.; Sura, K.; Włodyga, K.; Dzięcielewski, I.; Jones, S.T.; Sliwa, A.; Wybrańska, I.; Hołyst, R.; et al. Gold-oxoborate nanocomposites and their biomedical applications. ACS Appl. Mater. Interfaces 2015, 7, 3931–3939. [Google Scholar] [CrossRef]
- Tripathy, A.; Sen, P.; Su, B.; Briscoe, W.H. Natural and bioinspired nanostructured bactericidal surfaces. Adv. Colloid Interface Sci. 2017, 248, 85–104. [Google Scholar] [CrossRef] [PubMed]
- Pillai, P.P.; Kowalczyk, B.; Kandere-Grzybowska, K.; Borkowska, M.; Grzybowski, B.A. Engineering Gram Selectivity of Mixed-Charge Gold Nanoparticles by Tuning the Balance of Surface Charges. Angew. Chemie Int. Ed. 2016, 55, 8610–8614. [Google Scholar] [CrossRef]
- Vallet-Regí, M.; González, B.; Izquierdo-Barba, I. Nanomaterials as promising alternative in the infection treatment. Int. J. Mol. Sci. 2019, 20, 3806. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Alavidze, Z.J.; Glenn Morris, J. Bacteriophage Therapy. Antimicrob. Agents Chemother. 2001, 45, 649–659. [Google Scholar] [CrossRef]
- Parfitt, T. Georgia: An unlikely stronghold for bacteriophage therapy. Lancet 2005, 365, 2166–2167. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [PubMed]
- McCallin, S.; Alam Sarker, S.; Barretto, C.; Sultana, S.; Berger, B.; Huq, S.; Krause, L.; Bibiloni, R.; Schmitt, B.; Reuteler, G.; et al. Safety analysis of a Russian phage cocktail: From MetaGenomic analysis to oral application in healthy human subjects. Virology 2013, 443, 187–196. [Google Scholar] [CrossRef]
- Novickij, V.; Stanevičiene, R.; Vepštaite-Monstaviče, I.; Gruškiene, R.; Krivorotova, T.; Sereikaite, J.; Novickij, J.; Serviene, E. Overcoming antimicrobial resistance in bacteria using bioactive magnetic nanoparticles and pulsed electromagnetic fields. Front. Microbiol. 2018, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Qian, X.; Gu, J.; Wang, X.J.; Duan, M.L. Mechanism and Effect of Temperature on Variations in Antibiotic Resistance Genes during Anaerobic Digestion of Dairy Manure. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef]
- Li, H.; Gänzle, M. Some like it hot: Heat resistance of Escherichia coli in food. Front. Microbiol. 2016, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Loraine, G.; Chahine, G.; Hsiao, C.T.; Choi, J.K.; Aley, P. Disinfection of gram-negative and gram-positive bacteria using DynaJets® hydrodynamic cavitating jets. Ultrason. Sonochem. 2012, 19, 710–717. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Cebrián, G.; Condón, S.; Mañas, P. Physiology of the Inactivation of Vegetative Bacteria by Thermal Treatments: Mode of Action, Influence of Environmental Factors and Inactivation Kinetics. Foods 2017, 6, 107. [Google Scholar] [CrossRef]
- Yoon, Y.; Lee, H.; Lee, S.; Kim, S.; Choi, K.H. Membrane fluidity-related adaptive response mechanisms of foodborne bacterial pathogens under environmental stresses. Food Res. Int. 2015, 72, 25–36. [Google Scholar] [CrossRef]
- Cebrián, G.; Mañas, P.; Condón, S. Comparative resistance of bacterial foodborne pathogens to non-thermal technologies for food preservation. Front. Microbiol. 2016, 7, 1–17. [Google Scholar] [CrossRef]
- René, O.; Alix, J.H. Late steps of ribosome assembly in E. coli are sensitive to a severe heat stress but are assisted by the HSP70 chaperone machine. Nucleic Acids Res. 2011, 39, 1855–1867. [Google Scholar] [CrossRef] [PubMed]
- Guyot, S.; Pottier, L.; Ferret, E.; Gal, L.; Gervais, P. Physiological responses of Escherichia coli exposed to different heat-stress kinetics. Arch. Microbiol. 2010, 192, 651–661. [Google Scholar] [CrossRef]
- Szeto, W.; Yam, W.C.; Huang, H.; Leung, D.Y.C. The efficacy of vacuum-ultraviolet light disinfection of some common environmental pathogens. BMC Infect. Dis. 2020, 20, 1–9. [Google Scholar] [CrossRef]
- Maclean, M.; MacGregor, S.J.; Anderson, J.G.; Woolsey, G. Inactivation of bacterial pathogens following exposure to light from a 405-nanometer light-emitting diode array. Appl. Environ. Microbiol. 2009, 75, 1932–1937. [Google Scholar] [CrossRef]
- Kovach, C.R.; Taneli, Y.; Neiman, T.; Dyer, E.M.; Arzaga, A.J.A.; Kelber, S.T. Evaluation of an ultraviolet room disinfection protocol to decrease nursing home microbial burden, infection and hospitalization rates. BMC Infect. Dis. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.; Castillo, G.; Callejas, L.; López, H.; Olmos, J. Frequency of transferable multiple antibiotic resistance amongst coliform bacteria isolated from a treated sewage effluent in Antofagasta, Chile. Electron. J. Biotechnol. 2006, 9, 533–540. [Google Scholar] [CrossRef]
- Lamprecht-Grandío, M.; Cortesão, M.; Mirete, S.; de la Cámara, M.B.; de Figueras, C.G.; Pérez-Pantoja, D.; White, J.J.; Farías, M.E.; Rosselló-Móra, R.; González-Pastor, J.E. Novel Genes Involved in Resistance to both Ultraviolet Radiation and Perchlorate from the Metagenomes of Hypersaline Environments. Front. Microbiol. 2020, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Mofidi, A.A.; Rochelle, P.A.; Chou, C.I.; Mehta, H.M.; Verne, L.; Linden, K.G. Bacterial Survival After Ultraviolet Light Disinfection: Resistance, Regrowth and Repair. Am. Water Work. Assoc. Annu. Conf. Exhib. 2002, 1–11. [Google Scholar]
- Pérez, V.; Hengst, M.; Kurte, L.; Dorador, C.; Jeffrey, W.H.; Wattiez, R.; Molina, V.; Matallana-Surget, S. Bacterial survival under extreme UV radiation: A comparative proteomics study of Rhodobacter sp., isolated from high altitude wetlands in Chile. Front. Microbiol. 2017, 8, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Alcántara-Díaz, D.; Breña-Valle, M.; Serment-Guerrero, J. Divergent adaptation of Escherichia coli to cyclic ultraviolet light exposures. Mutagenesis 2004, 19, 349–354. [Google Scholar] [CrossRef]
- Marizcurrena, J.J.; Morel, M.A.; Braña, V.; Morales, D.; Martinez-López, W.; Castro-Sowinski, S. Searching for novel photolyases in UVC-resistant Antarctic bacteria. Extremophiles 2017, 21, 409–418. [Google Scholar] [CrossRef]
