Lefamulin in Patients with Community-Acquired Bacterial Pneumonia Caused by Atypical Respiratory Pathogens: Pooled Results from Two Phase 3 Trials
Abstract
1. Introduction
2. Results
2.1. Patients
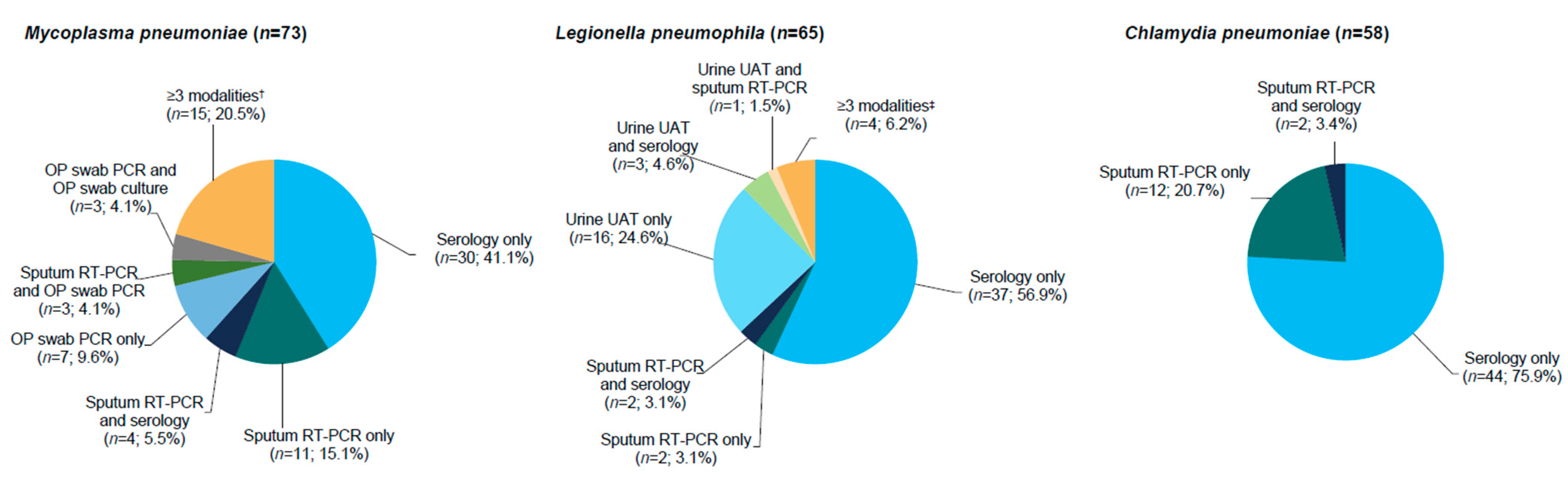
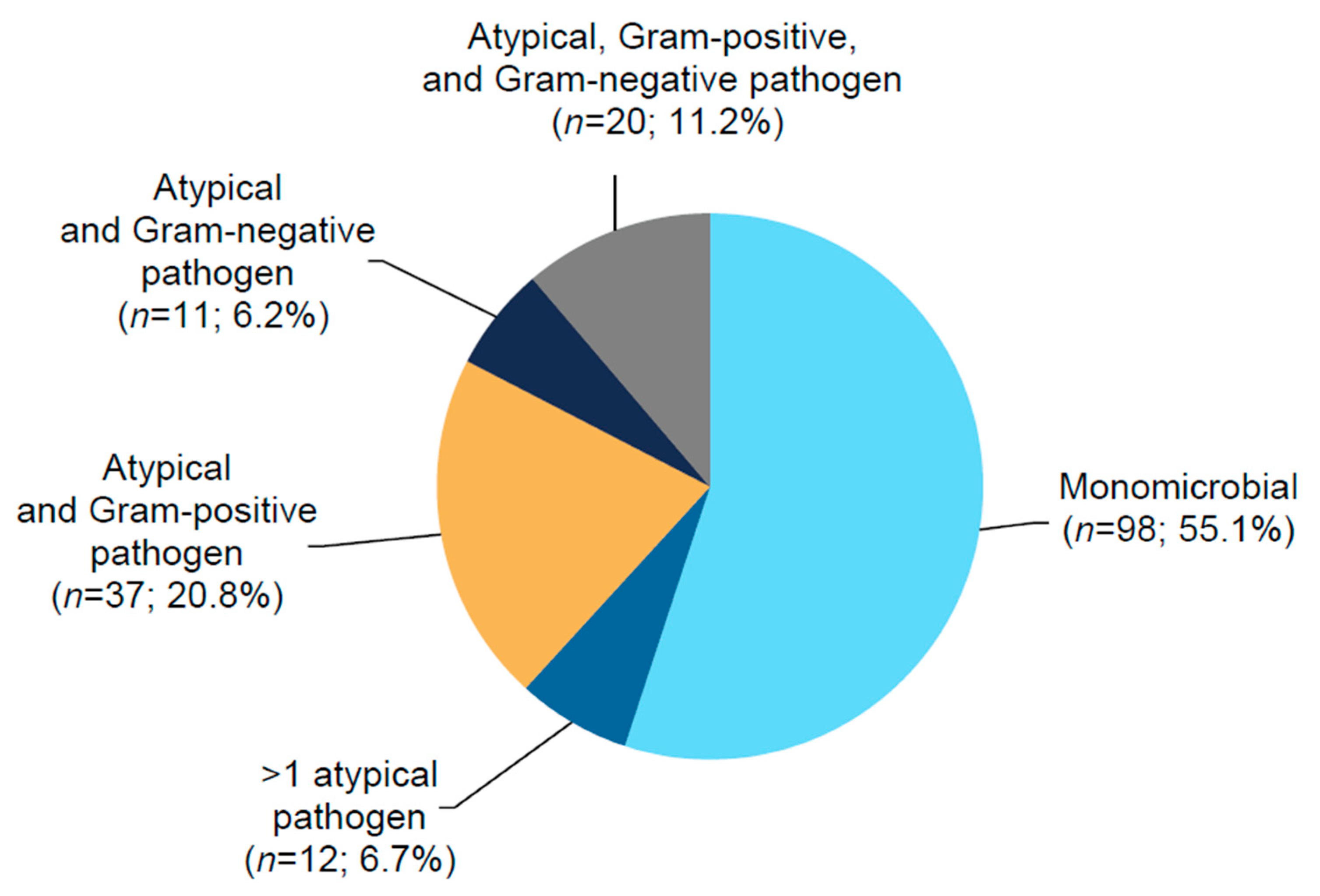
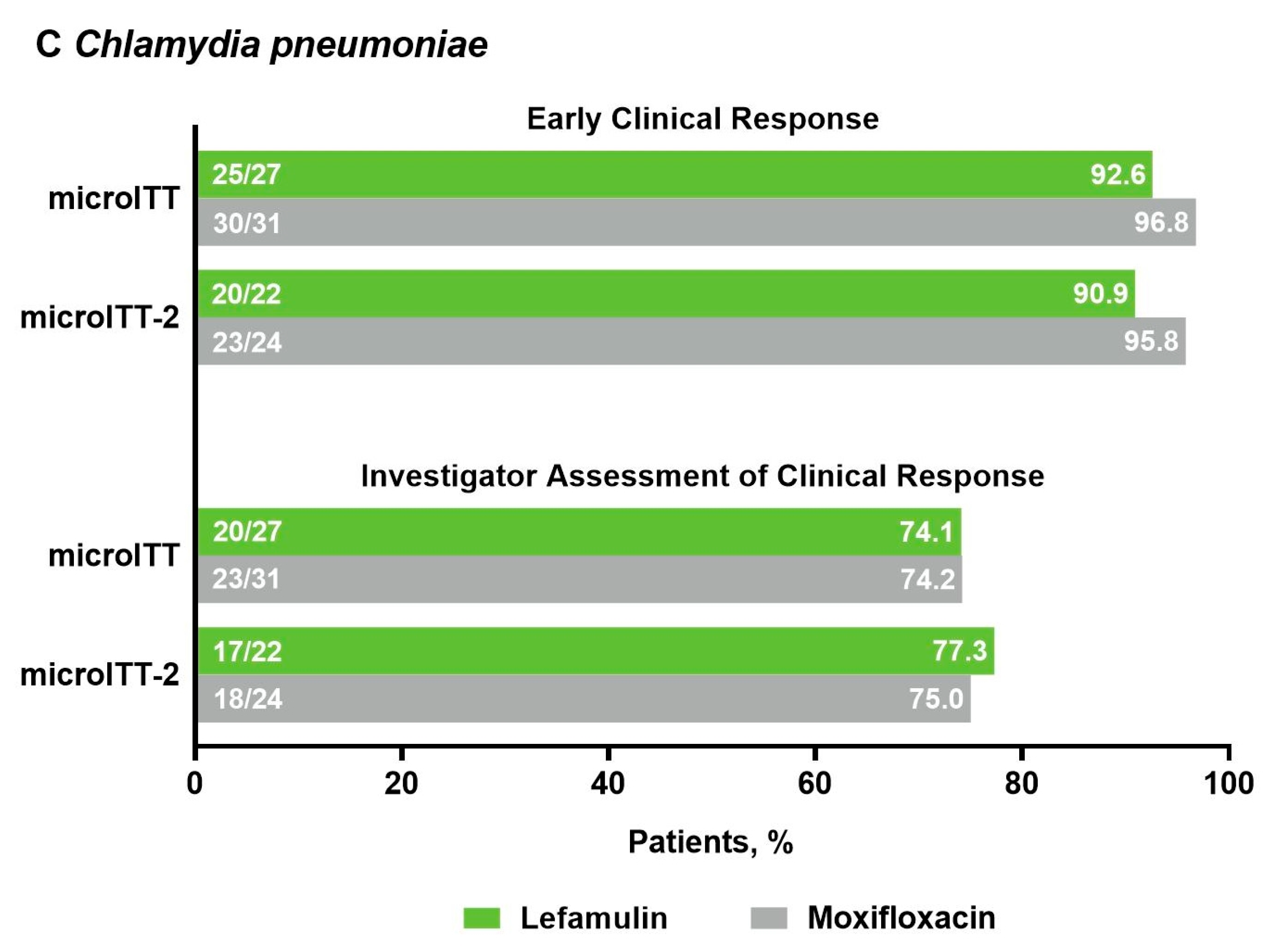
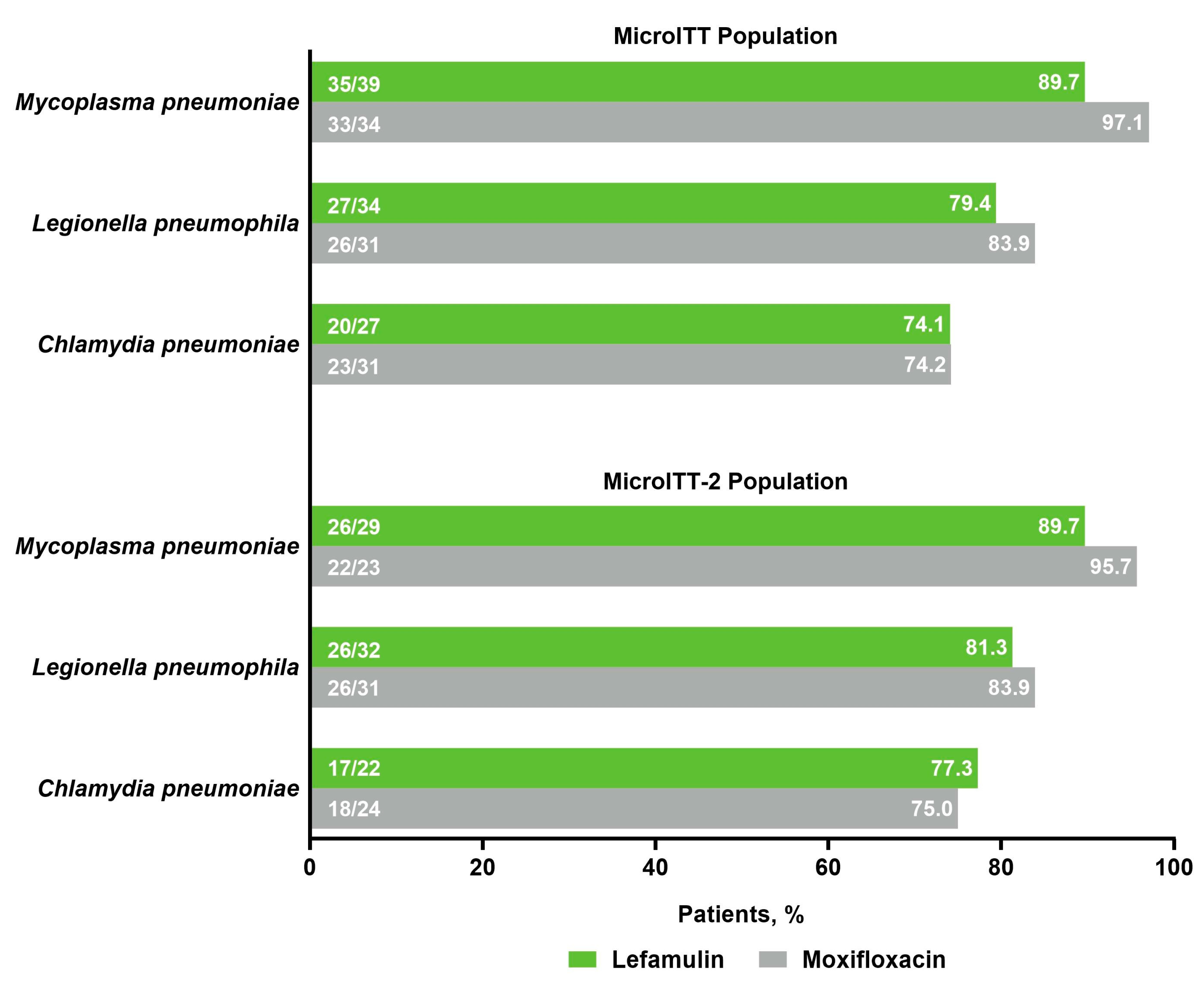
2.2. Efficacy
2.3. Safety
3. Discussion
4. Materials and Methods
4.1. Study Design and Participants
4.2. Microbiological Assessments
4.3. Efficacy Assessments
4.4. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Prior Presentations
References
- Cavallazzi, R.; Furmanek, S.; Arnold, F.W.; Beavin, L.A.; Wunderink, R.G.; Niederman, M.S.; Ramirez, J.A. The burden of community-acquired pneumonia requiring admission to an intensive care unit in the United States. Chest 2020, 158, 1008–1016. [Google Scholar] [CrossRef] [PubMed]
- Welte, T.; Torres, A.; Nathwani, D. Clinical and economic burden of community-acquired pneumonia among adults in Europe. Thorax 2012, 67, 71–79. [Google Scholar] [CrossRef]
- File, T.M.; Marrie, T.J. Burden of community-acquired pneumonia in North American adults. Postgrad. Med. 2010, 122, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Murphy, S.L.; Kochanek, K.D.; Arias, E. Mortality in the United States. 2018. Available online: https://www.cdc.gov/nchs/data/databriefs/db355-h.pdf (accessed on 1 November 2021).
