Antibiotic Prescribing Trends in Belgian Out-of-Hours Primary Care during the COVID-19 Pandemic: Observational Study Using Routinely Collected Health Data
Abstract
1. Introduction
2. Results
2.1. Population Characteristics
2.2. Trends in Antibiotic Prescribing
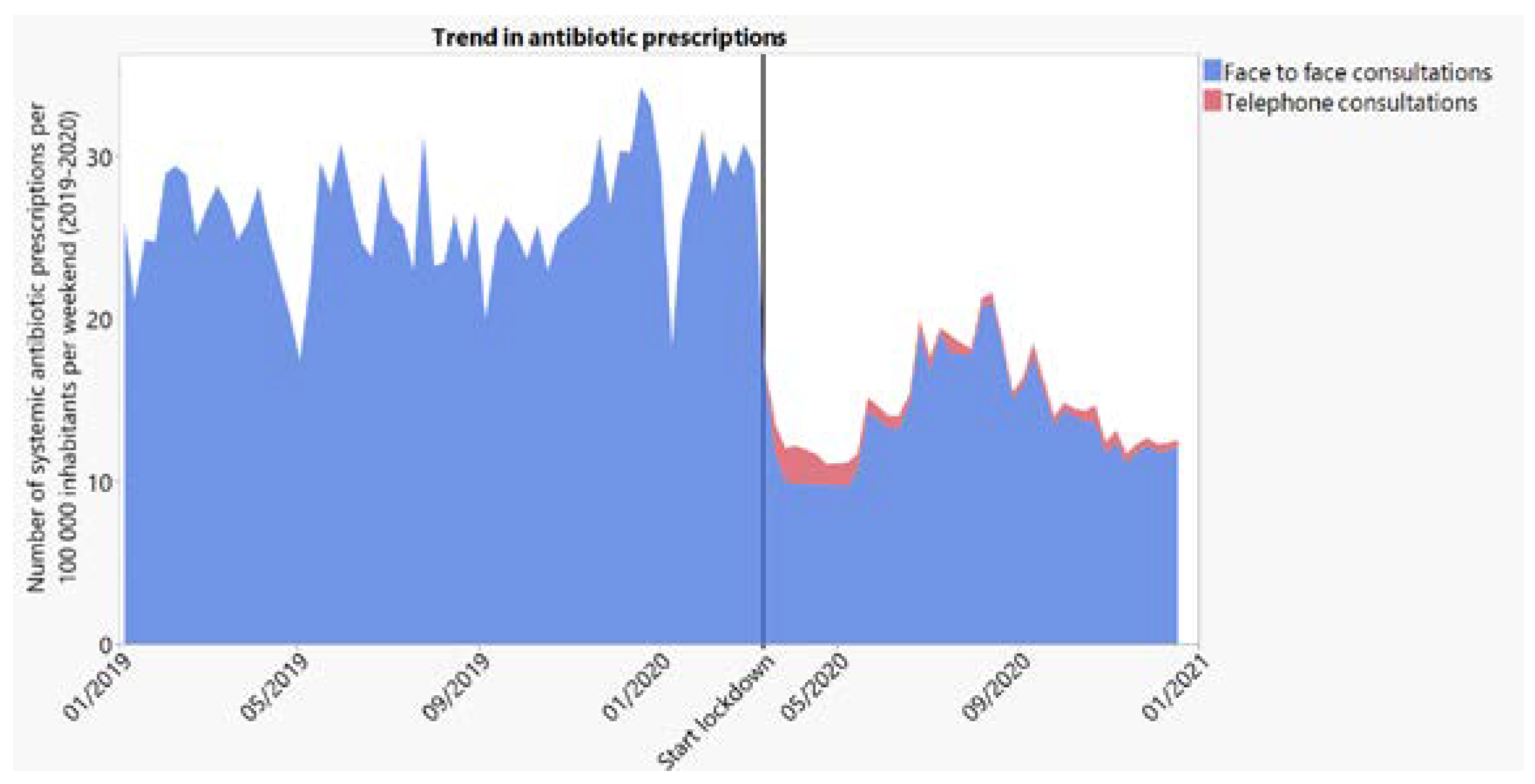
2.2.1. General Trend in Antibiotic Prescribing
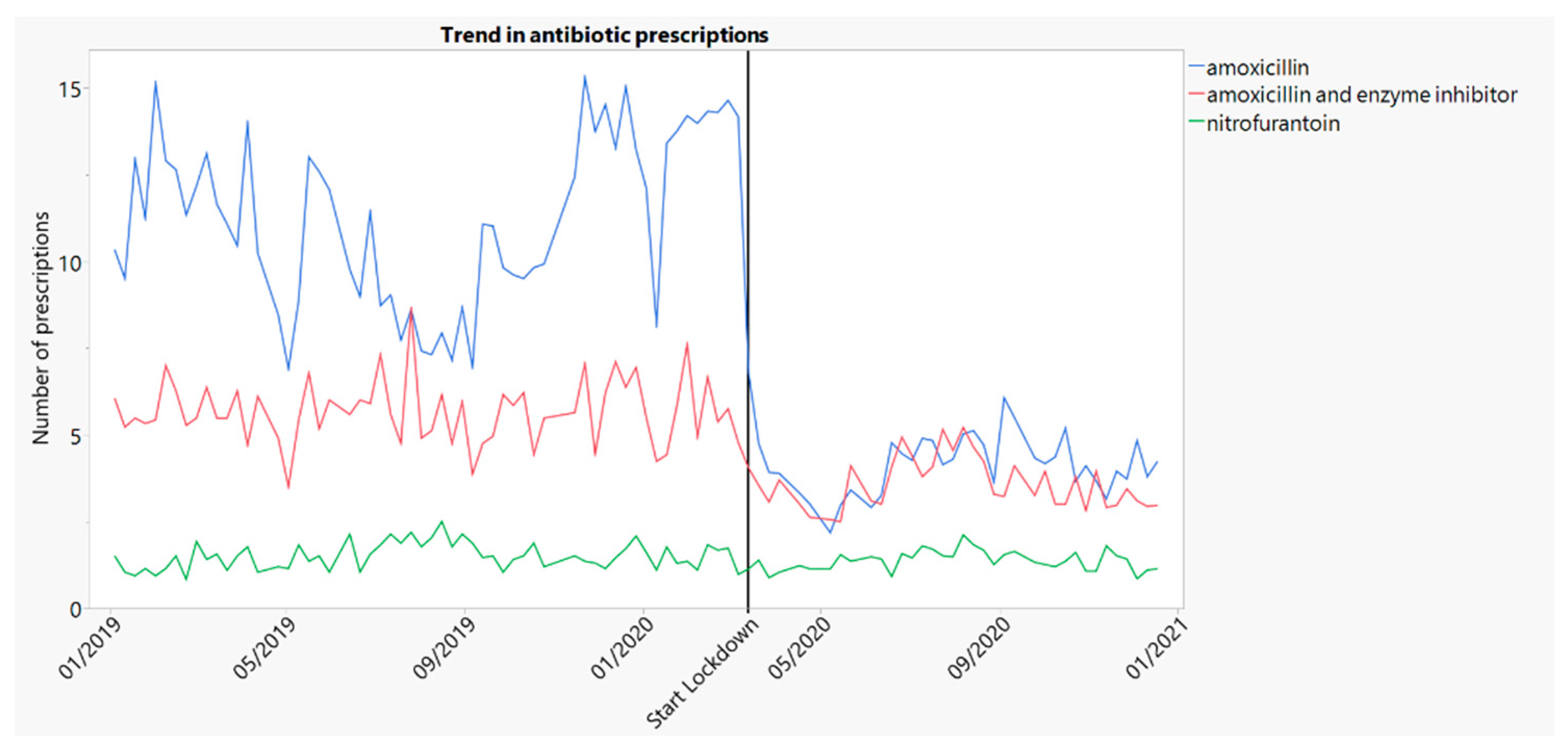
2.2.2. Trends in Prescribing of Amoxicillin and Amoxicillin/Clavulanate
2.2.3. Trends in Prescribing of Nitrofurantoin
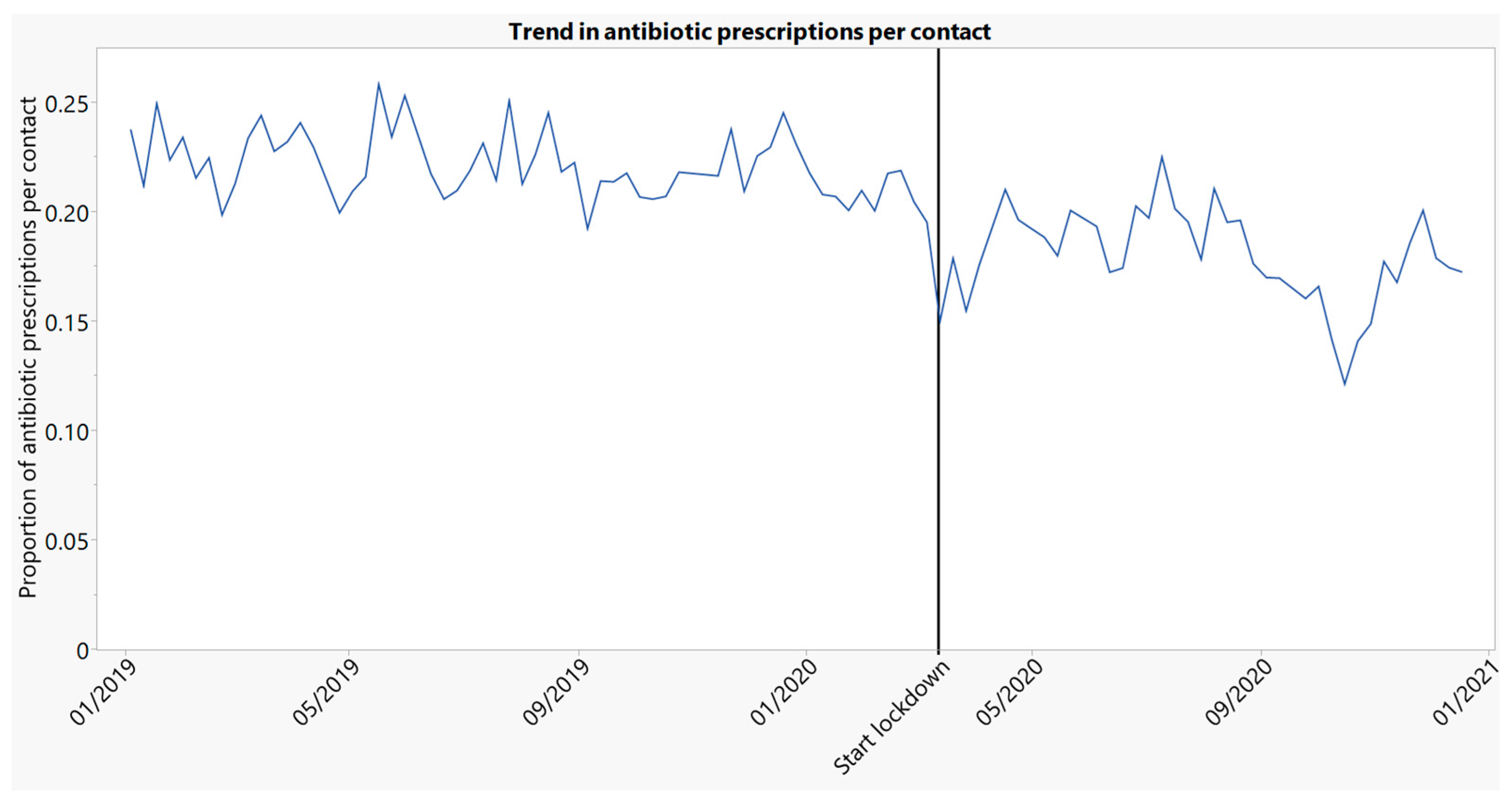
2.2.4. Trend in Antibiotic Prescribing per Contact Type (Face-to-Face and Face-to-Face + Telephone)
3. Discussion
Strengths and Limitations
4. Materials and Methods
4.1. Study Design and Setting
4.2. Data Sources
4.3. Studied Contacts
4.4. Variables
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verhoeven, V.; Tsakitzidis, G.; Philips, H.; Van Royen, P. Impact of the COVID-19 pandemic on the core functions of primary care: Will the cure be worse than the disease? A qualitative interview study in Flemish GPs. BMJ Open 2020, 10, e039674. [Google Scholar] [CrossRef]
- Wanat, M.; Hoste, M.; Gobat, N.; Anastasaki, M.; Bohmer, F.; Chlabicz, S.; Colliers, A.; Farrell, K.; Karkana, M.N.; Kinsman, J.; et al. Transformation of primary care during the COVID-19 pandemic: Experiences of healthcare professionals in eight European countries. Br. J. Gen. Pract. 2021, 71, e634–e642. [Google Scholar] [CrossRef]
- Krist, A.H.; DeVoe, J.E.; Cheng, A.; Ehrlich, T.; Jones, S.M. Redesigning primary care to address the COVID-19 pandemic in the midst of the pandemic. Ann. Fam. Med. 2020, 18, 349–354. [Google Scholar] [CrossRef] [PubMed]