- Monsalves, M.T.; Ollivet-Besson, G.P.; Amenabar, M.J.; Blamey, J.M. Isolation of a psychrotolerant and UV-C-resistant bacterium from elephant island, antarctica with a highly thermoactive and thermostable catalase. Microorganisms 2020, 8, 95. [Google Scholar] [CrossRef]
- Díaz-Riaño, J.; Posada, L.; Acosta, I.C.; Ruíz-Pérez, C.; García-Castillo, C.; Reyes, A.; Zambrano, M.M. Computational search for UV radiation resistance strategies in Deinococcus swuensis isolated from Paramo ecosystems. PLoS ONE 2019, 14, 1–17. [Google Scholar] [CrossRef]
- Moreirinha, C.; Almeida, A.; Saraiva, J.A.; Delgadillo, I. High-pressure processing effects on foodborne bacteria by mid-infrared spectroscopy analysis. LWT Food Sci. Technol. 2016, 73, 212–218. [Google Scholar] [CrossRef]
- Chikuma, S.; Kasahara, R.; Kato, C.; Tamegai, H. Bacterial adaptation to high pressure: A respiratory system in the deep-sea bacterium Shewanella violacea DSS12. FEMS Microbiol. Lett. 2007, 267, 108–112. [Google Scholar] [CrossRef][Green Version]
- Masson, P.; Tonello, C.; Balny, C. High-pressure biotechnology in medicine and pharmaceutical science. J. Biomed. Biotechnol. 2001, 2001, 85–88. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H. Review of disinfection and sterilization—Back to the basics. Infect. Chemother. 2018, 50, 101–109. [Google Scholar] [CrossRef] [PubMed]
- Reineke, K.; Mathys, A.; Knorr, D. The impact of high pressure and temperature on bacterial spores: Inactivation mechanisms of Bacillus subtilis above 500 MPa. J. Food Sci. 2011, 76. [Google Scholar] [CrossRef]
- Vanlint, D.; Rutten, N.; Michiels, C.W.; Aertsen, A. Emergence and stability of high-pressure resistance in different food-borne pathogens. Appl. Environ. Microbiol. 2012, 78, 3234–3241. [Google Scholar] [CrossRef] [PubMed]
- Mota, M.J.; Lopes, R.P.; Delgadillo, I.; Saraiva, J.A. Microorganisms under high pressure—Adaptation, growth and biotechnological potential. Biotechnol. Adv. 2013, 31, 1426–1434. [Google Scholar] [CrossRef]
- Pudasaini, S.; Perera, A.T.K.; Ng, S.H.; Yang, C. Bacterial inactivation via microfluidic electroporation device with insulating micropillars. Electrophoresis 2021, 1–9. [Google Scholar] [CrossRef]
- Kotnik, T.; Frey, W.; Sack, M.; Haberl Meglič, S.; Peterka, M.; Miklavčič, D. Electroporation-based applications in biotechnology. Trends Biotechnol. 2015, 33, 480–488. [Google Scholar] [CrossRef]
- Martens, S.L.; Klein, S.; Barnes, R.A.; TrejoSanchez, P.; Roth, C.C.; Ibey, B.L. 600-ns pulsed electric fields affect inactivation and antibiotic susceptibilities of Escherichia coli and Lactobacillus acidophilus. AMB Express 2020, 10. [Google Scholar] [CrossRef]
- Escoffre, J.M.; Portet, T.; Wasungu, L.; Teissié, J.; Dean, D.; Rols, M.P. What is (Still not) known of the mechanism by which electroporation mediates gene transfer and expression in cells and tissues. Mol. Biotechnol. 2009, 41, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Garcia, P.A.; Ge, Z.; Moran, J.L.; Buie, C.R. Microfluidic screening of electric fields for electroporation. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Cannon, R.; Ellis, S.; Hayes, D.; Narayanan, G.; Martin, R.C.G. Safety and early efficacy of irreversible electroporation for hepatic tumors in proximity to vital structures. J. Surg. Oncol. 2013, 107, 544–549. [Google Scholar] [CrossRef]
- Pillet, F.; Formosa-Dague, C.; Baaziz, H.; Dague, E.; Rols, M.P. Cell wall as a target for bacteria inactivation by pulsed electric fields. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Costello, S.R. Effect of Electric Field on Growth Kinetics of Yogurt Starter Cultures, Lactobacillus Bulgaricus and Streptococcus Thermophilus. Master’s Thesis, The Ohio State University, Columbus, OH, USA, 2012. [Google Scholar]
- Gall, I.; Herzberg, M.; Oren, Y. The effect of electric fields on bacterial attachment to conductive surfaces. Soft Matter 2013, 9, 2443–2452. [Google Scholar] [CrossRef]
- Yonemoto, Y.; Yamashita, T.; Muraji, M.; Tatebe, W.; Ooshima, H.; Kato, J.; Kimura, A.; Murata, K. Resistance of yeast and bacterial spores to high voltage electric pulses. J. Ferment. Bioeng. 1993, 75, 99–102. [Google Scholar] [CrossRef]
- Puligundla, P.; Pyun, Y.R.; Mok, C. Pulsed electric field (PEF) technology for microbial inactivation in low-alcohol red wine. Food Sci. Biotechnol. 2018, 27, 1691–1696. [Google Scholar] [CrossRef]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nat. Rev. Microbiol. 2020. [Google Scholar] [CrossRef]
- Pelgrift, R.Y.; Friedman, A.J. Nanotechnology as a therapeutic tool to combat microbial resistance. Adv. Drug Deliv. Rev. 2013, 65, 1803–1815. [Google Scholar] [CrossRef]
- Stark, W.J. Nanoparticles in biological systems. Angew. Chemie Int. Ed. 2011, 50, 1242–1258. [Google Scholar] [CrossRef] [PubMed]
- Doane, T.; Burda, C. Nanoparticle mediated non-covalent drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 607–621. [Google Scholar] [CrossRef]
- Limbach, L.K.; Li, Y.; Grass, R.N.; Brunner, T.J.; Hintermann, M.A.; Muller, M.; Gunther, D.; Stark, W.J. Oxide Nanoparticle Uptake in Human Lung Fibroblasts: Effects of Particle Size, Agglomeration, and Diffusion at Low Concentrations. Environ. Sci. Technol. 2005, 39, 9370–9376. [Google Scholar] [CrossRef]
- Studer, A.M.; Limbach, L.K.; Van Duc, L.; Krumeich, F.; Athanassiou, E.K.; Gerber, L.C.; Moch, H.; Stark, W.J. Nanoparticle cytotoxicity depends on intracellular solubility: Comparison of stabilized copper metal and degradable copper oxide nanoparticles. Toxicol. Lett. 2010, 197, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Treuel, L.; Jiang, X.; Nienhaus, G.U. New views on cellular uptake and trafficking of manufactured nanoparticles. J. R. Soc. Interface 2013, 10, 20120939. [Google Scholar] [CrossRef]
- Ji, Z.; Wang, X.; Zhang, H.; Lin, S.; Meng, H.; Sun, B.; George, S.; Xia, T.; Nel, A.E.; Zink, J.I. Designed synthesis of CeO2 nanorods and nanowires for studying toxicological effects of high aspect ratio nanomaterials. ACS Nano 2012, 6, 5366–5380. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.J.P.; Mohammed, H.; Murali, K.; Kamarudeen, M. Synthesis of silver nanorods using Coscinium fenestratum extracts and its cytotoxic activity against Hep-2 cell line. Colloids Surf. B. Biointerfaces 2012, 98, 7–11. [Google Scholar] [CrossRef]