- McDermott, K.W.; Elixhauser, A.; Sun, R. Trends in hospital inpatient stays in the United States, 2005-2014: Statistical brief #225. In Healthcare Cost and Utilization Project (HCUP) Statistical Briefs; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2017. [Google Scholar]
- Marchello, C.; Dale, A.P.; Thai, T.N.; Han, D.S.; Ebell, M.H. Prevalence of atypical pathogens in patients with cough and community-acquired pneumonia: A meta-analysis. Ann. Fam. Med. 2016, 14, 552–566. [Google Scholar] [CrossRef] [PubMed]
- Arnold, F.W.; Summersgill, J.T.; Ramirez, J.A. Role of atypical pathogens in the etiology of community-acquired pneumonia. Semin. Respir. Crit. Care Med. 2016, 37, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Gramegna, A.; Sotgiu, G.; Di Pasquale, M.; Radovanovic, D.; Terraneo, S.; Reyes, L.F.; Vendrell, E.; Neves, J.; Menzella, F.; Blasi, F.; et al. Atypical pathogens in hospitalized patients with community-acquired pneumonia: A worldwide perspective. BMC Infect. Dis. 2018, 18, 677. [Google Scholar] [CrossRef] [PubMed]
- Gatsura, S.; Deriushkin, V.; Gatsura, O.; Ulyanova, E. Will mild atypical pneumonia remain underdiagnosed and undertreated in primary care setting? Eur. J. Public Health 2020, 30, ckaa166.823. [Google Scholar] [CrossRef]
- Khoury, T.; Sviri, S.; Rmeileh, A.A.; Nubani, A.; Abutbul, A.; Hoss, S.; van Heerden, P.V.; Bayya, A.E.; Hidalgo-Grass, C.; Moses, A.E.; et al. Increased rates of intensive care unit admission in patients with Mycoplasma pneumoniae: A retrospective study. Clin. Microbiol. Infect. 2016, 22, 711–714. [Google Scholar] [CrossRef]
- Cillóniz, C.; Ewig, S.; Polverino, E.; Marcos, M.A.; Esquinas, C.; Gabarrús, A.; Mensa, J.; Torres, A. Microbial aetiology of community-acquired pneumonia and its relation to severity. Thorax 2011, 66, 340–346. [Google Scholar] [CrossRef]
- Valade, S.; Biard, L.; Lemiale, V.; Argaud, L.; Pène, F.; Papazian, L.; Bruneel, F.; Seguin, A.; Kouatchet, A.; Oziel, J.; et al. Severe atypical pneumonia in critically ill patients: A retrospective multicenter study. Ann. Intensive Care 2018, 8, 81. [Google Scholar] [CrossRef]
- Zubairi, A.B.; Zafar, A.; Salahuddin, N.; Haque, A.S.; Waheed, S.; Khan, J.A. Atypical pathogens causing community-acquired pneumonia in adults. J. Pak. Med. Assoc. 2012, 62, 653–656. [Google Scholar] [PubMed]
- Capelastegui, A.; España, P.P.; Bilbao, A.; Gamazo, J.; Medel, F.; Salgado, J.; Gorostiaga, I.; Lopez de Goicoechea, M.J.; Gorordo, I.; Esteban, C.; et al. Etiology of community-acquired pneumonia in a population-based study: Link between etiology and patients characteristics, process-of-care, clinical evolution and outcomes. BMC Infect. Dis. 2012, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Arancibia, F.; Cortes, C.P.; Valdés, M.; Cerda, J.; Hernández, A.; Soto, L.; Torres, A. Importance of Legionella pneumophila in the etiology of severe community-acquired pneumonia in Santiago, Chile. Chest 2014, 145, 290–296. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. What Clinicians Need to Know about Legionnaires’ Disease. Available online: https://www.cdc.gov/legionella/downloads/fs-legionella-clinicians.pdf (accessed on 1 November 2021).
- Mandell, L.A.; Wunderink, R.G.; Anzueto, A.; Bartlett, J.G.; Campbell, G.D.; Dean, N.C.; Dowell, S.F.; File, T.M., Jr.; Musher, D.M.; Niederman, M.S.; et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin. Infect. Dis. 2007, 44, S27–S72. [Google Scholar] [CrossRef]
- Sharma, L.; Losier, A.; Tolbert, T.; Dela Cruz, C.S.; Marion, C.R. Atypical pneumonia: Updates on Legionella, Chlamydophila, and Mycoplasma pneumonia. Clin. Chest Med. 2017, 38, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Massip, C.; Descours, G.; Ginevra, C.; Doublet, P.; Jarraud, S.; Gilbert, C. Macrolide resistance in Legionella pneumophila: The role of LpeAB efflux pump. J. Antimicrob. Chemother. 2017, 72, 1327–1333. [Google Scholar] [CrossRef]
- Shadoud, L.; Almahmoud, I.; Jarraud, S.; Etienne, J.; Larrat, S.; Schwebel, C.; Timsit, J.F.; Schneider, D.; Maurin, M. Hidden selection of bacterial resistance to fluoroquinolones in vivo: The case of Legionella pneumophila and humans. EBioMedicine 2015, 2, 1179–1185. [Google Scholar] [CrossRef]