- van der Velden, A.W.; Bax, E.A.; Bongard, E.; Aabenhus, R.M.; Anastasaki, M.; Anthierens, S.; Balan, A.; Böhmer, F.; Bruno, P.; Chlabicz, S. Primary care for patients with respiratory tract infection before and early on in the COVID-19 pandemic: An observational study in 16 European countries. BMJ Open 2021, 11, e049257. [Google Scholar] [CrossRef] [PubMed]
- van Olmen, J.; Remmen, R.; Van Royen, P.; Philips, H.; Verhoeven, V.; Anthierens, S. Regional coordination and bottom-up response of general practitioners in Belgium and the Netherlands. BMJ 2020, 369, m1377. [Google Scholar] [CrossRef] [PubMed]
- Morreel, S.; Philips, H.; Verhoeven, V. Organisation and characteristics of out-of-hours primary care during a COVID-19 outbreak: A real-time observational study. PLoS ONE 2020, 15, e0237629. [Google Scholar] [CrossRef]
- Huttner, B.; Catho, G.; Pano-Pardo, J.R.; Pulcini, C.; Schouten, J. COVID-19: Don’t neglect antimicrobial stewardship principles! Clin. Microbiol. Infect. 2020, 26, 808–810. [Google Scholar] [CrossRef]
- Colliers, A.; Adriaenssens, N.; Anthierens, S.; Bartholomeeusen, S.; Philips, H.; Remmen, R.; Coenen, S. Antibiotic Prescribing Quality in Out-of-Hours Primary Care and Critical Appraisal of Disease-Specific Quality Indicators. Antibiotics 2019, 8, 79. [Google Scholar] [CrossRef]
- Smits, M.; Colliers, A.; Jansen, T.; Remmen, R.; Bartholomeeusen, S.; Verheij, R. Examining differences in out-of-hours primary care use in Belgium and the Netherlands: A cross-sectional study. Eur. J. Public Health 2019, 29, 1018–1024. [Google Scholar] [CrossRef] [PubMed]