- Rai, M.K.; Deshmukh, S.D.; Ingle, A.P.; Gade, A.K. Silver nanoparticles: The powerful nanoweapon against multidrug-resistant bacteria. J. Appl. Microbiol. 2012, 112, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Haider, A.; Kang, I.-K. Preparation of Silver Nanoparticles and Their Industrial and Biomedical Applications: A Comprehensive Review. Adv. Mater. Sci. Eng. 2015, 2015, e165257. [Google Scholar] [CrossRef]
- Rauwel, P.; Rauwel, E.; Ferdov, S.; Singh, M.P. Silver Nanoparticles: Synthesis, Properties, and Applications. Adv. Mater. Sci. Eng. 2015, 2015, e624394. [Google Scholar] [CrossRef]
- Tubert-Brohman, I.; Sherman, W.; Repasky, M.; Beuming, T. Improved Docking of Polypeptides with Glide. J. Chem. Inf. Model. 2013, 53, 1689–1699. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Dong, Y.; Yang, Y.; Yu, P.; Zhang, Y.; Hu, J.; Li, T.; Zhang, X.; Liu, X.; Xu, Q.; et al. Rational Design of Silver Gradient for Studying Size Effect of Silver Nanoparticles on Contact Killing. ACS Biomater. Sci. Eng. 2019, 5, 425–431. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, E.P.; Hasan, J.; Webb, H.K.; Truong, V.K.; Watson, G.S.; Watson, J.A.; Baulin, V.A.; Pogodin, S.; Wang, J.Y.; Tobin, M.J.; et al. Natural bactericidal surfaces: Mechanical rupture of pseudomonas aeruginosa cells by cicada wings. Small 2012, 8, 2489–2494. [Google Scholar] [CrossRef] [PubMed]
- Pogodin, S.; Hasan, J.; Baulin, V.A.; Webb, H.K.; Truong, V.K.; Phong Nguyen, T.H.; Boshkovikj, V.; Fluke, C.J.; Watson, G.S.; Watson, J.A.; et al. Biophysical model of bacterial cell interactions with nanopatterned cicada wing surfaces. Biophys. J. 2013, 104, 835–840. [Google Scholar] [CrossRef]
- Johannes, L.; Mayor, S. Induced domain formation in endocytic invagination, lipid sorting, and scission. Cell 2010, 142, 507–510. [Google Scholar] [CrossRef]
- Silhavy, T.J.; Kahne, D.; Walker, S. The Bacterial Cell Envelope. Cold Spring Harb Perspect. Biol. 2010, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Fuerst, J.A.; Sagulenko, E. Protein uptake by bacteria. Commun. Integr. Biol. 2010, 3, 572–575. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; de Jimenez Aberasturi, D.; de Larramendi, I.R.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef]
- Nel, A.; Xia, T.; Mädler, L.; Li, N. Toxic potential of materials at the nanolevel. Science 2006, 311, 622–627. [Google Scholar] [CrossRef] [PubMed]
- Xia, Q.; Hwang, H.-M.; Ray, P.C.; Yu, H. Mechanisms of nanotoxicity: Generation of reactive oxygen species. J. Food Drug Anal. 2014, 22, 64–75. [Google Scholar] [CrossRef]
- Walters, C.; Pool, E.; Somerset, V. Nanotoxicology: A Review. In Toxicology—New Aspects to This Scientific Conundrum; InTech: London, UK, 2016. [Google Scholar]
- Lewinski, N.; Colvin, V.; Drezek, R. Cytotoxicity of Nanoparticles. Small 2008, 4, 26–49. [Google Scholar] [CrossRef]
- Vincent, M.; Duval, R.E.; Hartemann, P.; Engels-Deutsch, M. Contact killing and antimicrobial properties of copper. J. Appl. Microbiol. 2018, 124, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Mathews, S.; Hans, M.; Mücklich, F.; Solioz, M. Contact killing of bacteria on copper is suppressed if bacterial-metal contact is prevented and is induced on iron by copper ions. Appl. Environ. Microbiol. 2013, 79, 2605–2611. [Google Scholar] [CrossRef]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interface Sci. 2004, 275, 177–182. [Google Scholar] [CrossRef]
- Hans, M.; Mathews, S.; Mücklich, F.; Solioz, M. Physicochemical properties of copper important for its antibacterial activity and development of a unified model. Biointerphases 2016, 11, 018902. [Google Scholar] [CrossRef]
- Vincent, M.; Hartemann, P.; Engels-Deutsch, M. Antimicrobial applications of copper. Int. J. Hyg. Environ. Health 2016, 219, 585–591. [Google Scholar] [CrossRef]
- Slavin, Y.N.; Asnis, J.; Häfeli, U.O.; Bach, H. Metal nanoparticles: Understanding the mechanisms behind antibacterial activity. J. Nanobiotechnol. 2017, 15, 65. [Google Scholar] [CrossRef] [PubMed]
- Tao, B.; Lin, C.; Deng, Y.; Yuan, Z.; Shen, X.; Chen, M.; He, Y.; Peng, Z.; Hu, Y.; Cai, K. Copper-nanoparticle-embedded hydrogel for killing bacteria and promoting wound healing with photothermal therapy. J. Mater. Chem. B 2019, 7, 2534–2548. [Google Scholar] [CrossRef] [PubMed]
- Agnihotri, S.; Mukherji, S.; Mukherji, S. Immobilized silver nanoparticles enhance contact killing and show highest efficacy: Elucidation of the mechanism of bactericidal action of silver. Nanoscale 2013, 5, 7328. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Lee, D.; Sheng, X.; Cohen, R.E.; Rubner, M.F. Two-Level Antibacterial Coating with Both Release-Killing and Contact-Killing Capabilities. Langmuir 2006, 22, 9820–9823. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Juodkazis, S.; Crawford, R.J.; Stoodley, P.; Ivanova, E.P. Mechano-bactericidal actions of nanostructured surfaces. Nat. Rev. Microbiol. 2021, 19, 8–22. [Google Scholar] [CrossRef] [PubMed]
- Eleraky, N.E.; Allam, A.; Hassan, S.B.; Omar, M.M. Nanomedicine fight against antibacterial resistance: An overview of the recent pharmaceutical innovations. Pharmaceutics 2020, 12, 142. [Google Scholar] [CrossRef]
- Horikoshi, S.; Serpone, N. Microwaves in Nanoparticle Synthesis: Fundamentals and Applications; Wiley-VCH: Weinheim, Germany, 2013; ISBN 9783527331970. [Google Scholar]
- Salas Orozco, M.F.; Niño-Martínez, N.; Martínez-Castañón, G.A.; Méndez, F.T.; Ruiz, F. Molecular mechanisms of bacterial resistance to metal and metal oxide nanoparticles. Int. J. Mol. Sci. 2019, 20, 2808. [Google Scholar] [CrossRef]
- Reyes-Esparza, J.; Martínez-Mena, A.; Gutiérrez-Sancha, I.; Rodríguez-Fragoso, P.; Cruz, G.G.; Mondragón, R.; Rodríguez-Fragoso, L. Synthesis, characterization and biocompatibility of cadmium sulfide nanoparticles capped with dextrin for in vivo and in vitro imaging application. J. Nanobiotechnology 2015, 13, 1–14. [Google Scholar] [CrossRef]