- Bruin, J.P.; Koshkolda, T.; IJzerman, E.P.F.; Lück, C.; Diederen, B.M.W.; Den Boer, J.W.; Mouton, J.W. Isolation of ciprofloxacin-resistant Legionella pneumophila in a patient with severe pneumonia. J. Antimicrob. Chemother. 2014, 69, 2869–2871. [Google Scholar] [CrossRef]
- Beeton, M.L.; Zhang, X.S.; Uldum, S.A.; Bébéar, C.; Dumke, R.; Gullsby, K.; Ieven, M.; Loens, K.; Nir-Paz, R.; Pereyre, S.; et al. Mycoplasma pneumoniae infections, 11 countries in Europe and Israel, 2011 to 2016. Euro. Surveill. 2020, 25, 1900112. [Google Scholar] [CrossRef]
- Brown, R.J.; Macfarlane-Smith, L.; Phillips, S.; Chalker, V.J. Detection of macrolide resistant Mycoplasma pneumoniae in England, September 2014 to September 2015. Euro. Surveill. 2015, 20, 30078. [Google Scholar] [CrossRef]
- Dumke, R.; Ziegler, T. Long-term low rate of macrolide-resistant Mycoplasma pneumoniae strains in Germany. Antimicrob. Agents Chemother. 2019, 63, e00455-19. [Google Scholar] [CrossRef]
- Loconsole, D.; De Robertis, A.L.; Mallamaci, R.; Sallustio, A.; Morea, A.; Prato, R.; Quarto, M.; Martinelli, D.; Chironna, M. First description of macrolide-resistant Mycoplasma pneumoniae in adults with community-acquired pneumonia in Italy. Biomed. Res. Int. 2019, 2019, 7168949. [Google Scholar] [CrossRef] [PubMed]
- Principi, N.; Esposito, S. Macrolide-resistant Mycoplasma pneumoniae: Its role in respiratory infection. J. Antimicrob. Chemother. 2013, 68, 506–511. [Google Scholar] [CrossRef] [PubMed]
- Diaz, M.H.; Benitez, A.J.; Winchell, J.M. Investigations of Mycoplasma pneumoniae infections in the United States: Trends in molecular typing and macrolide resistance from 2006 to 2013. J. Clin. Microbiol. 2015, 53, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Lee, S.; Selvarangan, R.; Qin, X.; Tang, Y.W.; Stiles, J.; Hong, T.; Todd, K.; Ratliff, A.E.; Crabb, D.M.; et al. Macrolide-resistant Mycoplasma pneumoniae, United States. Emerg. Infect. Dis. 2015, 21, 1470–1472. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Ratliff, A.; Crabb, D.M.; Xiao, L.; Qin, X.; Selvarangan, R.; Tang, Y.W.; Zheng, X.; Dien Bard, J.; Hong, T.; et al. Macrolide-resistant Mycoplasma pneumoniae in the United States as determined from a national surveillance program. J. Clin. Microbiol. 2019, 57, e00968-19. [Google Scholar] [CrossRef] [PubMed]
- Peyrani, P.; Mandell, L.; Torres, A.; Tillotson, G.S. The burden of community-acquired bacterial pneumonia in the era of antibiotic resistance. Expert Rev. Respir. Med. 2019, 13, 139–152. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration. FDA Drug Safety Communication: FDA Updates Warnings for Oral and Injectable Fluoroquinolone Antibiotics Due to Disabling Side Effects. Available online: https://www.fda.gov/Drugs/DrugSafety/ucm511530.htm (accessed on 1 November 2021).
- US Food and Drug Administration. FDA Drug Safety Communication: FDA Reinforces Safety Information About Serious Low Blood Sugar Levels and Mental Health Side Effects with Fluoroquinolone Antibiotics; Requires Label Changes. Available online: https://www.fda.gov/downloads/Drugs/DrugSafety/UCM612834.pdf (accessed on 1 November 2021).
- Nabriva Therapeutics US, Inc. Xenleta™ (Lefamulin). Full Prescribing Information; Nabriva Therapeutics US, Inc.: Fort Washington, PA, USA, 2021. [Google Scholar]
- Paukner, S.; Gelone, S.P.; Arends, S.J.R.; Flamm, R.K.; Sader, H.S. Antibacterial activity of lefamulin against pathogens most commonly causing community-acquired bacterial pneumonia: SENTRY antimicrobial surveillance program (2015–2016). Antimicrob. Agents Chemother. 2019, 63, e02161-18. [Google Scholar] [CrossRef]
- Waites, K.B.; Crabb, D.M.; Duffy, L.B.; Jensen, J.S.; Liu, Y.; Paukner, S. In vitro activities of lefamulin and other antimicrobial agents against macrolide-susceptible and macrolide-resistant Mycoplasma pneumoniae from the United States, Europe, and China. Antimicrob. Agents Chemother. 2017, 61, e02008-16. [Google Scholar] [CrossRef]
- Sader, H.S.; Paukner, S.; Ivezic-Schoenfeld, Z.; Biedenbach, D.J.; Schmitz, F.J.; Jones, R.N. Antimicrobial activity of the novel pleuromutilin antibiotic BC-3781 against organisms responsible for community-acquired respiratory tract infections (CARTIs). J. Antimicrob. Chemother. 2012, 67, 1170–1175. [Google Scholar] [CrossRef]
- Wicha, W.W.; Craig, W.A.; Andes, D. In vivo pharmacodynamics of lefamulin, the first systemic pleuromutilin for human use, in a neutropenic murine thigh infection model. J. Antimicrob. Chemother. 2019, 74, iii5–iii10. [Google Scholar] [CrossRef]