- Colliers, A.; Coenen, S.; Remmen, R.; Philips, H.; Anthierens, S. How do general practitioners and pharmacists experience antibiotic use in out-of-hours primary care? An exploratory qualitative interview study to inform a participatory action research project. BMJ Open 2018, 8, e023154. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.; Halls, A.; Tonkin-Crine, S.; Moore, M.; Latter, S.; Little, P.; Eyles, C.; Postle, K.; Leydon, G. General practitioner and nurse prescriber experiences of prescribing antibiotics for respiratory tract infections in UK primary care out-of-hours services (the UNITE study). J. Antimicrob. Chemother. 2018, 73, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Gautret, P.; Lagier, J.C.; Parola, P.; Hoang, V.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and azithromycin as a treatment of COVID-19: Results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef]
- Gbinigie, K.; Frie, K. Should azithromycin be used to treat COVID-19? A rapid review. BJGP Open 2020, 4, bjgpopen20X101094. [Google Scholar] [CrossRef] [PubMed]
- Adler, H.; Ball, R.; Fisher, M.; Mortimer, K.; Vardhan, M.S. Low rate of bacterial co-infection in patients with COVID-19. Lancet Microbe 2020, 1, e62. [Google Scholar] [CrossRef]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-infections: Potentially lethal and unexplored in COVID-19. Lancet Microbe 2020, 1, e11. [Google Scholar] [CrossRef]