- Abo-Zeid, Y.; Williams, G.R. The potential anti-infective applications of metal oxide nanoparticles: A systematic review. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnology 2020, 12, 1–36. [Google Scholar] [CrossRef]
- Xin, Q.; Shah, H.; Nawaz, A.; Xie, W.; Akram, M.Z.; Batool, A.; Tian, L.; Jan, S.U.; Boddula, R.; Guo, B.; et al. Antibacterial Carbon-Based Nanomaterials. Adv. Mater. 2019, 31, 1–15. [Google Scholar] [CrossRef]
- Kakkar, A.; Traverso, G.; Farokhzad, O.C.; Weissleder, R.; Langer, R. Evolution of macromolecular complexity in drug delivery systems. Nat. Rev. Chem. 2017, 1, 63. [Google Scholar] [CrossRef]
- Ishihara, K.; Chen, W.; Liu, Y.; Tsukamoto, Y.; Inoue, Y. Cytocompatible and multifunctional polymeric nanoparticles for transportation of bioactive molecules into and within cells. Sci. Technol. Adv. Mater. 2016, 17, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Xie, C.; Zhen, X.; Lyu, Y.; Duan, H.; Liu, X.; Jokerst, J.V.; Pu, K. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 2017, 35, 1102. [Google Scholar] [CrossRef]
- Pu, K.; Chattopadhyay, N.; Rao, J. Recent advances of semiconducting polymer nanoparticles in in vivo molecular imaging. J. Control. Release 2016, 240, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Kairdolf, B.A.; Qian, X.; Nie, S. Bioconjugated Nanoparticles for Biosensing, In Vivo Imaging, and Medical Diagnostics. Anal. Chem. 2017, 89, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Van Rijt, S.; Habibovic, P. Enhancing regenerative approaches with nanoparticles. J. R. Soc. Interface 2017, 14. [Google Scholar] [CrossRef]
- Lam, S.J.; Wong, E.H.H.; Boyer, C.; Qiao, G.G. Antimicrobial polymeric nanoparticles. Prog. Polym. Sci. 2018, 76, 40–64. [Google Scholar] [CrossRef]
- Korschelt, K.; Tahir, M.N.; Tremel, W. A Step into the Future: Applications of Nanoparticle Enzyme Mimics. Chemistry 2018, 24, 9703–9713. [Google Scholar] [CrossRef]
- Cao, F.; Zhang, L.; Wang, H.; You, Y.; Wang, Y.; Gao, N.; Ren, J.; Qu, X. Defect-Rich Adhesive Nanozymes as Efficient Antibiotics for Enhanced Bacterial Inhibition. Angew. Chemie Int. Ed. 2019, 58, 16236–16242. [Google Scholar] [CrossRef]
- Singh, S. Nanomaterials exhibiting enzyme-like properties (Nanozymes): Current advances and future perspectives. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Li, W.; Pan, X.; Gadd, G.M. Applications of nanozymes in the environment. Environ. Sci. Nano 2020, 7, 1305–1318. [Google Scholar] [CrossRef]
- Gao, F.; Shao, T.; Yu, Y.; Xiong, Y.; Yang, L. Surface-bound reactive oxygen species generating nanozymes for selective antibacterial action. Nat. Commun. 2021, 12, 1–18. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Z.; Gao, Z.; Tammina, S.K.; Yang, Y. Nanozymes used for antimicrobials and their applications. Colloids Surfaces B Biointerfaces 2020, 195, 111252. [Google Scholar] [CrossRef]
- Rao, J.P.; Geckeler, K.E. Polymer nanoparticles: Preparation techniques and size-control parameters. Prog. Polym. Sci. 2011, 36, 887–913. [Google Scholar] [CrossRef]
- Forier, K.; Raemdonck, K.; De Smedt, S.C.; Demeester, J.; Coenye, T.; Braeckmans, K. Lipid and polymer nanoparticles for drug delivery to bacterial biofilms. J. Control. Release 2014, 190, 607–623. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Landis, R.F.; Li, C.H.; Schnurr, M.; Das, R.; Lee, Y.W.; Yazdani, M.; Liu, Y.; Kozlova, A.; Rotello, V.M. Engineered Polymer Nanoparticles with Unprecedented Antimicrobial Efficacy and Therapeutic Indices against Multidrug-Resistant Bacteria and Biofilms. J. Am. Chem. Soc. 2018, 140, 12137–12143. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alhabib, H.; Alsaif, R.; Shazly, G.; Alqahtani, H.; Alsarra, I.A.; Mahdavi, J. Antibacterial activity of chitosan nanoparticles against pathogenic N. gonorrhoea. Int. J. Nanomed. 2020, 15, 7877–7887. [Google Scholar] [CrossRef]
- Alqahtani, F.Y.; Aleanizy, F.S.; El Tahir, E.; Alquadeib, B.T.; Alsarra, I.A.; Alanazi, J.S.; Abdelhady, H.G. Preparation, characterization, and antibacterial activity of diclofenac-loaded chitosan nanoparticles. Saudi Pharm. J. 2019, 27, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Divya, K.; Vijayan, S.; George, T.K.; Jisha, M.S. Antimicrobial properties of chitosan nanoparticles: Mode of action and factors affecting activity. Fibers Polym. 2017, 18, 221–230. [Google Scholar] [CrossRef]
- Smiechowicz, E.; Niekraszewicz, B.; Kulpinski, P.; Dzitko, K. Antibacterial composite cellulose fibers modified with silver nanoparticles and nanosilica. Cellulose 2018, 25, 3499–3517. [Google Scholar] [CrossRef]
- Barud, H.S.; Regiani, T.; Marques, R.F.C.; Lustri, W.R.; Messaddeq, Y.; Ribeiro, S.J.L. Antimicrobial bacterial cellulose-silver nanoparticles composite membranes. J. Nanomater. 2011, 2011. [Google Scholar] [CrossRef]
- Mei, L.; Cao, F.; Zhang, L.; Xu, J.; Xu, Z.; Yu, Y.; Zhang, X.; Shi, Y.; Li, X.; Cheng, K.; et al. Ag-Conjugated graphene quantum dots with blue light-enhanced singlet oxygen generation for ternary-mode highly-efficient antimicrobial therapy. J. Mater. Chem. B 2020, 8, 1371–1382. [Google Scholar] [CrossRef]
- Dong, X.; Liang, W.; Meziani, M.J.; Sun, Y.P.; Yang, L. Carbon dots as potent antimicrobial agents. Theranostics 2020, 10, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef]
- Baptista, P.V.; McCusker, M.P.; Carvalho, A.; Ferreira, D.A.; Mohan, N.M.; Martins, M.; Fernandes, A.R. Nano-strategies to fight multidrug resistant bacteria—“A Battle of the Titans”. Front. Microbiol. 2018, 9, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Arango-Santander, S.; Pelaez-Vargas, A.; Freitas, S.C.; García, C. A novel approach to create an antibacterial surface using titanium dioxide and a combination of dip-pen nanolithography and soft lithography. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Hussain Bhat, A.; Khan, I.; Jawaid, M.; Suliman, F.O.; Al-Lawati, H.; Muhamed, S.; Editors, A.-K. Advanced Structured Materials. In Nanomaterials for Healthcare, Energy and Environment; Springer: Singapore; ISBN 9789811398322.