- Wicha, W.W.; Strickmann, D.B.; Paukner, S. Pharmacokinetics/pharmacodynamics of lefamulin in a neutropenic murine pneumonia model with Staphylococcus aureus and Streptococcus pneumoniae. J. Antimicrob. Chemother. 2019, 74, iii11–iii18. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Torres, A.; Niederman, M.; van der Eerden, M.; Chalmers, J.; Welte, T.; Blasi, F. Community-acquired pneumonia related to intracellular pathogens. Intensive Care Med. 2016, 42, 1374–1386. [Google Scholar] [CrossRef] [PubMed]
- File, T.M., Jr.; Goldberg, L.; Das, A.; Sweeney, C.; Saviski, J.; Gelone, S.P.; Seltzer, E.; Paukner, S.; Wicha, W.W.; Talbot, G.H.; et al. Efficacy and safety of intravenous-to-oral lefamulin, a pleuromutilin antibiotic, for the treatment of community-acquired bacterial pneumonia: The phase III Lefamulin Evaluation Against Pneumonia (LEAP 1) trial. Clin. Infect. Dis. 2019, 69, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.; Goldberg, L.; Das, A.F.; Moran, G.J.; Sandrock, C.; Gasink, L.B.; Spera, P.; Sweeney, C.; Paukner, S.; Wicha, W.W.; et al. Oral lefamulin vs moxifloxacin for early clinical response among adults with community-acquired bacterial pneumonia: The LEAP 2 randomized clinical trial. JAMA 2019, 322, 1661–1671. [Google Scholar] [CrossRef] [PubMed]
- File, T.M., Jr.; Alexander, E.; Goldberg, L.; Das, A.F.; Sandrock, C.; Paukner, S.; Moran, G.J. Lefamulin efficacy and safety in a pooled phase 3 clinical trial population with community-acquired bacterial pneumonia and common clinical comorbidities. BMC Pulm. Med. 2021, 21, 154. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Guo, Q.; Song, W.D.; Zhou, Y.P.; Li, M.; Chen, X.K.; Liu, H.; Peng, H.L.; Yu, H.Q.; Chen, X.; et al. Modified IDSA/ATS minor criteria for severe community-acquired pneumonia best predicted mortality. Medicine 2015, 94, e1474. [Google Scholar] [CrossRef]
- Phin, N.; Parry-Ford, F.; Harrison, T.; Stagg, H.R.; Zhang, N.; Kumar, K.; Lortholary, O.; Zumla, A.; Abubakar, I. Epidemiology and clinical management of Legionnaires’ disease. Lancet Infect. Dis. 2014, 14, 1011–1021. [Google Scholar] [CrossRef]
- Cunha, B.A. The atypical pneumonias: Clinical diagnosis and importance. Clin. Microbiol. Infect. 2006, 12 (Suppl. S3), 12–24. [Google Scholar] [CrossRef]
- Ishiguro, T.; Takayanagi, N.; Yamaguchi, S.; Yamakawa, H.; Nakamoto, K.; Takaku, Y.; Miyahara, Y.; Kagiyama, N.; Kurashima, K.; Yanagisawa, T.; et al. Etiology and factors contributing to the severity and mortality of community-acquired pneumonia. Intern. Med. 2013, 52, 317–324. [Google Scholar] [CrossRef]
- Tao, L.L.; Hu, B.J.; He, L.X.; Wei, L.; Xie, H.M.; Wang, B.Q.; Li, H.Y.; Chen, X.H.; Zhou, C.M.; Deng, W.W. Etiology and antimicrobial resistance of community-acquired pneumonia in adult patients in China. Chin. Med. J. 2012, 125, 2967–2972. [Google Scholar] [CrossRef] [PubMed]
- Cillóniz, C.; Civljak, R.; Nicolini, A.; Torres, A. Polymicrobial community-acquired pneumonia: An emerging entity. Respirology 2016, 21, 65–75. [Google Scholar] [CrossRef]
- Eljaaly, K.; Alshehri, S.; Aljabri, A.; Abraham, I.; Al Mohajer, M.; Kalil, A.C.; Nix, D.E. Clinical failure with and without empiric atypical bacteria coverage in hospitalized adults with community-acquired pneumonia: A systematic review and meta-analysis. BMC Infect. Dis. 2017, 17, 385. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Ma, J.; Hu, B.; Gao, X.; He, L.; Shen, W.; Weng, L.; Cai, L.; Huang, Y.; Hu, Z.; et al. Improvement in clinical and economic outcomes with empiric antibiotic therapy covering atypical pathogens for community-acquired pneumonia patients: A multicenter cohort study. Int. J. Infect. Dis. 2015, 40, 102–107. [Google Scholar] [CrossRef][Green Version]
- Mendes, R.E.; Farrell, D.J.; Flamm, R.K.; Talbot, G.H.; Ivezic-Schoenfeld, Z.; Paukner, S.; Sader, H.S. In vitro activity of lefamulin tested against Streptococcus pneumoniae with defined serotypes, including multidrug-resistant isolates causing lower respiratory tract infections in the United States. Antimicrob. Agents Chemother. 2016, 60, 4407–4411. [Google Scholar] [CrossRef] [PubMed][Green Version]