- Down, B.; Kulkarni, S.; Khan, A.H.A.; Barker, B.; Tang, I. Novel coronavirus (COVID-19) infection: What a doctor on the frontline needs to know. Ann. Med. Surg. 2020, 55, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Phua, J.; Weng, L.; Ling, L.; Egi, M.; Lim, C.-M.; Divatia, J.V.; Shrestha, B.R.; Arabi, Y.M.; Ng, J.; Gomersall, C.D. Intensive care management of coronavirus disease 2019 (COVID-19): Challenges and recommendations. Lancet Respir. Med. 2020, 8, 506–517. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, L.; Yang, Y.; Peng, W.; Wang, W.; Chen, X. Therapeutic and triage strategies for 2019 novel coronavirus disease in fever clinics. Lancet Respir. Med. 2020, 8, e11–e12. [Google Scholar] [CrossRef]
- Clancy, C.J.; Buehrle, D.J.; Nguyen, M.H. PRO: The COVID-19 pandemic will result in increased antimicrobial resistance rates. JAC Antimicrob. Resist. 2020, 2, dlaa049. [Google Scholar] [CrossRef]
- Grau, S.; Echeverria-Esnal, D.; Gómez-Zorrilla, S.; Navarrete-Rouco, M.E.; Masclans, J.R.; Espona, M.; Gracia-Arnillas, M.P.; Duran, X.; Comas, M.; Horcajada, J.P. Evolution of antimicrobial consumption during the first wave of COVID-19 pandemic. Antibiotics 2021, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.D.; Fairfield, C.J.; Drake, T.M.; Turtle, L.; Seaton, R.A.; Wootton, D.G.; Sigfrid, L.; Harrison, E.M.; Docherty, A.B.; de Silva, T.I. Co-infections, secondary infections, and antimicrobial use in patients hospitalised with COVID-19 during the first pandemic wave from the ISARIC WHO CCP-UK study: A multicentre, prospective cohort study. Lancet Microbe 2021, 2, e354–e365. [Google Scholar] [CrossRef]