- Annunziato, G. Strategies to overcome antimicrobial resistance (AMR) making use of non-essential target inhibitors: A review. Int. J. Mol. Sci. 2019, 20, 5844. [Google Scholar] [CrossRef]
- Díaz, C.; Schilardi, P.L.; Salvarezza, R.C.; De Mele, M.F.L. Nano/microscale order affects the early stages of biofilm formation on metal surfaces. Langmuir 2007, 23, 11206–11210. [Google Scholar] [CrossRef] [PubMed]
- Behravan, M.; Hossein Panahi, A.; Naghizadeh, A.; Ziaee, M.; Mahdavi, R.; Mirzapour, A. Facile green synthesis of silver nanoparticles using Berberis vulgaris leaf and root aqueous extract and its antibacterial activity. Int. J. Biol. Macromol. 2019, 124, 148–154. [Google Scholar] [CrossRef]
- Bhardwaj, K.A.; Vinothkumar, K.; Rajpara, N. Bacterial Quorum Sensing Inhibitors: Attractive Alternatives for Control of Infectious Pathogens Showing Multiple Drug Resistance. Recent Pat. Antiinfect. Drug Discov. 2013, 8, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.T.; Bassler, B.L. Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb. Perspect. Med. 2012, 2. [Google Scholar] [CrossRef]
- Sajid, M.; Khan, M.S.A.; Cameotra, S.S.; Ahmad, I. Drug Delivery Systems That Eradicate and/or Prevent Biofilm Formation. In Antibiofilm Agents; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Alshehrei, F.; Al-Ghamdi, S.B.; Bamaga, M.A.; Al-Thubiani, A.S.; Alam, M.Z. Virulence and biofilms as promising targets in developing antipathogenic drugs against candidiasis. Futur. Sci. OA 2020, 6. [Google Scholar] [CrossRef]
- Qais, F.A.; Khan, M.S.; Ahmad, I. Nanoparticles as quorum sensing inhibitor: Prospects and limitations. Biotechnol. Appl. Quor. Sens. Inhib. 2018, 227–244. [Google Scholar] [CrossRef]
- Srivastava, P.; Kowshik, M. Mechanisms of metal resistance and homeostasis in Haloarchaea. Archaea 2013, 2013. [Google Scholar] [CrossRef]
- Salas-Orozco, M.; Niño-Martínez, N.; Martínez-Castañón, G.A.; Méndez, F.T.; Jasso, M.E.C.; Ruiz, F. Mechanisms of resistance to silver nanoparticles in endodontic bacteria: A literature review. J. Nanomater. 2019, 2019. [Google Scholar] [CrossRef]
- Zhang, R.; Carlsson, F.; Edman, M.; Hummelgård, M.; Jonsson, B.G.; Bylund, D.; Olin, H. Escherichia coli Bacteria Develop Adaptive Resistance to Antibacterial ZnO Nanoparticles. Adv. Biosyst. 2018, 2, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Siemer, S.; Westmeier, D.; Barz, M.; Eckrich, J.; Wünsch, D.; Seckert, C.; Thyssen, C.; Schilling, O.; Hasenberg, M.; Pang, C.; et al. Biomolecule-corona formation confers resistance of bacteria to nanoparticle-induced killing: Implications for the design of improved nanoantibiotics. Biomaterials 2019, 192, 551–559. [Google Scholar] [CrossRef] [PubMed]
- De Lima, R.; Seabra, A.B.; Durán, N. Silver nanoparticles: A brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J. Appl. Toxicol. JAT 2012, 32, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Hendry, A.T.; Stewart, I.O. Silver-resistant Enterobacteriaceae from hospital patients. Can. J. Microbiol. 1979, 25, 915–921. [Google Scholar] [CrossRef] [PubMed]
- Li, X.Z.; Nikaido, H.; Williams, K.E. Silver-resistant mutants of Escherichia coli display active efflux of Ag+ and are deficient in porins. J. Bacteriol. 1997, 179, 6127–6132. [Google Scholar] [CrossRef]
- Gupta, A.; Silver, S. Silver as a biocide: Will resistance become a problem? Nat. Biotechnol. 1998, 16, 888. [Google Scholar] [CrossRef]
- Graves, J.L.; Tajkarimi, M.; Cunningham, Q.; Campbell, A.; Nonga, H.; Harrison, S.H.; Barrick, J.E. Rapid evolution of silver nanoparticle resistance in Escherichia coli. Front. Genet. 2015, 5, 1–13. [Google Scholar] [CrossRef]
- Hachicho, N.; Hoffmann, P.; Ahlert, K.; Heipieper, H.J. Effect of silver nanoparticles and silver ions on growth and adaptive response mechanisms of Pseudomonas putida mt-2. FEMS Microbiol. Lett. 2014, 355, 71–77. [Google Scholar] [CrossRef]
- Cui, L.; Wang, X.; Huang, D.; Zhao, Y.; Feng, J.; Lu, Q.; Pu, Q.; Wang, Y.; Cheng, G.; Wu, M.; et al. CRISPR-cas3 of Salmonella upregulates bacterial biofilm formation and virulence to host cells by targeting quorum-sensing systems. Pathogens 2020, 9, 53. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Köse, Ş.; Dao, S.; Ganbarov, K.; Tanomand, A.; Dal, T.; Aghazadeh, M.; Ghotaslou, R.; Rezaee, M.A.; Yousefi, B.; et al. How CRISPR-Cas system could be used to combat antimicrobial resistance. Infect. Drug Resist. 2020, 13, 1111–1121. [Google Scholar] [CrossRef]
- Valentin, E.; Bottomley, A.L.; Chilambi, G.S.; Harry, E.J.; Amal, R.; Sotiriou, G.A.; Rice, S.A.; Gunawan, C. Heritable nanosilver resistance in priority pathogen: A unique genetic adaptation and comparison with ionic silver and antibiotics. Nanoscale 2020, 12, 2384–2392. [Google Scholar] [CrossRef] [PubMed]
- Faghihzadeh, F.; Anaya, N.M.; Astudillo-Castro, C.; Oyanedel-Craver, V. Kinetic, metabolic and macromolecular response of bacteria to chronic nanoparticle exposure in continuous culture. Environ. Sci. Nano 2018, 5, 1386–1396. [Google Scholar] [CrossRef]
- Finley, P.J.; Norton, R.; Austin, C.; Mitchell, A.; Zank, S.; Durham, P. Unprecedented silver resistance in clinically isolated Enterobacteriaceae: Major implications for burn and wound management. Antimicrob. Agents Chemother. 2015, 59, 4734–4741. [Google Scholar] [CrossRef] [PubMed]