- LoVecchio, F.; Schranz, J.; Alexander, E.; Mariano, D.; Meads, A.; Sandrock, C.; Moran, G.J.; Giordano, P.A. Oral 5-day lefamulin for outpatient management of community-acquired bacterial pneumonia: Post-hoc analysis of the Lefamulin Evaluation Against Pneumonia (LEAP) 2 trial. J. Emerg. Med. 2021, 60, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Shorr, A.F.; Schranz, J.; Goldberg, L.; Paukner, S.; Alexander, E.; Moran, G.J.; Sandrock, C.; Gelone, S.P. Efficacy and safety of lefamulin vs moxifloxacin for atypical respiratory pathogens in adults with community-acquired bacterial pneumonia: Pooled results from the Lefamulin Evaluation Against Pneumonia (LEAP) 1 and LEAP 2 double-blind noninferiority phase 3 clinical trials. Chest 2019, 156, A1133–A1134. [Google Scholar] [CrossRef]
- Shorr, A.F.; Schranz, J.; Goldberg, L.; Paukner, S.; Das, A.; Moran, G.J.; Sandrock, C.; File Jr, T.M.; Alexander, E.; Gelone, S.P. Efficacy and safety of lefamulin (LEF) vs. moxifloxacin (MOX) for Legionella pneumophila (LP) in patients with community-acquired bacterial pneumonia (CABP): Pooled results from the Lefamulin Evaluation Against Pneumonia (LEAP) 1 and LEAP 2 phase 3 clinical trials. Open Forum Infect. Dis. 2019, 6, S303–S304. [Google Scholar] [CrossRef]
- Paukner, S.; Goldberg, L.; Alexander, E.; Das, A.F.; Heinrich, S.; Patel, P.; Moran, G.J.; Sandrock, C.; File, T.M., Jr.; Vidal, J.E.; et al. Pooled microbiological findings and efficacy outcomes by pathogen in adults with community-acquired bacterial pneumonia from the Lefamulin Evaluation Against Pneumonia (LEAP) 1 and LEAP 2 phase 3 trials of lefamulin versus moxifloxacin. J. Glob. Antimicrob. Resist. 2021. Published online ahead of print November 14. [Google Scholar] [CrossRef] [PubMed]
- Waites, K.B.; Duffy, L.B.; Xiao, L. 3.15 Detection of Human Mycoplasmas and Ureaplasmas from Clinical Specimens by Culture and PCR. In Clinical Microbiology Procedures Handbook, 4th ed.; Leber, A.L., Ed.; ASM Press: Washington, DC, USA, 2016. [Google Scholar]
- Thurman, K.A.; Warner, A.K.; Cowart, K.C.; Benitez, A.J.; Winchell, J.M. Detection of Mycoplasma pneumoniae, Chlamydia pneumoniae, and Legionella spp. in clinical specimens using a single-tube multiplex real-time PCR assay. Diagn. Microbiol. Infect. Dis. 2011, 70, 1–9. [Google Scholar] [CrossRef]
- Waites, K.B.; Xiao, L.; Paralanov, V.; Viscardi, R.M.; Glass, J.I. Molecular methods for the detection of Mycoplasma and Ureaplasma infections in humans: A paper from the 2011 William Beaumont Hospital symposium on molecular pathology. J. Mol. Diagn. 2012, 14, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Dumke, R.; Schurwanz, N.; Lenz, M.; Schuppler, M.; Lück, C.; Jacobs, E. Sensitive detection of Mycoplasma pneumoniae in human respiratory tract samples by optimized real-time PCR approach. J. Clin. Microbiol. 2007, 45, 2726–2730. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Atkinson, T.P.; Hagood, J.; Makris, C.; Duffy, L.B.; Waites, K.B. Emerging macrolide resistance in Mycoplasma pneumoniae in children: Detection and characterization of resistant isolates. Pediatr. Infect. Dis. J. 2009, 28, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing, 26th ed.; M100Ed26; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2016. [Google Scholar]
- European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 6.0, 2016. TECoAS Testing. Available online: https://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Breakpoint_tables/v_6.0_Breakpoint_table.xls (accessed on 1 November 2021).

| Parameter | All Patients (Pooled ITT Population) | Patients with Atypical Pathogens * (Pooled microITT Population) | ||
|---|---|---|---|---|
| Lefamulin (n = 646) | Moxifloxacin (n = 643) | Lefamulin (n = 91) | Moxifloxacin (n = 87) | |
| Age, y, mean (SD) | 58.9 (16.5) | 58.5 (15.7) | 54.7 (17.8) | 55.6 (17.5) |
| Age ≥ 65 y, n (%) | 268 (41.5) | 249 (38.7) | 28 (30.8) | 32 (36.8) |
| Male, n (%) | 377 (58.4) | 340 (52.9) | 53 (58.2) | 49 (56.3) |
| White, n (%) | 513 (79.4) | 509 (79.2) | 84 (92.3) | 74 (85.1) |
| PORT risk class, † n (%) | ||||
| I | 1 (0.2) | 2 (0.3) | 0 | 0 |
| II | 183 (28.3) | 190 (29.5) | 26 (28.6) | 21 (24.1) |
| III | 341 (52.8) | 334 (51.9) | 49 (53.8) | 44 (50.6) |
| IV | 116 (18.0) | 112 (17.4) | 16 (17.6) | 22 (25.3) |
| V | 5 (0.8) | 5 (0.8) | 0 | 0 |
| CURB-65 score, ‡ n (%) | ||||
| 0–2 | 610 (94.4) | 604 (93.9) | 87 (95.6) | 80 (92.0) |