- Al-Azzam, S.; Mhaidat, N.M.; Banat, H.A.; Alfaour, M.; Ahmad, D.S.; Muller, A.; Al-Nuseirat, A.; Lattyak, E.A.; Conway, B.R.; Aldeyab, M.A. An Assessment of the Impact of Coronavirus Disease (COVID-19) Pandemic on National Antimicrobial Consumption in Jordan. Antibiotics 2021, 10, 690. [Google Scholar] [CrossRef]
- Butler, C.C.; Dorward, J.; Yu, L.M.; Gbinigie, O.; Hayward, G.; Saville, B.R.; Van Hecke, O.; Berry, N.; Detry, M.; Saunders, C.; et al. Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): A randomised, controlled, open-label, adaptive platform trial. Lancet 2021, 397, 1063–1074. [Google Scholar] [CrossRef]
- BAPCOC. COVID-19: BAPCOC: Geen Plaats Voor Antibiotica Bij Thuis Behandelde COVID-19 Patiënten; Bij Vermoeden Van Bacteriële Pneumonie: Amoxicilline of Amoxi-Clav. 2020. Available online: https://www.bcfi.be/nl/gows/3308 (accessed on 3 December 2021).
- Loogman, M.; de Jong, N.; Platteel, T.N.; Bouma, M.; Verheij, T.J.; Opstelten, W. Luchtwegklachten in tijden van corona. Ned. Tijdschr. Geneeskd 2020, 164, D4999. [Google Scholar] [PubMed]
- WHO. Clinical Management of Severe Acute Respiratory Infection (SARI) When COVID-19 Disease Is Suspected. Available online: https://apps.who.int/iris/bitstream/handle/10665/331446/WHO-2019-nCoV-clinical-2020.4-eng.pdf?sequence=1&isAllowed=y (accessed on 3 December 2021).
- WHO. Clinical Management of COVID-19—Interim Guidance. Available online: https://apps.who.int/iris/bitstream/handle/10665/332196/WHO-2019-nCoV-clinical-2020.5-eng.pdf (accessed on 3 December 2021).