- Matuła, K.; Richter, Ł.; Janczuk-Richter, M.; Nogala, W.; Grzeszkowiak, M.; Peplińska, B.; Jurga, S.; Wyroba, E.; Suski, S.; Bilski, H.; et al. Phenotypic plasticity of Escherichia coli upon exposure to physical stress induced by ZnO nanorods. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Liu, S.B.; Ng, A.K.; Xu, R.; Wei, J.; Tan, C.M.; Yang, Y.H.; Chen, Y.Y.; Kang, S.; Pinault, M.; Pfefferle, L.D.; et al. Antibacterial action of dispersed single-walled carbon nanotubes on Escherichia coli and Bacillus subtilis investigated by atomic force microscopy. Nanoscale 2010, 2, 2744. [Google Scholar] [CrossRef]
- Baym, M.; Lieberman, T.D.; Kelsic, E.D.; Chait, R.; Gross, R.; Yelin, I.; Kishony, R. Spatiotemporal microbial evolution on antibiotic landscapes. Science 2016, 353, 1147–1151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Lambert, G.; Liao, D.; Kim, H.; Robin, K.; Tung, C.K.; Pourmand, N.; Austin, R.H. Acceleration of emergence of bacterial antibiotic resistance in connected microenvironments. Science 2011, 333, 1764–1767. [Google Scholar] [CrossRef]
- Moghadam, M.T.; Amirmozafari, N.; Shariati, A.; Hallajzadeh, M.; Mirkalantari, S.; Khoshbayan, A.; Jazi, F.M. How phages overcome the challenges of drug resistant bacteria in clinical infections. Infect. Drug Resist. 2020, 13, 45–61. [Google Scholar] [CrossRef]
- Brives, C.; Pourraz, J. Phage therapy as a potential solution in the fight against AMR: Obstacles and possible futures. Palgrave Commun. 2020, 6, 1–11. [Google Scholar] [CrossRef]
- Hay, I.D.; Lithgow, T. Filamentous phages: Masters of a microbial sharing economy. EMBO Rep. 2019, 20. [Google Scholar] [CrossRef]
- Pirnay, J.P. Phage Therapy in the Year 2035. Front. Microbiol. 2020, 11, 1–8. [Google Scholar] [CrossRef]
- Wright, A.; Hawkins, C.H.; Änggård, E.E.; Harper, D.R. A controlled clinical trial of a therapeutic bacteriophage preparation in chronic otitis due to antibiotic-resistant Pseudomonas aeruginosa; A preliminary report of efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef]
- Leitner, L.; Ujmajuridze, A.; Chanishvili, N.; Goderdzishvili, M.; Chkonia, I.; Rigvava, S.; Chkhotua, A.; Changashvili, G.; McCallin, S.; Schneider, M.P.; et al. Intravesical bacteriophages for treating urinary tract infections in patients undergoing transurethral resection of the prostate: A randomised, placebo-controlled, double-blind clinical trial. Lancet Infect. Dis. 2020, 3099, 1–10. [Google Scholar] [CrossRef]
- Speck, P.; Smithyman, A. Safety and efficacy of phage therapy via the intravenous route. FEMS Microbiol. Lett. 2015, 363, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons learned from the first 10 consecutive cases of intravenous bacteriophage therapy to treat multidrug-resistant bacterial infections at a single center in the United States. Open Forum Infect. Dis. 2020, 7. [Google Scholar] [CrossRef]
- Voelker, R. FDA Approves Bacteriophage Trial. JAMA 2019, 321, 638. [Google Scholar] [CrossRef] [PubMed]
- Cisek, A.A.; Dąbrowska, I.; Gregorczyk, K.P.; Wyżewski, Z. Phage Therapy in Bacterial Infections Treatment: One Hundred Years after the Discovery of Bacteriophages. Curr. Microbiol. 2017, 74, 277–283. [Google Scholar] [CrossRef]
- Gordillo Altamirano, F.L.; Barr, J.J. Phage therapy in the postantibiotic era. Clin. Microbiol. Rev. 2019, 32, 1–25. [Google Scholar] [CrossRef]
- Gazeev, S. Fagterapi och dess Tillämpning inom Veterinärmedicin. In Applications of Phage Therapy in Veterinary Medicine Applications of Phage Therapy in Veterinary Medicine; Swedish University of Agricultural Sciences: Uppsala, Sweden, 2018; p. 18. [Google Scholar]
- Smirnov, D.D.; Kapustin, A.V.; Yakimova, E.A.; Savinov, V.A.; Laishevtsev, A.I. Perspectives of the use of bacteriophages in agriculture, food and processing industries. IOP Conf. Ser. Earth Environ. Sci. 2020, 548. [Google Scholar] [CrossRef]
- Endersen, L.; Coffey, A. The use of bacteriophages for food safety. Curr. Opin. Food Sci. 2020, 36, 1–8. [Google Scholar] [CrossRef]
- Summers, W.C. The strange history of phage therapy. Bacteriophage 2012, 2, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.M.; Koskella, B.; Lin, H.C. Phage therapy: An alternative to antibiotics in the age of multi-drug resistance. World J. Gastrointest. Pharmacol. Ther. 2017, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Van Belleghem, J.D.; Dąbrowska, K.; Vaneechoutte, M.; Barr, J.J.; Bollyky, P.L. Interactions between bacteriophage, bacteria, and the mammalian immune system. Viruses 2019, 11, 10. [Google Scholar] [CrossRef]
- Lehti, T.A.; Pajunen, M.I.; Skog, M.S.; Finne, J. Internalization of a polysialic acid-binding Escherichia coli bacteriophage into eukaryotic neuroblastoma cells. Nat. Commun. 2017. [Google Scholar] [CrossRef]
- Di Giovine, M.; Salone, B.; Martina, Y.; Amati, V.; Zambruno, G.; Cundari, E.; Failla, C.M.; Saggio, I. Binding properties, cell delivery, and gene transfer of adenoviral penton base displaying bacteriophage. Virology 2001. [Google Scholar] [CrossRef]
- Weber-Dabrowska, B.; Zimecki, M.; Mulczyk, M. Effective phage therapy is associated with normalization of cytokine production by blood cell cultures. Arch. Immunol. Ther. Exp. 2000, 48, 31–37. [Google Scholar]