| 3–5 | 36 (5.6) | 39 (6.1) | 4 (4.4) | 7 (8.0) |
| Minor ATS severity criteria, § n (%) | 85 (13.2) | 85 (13.2) | 15 (16.5) | 9 (10.3) |
| Modified ATS severity criteria, || n (%) | 53 (8.2) | 57 (8.9) | 8 (8.8) | 7 (8.0) |
| Multilobar pneumonia, n (%) | 170 (26.3) | 177 (27.5) | 20 (22.0) | 17 (19.5) |
| SIRS, n (%) | 621 (96.1) | 609 (94.7) | 89 (97.8) | 82 (94.3) |
| Bacteremic, n (%) | 13 (2.0) | 12 (1.9) | 0 | 1 (1.1) |
| Prior antibiotic use, # n (%) | 147 (22.8) | 145 (22.6) | 28 (30.8) | 23 (26.4) |
| Renal status, ** n (%) | ||||
| Normal | 311 (48.1) | 312 (48.5) | 56 (61.5) | 46 (52.9) |
| Mild impairment | 201 (31.1) | 192 (29.9) | 25 (27.5) | 26 (29.9) |
| Moderate impairment | 125 (19.3) | 132 (20.5) | 8 (8.8) | 15 (17.2) |
| Severe impairment | 7 (1.1) | 6 (0.9) | 2 (2.2) | 0 |
| Missing | 2 (0.3) | 1 (0.2) | 0 | 0 |
| Medical history, †† n (%) | ||||
| Smoking history | 284 (44.0) | 242 (37.6) | 35 (38.5) | 25 (28.7) |
| Hypertension | 248 (38.4) | 253 (39.3) | 35 (38.5) | 29 (33.3) |
| Asthma/COPD | 119 (18.4) | 113 (17.6) | 10 (11.0) | 10 (11.5) |
| Diabetes mellitus | 80 (12.4) | 88 (13.7) | 7 (7.7) | 12 (13.8) |
| Baseline pathogen, ‡‡ n (%) | ||||
| Mycoplasma pneumoniae | 39 (6.0) | 34 (5.3) | 39 (42.9) | 34 (39.1) |
| Legionella pneumophila | 34 (5.3) | 31 (4.8) | 34 (37.4) | 31 (35.6) |
| Chlamydia pneumoniae | 27 (4.2) | 31 (4.8) | 27 (29.7) | 31 (35.6) |
| Streptococcus pneumoniae | 216 (33.4) | 223 (34.7) | 24 (26.4) | 29 (33.3) |
| Haemophilus influenzae | 107 (16.6) | 105 (16.3) | 8 (8.8) | 13 (14.9) |
| Moraxella catarrhalis | 46 (7.1) | 22 (3.4) | 7 (7.7) | 1 (1.1) |
| Staphylococcus aureus | 23 (3.6) | 10 (1.6) | 5 (5.5) | 1 (1.1) |
| Patients, n (%) | All Patients (Pooled Safety Population) | Patients with Atypical Pathogens * at Baseline (Pooled microITT Population) | ||
|---|---|---|---|---|
| Lefamulin (n = 641) | Moxifloxacin (n = 641) | Lefamulin (n = 91) | Moxifloxacin (n = 87) | |
| Any TEAE † | 224 (34.9) | 195 (30.4) | 31 (34.1) | 28 (32.2) |
| Mild | 119 (18.6) | 117 (18.3) | 16 (17.6) | 16 (18.4) |
| Moderate | 78 (12.2) | 55 (8.6) | 12 (13.2) | 7 (8.0) |
| Severe | 27 (4.2) | 23 (3.6) | 3 (3.3) | 5 (5.7) |
| Related TEAE ‡ | 99 (15.4) | 68 (10.6) | 8 (8.8) | 7 (8.0) |
| Serious TEAE | 36 (5.6) | 31 (4.8) | 6 (6.6) | 5 (5.7) |
| Related serious TEAE | 3 (0.5) | 2 (0.3) | 0 | 0 |
| TEAE leading to study drug discontinuation | 20 (3.1) | 21 (3.3) | 0 | 4 (4.6) |
| TEAE leading to death (over entire study duration) | 11 (1.7) | 8 (1.2) | 1 (1.1) § | 0 |
| 28d all-cause mortality—deceased at Day 28 || | 8 (1.2) | 7 (1.1) | 0 | 0 |
| TEAEs by SOC in ≥5% of patients in any treatment group | ||||
| Gastrointestinal disorders | 84 (13.1) | 65 (10.1) | 7 (7.7) | 7 (8.0) |
| Infections and infestations | 47 (7.3) | 40 (6.2) | 7 (7.7) | 7 (8.0) |
| Investigations | 31 (4.8) | 26 (4.1) | 5 (5.5) | 4 (4.6) |
| Respiratory, thoracic, and mediastinal disorders | 29 (4.5) | 28 (4.4) | 5 (5.5) | 2 (2.3) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paukner, S.; Mariano, D.; Das, A.F.; Moran, G.J.; Sandrock, C.; Waites, K.B.; File, T.M., Jr. Lefamulin in Patients with Community-Acquired Bacterial Pneumonia Caused by Atypical Respiratory Pathogens: Pooled Results from Two Phase 3 Trials. Antibiotics 2021, 10, 1489. https://doi.org/10.3390/antibiotics10121489
Paukner S, Mariano D, Das AF, Moran GJ, Sandrock C, Waites KB, File TM Jr. Lefamulin in Patients with Community-Acquired Bacterial Pneumonia Caused by Atypical Respiratory Pathogens: Pooled Results from Two Phase 3 Trials. Antibiotics. 2021; 10(12):1489. https://doi.org/10.3390/antibiotics10121489
Chicago/Turabian StylePaukner, Susanne, David Mariano, Anita F. Das, Gregory J. Moran, Christian Sandrock, Ken B. Waites, and Thomas M. File, Jr. 2021. "Lefamulin in Patients with Community-Acquired Bacterial Pneumonia Caused by Atypical Respiratory Pathogens: Pooled Results from Two Phase 3 Trials" Antibiotics 10, no. 12: 1489. https://doi.org/10.3390/antibiotics10121489
APA StylePaukner, S., Mariano, D., Das, A. F., Moran, G. J., Sandrock, C., Waites, K. B., & File, T. M., Jr. (2021). Lefamulin in Patients with Community-Acquired Bacterial Pneumonia Caused by Atypical Respiratory Pathogens: Pooled Results from Two Phase 3 Trials. Antibiotics, 10(12), 1489. https://doi.org/10.3390/antibiotics10121489