- Nahler, G. Anatomical therapeutic chemical classification system (ATC). In Dictionary of Pharmaceutical Medicine; Springer: Cham, Switzerland, 2009; p. 8. [Google Scholar]
- Dunlop, C.; Howe, A.; Li, D.; Allen, L.N. The coronavirus outbreak: The central role of primary care in emergency preparedness and response. BJGP Open 2020, 4, bjgpopen20X101041. [Google Scholar] [CrossRef]
- Wanat, M.; Hoste, M.; Gobat, N.; Anastasaki, M.; Bohmer, F.; Chlabicz, S.; Colliers, A.; Farrell, K.; Karkana, M.N.; Kinsman, J.; et al. Supporting Primary Care Professionals to Stay in Work During the COVID-19 Pandemic: Views on Personal Risk and Access to Testing During the First Wave of Pandemic in Europe. Front. Med. 2021, 8, 726319. [Google Scholar] [CrossRef]
- Lazzerini, M.; Barbi, E.; Apicella, A.; Marchetti, F.; Cardinale, F.; Trobia, G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc. Health 2020, 4, e10–e11. [Google Scholar] [CrossRef]
- van Ballegooijen, H.; Goossens, L.; Bruin, R.H.; Michels, R.; Krol, M. Concerns, quality of life, access to care and productivity of the general population during the first 8 weeks of the coronavirus lockdown in Belgium and the Netherlands. BMC Health Serv. Res. 2021, 21, 227. [Google Scholar] [CrossRef] [PubMed]
- Moynihan, R.; Sanders, S.; Michaleff, Z.A.; Scott, A.M.; Clark, J.; To, E.J.; Jones, M.; Kitchener, E.; Fox, M.; Johansson, M. Impact of COVID-19 pandemic on utilisation of healthcare services: A systematic review. BMJ Open 2021, 11, e045343. [Google Scholar] [CrossRef]
- King, L.M.; Lovegrove, M.C.; Shehab, N.; Tsay, S.; Budnitz, D.S.; Geller, A.I.; Lind, J.N.; Roberts, R.M.; Hicks, L.A.; Kabbani, S. Trends in US outpatient antibiotic prescriptions during the coronavirus disease 2019 pandemic. Clin. Infect. Dis. 2021, 73, e652–e660. [Google Scholar] [CrossRef]
- Sigurdsson, E.L.; Blondal, A.B.; Jonsson, J.S.; Tomasdottir, M.O.; Hrafnkelsson, H.; Linnet, K.; Sigurdsson, J.A. How primary healthcare in Iceland swiftly changed its strategy in response to the COVID-19 pandemic. BMJ Open 2020, 10, e043151. [Google Scholar] [CrossRef]
- Hussain, A.Z.; Paudyal, V.; Hadi, M.A. Impact of the COVID-19 Pandemic on the Prescribing Patterns of First-Line Antibiotics in English Primary Care: A Longitudinal Analysis of National Prescribing Dataset. Antibiotics 2021, 10, 591. [Google Scholar] [CrossRef]
- Zhu, N.; Aylin, P.; Rawson, T.; Gilchrist, M.; Majeed, A.; Holmes, A. Investigating the impact of COVID-19 on primary care antibiotic prescribing in North West London across two epidemic waves. Clin. Microbiol. Infect. 2021, 27, 762–768. [Google Scholar] [CrossRef]
- Zhu, N.; McLeod, M.; McNulty, C.; Lecky, D.; Holmes, A.; Ahmad, R. Trends in Antibiotic Prescribing in Out-of-Hours Primary Care in England from January 2016 to June 2020 to Understand Behaviours during the First Wave of COVID-19. Antibiotics 2021, 10, 32. [Google Scholar] [CrossRef]
- Armitage, R.; Nellums, L.B. Antibiotic prescribing in general practice during COVID-19. Lancet Infect. Dis. 2021, 21, e144. [Google Scholar] [CrossRef]
- van de Pol, A.C.; Boeijen, J.A.; Venekamp, R.P.; Platteel, T.; Damoiseaux, R.; Kortekaas, M.F.; van der Velden, A.W. Impact of the COVID-19 Pandemic on Antibiotic Prescribing for Common Infections in The Netherlands: A Primary Care-Based Observational Cohort Study. Antibiotics 2021, 10, 196. [Google Scholar] [CrossRef] [PubMed]
- Getahun, H.; Smith, I.; Trivedi, K.; Paulin, S.; Balkhy, H.H. Tackling antimicrobial resistance in the COVID-19 pandemic. Bull. World Health Organ. 2020, 98, 442. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.M.; Moore, L.S.P.; Castro-Sanchez, E.; Charani, E.; Davies, F.; Satta, G.; Ellington, M.J.; Holmes, A.H. COVID-19 and the potential long-term impact on antimicrobial resistance. J. Antimicrob. Chemother. 2020, 75, 1681–1684. [Google Scholar] [CrossRef] [PubMed]
- Tomczyk, S.; Taylor, A.; Brown, A.; de Kraker, M.E.; El-Saed, A.; Alshamrani, M.; Hendriksen, R.S.; Jacob, M.; Löfmark, S.; Perovic, O. Impact of the COVID-19 pandemic on the surveillance, prevention and control of antimicrobial resistance: A global survey. J. Antimicrob. Chemother. 2021, 76, 3045–3058. [Google Scholar] [CrossRef] [PubMed]
- Bernal, J.L.; Cummins, S.; Gasparrini, A. Interrupted time series regression for the evaluation of public health interventions: A tutorial. Int. J. Epidemiol. 2017, 46, 348–355. [Google Scholar] [CrossRef]
- September 2020 Report of ‘lnstitut Pharmaco-Epidémiologique Belge’. Available online: https://docplayer.nl/202775660-Ipheb-report-september-2020-publication-geheel-van-de-terugbetaalde-geneesmiddelen.html (accessed on 3 December 2021).