- Park, K.; Cha, K.E.; Myung, H. Observation of inflammatory responses in mice orally fed with bacteriophage T7. J. Appl. Microbiol. 2014. [Google Scholar] [CrossRef]
- Szermer-Olearnik, B.; Boratyński, J. Removal of endotoxins from bacteriophage preparations by extraction with organic solvents. PLoS ONE 2015. [Google Scholar] [CrossRef]
- Manohar, P.; Tamhankar, A.J.; Leptihn, S.; Ramesh, N. Pharmacological and Immunological Aspects of Phage Therapy. Infect. Microbes Dis. 2019, 1, 34–42. [Google Scholar] [CrossRef]
- Maciejewska, B.; Olszak, T.; Drulis-Kawa, Z. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: An ambitious and also a realistic application? Appl. Microbiol. Biotechnol. 2018, 102, 2563–2581. [Google Scholar] [CrossRef] [PubMed]
- Łusiak-Szelachowska, M.; Zaczek, M.; Weber-Dabrowska, B.; Miȩdzybrodzki, R.; Kłak, M.; Fortuna, W.; Letkiewicz, S.; Rogóz, P.; Szufnarowski, K.; Jończyk-Matysiak, E.; et al. Phage neutralization by sera of patients receiving phage therapy. Viral Immunol. 2014, 27, 295–304. [Google Scholar] [CrossRef]
- Górski, A.; Miedzybrodzki, R.; Borysowski, J.; Dabrowska, K.; Wierzbicki, P.; Ohams, M.; Korczak-Kowalska, G.; Olszowska-Zaremba, N.; Łusiak-Szelachowska, M.; Kłak, M.; et al. Phage as a Modulator of Immune Responses. Practical Implications for Phage Therapy. Adv. Virus Res. 2012, 83, 41–47. [Google Scholar]
- Hess, K.L.; Jewell, C.M. Phage display as a tool for vaccine and immunotherapy development. Bioeng. Transl. Med. 2020, 5, 1–15. [Google Scholar] [CrossRef]
- Vukotic, G.; Obradovic, M.; Novovic, K.; Di Luca, M.; Jovcic, B.; Fira, D.; Neve, H.; Kojic, M.; McAuliffe, O. Characterization, Antibiofilm, and Depolymerizing Activity of Two Phages Active on Carbapenem-Resistant Acinetobacter baumannii. Front. Med. 2020, 7, 1–12. [Google Scholar] [CrossRef]
- Ferriol-González, C.; Domingo-Calap, P. Phages for Biofilm Removal. Antibiotics 2020, 9, 268. [Google Scholar] [CrossRef]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.P. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Gonzalez, R.A.; Leung, C.Y.; Chan, B.K.; Turner, P.E.; Weitz, J.S. Quantitative Models of Phage-Antibiotics Combination Therapy. BioRxiv 2019, 5. [Google Scholar] [CrossRef]
- Morrisette, T.; Kebriaei, R.; Morales, S.; Rybak, M.J. Bacteriophage-Antibiotic Combinations: A Promising Alternative for Refractory Infections? Contagion 2020, 5, 1. [Google Scholar]
- Liu, C.G.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplan, H.B.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Phage-antibiotic synergy is driven by a unique combination of antibacterial mechanism of action and stoichiometry. MBio 2020, 11, 1–19. [Google Scholar] [CrossRef]
- Paczesny, J.; Richter, Ł.; Hołyst, R. Recent progress in the detection of bacteria using bacteriophages: A review. Viruses 2020, 12, 845. [Google Scholar] [CrossRef] [PubMed]
- Banuelos, S.; Gulbudak, H.; Horn, M.A.; Huang, Q.; Nandi, A.; Ryu, H.; Segal, R. Investigating the impact of combination phage and antibiotic therapy: A modeling study. BioRxiv 2020. [Google Scholar] [CrossRef]
- Górski, A.; Borysowski, J.; Międzybrodzki, R. Phage therapy: Towards a successful clinical trial. Antibiotics 2020, 9, 827. [Google Scholar] [CrossRef]
- Peng, H.; Borg, R.E.; Dow, L.P.; Pruitt, B.L.; Chen, I.A. Controlled phage therapy by photothermal ablation of specific bacterial species using gold nanorods targeted by chimeric phages. Proc. Natl. Acad. Sci. USA 2020, 117, 1951–1961. [Google Scholar] [CrossRef]
- Paczesny, J.; Bielec, K. Application of bacteriophages in nanotechnology. Nanomaterials 2020, 10, 944. [Google Scholar] [CrossRef] [PubMed]
- Quispe-Tintaya, W. Phage-Enabled Nanomedicine: From Probes to Therapeutics in Precision Medicine. Physiol. Behav. 2017, 176, 139–148. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef]
- Foster, T.J. Immune evasion by staphylococci. Nat. Rev. Microbiol. 2005, 3, 948–958. [Google Scholar] [CrossRef]
- Seed, K.D. Battling Phages: How Bacteria Defend against Viral Attack. PLoS Pathog. 2015, 11, 1–5. [Google Scholar] [CrossRef]
- Destoumieux-Garzón, D.; Duquesne, S.; Peduzzi, J.; Goulard, C.; Desmadril, M.; Letellier, L.; Rebuffat, S.; Boulanger, P. The iron-siderophore transporter FhuA is the receptor for the antimicrobial peptide microcin J25: Role of the microcin Val11-Pro16 β-hairpin region in the recognition mechanism. Biochem. J. 2005, 389, 869–876. [Google Scholar] [CrossRef]
- Vasu, K.; Nagaraja, V. Diverse Functions of Restriction-Modification Systems in Addition to Cellular Defense. Microbiol. Mol. Biol. Rev. 2013, 77, 53–72. [Google Scholar] [CrossRef]
- Goldfarb, T.; Sberro, H.; Weinstock, E.; Cohen, O.; Doron, S.; Charpak-Amikam, Y.; Afik, S.; Ofir, G.; Sorek, R. BREX is a novel phage resistance system widespread in microbial genomes. EMBO J. 2015, 34, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, K. BacteRiophage EXclusion (BREX): A novel anti-phage mechanism in the arsenal of bacterial defense system. J. Cell. Physiol. 2018, 233, 771–773. [Google Scholar] [CrossRef] [PubMed]
- Ofir, G.; Melamed, S.; Sberro, H.; Mukamel, Z.; Silverman, S.; Yaakov, G.; Doron, S.; Sorek, R. DISARM is a widespread bacterial defence system with broad anti-phage activities. Nat. Microbiol. 2018, 3, 90–98. [Google Scholar] [CrossRef]
- Swarts, D.C.; Jore, M.M.; Westra, E.R.; Zhu, Y.; Janssen, J.H.; Snijders, A.P.; Wang, Y.; Patel, D.J.; Berenguer, J.; Brouns, S.J.J.; et al. DNA-guided DNA interference by a prokaryotic Argonaute. Nature 2014, 507, 258–261. [Google Scholar] [CrossRef]
- Xiong, L.; Liu, S.; Chen, S.; Xiao, Y.; Zhu, B.; Gao, Y.; Zhang, Y.; Chen, B.; Luo, J.; Deng, Z.; et al. A new type of DNA phosphorothioation-based antiviral system in archaea. Nat. Commun. 2019, 10, 1–11. [Google Scholar] [CrossRef]
- Kronheim, S.; Daniel-Ivad, M.; Duan, Z.; Hwang, S.; Wong, A.I.; Mantel, I.; Nodwell, J.R.; Maxwell, K.L. A chemical defence against phage infection. Nature 2018, 564, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Phumyen, A.; Jantasorn, S.; Jumnainsong, A.; Leelayuwat, C. Doxorubicin-conjugated bacteriophages carrying anti-MHC class I chain-related A for targeted cancer therapy in vitro. Onco. Targets. Ther. 2014, 7, 2183–2195. [Google Scholar] [CrossRef][Green Version]
- Lau, R.K.; Ye, Q.; Birkholz, E.A.; Berg, K.R.; Patel, L.; Mathews, I.T.; Watrous, J.D.; Ego, K.; Whiteley, A.T.; Lowey, B.; et al. Structure and Mechanism of a Cyclic Trinucleotide-Activated Bacterial Endonuclease Mediating Bacteriophage Immunity. Mol. Cell 2020, 77, 723–733.e6. [Google Scholar] [CrossRef] [PubMed]
- Millman, A.; Melamed, S.; Amitai, G.; Sorek, R. Diversity and classification of cyclic-oligonucleotide-based anti-phage signalling systems. Nat. Microbiol. 2020, 5, 1608–1615. [Google Scholar] [CrossRef]
- Hampton, H.G.; Watson, B.N.J.; Fineran, P.C. The arms race between bacteria and their phage foes. Nature 2020, 577, 327–336. [Google Scholar] [CrossRef]
- Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. Against Viruses in Prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar]
- Broniewski, J.M.; Chisnall, M.A.W.; Molin, N.; Westra, E.R. The effect of Quorum sensing inhibitors on the evolution of CRISPR-based phage immunity in Pseudomonas aeruginosa. ISME J. 2021. [Google Scholar] [CrossRef] [PubMed]
- Shehreen, S.; Chyou, T.Y.; Fineran, P.C.; Brown, C.M. Genome-wide correlation analysis suggests different roles of CRISPR-Cas systems in the acquisition of antibiotic resistance genes in diverse species. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374. [Google Scholar] [CrossRef] [PubMed]
- Trasanidou, D.; Gerós, A.S.; Mohanraju, P.; Nieuwenweg, A.C.; Nobrega, F.L.; Staals, R.H.J. Keeping crispr in check: Diverse mechanisms of phage-encoded anti-crisprs. FEMS Microbiol. Lett. 2019, 366, 1–14. [Google Scholar] [CrossRef]
- Landsberger, M.; Gandon, S.; Meaden, S.; Rollie, C.; Chevallereau, A.; Chabas, H.; Buckling, A.; Westra, E.R.; van Houte, S. Anti-CRISPR Phages Cooperate to Overcome CRISPR-Cas Immunity. Cell 2018, 174, 908–916.e12. [Google Scholar] [CrossRef] [PubMed]
- Gussow, A.B.; Park, A.E.; Borges, A.L.; Shmakov, S.A.; Makarova, K.S.; Wolf, Y.I.; Bondy-Denomy, J.; Koonin, E.V. Machine-learning approach expands the repertoire of anti-CRISPR protein families. Nat. Commun. 2020, 11, 1–12. [Google Scholar] [CrossRef]
- Matsumoto, D.; Tamamura, H.; Nomura, W. A cell cycle-dependent CRISPR-Cas9 activation system based on an anti-CRISPR protein shows improved genome editing accuracy. Commun. Biol. 2020, 3, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Stanley, S.Y.; Borges, A.L.; Chen, K.H.; Swaney, D.L.; Krogan, N.J.; Bondy-Denomy, J.; Davidson, A.R. Anti-CRISPR-Associated Proteins Are Crucial Repressors of Anti-CRISPR Transcription. Cell 2019, 178, 1452–1464.e13. [Google Scholar] [CrossRef]
- Rostøl, J.T.; Marraffini, L. (Ph)ighting Phages: How Bacteria Resist Their Parasites. Cell Host Microbe 2019, 25, 184–194. [Google Scholar] [CrossRef]
- Richter, Ł.; Księżarczyk, K.; Paszkowska, K.; Janczuk-Richter, M.; Niedziółka-Jönnson, J.; Gapiński, J.; Łoś, M.; Hołyst, R.; Paczesny, J. Adsorption of bacteriophages on polypropylene labware affects reproducibility of phage research. Sci. Rep. 2021, 1–12. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, S.; Matuła, K.; Karoń, S.; Paczesny, J. Resistance and Adaptation of Bacteria to Non-Antibiotic Antibacterial Agents: Physical Stressors, Nanoparticles, and Bacteriophages. Antibiotics 2021, 10, 435. https://doi.org/10.3390/antibiotics10040435
Raza S, Matuła K, Karoń S, Paczesny J. Resistance and Adaptation of Bacteria to Non-Antibiotic Antibacterial Agents: Physical Stressors, Nanoparticles, and Bacteriophages. Antibiotics. 2021; 10(4):435. https://doi.org/10.3390/antibiotics10040435
Chicago/Turabian StyleRaza, Sada, Kinga Matuła, Sylwia Karoń, and Jan Paczesny. 2021. "Resistance and Adaptation of Bacteria to Non-Antibiotic Antibacterial Agents: Physical Stressors, Nanoparticles, and Bacteriophages" Antibiotics 10, no. 4: 435. https://doi.org/10.3390/antibiotics10040435
APA StyleRaza, S., Matuła, K., Karoń, S., & Paczesny, J. (2021). Resistance and Adaptation of Bacteria to Non-Antibiotic Antibacterial Agents: Physical Stressors, Nanoparticles, and Bacteriophages. Antibiotics, 10(4), 435. https://doi.org/10.3390/antibiotics10040435