- Colliers, A.; Bartholomeeusen, S.; Remmen, R.; Coenen, S.; Michiels, B.; Bastiaens, H.; Van Royen, P.; Verhoeven, V.; Holmgren, P.; De Ruyck, B.; et al. Improving Care and Research Electronic Data Trust Antwerp (iCAREdata): A research database of linked data on out-of-hours primary care. BMC Res. Notes 2016, 9, 259. [Google Scholar] [CrossRef][Green Version]
- Bartholomeeusen, S.; Philips, H.; Van Royen, P.; Remmen, R.; Coenen, S. iCAREdata: Improving Care and Research Electronic Data Trust Antwerp. Zenodo 2017. [Google Scholar] [CrossRef]
- WONCA. ICPC-2-R: International Classification of Primary Care; Oxford University Press: Oxford, UK, 2005. [Google Scholar]
- Schaffer, A.L.; Dobbins, T.A.; Pearson, S.-A. Interrupted time series analysis using autoregressive integrated moving average (ARIMA) models: A guide for evaluating large-scale health interventions. BMC Med. Res. Methodol. 2021, 21, 58. [Google Scholar] [CrossRef] [PubMed]
- Hyndman, R.J.; Khandakar, Y. Automatic time series forecasting: The forecast package for R. J. Stat. Softw. 2008, 27, 1–22. [Google Scholar] [CrossRef]
| Before Lockdown | After Lockdown | |
|---|---|---|
| Number of contacts per weekend, per 100,000 population (SD) | ||
| - All | 121 (15) | 160 (39) |
| - Face-to-face | 120 (15) | 85 (17) |
| - Telephone | 1 (0) | 75 (29) |
| Number of antibiotic prescriptions per weekend, per 100,000 population (SD) | ||
| - All (J01) | 26 (3) | 15 (3) |
| - Amoxicillin (J01CA04) | 11 (2) | 4 (1) |
| - Amoxicillin/clavulanate (J01CR02) | 6 (1) | 4 (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colliers, A.; De Man, J.; Adriaenssens, N.; Verhoeven, V.; Anthierens, S.; De Loof, H.; Philips, H.; Coenen, S.; Morreel, S. Antibiotic Prescribing Trends in Belgian Out-of-Hours Primary Care during the COVID-19 Pandemic: Observational Study Using Routinely Collected Health Data. Antibiotics 2021, 10, 1488. https://doi.org/10.3390/antibiotics10121488
Colliers A, De Man J, Adriaenssens N, Verhoeven V, Anthierens S, De Loof H, Philips H, Coenen S, Morreel S. Antibiotic Prescribing Trends in Belgian Out-of-Hours Primary Care during the COVID-19 Pandemic: Observational Study Using Routinely Collected Health Data. Antibiotics. 2021; 10(12):1488. https://doi.org/10.3390/antibiotics10121488
Chicago/Turabian StyleColliers, Annelies, Jeroen De Man, Niels Adriaenssens, Veronique Verhoeven, Sibyl Anthierens, Hans De Loof, Hilde Philips, Samuel Coenen, and Stefan Morreel. 2021. "Antibiotic Prescribing Trends in Belgian Out-of-Hours Primary Care during the COVID-19 Pandemic: Observational Study Using Routinely Collected Health Data" Antibiotics 10, no. 12: 1488. https://doi.org/10.3390/antibiotics10121488
APA StyleColliers, A., De Man, J., Adriaenssens, N., Verhoeven, V., Anthierens, S., De Loof, H., Philips, H., Coenen, S., & Morreel, S. (2021). Antibiotic Prescribing Trends in Belgian Out-of-Hours Primary Care during the COVID-19 Pandemic: Observational Study Using Routinely Collected Health Data. Antibiotics, 10(12), 1488. https://doi.org/10.3390/antibiotics10121488








